Anterior Mandibular Displacement in Growing Rats Enhances Growth—A 3D Analysis
Abstract
1. Introduction
1.1. Clinical Problem and Importance
1.2. Mechanistic Backround and Current Interventions
1.3. Research Gaps
2. Materials and Methods
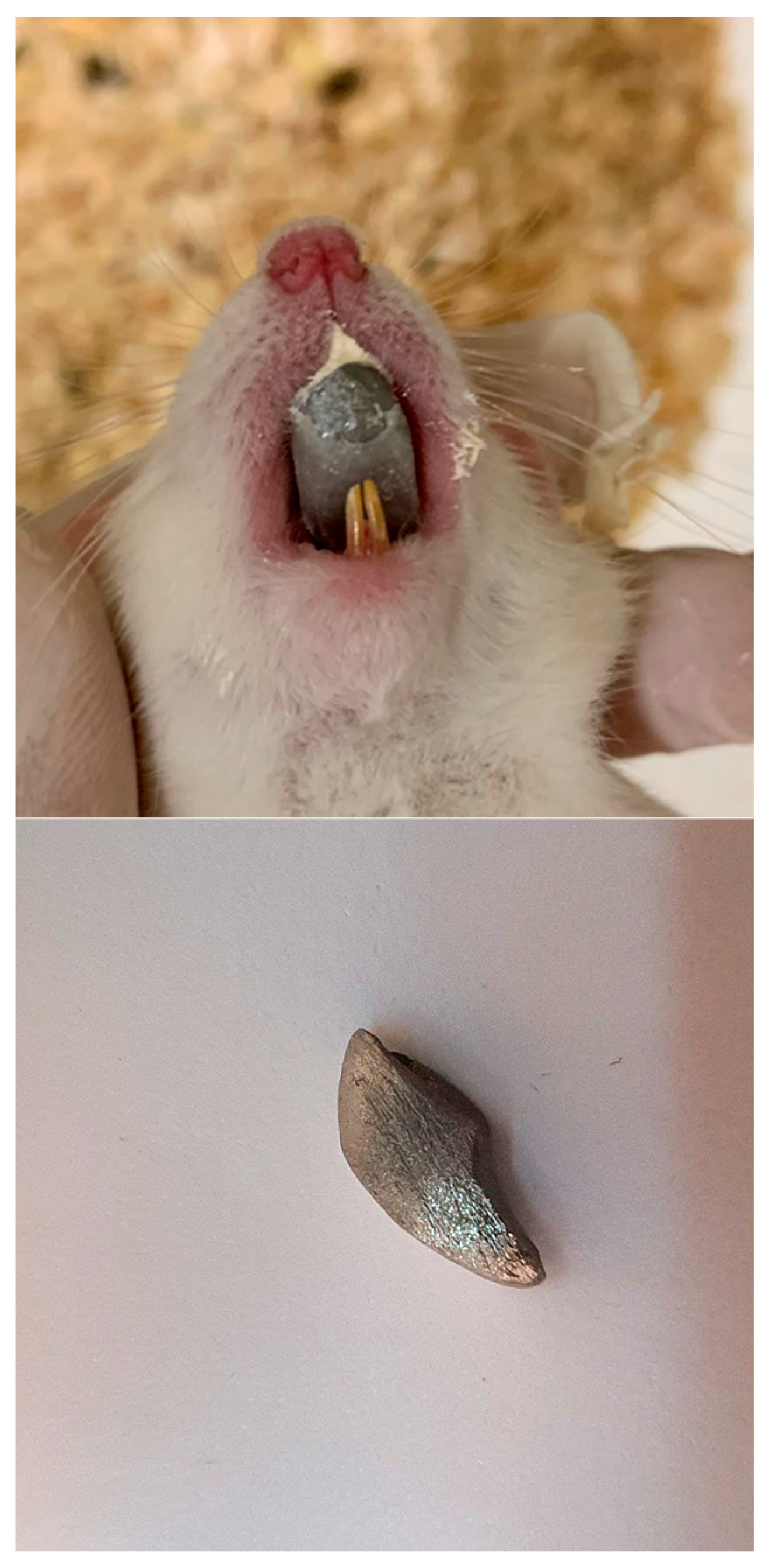
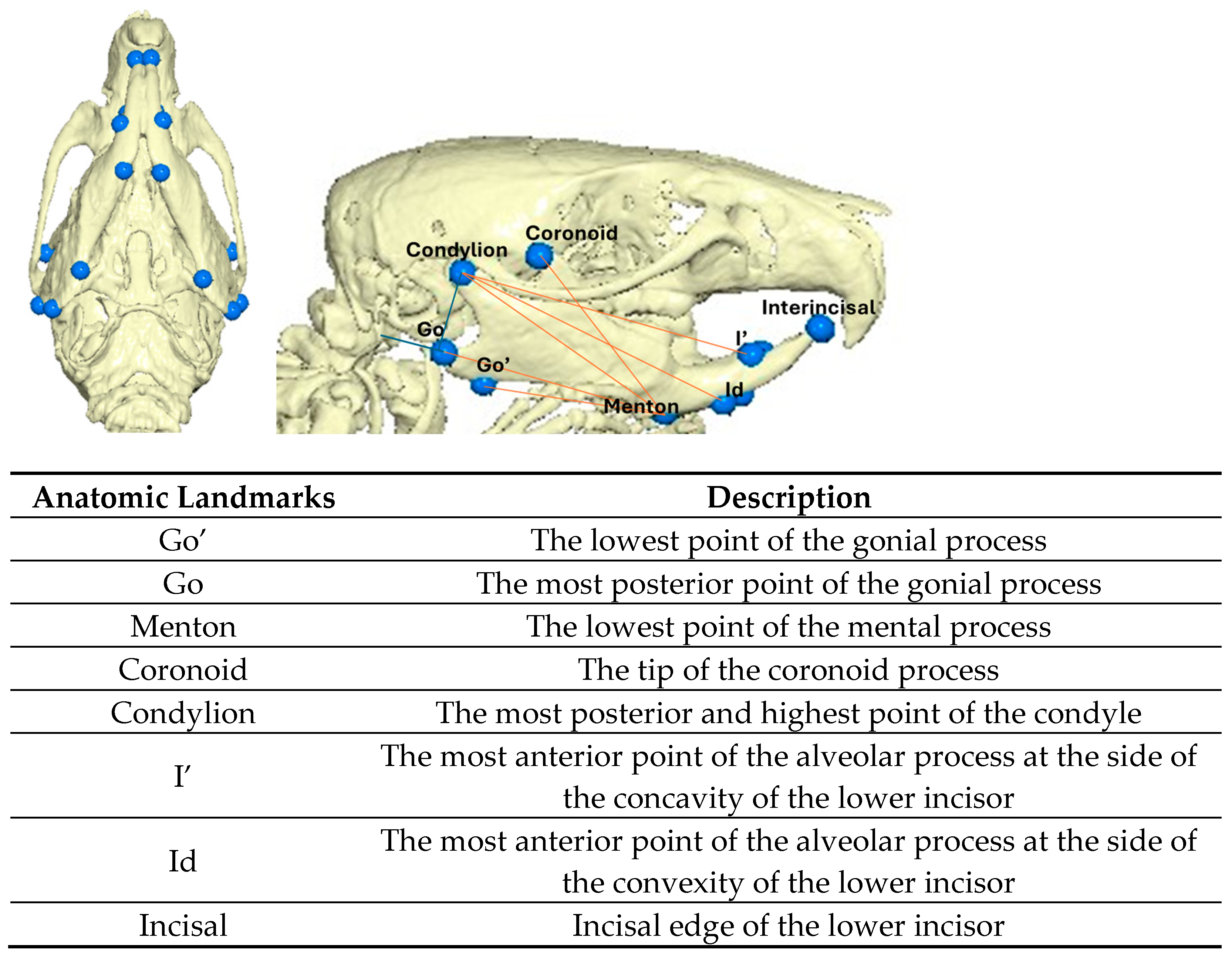
2.1. Experimental Design
2.2. Statistical Analysis
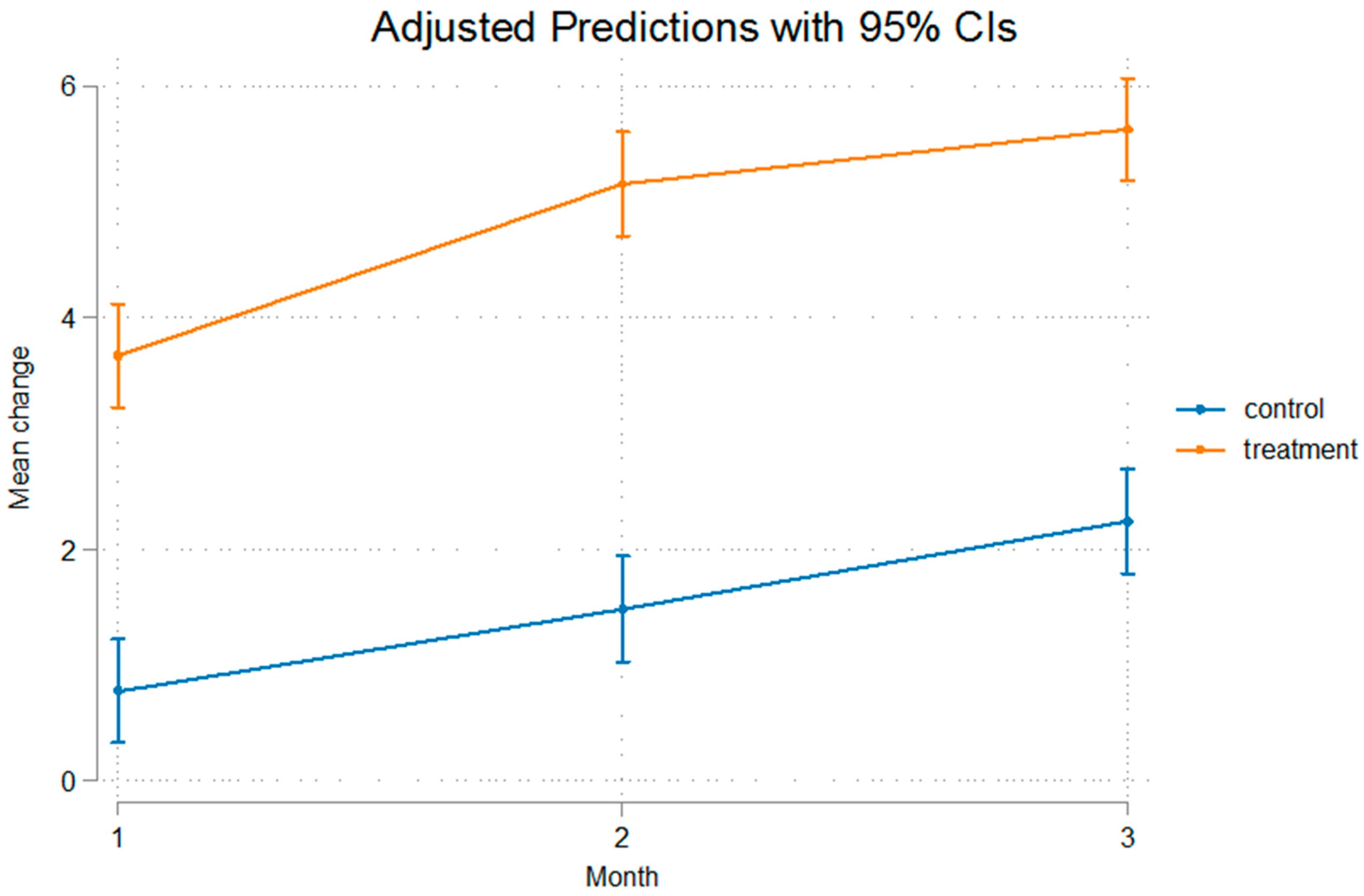
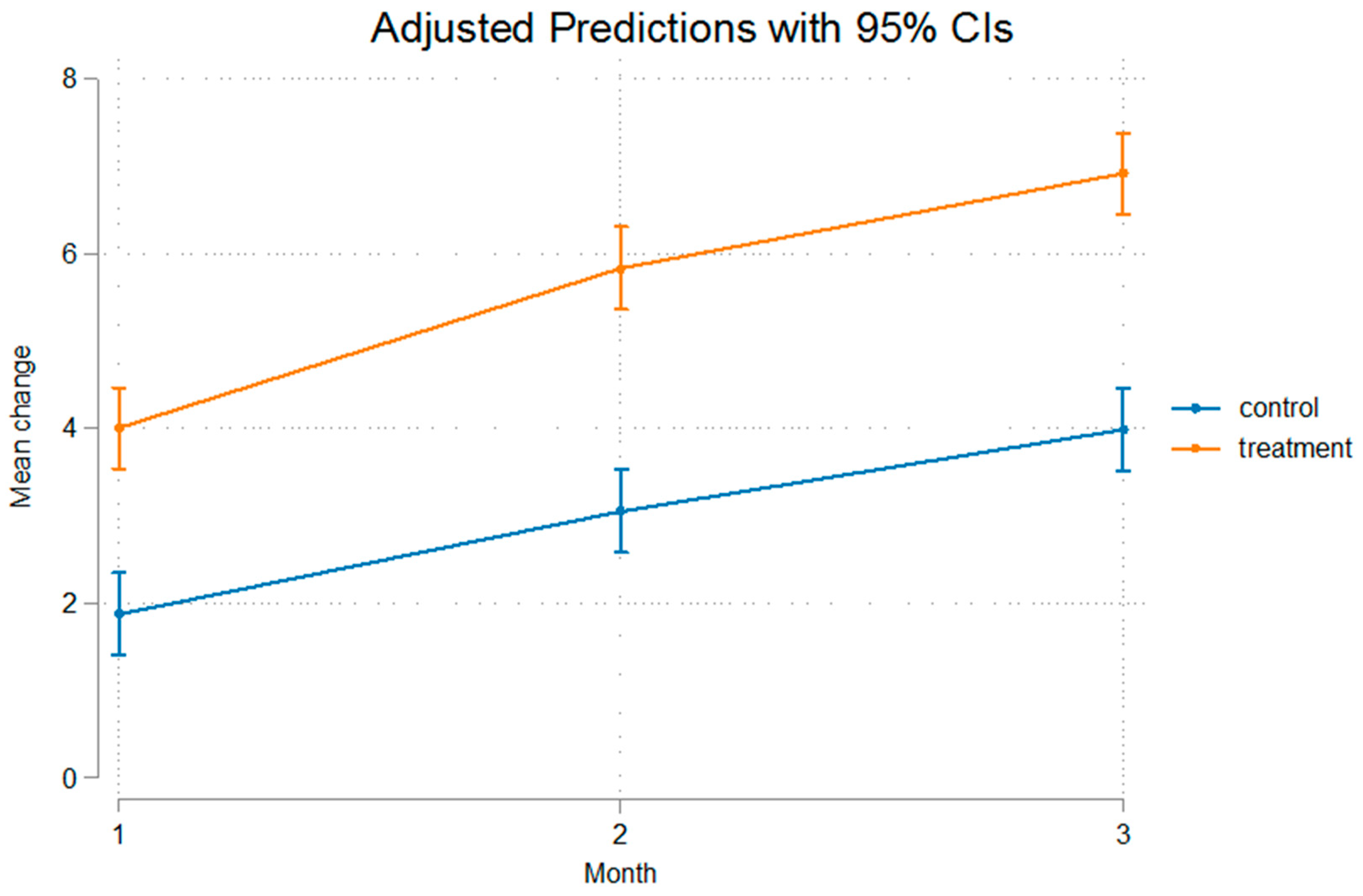
3. Results
4. Discussion
4.1. Comparison with Existing Literature
4.2. Mechanisms of Mandibular Growth
4.3. Methodological Considerations
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sassouni, V. A classification of skeletal facial types. Am. J. Orthod. 1969, 55, 109–123. [Google Scholar] [CrossRef]
- Tulloch, J.F.C.; Medland, W.; Tuncay, O.C. Methods used to evaluate growth modification in Class II malocclusion. Am. J. Orthod. Dentofac. Orthop. 1990, 98, 340–347. [Google Scholar] [CrossRef]
- Stahl, F.; Baccetti, T.; Franchi, L.; McNamara, J.A. Longitudinal growth changes in untreated subjects with Class II Division I malocclusion. Am. J. Orthod. Dentofac. Orthop. 2008, 134, 125–137. [Google Scholar] [CrossRef] [PubMed]
- DeVincenzo, J.P. Changes in mandibular length before, during, and after successful orthopedic correction of Class II malocclusions, using a functional appliance. Am. J. Orthod. Dentofac. Orthop. 1991, 99, 241–257. [Google Scholar] [CrossRef]
- Tulloch, J.F.C.; Proffit, W.R.; Phillips, C. Outcomes in a 2-phase randomized clinical trial of early class II treatment. Am. J. Orthod. Dentofac. Orthop. 2004, 125, 657–667. [Google Scholar] [CrossRef]
- Patel, H.P.; Moseley, H.C.; Noar, J.H. Cephalometric determinants of successful functional appliance therapy. Angle Orthod. 2002, 72, 410–417. [Google Scholar] [CrossRef]
- Panigrahi, P.; Vineeth, V. Biomechanical effects of fixed functional appliance on craniofacial structures. Angle Orthod. 2009, 79, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Otwad, P.; Potres, Z.; Shen, G.; Petocz, P.; Darendeliler, M.A. A histochemical study on condylar cartilage and glenoid fossa during mandibular advancement. Angle Orthod. 2011, 81, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Rabie, A.B.; She, T.T.; Hagg, U. Functional appliance therapy accelerates and enhances condylar growth. Am. J. Orthod. Dentofac. Orthop. 2003, 123, 40–88. [Google Scholar] [CrossRef]
- Rabie, A.B.; Xiong, H.; Hagg, U. Forward mandibular positioning enhances condylar adaptation in adult rats. Eur. J. Orthod. 2004, 26, 353–358. [Google Scholar] [CrossRef]
- Shen, G.; Rabie, A.B.; Zhao, Z.H.; Kaluarachchi, K. Forward deviation of the mandibular condyle enhances endochondral ossification of condylar cartilage indicated by increased expression of type X collagen. Arch. Oral. Biol. 2006, 51, 315–324. [Google Scholar] [CrossRef]
- Fuentes, M.A.; Opperman, L.A.; Buschang, P.; Bellinger, L.L.; Carlson, D.S.; Hinton, R.J. Lateral functional shift of the mandible: Part II. Effects on gene expression in condylar cartilage. Am. J. Orthod. Dentofac. Orthop. 2003, 123, 160–166. [Google Scholar] [CrossRef]
