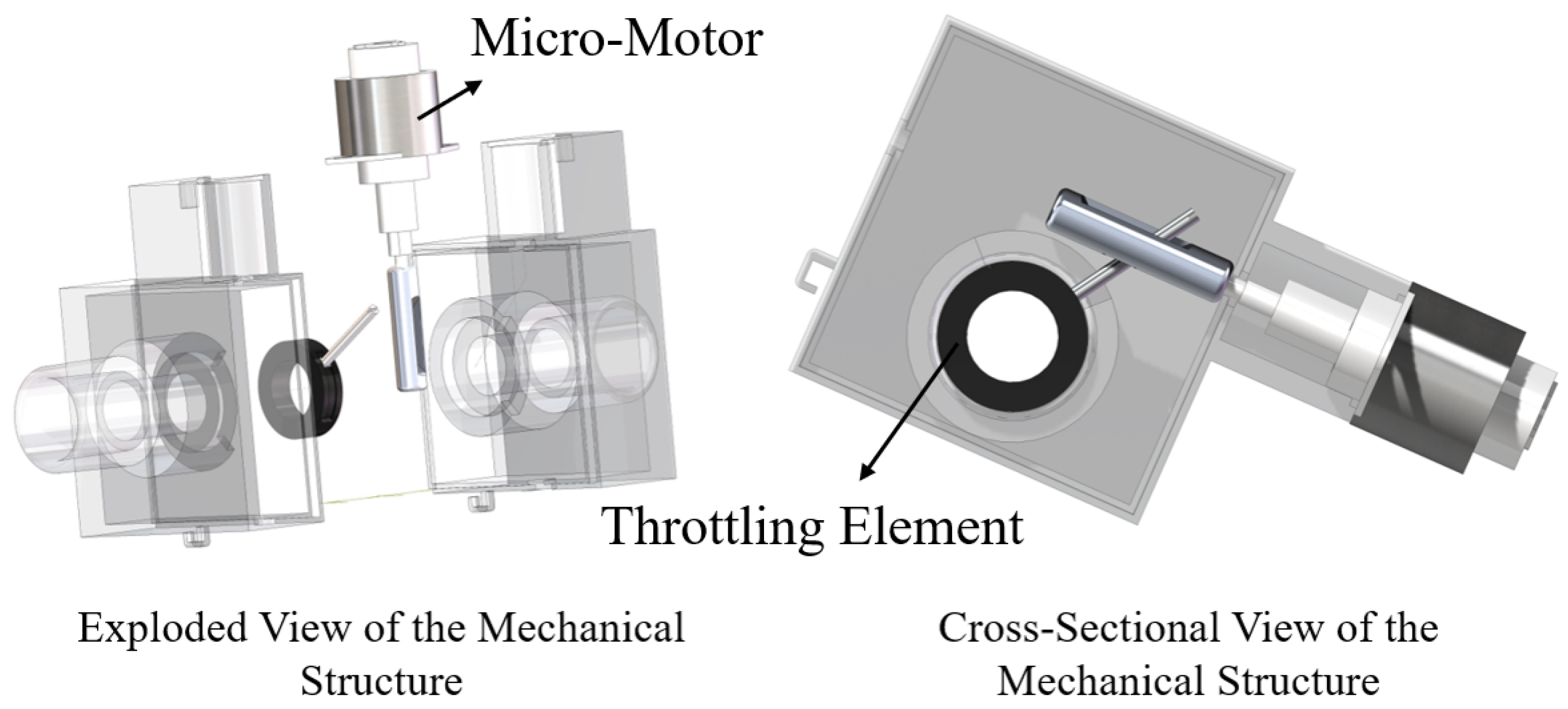
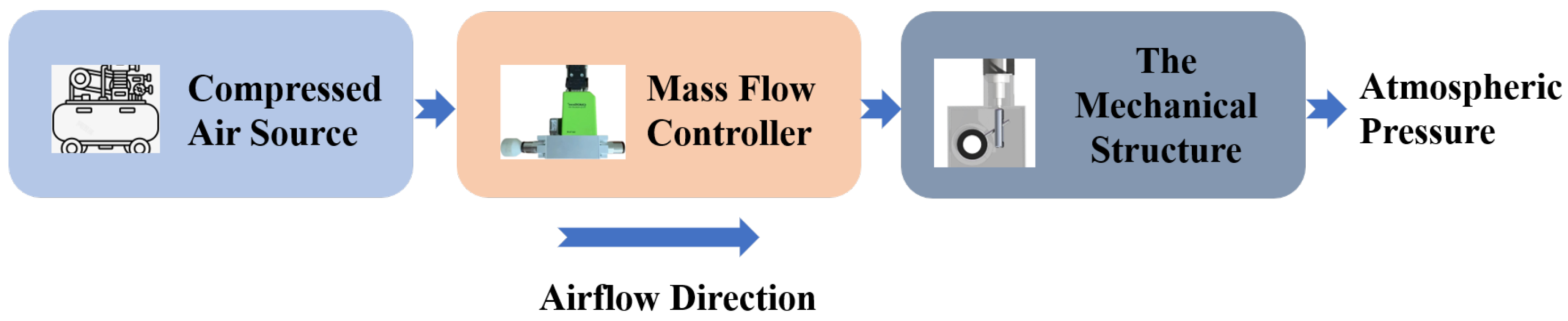
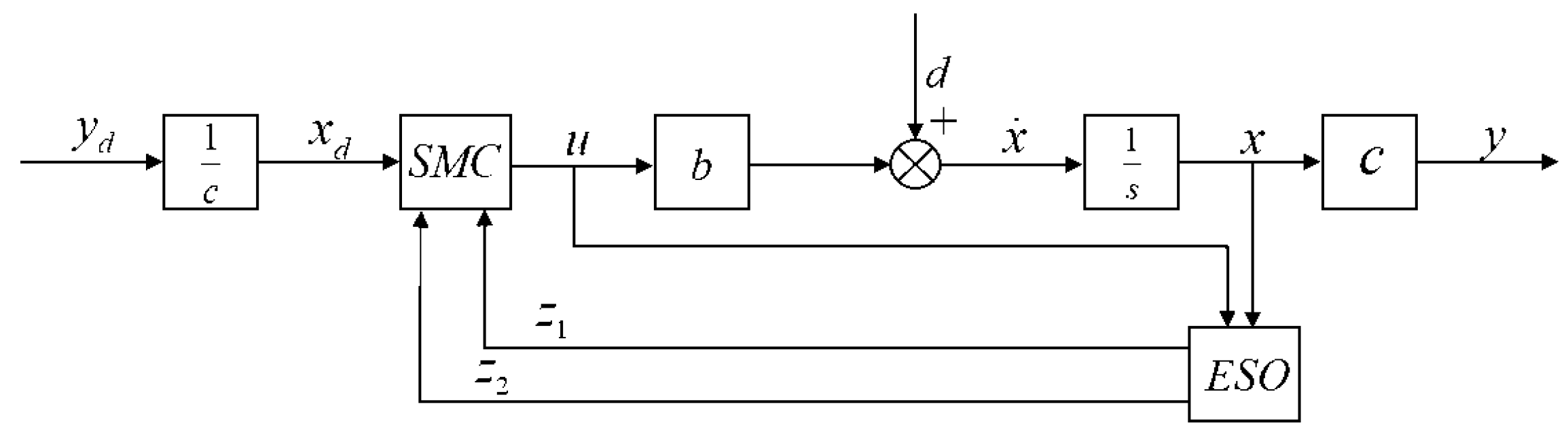
1. Introduction
The global population’s accelerated aging and the high prevalence of pulmonary diseases have made ventilators indispensable in contemporary clinical practice. The precision and appropriateness of ventilator parameter settings directly influence the safety and efficacy of mechanical ventilation, imposing more stringent demands on ventilator design, manufacturing, and performance testing [
1,
2,
3,
4,
5,
6,
7]. Lung simulators not only function as essential tools for evaluating ventilator performance but are also widely applied in clinical training and respiratory research [
8,
9,
10,
11,
12,
13]. Although animal experiments have historically played a crucial role in investigating respiratory physiology and verifying ventilator functionality, concerns related to ethics, costs, and reproducibility have increasingly driven the development of viable alternatives. Lung simulators offer a promising substitute by providing precisely controllable parameters such as tidal volume, airway pressure, and the respiratory rate. In contrast to animal models, which inherently vary due to differences in body weight, age, and health status, lung simulators facilitate standardized and repeatable testing across a wide range of respiratory conditions. The COVID-19 pandemic has further underscored such demands, driving extensive clinical trials and exploring AI-optimized combination therapies [
14]. These advantages enhance experimental consistency, lower costs, and accelerate device development, aligning with current regulatory trends that advocate for minimizing or replacing animal testing in biomedical research [
15,
16,
17].
Lung simulators are generally classified into two categories: passive and active systems. At present, passive lung simulators are more widely used in ventilator testing platforms due to their low cost and structural simplicity. These devices typically employ a splinted structure with fixed elasticity to simulate lung compliance and integrate ball valves at the air inlet to replicate airway resistance. They support ventilator performance assessment by enabling pressure and flow measurements at both the inlet and outlet. One prominent example is the SmartLung Adult lung simulator, developed by IMT Analytics Inc. However, despite their affordability and ease of construction, passive lung simulators rely heavily on the inherent physical characteristics of their constituent materials. As a result, they lack the ability to replicate patient-initiated spontaneous breathing and do not support continuous modulation of respiratory mechanics. Consequently, their use is limited in evaluating ventilator–patient synchrony performance under dynamic conditions [
