Current Topics in OCT Applications in Vitreoretinal Surgery
Abstract
1. Introduction
2. Overview of OCT Technology in Retinal Imaging
3. Clinical Applications of OCT in Vitreoretinal Diseases
3.1. Macular Diseases
3.2. Retinal Detachment
3.3. Diabetic Retinopathy
4. Contemporary Topics in OCT-Based Assessment in Vitreoretinal Surgery
4.1. Macular Hole (MH)
4.1.1. Novel Biomarkers of MH in Vitreoretinal Surgery
4.1.2. Advanced Surgical Techniques of MH and OCT (Table 1)
| Technique | Indications | Characteristics | References |
|---|---|---|---|
| Inverted ILM flap | Large FTMHs, lamellar ILMHs | High closure rate, functionally good outcome | [57,59,60,61,62,63,64,65] |
| ILM insertion | Large FTMHs, lamellar ILMHs | High closure rate | [57,64] |
| Amniotic membrane transplantation, autologous tissue patch | Large FTMHs | High closure rate, requiring equipment | [69,70,71,72,73,74,75,76,77] |
| Hemitemporal ILM peeling | FTMHs | Reducing damage of retinal nerve fiber layer | [78,79] |
| Intraoperative closure with PFCL | FTMHs | Original method | [82] |
4.1.3. More Insights into MH and OCT
4.2. Epiretinal Membrane (ERM)
4.2.1. Biomarkers of ERM in Vitreoretinal Surgery
4.2.2. Novel Topics in ERM Surgery and OCT
4.3. Rhegmatogenous Retinal Detachment (RRD)
4.3.1. Morphologic Stages of RRD and Retinal Displacement Following Surgery
4.3.2. Biomarkers of RRD and Vitreoretinal Surgery (Table 3)
| Biomarkers | Major outcomes | References |
|---|---|---|
| EZ/ELM, IS/OS reflectivity | Postoperative VA, macular sensitivity, multifocal-ERG amplitude | [128,129,130] |
| HRDs in outer retina | Preoperative morphologic stage, postoperative VA, CME | [131] |
| Epiretinal HRDs or layers | Epiretinal membrane, VCRs | [133,134] |
| Subretinal HRPs, multiple SRPs, postoperative outer retinal folds, retinal displacement, discontinuity of ELM | Postoperative reduced BCVA | [125,132,135,136] |
| Discontinuity of interdigitation zone | Metamorphosia | [125] |
4.3.3. Current Additional Topics in RRD and OCT
4.4. Miscellaneous Topics in OCT and Vitreoretinal Surgery
5. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| OCT | Optical coherence tomography |
| SD | Spectral domain |
| SS | Swept source |
| ED | Enhanced depth |
| OCTA | OCT angiography |
| AMD | Age-related macular degeneration |
| MNV | Macular neovascularization |
| MH | Macular hole |
| RPE | Retinal pigment epithelium |
| RRD | Rhegmatogenous retinal detachment |
| TRD | Tractional retinal detachment |
| RS | Retinoschisis |
| DR | Diabetic retinopathy |
| NPDR | Non-proliferative diabetic retinopathy |
| PDR | Proliferative diabetic retinopathy |
| MAs | Microaneurysms |
| FA | Fluorescein angiography |
| UWF | Ultra-widefield |
| FTMH | Full-thickness MH |
| DONFL | Dissociated optic nerve fiber layer |
| ILM | Internal limiting membrane |
| FAZ | Foveal avascular zone |
| ERM | Epiretinal membrane |
| iERM | Idiopathic epiretinal membrane |
| EIFL | Ectopic inner foveal layer |
| EZ | Ellipsoid zone |
| ELM | External limiting membrane |
| SCP | Superficial capillary plexus |
| RPC | Radial peripapillary capillary |
| AI | Artificial intelligence |
| DRIL | Disorganization of retinal inner layer |