- Woodside, D.G.; Metaxas, A.; Altuna, G. The influence of functional appliance therapy on glenoid fossa remodeling. Am. J. Orthod. Dentofac. Orthop. 1987, 92, 181–198. [Google Scholar] [CrossRef]
- Spyropoulos, M.N.; Tsolakis, A.I.; Alexandridis, C.; Katsavrias, E.; Dontas, I. Role of suprahyoid musculature on mandibular morphology and growth orientation in rats. Am. J. Orthod. Dentofac. Orthop. 2002, 122, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Meikle, M.C. Remodeling the dentofacial skeleton: The biological basis of orthodontics and dentofacial orthopedics. J. Dent. Res. 2007, 86, 12–24. [Google Scholar] [CrossRef]
- Lyros, I.; Tsolakis, I.A.; Kotantoula, G.; Tosios, K.; George, V.; Nikitakis, N.; Ferdianakis, E.; Lykogeorgos, T.; Fora, E.; Tsolakis, A.I. Immunohistochemical Evaluation of Bone Remodeling Following Compressive Force on Mandibular Condyle. Biology 2025, 14, 228. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. A 2003 update of bone physiology and Wolff’s Law for clinicians. Angle Orthod. 2004, 74, 3–15. [Google Scholar] [PubMed]
- Matyas, J.; Edwards, P.; Miniaci, A.; Shrive, N.; Wilson, J.; Bray, R.; Frank, C. Ligament tension affects nuclear shape in situ: An in vitro study. Connect. Tissue Res. 1994, 31, 45–53. [Google Scholar] [CrossRef]
- Sugawara, Y.; Ando, R.; Kamioka, H.; Ishihara, Y.; Murshid, S.A.; Hashimoto, K.; Kataoka, N.; Tsujioka, K.; Kajiya, F.; Yamashiro, T.; et al. The alteration of a mechanical property of bone cells during the process of changing from osteoblasts to osteocytes. Bone 2008, 43, 19–24. [Google Scholar] [CrossRef]
- Lyros, I.; Perrea, D.; Tosios, K.; Nikitakis, N.; Tsolakis, I.A.; Ferdianakis, E.; Fora, E.; Lykogeorgos, T.; Maroulakos, M.P.; Vardas, E.; et al. Histological and Biochemical Analysis after Posterior Mandibular Displacement in Rats. Vet. Sci. 2022, 9, 625. [Google Scholar] [CrossRef]
- Shen, G.; Hagg, U.; Darendeliler, M.A. Skeletal effects of bite jumping therapy on the mandible- removable vs. fixed functional appliances. Orthod. Craniofacial Res. 2005, 8, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, D.G.; Santos, M.; Mendes, S.M.; Normando, D. Mandibular propulsion appliance for adults with Class II malocclusion: A systematic review and meta-analysis. Eur. J. Orthod. 2020, 42, 163–173. [Google Scholar] [CrossRef]
- Lyros, I.; Makrygiannakis, M.A.; Lykogeorgos, T.; Ferdianakis, E.; Tsolakis, A.I. Posterior Mandibular Displacement—A Systematic Review Based on Animal Studies. Animals 2021, 11, 823. [Google Scholar] [CrossRef] [PubMed]
- Lyros, I.; Ferdianakis, E.; Halazonetis, D.; Lykogeorgos, T.; Alexiou, A.; Alexiou, K.-E.; Georgaki, M.; Vardas, E.; Yfanti, Z.; Tsolakis, A.I. Three-Dimensional Analysis of Posterior Mandibular Displacement in Rats. Vet. Sci. 2022, 9, 144. [Google Scholar] [CrossRef]
- Rygh, P.; Moyers, R.E. Force systems and tissue responses to forces in orthodontics and facial orthopedics. In Handbook of Orthodontics, 4th ed.; Moyers, R.E., Ed.; Year Book Medical Publishers: Chicago, IL, USA, 1988; pp. 306–331. [Google Scholar]