18,
19,
20].
In contrast, active lung simulators have been under development for nearly two decades, with the ASL 5000 by Ingmar Medical representing one of the most prominent examples. Dexter [
21] validated the ASL 5000’s effectiveness in replicating neonatal and pediatric lung conditions, thereby underscoring its pivotal role in respiratory research. It has been utilized to assess the performance of innovative respiratory flow sensors by reproducing realistic breathing scenarios, thus providing a controlled environment to ensure sensor reliability under practical conditions [
22]. Moreover, the ASL 5000 has been extensively applied in studies of assisted ventilation to simulate scenarios such as apnea and to examine the interaction between ventilators and lung models, facilitating the refinement of assisted ventilation techniques [
23]. Consequently, the ASL 5000 makes a substantial contribution to the advancement of respiratory monitoring technologies and serves as a critical experimental platform for optimizing clinical ventilation strategies.
In addition to the ASL 5000, numerous researchers have explored various designs for active lung simulators. For example, Bunburaphong [
24] developed an active lung simulator utilizing an airbag-spring mechanism and assessed its performance under Bi-Level Positive Airway Pressure (BiPAP) therapy. Elmaati [
25] introduced a box–airbag configuration, derived transfer functions for three representative lung conditions—healthy lungs, acute respiratory distress syndrome (ARDS), and chronic obstructive pulmonary disease (COPD)—and conducted simulations using the developed simulator. Knöbel [
26] implemented a cylinder–piston design to reproduce the respiratory process, assuming linear lung compliance, while Dong [
27] from Nankai University employed a similar cylinder-piston architecture to construct a lung simulator for testing industrial oxygen masks. Notably, soft robotic devices for respiratory assistance, such as a diaphragm-assist device validated using a clinically relevant respiratory simulator, have also advanced respiratory care [
28].