| BCVA | Best corrected visual acuity |
| CVI | Choroidal vascular index |
| IS/OS | Inner segment/outer segment |
| ICCs | Intraretinal cystic cavities |
| HRDs | Hyperreflective dots |
| HRPs | Hyperreflective points |
References
- Drexler, W.; Fujimoto, J.G. State-of-the-art retinal optical coherence tomography. Prog. Retin. Eye Res. 2008, 27, 45–88. [Google Scholar] [CrossRef]
- Swanson, E.A.; Izatt, J.A.; Hee, M.R.; Huang, D.; Lin, C.P.; Schuman, J.S.; Puliafito, C.A.; Fujimoto, J.G. In vivo retinal imaging by optical coherence tomography. Opt. Lett. 1993, 18, 1864–1866. [Google Scholar] [CrossRef]
- Fujimoto, J.; Swanson, E. The Development, Commercialization, and Impact of Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT1–OCT13. [Google Scholar] [CrossRef] [PubMed]
- Reznicek, L.; Klein, T.; Wieser, W.; Kernt, M.; Wolf, A.; Haritoglou, C.; Kampik, A.; Huber, R.; Neubauer, A.S. Megahertz ultra-wide-field swept-source retina optical coherence tomography compared to current existing imaging devices. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1009–1016. [Google Scholar] [CrossRef]
- Ohno-Matsui, K.; Takahashi, H.; Mao, Z.; Nakao, N. Determining posterior vitreous structure by analysis of images obtained by AI-based 3D segmentation and ultrawidefield optical coherence tomography. Br J. Ophthalmol. 2023, 107, 732–737. [Google Scholar] [CrossRef]
- Takahashi, H.; Nakao, N.; Shinohara, K.; Sugisawa, K.; Uramoto, K.; Igarashi-Yokoi, T.; Yoshida, T.; Ohno-Matsui, K. Posterior vitreous detachment and paravascular retinoschisis in highly myopic young patients detected by ultra-widefield OCT. Sci. Rep. 2021, 11, 17330. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Uramoto, K.; Ohno-Matsui, K. Ultra-Widefield Optical Coherence Tomography For Retinal Detachment With Proliferative Vitreoretinopathy. Retin. Cases Brief. Rep. 2022, 16, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, A.; Kanclerz, P. Early Descriptions Of Vitreous Surgery. Retina 2021, 41, 1364–1372. [Google Scholar] [CrossRef]
- Machemer, R.; Parel, J.M.; Buettner, H. A new concept for vitreous surgery. I. Instrumentation. Am. J. Ophthalmol. 1972, 73, 1–7. [Google Scholar] [CrossRef]
- Aylward, G.W. 25th RCOphth Congress, President’s Session paper, 25 years of progress in vitreoretinal surgery. Eye 2014, 28, 1053–1059. [Google Scholar] [CrossRef]
- Romano, M.R.; Allegrini, D.; Della Guardia, C.; Schiemer, S.; Baronissi, I.; Ferrara, M.; Cennamo, G. Vitreous and intraretinal macular changes in diabetic macular edema with and without tractional components. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1–8. [Google Scholar] [CrossRef]
- Potsaid, B.; Baumann, B.; Huang, D.; Barry, S.; Cable, A.E.; Schuman, J.S.; Duker, J.S.; Fujimoto, J.G. Ultrahigh speed 1050nm swept source/Fourier domain OCT retinal and anterior segment imaging at 100,000 to 400,000 axial scans per second. Opt. Express. 2010, 18, 20029–20048. [Google Scholar] [CrossRef]