- McNamara, J.A.; Carlson, D.S. Quantitative analysis of temporomandibular joint adaptations to protrusive function. Am. J. Orthod. 1979, 76, 593–611. [Google Scholar] [CrossRef]
- Charlier, J.P.; Petrovic, A.; Stutzmann, J.H. Effects of mandibular hyperpropulsion on the prechondroblastic zone of young rat condyle. Am. J. Orthod. 1969, 55, 71–74. [Google Scholar] [CrossRef]
- Kantomaa, T.; Tuominen, M.; Pirttiniemi, P. Effect of mechanical forces on chondrocyte maturation and differentiation in the mandibular condyle of the rat. J. Dent. Res. 1994, 73, 1150–1156. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Xie, M.J.; Yang, H.X.; Liu, X.; Ren, H.T.; Zhang, M.; Lu, L.; Liu, X.D.; Zhang, J.; Wang, M.Q. Catabolic changes of rat temporomandibular joint discs induced by unilateral anterior crossbite. J. Oral. Rhabil. 2019, 46, 340–348. [Google Scholar] [CrossRef]
- Wang, S.; Ye, L.; Li, M.; Zhan, H.; Ye, R.; Li, Y.; Zhao, Z. Effects of growth hormone and functional appliance on mandibular growth in an adolescent rat model. Angle Orthod. 2018, 88, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.E. Functional appliances: A mortgage on mandibular position. Aust. Orthod. J. 1996, 14, 154–157. [Google Scholar] [CrossRef]
- Pancherz, H. Treatment of Class II malocclusions by jumping the bite with the Herbst appliance: A cephalometric investigation. Am. J. Orthod. 1979, 76, 423–442. [Google Scholar] [CrossRef]
- Wieslander, L. Intensive treatment of severe Class II malocclusions with a headgear-Herbst appliance in the early mixed dentition. Am. J. Orthod. 1984, 86, 1–13. [Google Scholar] [CrossRef]
- McNamara, J.A., Jr.; Bookstein, F.L.; Shaughnessy, T.G. Skeletal and dental changes following functional regulator therapy on Class II patients. Am. J. Orthod. 1985, 88, 91–110. [Google Scholar] [CrossRef]
- Vargervik, K.; Harvold, E.P. Response to activator treatment in Class II malocclusions. Am. J. Orthod. 1985, 88, 242–251. [Google Scholar] [CrossRef]
- Paulsen, H.U.; Karle, A.; Bakke, M.; Herskind, A. CT-scanning and radiographic analysis of temporomandibular joints and cephalometric analysis in a case of Herbst treatment in late puberty. Eur. J. Orthod. 1995, 17, 165–175. [Google Scholar] [CrossRef]
- Ruf, S.; Pancherz, H. Temporomandibular joint remodeling in adolescents and young adults during Herbst treatment: A prospective longitudinal magnetic resonance imaging and cephalometric radiographic investigation. Am. J. Orthod. Dentofac. Orthop. 1999, 115, 607–618. [Google Scholar] [CrossRef]
- Tsolakis, A.I.; Spyropoulos, M.N. An appliance designed for experimental mandibular hyperpropulsion in rats. Eur. J. Orthod. 1997, 19, 1–7. [Google Scholar] [CrossRef]
- Tsolakis, A.I.; Spyropoulos, M.N.; Katsavrias, E.; Alexandridis, K. Effects of altered mandibular function on mandibular growth after condylectomy. Eur. J. Orthod. 1997, 19, 9–19. [Google Scholar] [CrossRef]
- Bollen, M.A.; Makinen, K.K.; Makinen, P.L.; Carlson, D.S. Collagenolytic and phosphatase activity in the rat mandible after functional protrusion. Archs Oral. Biol. 1989, 34, 267–273. [Google Scholar] [CrossRef]