In addition, several researchers have explored hybrid active lung simulators that combine freshly excised porcine lungs with advanced inorganic materials to better replicate human respiratory physiology. While such approaches can improve biological fidelity, they are associated with considerable drawbacks, high operational costs and limited durability, as the excised pig lungs undergo irreversible degradation after only a few experimental cycles, necessitating frequent replacement [
29,
30,
31,
32].
Nevertheless, existing lung simulators continue to exhibit critical limitations. Specifically, (i) compliance and resistance are rarely adjustable in a continuous and precise manner, restricting their capacity to mimic diverse pathological states; (ii) dual active–passive operating modes are generally absent, limiting physiological versatility; and (iii) the ability to reproduce asymmetric dual-chamber dynamics and to evaluate patient–ventilator synchrony remains insufficient. These gaps highlight the urgent need for simulation systems that are both functionally adaptable and physiologically realistic.
In parallel, the growing demand for home care and portable ventilation has driven rapid progress in non-invasive ventilation devices and intelligent monitoring platforms. These developments not only broaden the clinical application landscape but also establish practical benchmarks for assessing lung simulators in terms of physiological fidelity and translational relevance. Although the present study is primarily oriented toward bench evaluation and research–teaching contexts, future work will aim to integrate the simulator with home care systems and portable ventilators, thereby expanding validation scenarios and enabling new directions in non-invasive ventilation design.
To address the aforementioned limitations, we propose a novel stepper motor-driven lung simulator. In this design, precise motor actuation directly controls airbag motion, effectively eliminating the hysteresis that is commonly observed in cylinder–piston mechanisms. By faithfully reproducing both active and passive respiratory behaviors, the simulator achieves enhanced accuracy, responsiveness, and configurability. These advantages make it a promising platform for ventilator performance testing and respiratory research, bridging the gap between existing simulation tools and the evolving demands of modern clinical practice. In future work, given the extensive dataset generated by the simulator, advanced deep learning time-series architectures such as LSTM–U-Net could be leveraged to classify ventilation modes and automatically detect anomalies, thereby enabling phase segmentation and the identification of patient–ventilator asynchrony patterns [