- Hillmann, D.; Pfäffle, C.; Spahr, H.; Sudkamp, H.; Franke, G.; Hüttmann, G. In Vivo FF-SS-OCT Optical Imaging of Physiological Responses to Photostimulation of Human Photoreceptor Cells. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics [Internet]; Bille, J.F., Ed.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Chiku, Y.; Hirano, T.; Takahashi, Y.; Tuchiya, A.; Nakamura, M.; Murata, T. Evaluating posterior vitreous detachment by widefield 23-mm swept-source optical coherence tomography imaging in healthy subjects. Sci. Rep. 2021, 11, 19754. [Google Scholar] [CrossRef]
- Wong, I.Y.; Koizumi, H.; Lai, W.W. Enhanced depth imaging optical coherence tomography. Ophthalmic Surg. Lasers Imaging Retin. 2011, 42, S75–S84. [Google Scholar] [CrossRef]
- Ohno-Matsui, K.; Igarashi-Yokoi, T.; Azuma, T.; Sugisawa, K.; Xiong, J.; Takahashi, T.; Uramoto, K.; Kamoi, K.; Okamoto, M.; Banerjee, S.; et al. Polarization-Sensitive OCT Imaging of Scleral Abnormalities in Eyes With High Myopia and Dome-Shaped Macula. JAMA Ophthalmol. 2024, 142, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Sugisawa, K.; Takahashi, H.; Yamanari, M.; Okamoto, M.; Igarashi-Yokoi, T.; Azuma, T.; Miki, T.; Lu, H.; Wu, Y.; Xiong, J.; et al. Visualization of the scleral structure changes at various stages of eyes with myopic maculopathy using polarization-sensitive OCT. Asia Pac. J. Ophthalmol. 2024, 13, 100117. [Google Scholar] [CrossRef]
- Ishibazawa, A.; Nagaoka, T.; Takahashi, A.; Omae, T.; Tani, T.; Sogawa, K.; Yokota, H.; Yoshida, A. Optical Coherence Tomography Angiography in Diabetic Retinopathy, A Prospective Pilot Study. Am. J. Ophthalmol. 2015, 160, 35–44.e1. [Google Scholar] [CrossRef]
- Salz, D.A.; de Carlo, T.E.; Adhi, M.; Moult, E.; Choi, W.; Baumal, C.R.; Witkin, A.J.; Duker, J.S.; Fujimoto, J.G.; Waheed, N.K. Select Features of Diabetic Retinopathy on Swept-Source Optical Coherence Tomographic Angiography Compared with Fluorescein Angiography and Normal Eyes. JAMA Ophthalmol. 2016, 134, 644–650. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, J.; Chen, Z. Advances in Doppler optical coherence tomography and angiography. Transl. Biophotonics. 2019, 1, e201900005. [Google Scholar] [CrossRef] [PubMed]
- Wartak, A.; Beer, F.; Desissaire, S.; Baumann, B.; Pircher, M.; Hitzenberger, C.K. Investigating spontaneous retinal venous pulsation using Doppler optical coherence tomography. Sci. Rep. 2019, 9, 4237. [Google Scholar] [CrossRef]
- Yee, P.; Sevgi, D.D.; Abraham, J.; Srivastava, S.K.; Le, T.; Uchida, A.; Figueiredo, N.; Rachitskaya, A.V.; Sharma, S.; Reese, J.; et al. iOCT-assisted macular hole surgery, Outcomes and utility from the DISCOVER study. Br. J. Ophthalmol. 2021, 105, 403–409. [Google Scholar] [CrossRef]
- Abraham, J.R.; Srivastava, S.K.; KLe, T.; Sharma, S.; Rachitskaya, A.; Reese, J.L.; Ehlers, J.P. Intraoperative OCT-Assisted Retinal Detachment Repair in the DISCOVER Study, Impact and Outcomes. Ophthalmol. Retin. 2020, 4, 378–383. [Google Scholar] [CrossRef]