- Hiiemae, K.A.; Ardan, G.M. A cinefluorographic study of mandibular movement during feeding in the rat (Rattus Norvegicus). J. Zool 1968, 154, 139–154. [Google Scholar] [CrossRef]
- Oksayan, R.; Sokucu, O.; Ucuncu, N. Effects of bite-jumping appliances on mandibular advancement in growing rats: A radiographic study. Eur. J. Dent. 2014, 8, 291–295. [Google Scholar] [CrossRef]
- Taira, K.; Iino, S.; Kubota, T.; Fukunaga, T.; Miyawaki, S. Effects of mandibular advancement plus prohibition of lower incisor movement on mandibular growth in rats. Angle Orthod. 2009, 79, 1095–1101. [Google Scholar] [CrossRef]
- Rabie, A.B.; Leung, F.Y.; Chayanupatkul, A.; Hägg, U. The correlation between neovascularization and bone formation in the condyle during forward mandibular positioning. Angle Orthod. 2002, 72, 431–438. [Google Scholar] [CrossRef]
- Hajjar, D.; Santos, M.F.; Kimura, E.T. Propulsive appliance stimulates the synthesis of insulin-like growth factors I and II in the mandibular condylar cartilage of young rats. Arch. Oral. Biol. 2003, 48, 635–642. [Google Scholar] [CrossRef]
- Marques, M.R.; Hajjar, D.; Crema, V.O.; Kimura, E.T.; Santos, M.F. A mandibular propulsive appliance modulates collagen-binding integrins distribution in the young rat condylar cartilage. Biorheology 2006, 43, 293–302. [Google Scholar] [CrossRef]
- Tang, G.H.; Rabie, A.B.; Hagg, U. Indian hedgehog: A mechanotransduction mediator in condylar cartilage. J. Dent. Res. 2004, 83, 434–438. [Google Scholar] [CrossRef]
- Smith, R.L.; Carter, D.R.; Schurman, D.J. Pressure and shear differentially alter human articular chondrocyte metabolism: A review. Clin. Orthop. Relat. Res. 2004, 427, S89–S95. [Google Scholar]
- Xiong, H.; Rabie, M.B.; Hagg, U. Neovascularization and mandibular condylar bone remodelling in adult rats under mechanical strain. Front. Biosci. 2005, 10, 74–82. [Google Scholar] [CrossRef]
- Hinton, R.J.; McNamara, J.A., Jr. Temporal bone adaptations in response to protrusive function in juvenile and young adult rhesus monkeys (Macaca mulatta). Eur. J. Orthod. 1984, 6, 155–174. [Google Scholar] [CrossRef]
- Rabie, A.B.; Al-Kalaly, A. Does the degree of advancement during functional appliance therapy matter? Eur. J. Orthod. 2008, 30, 274–282. [Google Scholar] [CrossRef]
- Adams, J.W. Correction of error in cephalometricroentograms. Angle Orthod. 1940, 10, 3–13. [Google Scholar]
- Baumrid, S.; Frantz, R.C. The reliability of head film measurements. 1. Landmark identification. Am. J. Orthod. 1971, 60, 111–127. [Google Scholar] [CrossRef]
- McWilliam, J.S.; Welander, U. The effect of image quality on the identification of cephalometric landmarks. Angle Orthod. 1978, 48, 49–56. [Google Scholar]
- Lin, L.I. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar] [CrossRef]
- Tsolakis, I.A.; Verikokos, C.; Perrea, D.; Bitsanis, E.; Tsolakis, A.I. Effects of diet consistency on mandibular growth. A review. J. Hell. Vet. Med. Soc. 2019, 70, 1603–1610. [Google Scholar] [CrossRef]
- Ferdianakis, E.; Lyros, I.; Tsolakis, I.A.; Alexiou, A.; Alexiou, K.; Tsolakis, A.I. Anterior Mandibular Displacement in Growing Rats-A Systematic Review. Animals 2022, 12, 2059. [Google Scholar] [CrossRef]