33].
4. Discussion
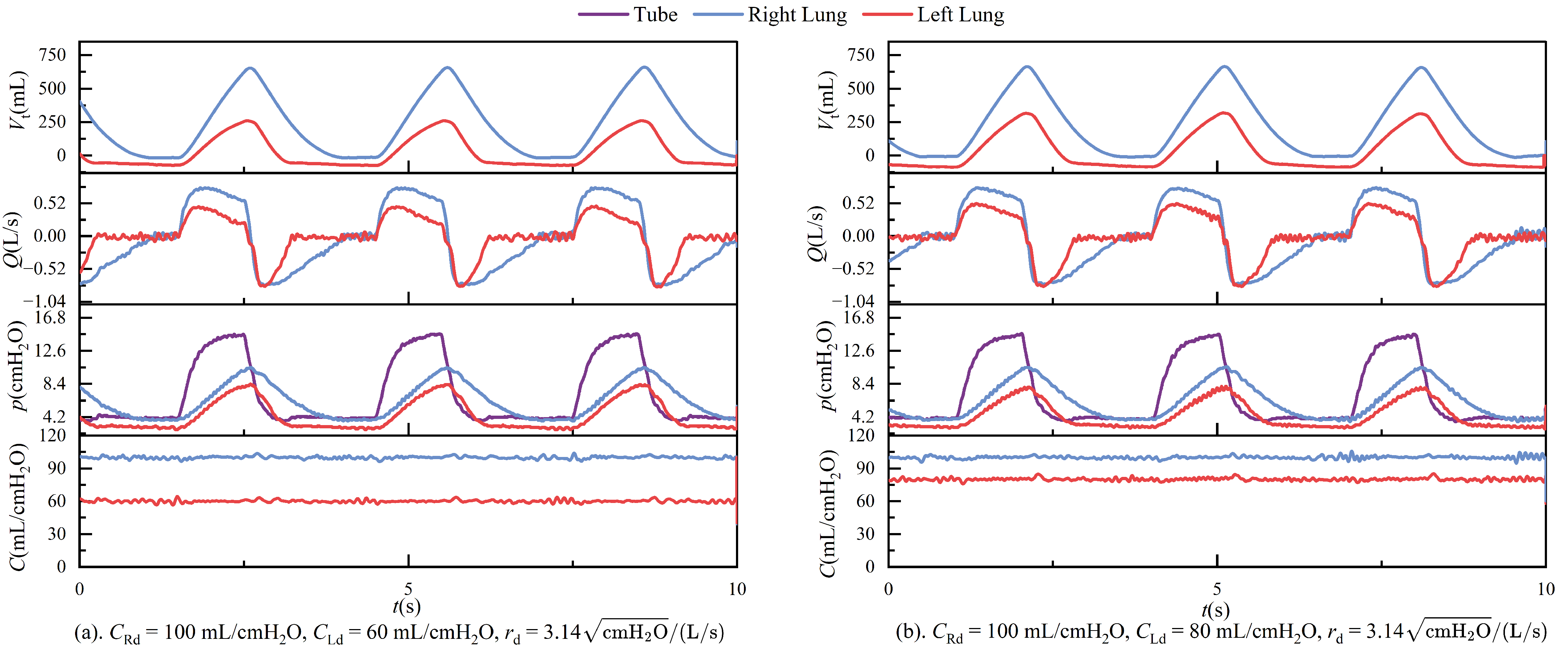
In passive mode, during ventilation, the ventilator connects to the left and right lungs via a three-way connector. Due to the elasticity of the tubing, even under conditions that strive to keep the tubing as straight as possible, airflow through the three-way connector can still experience some pressure imbalance, leading to different resistances on each side. These are illustrated in
Figure 11. The figure shows the respiratory parameter data for the left and right lungs under identical compliance and resistance settings. Although the parameters are similar, the respiratory parameter data for the two lungs differ. Specifically, the tidal volume on the right side is significantly larger than on the left side, indicating that the resistance on the left side is more significant than on the right. Typically, an increase in resistance would lead to a prolonged expiratory phase, as shown in
Figure 14. However, an increase in resistance shortens the expiratory phase. This phenomenon is caused by the effect illustrated in
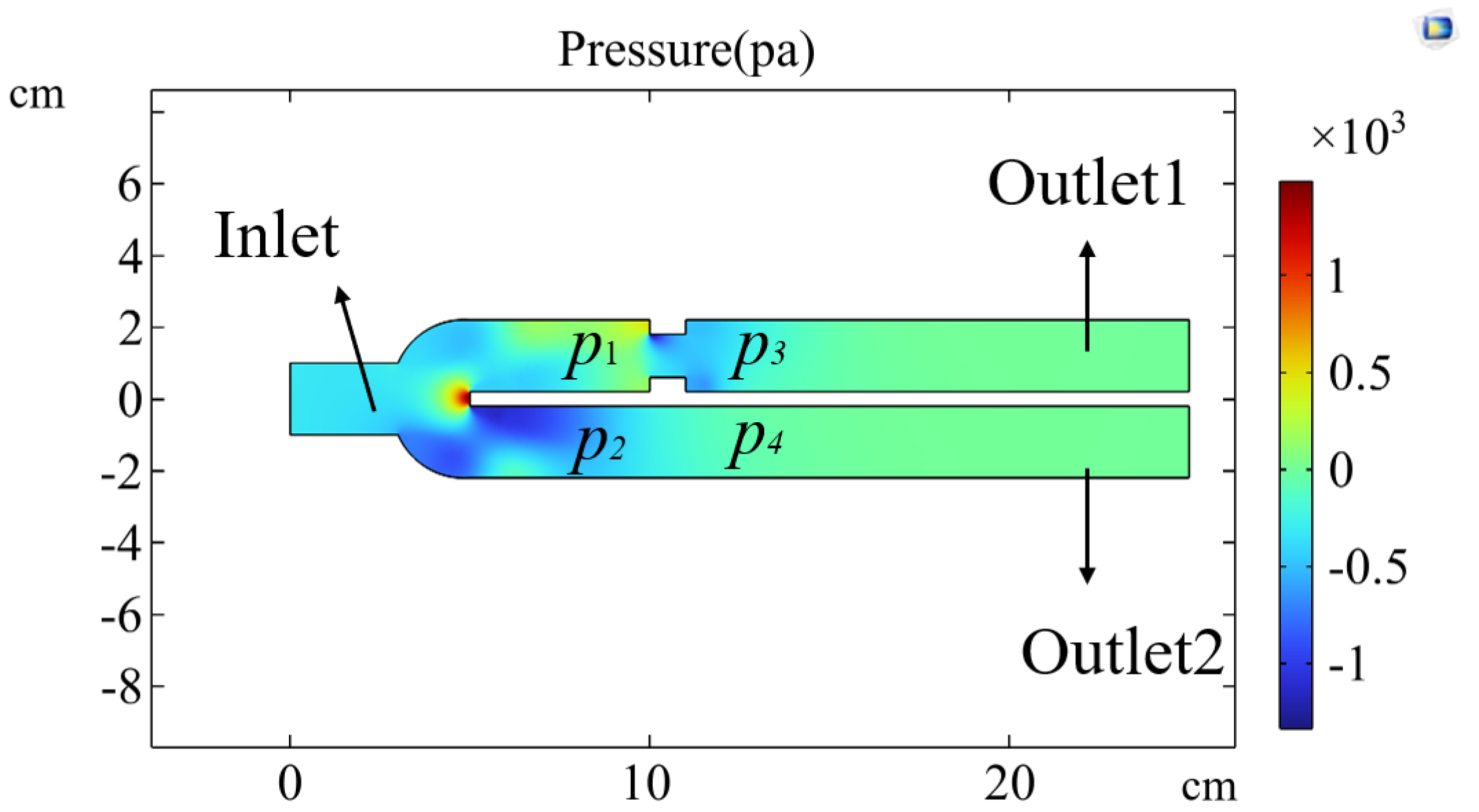
Figure 19.
Figure 19 shows the isobar distribution simulated using COMSOL software (6.2.0.658 Version) under conditions of unequal resistance on both sides of a three-way connector. Airflow enters at a certain speed from the inlet, and at the two outlet ducts, the pressure distribution results in
and
due to the unequal resistance. From
, it can be concluded that the pressure inside the lung on the side with higher resistance is lower, which aligns with the actual situation. When exhalation begins, the airflow exits the lung, and the pressure drop is more significant on the side with higher resistance, which also has a smaller tidal volume. These conditions ultimately lead to a shortened expiratory phase on the side with higher resistance.
The ventilator supplied initial pressure in experiments involving different compliance for the left and right lungs, and PEEP (positive end-expiratory pressure) was set to 4 cmH
2O. At this point, the pressure in both lungs could reach 4 cmH
2O. When the ventilator began ventilating the lung simulator, the difference in compliance between the two lungs caused the lung with lower compliance to get the PEEP first after the first respiratory cycle. In contrast, the pressure in the other lung continued to decrease. This led to the jetting phenomenon shown in
Figure 20.