- Foust, J.; McCloud, M.; Narawane, A.; Trout, R.M.; Chen, X.; Dhalla, A.H.; Li, J.D.; Viehland, C.; Draelos, M.; Vajzovic, L.; et al. New Directions for Ophthalmic OCT–Handhelds, Surgery, and Robotics. Transl. Vis. Sci. Technol. 2025, 14, 14. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, F.Y.; Cheung, C.Y.; Chen, H. Different Effect of Media Opacity on Vessel Density Measured by Different Optical Coherence Tomography Angiography Algorithms. Transl. Vis. Sci. Technol. 2020, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Darma, S.; Kok, P.H.; van den Berg, T.J.; Abràmoff, M.D.; Faber, D.J.; Hulsman, C.A.; Zantvoord, F.; Mourits, M.P.; Schlingemann, R.O.; Verbraak, F.D. Optical density filters modeling media opacities cause decreased SD-OCT retinal layer thickness measurements with inter- and intra-individual variation. Acta Ophthalmol. 2015, 93, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, M.; Schmitz-Valckenberg, S.; Chakravarthy, U. Age-Related Macular Degeneration, A Review. JAMA 2024, 331, 147–157. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Klimscha, S.; Waldstein, S.M.; Bogunović, H. A view of the current and future role of optical coherence tomography in the management of age-related macular degeneration. Eye 2017, 31, 26–44. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Waldstein, S.M. A paradigm shift in imaging biomarkers in neovascular age-related macular degeneration. Prog. Retin. Eye Res. 2016, 50, 1–24. [Google Scholar] [CrossRef]
- Taylor, T.R.P.; Menten, M.J.; Rueckert, D.; Sivaprasad, S.; Lotery, A.J. The role of the retinal vasculature in age-related macular degeneration, A spotlight on OCTA. Eye 2024, 38, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.; Ly, A.; Ohno-Matsui, K.; Kalloniatis, M.; Doig, G.S. Diagnostic accuracy of OCTA and OCT for myopic choroidal neovascularisation, A systematic review and meta-analysis. Eye 2023, 37, 21–29. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Li, B.; Yuan, M.; Chen, Y. Detection Rate and Diagnostic Value of Optical Coherence Tomography Angiography in the Diagnosis of Polypoidal Choroidal Vasculopathy, A Systematic Review and Meta-Analysis. J. Ophthalmol. 2019, 2019, 6837601. [Google Scholar] [CrossRef]
- Arevalo, J.F.; Sanchez, J.G.; Costa, R.A.; Farah, M.E.; Berrocal, M.H.; Graue-Wiechers, F.; Lizana, C.; Robledo, V.; Lopera, M. Optical coherence tomography characteristics of full-thickness traumatic macular holes. Eye 2008, 22, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Gass, J.D. Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am. J. Ophthalmol. 1995, 119, 752–759. [Google Scholar] [CrossRef]
- Duker, J.S.; Kaiser, P.K.; Binder, S.; de Smet, M.D.; Gaudric, A.; Reichel, E.; Sadda, S.R.; Sebag, J.; Spaide, R.F.; Stalmans, P. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology 2013, 120, 2611–2619. [Google Scholar] [CrossRef]
- Matoba, R.; Morizane, Y. Epiretinal membrane, An overview and update. Jpn. J. Ophthalmol. 2024, 68, 603–613. [Google Scholar] [CrossRef]
- Machemer, R. The importance of fluid absorption, traction, intraocular currents, and chorioretinal scars in the therapy of rhegmatogenous retinal detachments. XLI Edward Jackson memorial lecture. Am. J. Ophthalmol. 1984, 98, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, F.; Goud, R.; Belhouari, S.; Eng, K.T.; Mandelcorn, E.D.; da Costa, B.R.; Miranda, R.N.; Felfeli, T. Prognostic Features of Preoperative OCT in Retinal Detachments, A Systematic Review and Meta-analysis. Ophthalmol. Retin. 2023, 7, 383–397. [Google Scholar] [CrossRef]