- Kotantoula, G.; Tsolakis, I.A.; Lyros, I.; Makrygiannakis, M.A.; Kanareli, C.; Dalampira, M.; Tsolakis, A.I. Effects on Facial Growth Following Masseter Muscle Resection in Growing Rats-A Systematic Review. Animals 2023, 13, 1680. [Google Scholar] [CrossRef]
- Karamani, I.I.; Tsolakis, I.A.; Makrygiannakis, M.A.; Georgaki, M.; Tsolakis, A.I. Impact of Diet Consistency on the Mandibular Morphology: A Systematic Review of Studies on Rat Models. Int. J. Environ. Res. Public Health 2022, 19, 2706. [Google Scholar] [CrossRef]
- Tagliaro, M.L.; Guimaraes, M.L.R.; Padilha, D.M.P.; Callegari-Jaques, S.M.; Jeckel-Neto, E.A. Mandibular advancement and morphological changes in the mandibles of female mice of different ages. Exp. Gerontol. 2006, 419110, 1157–1164. [Google Scholar] [CrossRef]
- Hoyte, D.A.; Enlow, D.H. Wolff’s law and the problem of muscle attachment on resorptive surfaces of bone. Am. J. Phys. Anthropol. 1966, 24, 205–213. [Google Scholar] [CrossRef]
- McNamara, J.A. Neuromuscular and skeletal adaptation to altered function in the orofacial region. Am. J. Orthod. 1973, 64, 578–606. [Google Scholar] [CrossRef]
- Avis, V. The relation of the temporal muscle to the form of the coronoid process. Am. J. Phys. Anthropol. 1959, 17, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.J. An experimental study of functional components of growth in the rat mandible. Acta Anat. 1973, 85, 378–385. [Google Scholar] [CrossRef]
- McNamara, J.A.; Hinton, J.R.; Hoffman, D.L. Histologic analysis of temporomandibular joint adaptation to protrusive function in young adult rhesus monkeys (makaka mulata). Am. J. Orthod. 1982, 82, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Luder, H.U. Effects of activator treatment- evidence for the occurrence of two different types of reaction. Eur. J. Orthod. 1981, 3, 205–222. [Google Scholar] [CrossRef][Green Version]
- Rabie, A.B.; Zhao, Z.; Shen, G.; Hagg, E.U.; Robinson, W. Osteogenesis in the glenoid fossa in response to mandibular advancement. Am. J. Orthod. Dentofac. Orthop. 2001, 119, 390–400. [Google Scholar] [CrossRef]
- Killiany, D.M. An Experimental Demonsration of Condylar Plasticity. Master’s Thesis, St Louis University, St. Louis, MO, USA, 1984. [Google Scholar]
- Yozwiak, R.A. An Experimental Investigation of the Mechanical Properties of Condylar Growth in Young Guinea Pigs. Master’s Thesis, St Louis University, St. Louis, MO, USA, 1979. [Google Scholar]
- Tsolakis, A.I. Effects of Posterior Mandibular Traction in the Rabbit: A Cephalometric, Histologic, Electromyographic Study. Master’s Thesis, Case Western Reserve University, Cleveland, OH, USA, 1981. [Google Scholar]
- Ghafari, J.; Degroote, C. Condylar cartilage response to continuous mandibular displacement in the rat. Angle Orthod. 1986, 56, 49–57. [Google Scholar] [PubMed]
- Justice, M.J.; Dhillon, P. Using the mouse to model human disease: Increasing validity and reproducibility. Dis. Model Mech. 2016, 9, 101–103. [Google Scholar] [CrossRef]