Figure 20 illustrates the velocity distribution when airflow enters the three-way connector from point B at a constant speed. Although there is no airflow entering the symmetrical side at this time, it is evident that there are fluctuations in airflow velocity at point A, which will cause pressure changes on the opposite side. The jetting phenomenon caused the gas in the lung with lower compliance to continue flowing out, with the pressure dropping further below the PEEP set by the ventilator, ultimately resulting in a negative tidal volume in the lung with lower compliance. These conditions are also why the negative tidal volumes are observed in
Figure 12. It is important to note that the negative tidal volume here can be interpreted as a decrease in residual gas in the lung with lower compliance.
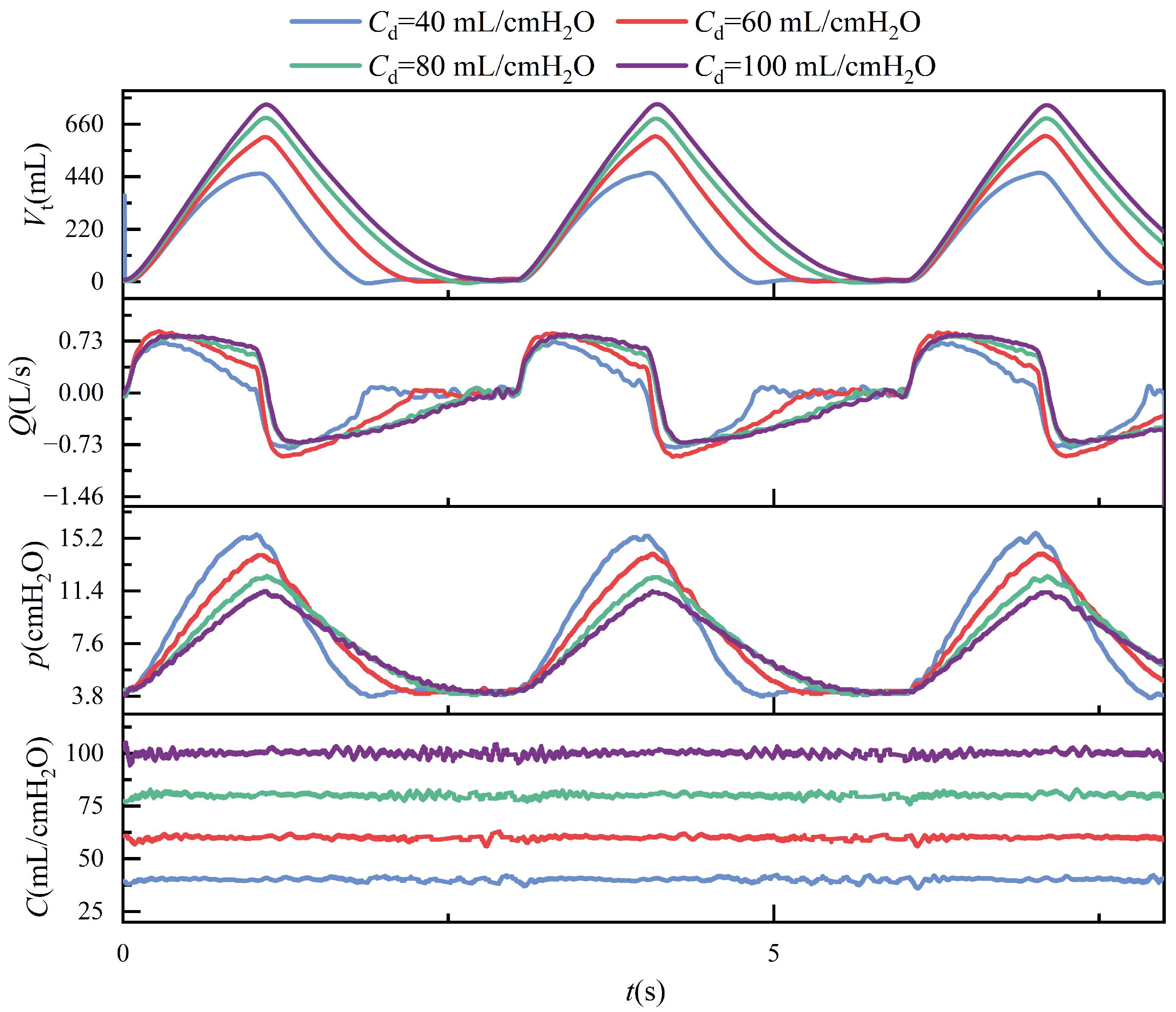
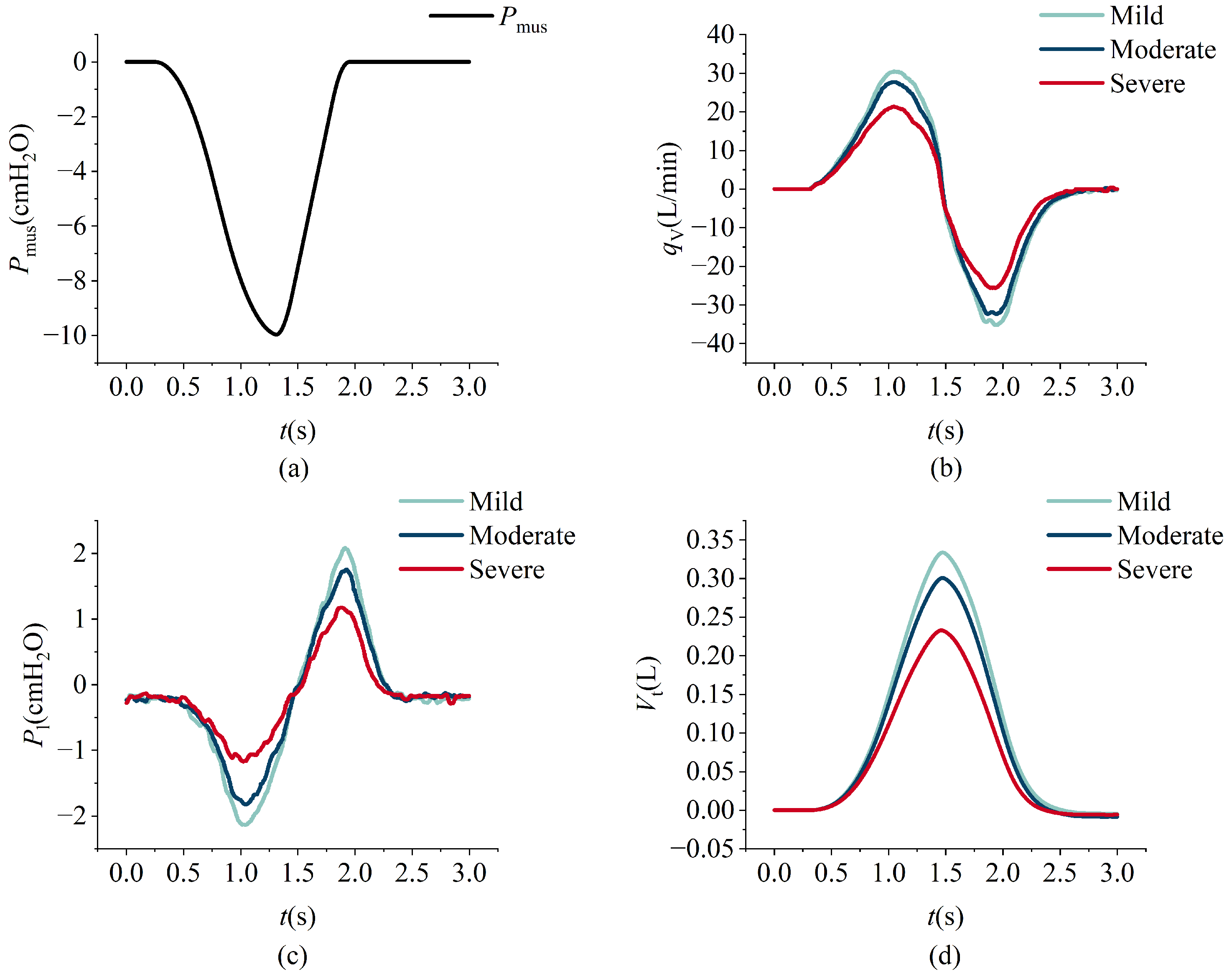
Under conditions of constant airway resistance, increasing lung compliance leads to substantial changes in respiratory mechanics. As compliance rises, tidal volume increases accordingly, since the lungs can accommodate a greater volume of air under the same pressure, as described by (
1). This enhanced elasticity reduces the mechanical load during inspiration, lowering the peak inspiratory pressure and decreasing overall respiratory effort. Furthermore, the resulting increase in tidal volume prolongs the pressure decay phase, which subsequently alters the airflow profile during the breathing cycle. These effects are depicted in
Figure 13, where the temporal evolution of key respiratory parameters under varying compliance is presented.
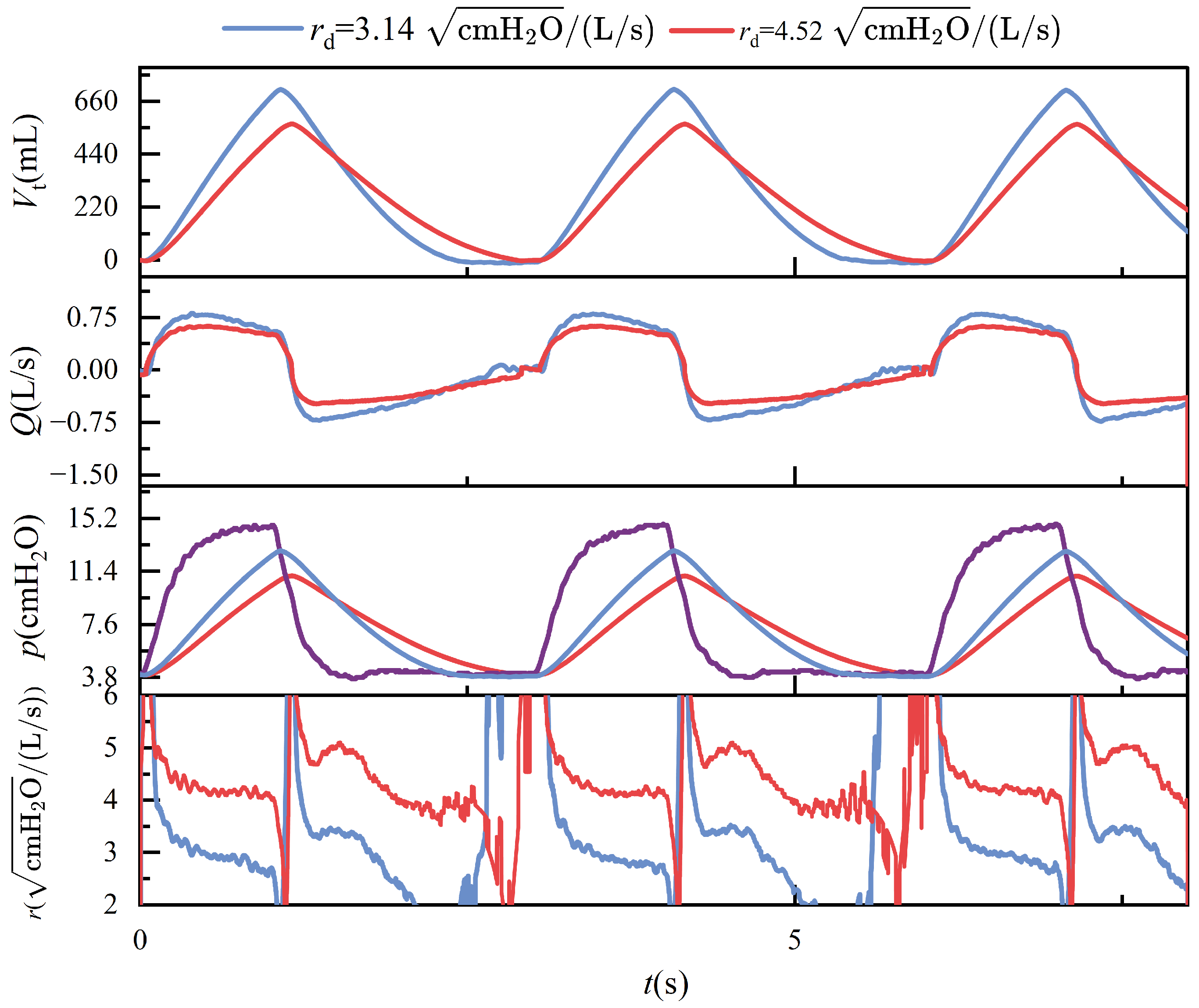
Maintaining constant compliance while progressively increasing airway resistance elicits distinct dynamic respiratory responses. As described in (
2), elevated airway resistance reduces tidal volume, since the increased resistance impedes airflow, thereby limiting the volume of gas delivered to the lungs under fixed pressure support. In the context of conscious, spontaneous breathing, greater airway resistance necessitates higher inspiratory pressure to achieve sufficient lung inflation, leading to increased