- Lyros, I.; Fora, E.; Damaskos, S.; Stanko, P.; Tsolakis, A. An incidental finding on a diagnostic CBCT: A case report. Aust. Orthod. J. 2014, 30, 67–71. [Google Scholar]
- Gribel, F.B.; Gribel, M.N.; Frazao, D.C.; McNamars, J.A., Jr.; Manzi, F.R. Accuracy and reliability of craniometric measurements on lateral cephalometry and 3D measurements on CBCT scans. Angle Orthod. 2011, 81, 26–35. [Google Scholar] [CrossRef]
- Sambaziotis, D. Comparison of Mandibular Linear Measurements and Evaluation of Technique Reliability Between 2D and 3D Imaging on Rats. Master’s Thesis, National and Kapodistrian University of Athens, Athens, Greece, 2020. [Google Scholar]
- Xiong, H.; Hägg, U.; Tang, G.H.; Rabie, A.B.; Robinson, W. The effect of continuous bite-jumping in adult rats: A morphological study. Angle Orthod. 2004, 74, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Tonge, E.A.; Heath, J.K.; Meikle, M.C. Anterior mandibular displacement and condylar growth. An experimental study in the rat. Am. J. Orthod. 1982, 82, 277–287. [Google Scholar] [CrossRef] [PubMed]

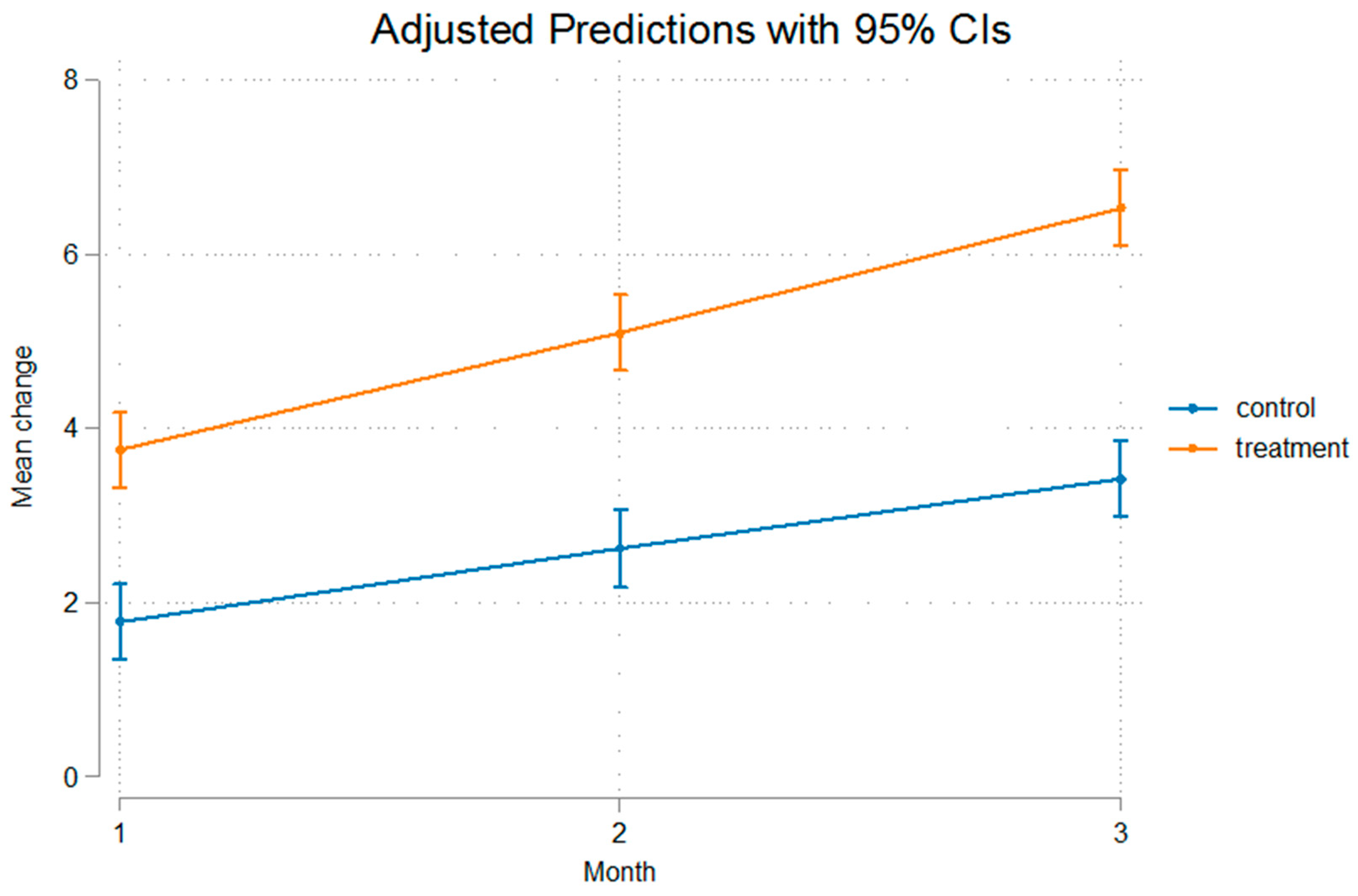
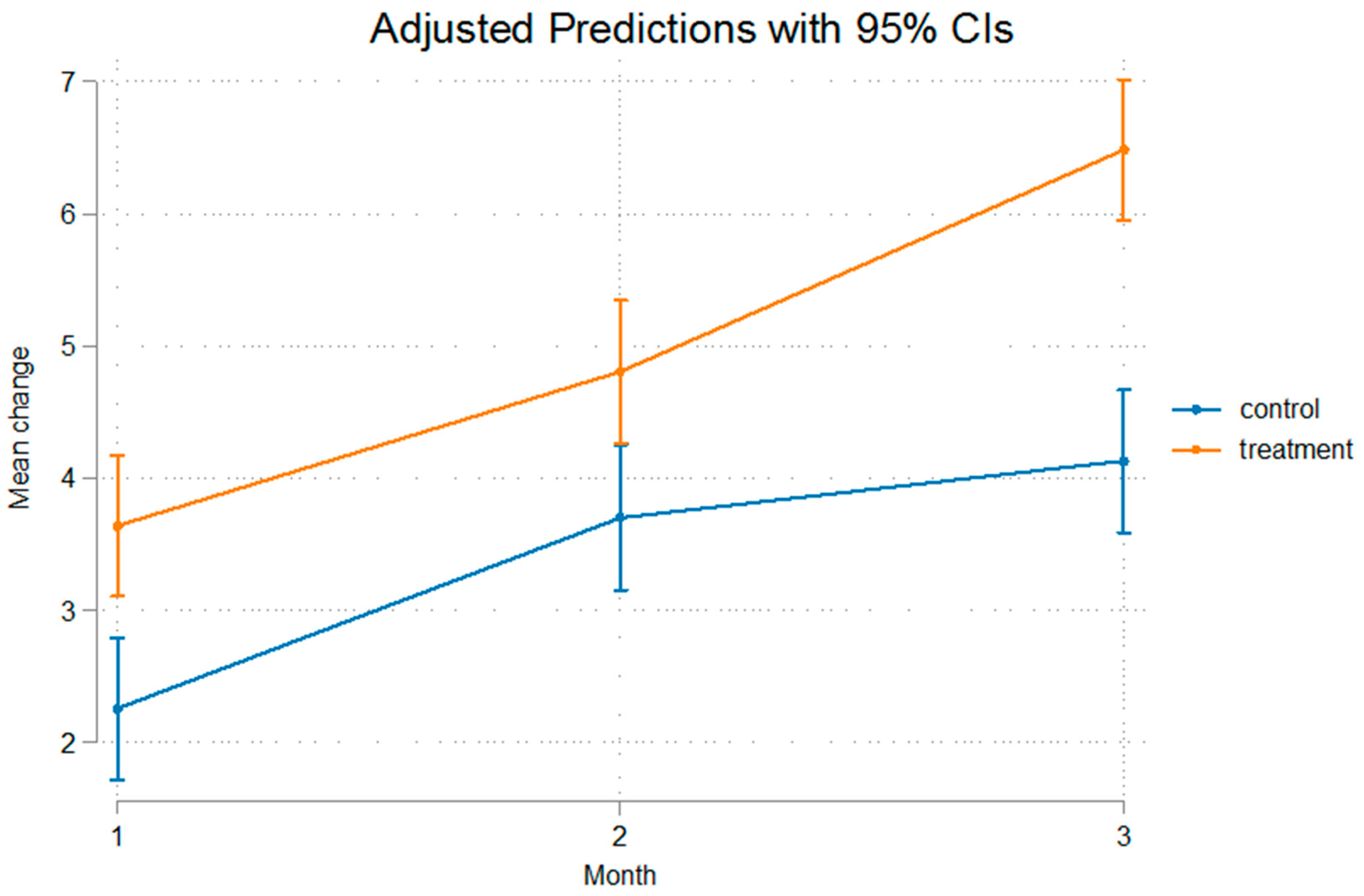
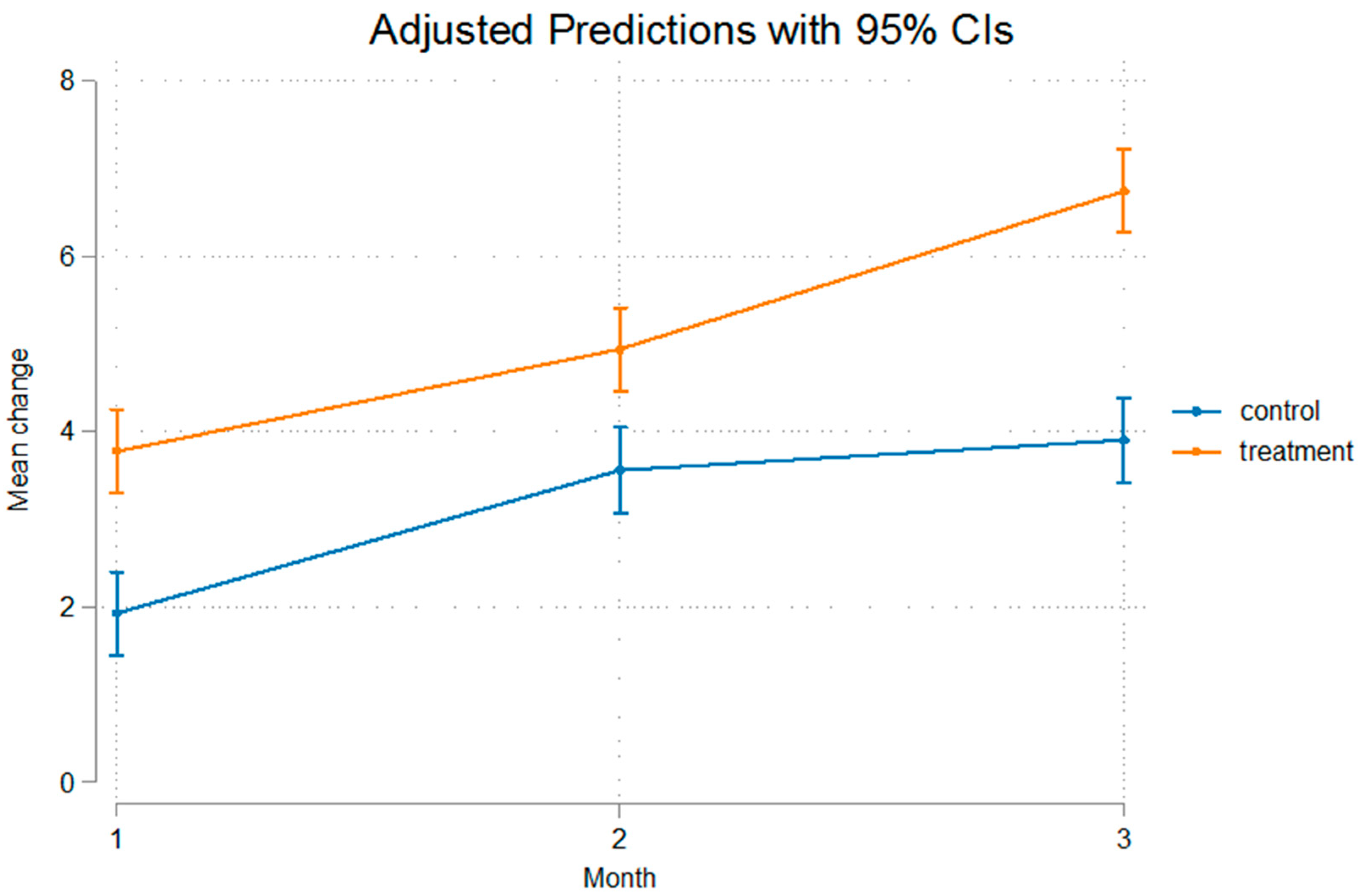
| Control Group | Mean (SD) | Mean (SD) | Mean (SD) | p-Value * |
|---|---|---|---|---|
| Subgroup | B1 | B2 | B3 | |
| Initial (mm) | ||||
| Weight (gr) | 109.42 (12.23) | 105.42 (22.12) | 121.67 (14.69) | 0.062 |
| Go’ Menton | 12.80 (0.56) | 13.04 (0.85) | 12.95 (0.67) | 0.698 |
| Go Menton | 16.51 (0.59) | 16.67 (0.73) | 16.75 (0.65) | 0.668 |
| Coronoid–Menton | 16.06 (0.56) | 16.07 (0.65) | 16.18 (0.55) | 0.850 |
| Condylion/Go-Menton | 8.02 (0.42) | 8.20 (0.39) | 8.43 (0.51) | 0.090 |
| Condylion–Menton | 18.27 (0.68) | 18.47 (0.71) | 18.61 (0.62) | 0.480 |
| Condylion-Id | 20.46 (0.74) | 20.36 (0.84) | 20.92 (0.85) | 0.213 |
| Condylion-I’ | 21.13 (0.85) | 21.04 (0.91) | 21.55 (0.63) | 0.277 |
| Final (mm) | ||||
| Weight (gr) | 282.25 (18.14) | 362.27 (35.06) | 430.17 (30.11) | <0.001 |
| Go’ Menton | 13.65 (0.47) | 14.65 (0.45) | 15.10 (0.74) | <0.001 |
| Go Menton | 18.44 (0.66) | 19.82 (0.59) | 20.67 (0.66) | <0.001 |
| Coronoid–Menton | 18.05 (0.52) | 19.06 (0.67) | 20.05 (0.53) | <0.001 |
| Condylion/Go-Menton | 9.25 (0.39) | 10.20 (0.58) | 10.36 (0.41) | <0.001 |
| Condylion–Menton | 20.13 (0.71) | 21.21 (0.74) | 21.95 (0.59) | <0.001 |
| Condylion-Id | 22.80 (0.72) | 24.21 (0.72) | 24.96 (0.83) | <0.001 |
| Condylion-I’ | 23.16 (0.68) | 24.78 (0.61) | 25.33 (0.53) | <0.001 |
| Difference (mm) | ||||
| Go’ Menton | 0.85 (0.70) | 1.62 (0.84) | 2.15 (0.84) | 0.001 |
| Go Menton | 1.93 (0.81) | 3.15 (0.89) | 3.93 (0.88) | <0.001 |
| Coronoid–Menton | 2.00 (0.68) | 2.99 (0.59) | 3.87 (0.77) | <0.001 |
| Condylion/Go-Menton | 1.23 (0.46) | 2.00 (0.64) | 1.93 (0.40) | 0.001 |
| Condylion–Menton | 1.85 (0.80) | 2.74 (0.71) | 3.35 (0.76) | <0.001 |
| Condylion-Id | 2.34 (0.87) | 3.85 (0.62) | 4.04 (1.25) | <0.001 |
| Condylion-I’ | 2.03 (1.03) | 3.74 (0.66) | 3.78 (0.86) | <0.001 |
| Treatment Group | Mean (SD) | Mean (SD) | Mean (SD) | |
|---|---|---|---|---|
| Subgroup Initial Measurements (mm) | A1 | A2 | A3 | p-Value * |
| Weight (gr) | 119.25 (13.71) | 121.75 (11.92) | 113.92 (14.90) | 0.363 |
| Go’ Menton | 11.04 (0.40) | 11.06 (0.72) | 11.36 (0.62) | 0.341 |
| Go Menton | 15.16 (0.42) | 15.20 (0.68) | 15.29 (0.67) | 0.876 |
| Coronoid–Menton | 15.05 (0.63) | 15.05 (0.50) | 15.11 (0.44) | 0.945 |
| Condylion/Go-Menton | 8.55 (0.42) | 8.55 (0.44) | 8.36 (0.40) | 0.442 |
| Condylion–Menton | 16.98 (0.53) | 17.00 (0.53) | 17.06 (0.57) | 0.929 |
| Condylion-Id | 19.45 (0.70) | 19.61 (0.56) | 19.55 (0.78) | 0.858 |
| Condylion-I’ | 19.76 (0.63) | 19.90 (0.49) | 19.74 (0.62) | 0.747 |
| Final measurements (mm) | ||||
| Weight (gr) | 182.25 (23.49) | 245.42 (36.08) | 315.92 (38.04) | <0.001 |
| Go’ Menton | 14.66 (0.54) | 16.12 (0.64) | 17.01 (0.90) | <0.001 |
| Go Menton | 19.13 (0.55) | 20.97 (0.69) | 22.22 (0.55) | <0.001 |
| Coronoid–Menton | 18.54 (0.48) | 20.00 (0.39) | 21.41 (0.49) | <0.001 |
| Condylion/Go-Menton | 9.32 (0.50) | 10.06 (0.44) | 10.70 (0.82) | <0.001 |
| Condylion–Menton | 20.69 (0.52) | 22.02 (0.55) | 23.61 (0.60) | <0.001 |
| Condylion-Id | 23.04 (0.41) | 24.32 (0.67) | 26.05 (0.87) | <0.001 |
| Condylion-I’ | 23.46 (0.53) | 24.72 (0.56) | 26.50 (0.89) | <0.001 |
| Difference (mm) | ||||
| Go’ Menton | 3.62 (0.69) | 5.07 (0.72) | 5.65 (0.92) | <0.001 |
| Go Menton | 3.97 (0.69) | 5.77 (0.62) | 6.93 (0.94) | <0.001 |
| Coronoid–Menton | 3.49 (0.61) | 4.95 (0.54) | 6.30 (0.66) | <0.001 |
| Condylion/Go-Menton | 0.77 (0.38) | 1.50 (0.45) | 2.34 (0.85) | <0.001 |
| Condylion–Menton | 3.70 (0.73) | 5.03 (0.63) | 6.55 (0.89) | <0.001 |
| Condylion-Id | 3.58 (0.72) | 4.71 (0.89) | 6.50 (1.12) | <0.001 |
| Condylion-I’ | 3.70 (0.73) | 4.82 (0.80) | 6.76 (1.01) | <0.001 |
| Mean Difference in mm | 95% CI * | p-Value * | |
|---|---|---|---|
| Go’ Menton | |||
| T 1 | 2.90 | (2.12, 3.69) | <0.001 |
| T 2 | 3.67 | (2.86, 4.48) | <0.001 |
| T 3 | 3.39 | (2.61, 4.17) | <0.001 |
| Go Menton | |||
| T 1 | 2.13 | (1.31, 2.95) | <0.001 |
| T 2 | 2.78 | (1.93, 3.62) | <0.001 |
| T 3 | 2.93 | (2.12, 3.75) | <0.001 |
| Coronoid–Menton | |||
| T 1 | 1.63 | (0.99, 2.26) | <0.001 |
| T 2 | 2.17 | (1.52, 2.82) | <0.001 |
| T 3 | 2.33 | (1.70, 2.96) | <0.001 |
| Condylion/Go-Menton | |||
| T 1 | −0.44 | (−1.01, 0.13) | 0.186 |
| T 2 | −0.47 | (−1.05, 0.12) | 0.166 |
| T 3 | 0.39 | (−0.17, 0.96) | 0.275 |
| Condylion–Menton | |||
| T 1 | 1.97 | (1.21, 2.73) | <0.001 |
| T 2 | 2.48 | (1.69, 3.26) | <0.001 |
| T 3 | 3.11 | (2.36, 3.87) | <0.001 |
| Condylion-Id | |||
| T 1 | 1.38 | (0.44, 2.32) | 0.002 |
| T 2 | 1.10 | (0.13, 2.07) | 0.020 |
| T 3 | 2.36 | (1.42, 3.29) | <0.001 |
| Condylion-I’ | |||
| T 1 | 1.85 | (1.01, 2.69) | <0.001 |
| T 2 | 1.37 | (0.51, 2.24) | 0.001 |
| T 3 | 2.84 | (2.01, 3.67) | <0.001 |
| Mean Difference (mm) | 95% CI * | p-Value * | |
|---|---|---|---|
| Go’ Menton | |||
| T 2 vs. 1, Β | 0.71 | (−0.10, 1.52) | 0.111 |
| T 3 vs. 1, Β | 1.47 | (0.64, 2.30) | <0.001 |
| T 3 vs. 2, Β | 0.76 | (−0.09, 1.60) | 0.097 |
| T 2 vs. 1, A | 1.48 | (0.67, 2.29) | <0.001 |
| T 3 vs. 1, A | 1.95 | (1.14, 2.76) | <0.001 |
| T 3 vs. 2, A | 0.47 | (−0.34, 1.29) | 0.565 |
| Go Menton | |||