peak airway pressures. However, in the device’s passive mode—which simulates unconscious, ventilator-dependent breathing with fixed pressure and inspiratory duration—rising airway resistance paradoxically results in a lower peak pressure, as less volume is delivered within the same time frame. During expiration, airflow decelerates and the overall breathing cycle is prolonged. These mechanical effects are further complicated by computational artifacts: when a parabolic resistance model is employed, the flow-dependent nature of resistance leads to significant numerical instabilities near zero-flow regions, especially during transitions between inspiration and expiration. These errors primarily arise from numerical discontinuities and reduced sensor resolution at low flow rates, ultimately undermining the accuracy of resistance estimation.
Figure 14 illustrates these phenomena, highlighting both the physiological and numerical consequences of increasing airway resistance.
Figure 15a illustrates the respiratory muscle force curve, while
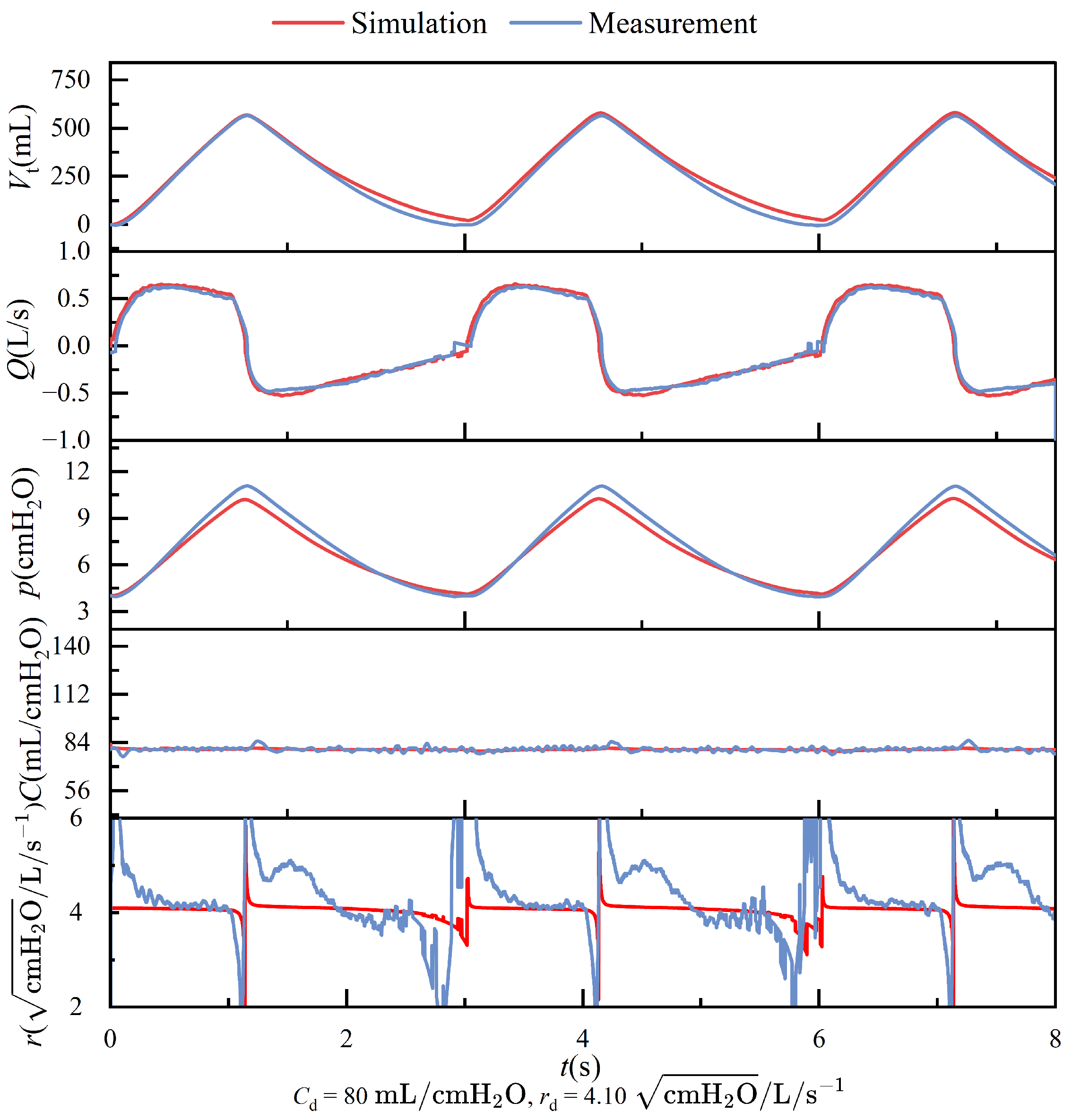
Figure 15b–d depict the comparison between simulation data (black dashed lines) and measured data (red solid lines). A noticeable lag is observed between the simulation and measured data, primarily attributable to the system architecture design of the lung simulator device. The simulation data curve is initially derived from predefined parameters during operation. Subsequently, motor movement is controlled according to the simulation curve, and flow and pressure signals are collected by sensors. These signals are then processed by the controller and displayed on the PC.
Figure 15b,d present the flow and tidal volume information, respectively, demonstrating that the measured and simulation data exhibit similar trends with closely matched amplitudes.
Figure 15c presents the pressure profiles, where the overall trends in the experimental and simulation data are generally consistent; however, a noticeable discrepancy in amplitude is observed. This difference primarily arises from structural distinctions between the simulation model and the physical system. Furthermore, as illustrated in
Figure 15b, the expiratory phase exhibits a rapid change in flow rate. The physical structure of the lung simulator is limited in its ability to accurately replicate this transient behavior, resulting in a higher peak expiratory flow in the simulation compared to that observed in the experimental data.
An increase in the maximum respiratory driving force results in elevated inspiratory and expiratory flow peaks, greater tidal volume, and larger absolute intrapulmonary pressure values. Notably, the expiratory peak consistently exceeds the inspiratory peak, a pattern that is attributable to the asymmetric time profile of the driving force, which rises gradually but decays rapidly. During the inspiratory phase, lung expansion is passive and constrained by elastic recoil and airway resistance, thereby limiting inspiratory flow. In contrast, expiration is predominantly driven by the swift release of stored elastic energy, with minimal muscular contribution. These dynamics are clearly depicted in
Figure 16.
Under identical respiratory driving force conditions, the progression of acute respiratory distress syndrome (ARDS) alters respiratory behavior substantially. As ARDS severity increases, lung compliance decreases due to widespread alveolar collapse, while resistance increases, reflecting a reduction in the functional residual capacity (FRC). These pathological changes lead to diminished tidal volume, reduced airflow, a shortened exhalation time, and lower peak positive and negative intrapulmonary pressures during expiration, as evidenced by the data shown in
Figure 17.
A similar trend is observed in chronic obstructive pulmonary disease (COPD), where escalating disease severity leads to increased upper airway and lung resistance alongside diminished elastic recoil. As a result, respiratory flow is reduced, exhalation is prolonged, and tidal volume decreases under the same respiratory muscle force conditions, as illustrated in
Figure 18b,d. Furthermore, while the absolute magnitude of intrapulmonary pressure rises during both inspiration and expiration with increasing COPD severity, a divergence emerges:
Figure 18c shows that inspiratory negative pressure increases, whereas expiratory positive pressure decreases. This counterintuitive outcome stems from the interaction between resistance and flow, as described by (
23); when flow decreases more than resistance increases, the resulting intrapulmonary pressure can decline despite elevated resistance.
The experiment verified that the designed lung simulation system exhibits effective compliance control. Compared to traditional passive lung simulators, it offers a more comprehensive adjustable range of compliance and meets the requirements for continuous regulation.
While the present study demonstrates that the proposed lung simulator achieves effective compliance control and stable device performance, several directions remain open for future research. To enhance the spatial fidelity of airflow and pressure distribution, we plan to extend CFD-based cross-checks under measured boundary conditions and leverage reserved hardware/timing interfaces for the integration of multi-point pressure sensors or electrical impedance tomography (EIT). For long-term structural reliability, since the airbag and resistance components are primarily polymer-based elastomers and conventional eddy current testing is less suitable, future designs involving conductive composites may incorporate eddy current or ultrasonic NDT techniques. To facilitate downstream applications, we will establish a standardized data/label interface and logging protocol, enabling the simulator’s output to be applied to advanced methods such as LSTM- or U-Net–based breathing phase segmentation and patient–ventilator asynchrony detection. Furthermore, the integration of electronics and intelligent monitoring represents another promising avenue. The current system already supports remote telemetry and structured logging, allowing for threshold- and SPC-based prototype alerts for abnormal events. With the accumulation of long-term operational data, predictive maintenance strategies based on drift detection and anomaly distribution modeling can be developed, thereby providing early warnings of performance degradation and complementing the physiological and mechanical extensions described above.