| T 2 vs. 1, Β | 1.18 | (0.33, 2.02) | 0.003 |
| T 3 vs. 1, Β | 2.11 | (1.24, 2.98) | <0.001 |
| T 3 vs. 2, Β | 0.93 | (0.05, 1.82) | 0.034 |
| T 2 vs. 1, A | 1.82 | (0.98, 2.67) | <0.001 |
| T 3 vs. 1, A | 2.91 | (2.07, 3.76) | <0.001 |
| T 3 vs. 2, A | 1.09 | (0.24, 1.94) | 0.007 |
| Coronoid–Menton | |||
| T 2 vs. 1, Β | 0.94 | (0.29, 1.59) | 0.002 |
| T 3 vs. 1, Β | 2.03 | (1.37, 2.70) | <0.001 |
| T 3 vs. 2, Β | 1.09 | (0.41, 1.77) | <0.001 |
| T 2 vs. 1, A | 1.49 | (0.84, 2.14) | <0.001 |
| T 3 vs. 1, A | 2.74 | (2.09, 3.39) | <0.001 |
| T 3 vs. 2, A | 1.25 | (0.59, 1.90) | <0.001 |
| Condylion/Go-Menton | |||
| T 2 vs. 1, Β | 0.76 | (0.18, 1.35) | 0.006 |
| T 3 vs. 1, Β | 0.73 | (0.12, 1.33) | 0.011 |
| T 3 vs. 2, Β | −0.04 | (−0.65, 0.58) | >0.999 |
| T 2 vs. 1, A | 0.74 | (0.15, 1.32) | 0.008 |
| T 3 vs. 1, A | 1.56 | (0.97, 2.15) | <0.001 |
| T 3 vs. 2, A | 0.82 | (0.23, 1.42) | 0.003 |
| Condylion–Menton | |||
| T 2 vs. 1, Β | 0.84 | (0.06, 1.62) | 0.030 |
| T 3 vs. 1, Β | 1.64 | (0.83, 2.44) | <0.001 |
| T 3 vs. 2, Β | 0.79 | (−0.02, 1.61) | 0.060 |
| T 2 vs. 1, A | 1.35 | (0.57, 2.13) | <0.001 |
| T 3 vs. 1, A | 2.78 | (2.00, 3.56) | <0.001 |
| T 3 vs. 2, A | 1.43 | (0.64, 2.22) | <0.001 |
| Condylion-Id | |||
| T 2 vs. 1, Β | 1.45 | (0.48, 2.41) | 0.001 |
| T 3 vs. 1, Β | 1.87 | (0.88, 2.86) | <0.001 |
| T 3 vs. 2, Β | 0.43 | (−0.59, 1.44) | >0.999 |
| T 2 vs. 1, A | 1.17 | (0.20, 2.13) | 0.011 |
| T 3 vs. 1, A | 1.87 | (0.88, 2.86) | <0.001 |
| T 3 vs. 2, A | 1.68 | (0.70, 2.65) | <0.001 |
| Condylion-I’ | |||
| T 2 vs. 1, Β | 1.64 | (0.77, 2.50) | <0.001 |
| T 3 vs. 1, Β | 1.97 | (1.09, 2.86) | <0.001 |
| T 3 vs. 2, Β | 0.34 | (−0.57, 1.24) | >0.999 |
| T 2 vs. 1, A | 1.16 | (0.30, 2.02) | 0.004 |
| T 3 vs. 1, A | 2.96 | (2.10, 3.83) | <0.001 |
| T 3 vs. 2, A | 1.80 | (0.93, 2.67) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferdianakis, E.; Lyros, I.; Halazonetis, D.; Kanavakis, G.; Perlea, P.; Yfanti, Z.; Alexiou, K.-E.; Doukaki, D.; Tsolakis, A.I. Anterior Mandibular Displacement in Growing Rats Enhances Growth—A 3D Analysis. Bioengineering 2025, 12, 982. https://doi.org/10.3390/bioengineering12090982
Ferdianakis E, Lyros I, Halazonetis D, Kanavakis G, Perlea P, Yfanti Z, Alexiou K-E, Doukaki D, Tsolakis AI. Anterior Mandibular Displacement in Growing Rats Enhances Growth—A 3D Analysis. Bioengineering. 2025; 12(9):982. https://doi.org/10.3390/bioengineering12090982
Chicago/Turabian StyleFerdianakis, Efstratios, Ioannis Lyros, Demetrios Halazonetis, Georgios Kanavakis, Paula Perlea, Zafeiroula Yfanti, Konstantina-Eleni Alexiou, Dafni Doukaki, and Apostolos I. Tsolakis. 2025. "Anterior Mandibular Displacement in Growing Rats Enhances Growth—A 3D Analysis" Bioengineering 12, no. 9: 982. https://doi.org/10.3390/bioengineering12090982
APA StyleFerdianakis, E., Lyros, I., Halazonetis, D., Kanavakis, G., Perlea, P., Yfanti, Z., Alexiou, K.-E., Doukaki, D., & Tsolakis, A. I. (2025). Anterior Mandibular Displacement in Growing Rats Enhances Growth—A 3D Analysis. Bioengineering, 12(9), 982. https://doi.org/10.3390/bioengineering12090982