- Shimada, N.; Ohno-Matsui, K.; Nishimuta, A.; Moriyama, M.; Yoshida, T.; Tokoro, T.; Mochizuki, M. Detection of paravascular lamellar holes and other paravascular abnormalities by optical coherence tomography in eyes with high myopia. Ophthalmology 2008, 115, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Ober, M.D.; Freund, K.B.; Shah, M.; Ahmed, S.; Mahmoud, T.H.; Aaberg TMJr Zacks, D.N.; Gao, H.; Mukkamala, K.; Desai, U.; Packo, K.H.; et al. Stellate nonhereditary idiopathic foveomacular retinoschisis. Ophthalmology 2014, 121, 1406–1413. [Google Scholar] [CrossRef]
- Ruiz-Medrano, J.; Montero, J.A.; Flores-Moreno, I.; Arias, L.; García-Layana, A.; Ruiz-Moreno, J.M. Myopic maculopathy, Current status and proposal for a new classification and grading system (ATN). Prog. Retin. Eye Res. 2019, 69, 80–115. [Google Scholar] [CrossRef]
- Jampol, L.M.; Glassman, A.R.; Sun, J. Evaluation and Care of Patients with Diabetic Retinopathy. N. Engl. J. Med. 2020, 382, 1629–1637. [Google Scholar] [CrossRef]
- Wilkinson, C.P.; Ferris, F.L., 3rd; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T.; et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Horie, S.; Ohno-Matsui, K. Progress of Imaging in Diabetic Retinopathy-From the Past to the Present. Diagnostics 2022, 12, 1684. [Google Scholar] [CrossRef]
- Szeto, S.K.; Lai, T.Y.; Vujosevic, S.; Sun, J.K.; Sadda, S.R.; Tan, G.; Sivaprasad, S.; Wong, T.Y.; Cheung, C.Y. Optical coherence tomography in the management of diabetic macular oedema. Prog. Retin. Eye Res. 2024, 98, 101220. [Google Scholar] [CrossRef]
- Horie, S.; Kukimoto, N.; Kamoi, K.; Igarashi-Yokoi, T.; Yoshida, T.; Ohno-Matsui, K. Blue Widefield Images of Scanning Laser Ophthalmoscope Can Detect Retinal Ischemic Areas in Eyes with Diabetic Retinopathy. Asia Pac. J. Ophthalmol. 2021, 10, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Horie, S.; Corradetti, G.; Esmaeilkhanian, H.; Sadda, S.R.; Cheung, C.M.G.; Ham, Y.; Chang, A.; Takahashi, T.; Ohno-Matsui, K. Microperimetry in Retinal Diseases. Asia Pac. J. Ophthalmol. 2023, 12, 211–227. [Google Scholar] [CrossRef]
- Waheed, N.K.; Rosen, R.B.; Jia, Y.; Munk, M.R.; Huang, D.; Fawzi, A.; Chong, V.; Nguyen, Q.D.; Sepah, Y.; Pearce, E. Optical coherence tomography angiography in diabetic retinopathy. Prog. Retin. Eye Res. 2023, 97, 101206. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.; Khalid, H.; Sivaprasad, S.; Nicholson, L.; Anikina, E.; Sullivan, P.; Patel, P.J.; Balaskas, K.; Keane, P.A. Objective Evaluation of Proliferative Diabetic Retinopathy Using OCT. Ophthalmol. Retin. 2020, 4, 164–174. [Google Scholar] [CrossRef]
- Guerin, G.M.; Pastore, M.R.; Guerin, P.L.; Grotto, A.; Inferrera, L.; Tognetto, D. External limiting membrane aperture as a reliable and predictive prognostic factor in macular hole surgery. Eur. J. Ophthalmol. 2025, 35, 1854–1862. [Google Scholar] [CrossRef]
- Quarta, A.; Govetto, A.; Porreca, A.; Toto, L.; Di Nicola, M.; Ruggeri, M.L.; Gironi, M.; Nubile, M.; Agnifili, L.; Romano, M.R.; et al. Development and preliminary evaluation of a novel preoperative index for quantitative analysis of photoreceptor loss in full-thickness macular holes. Graefes Arch. Clin. Exp. Ophthalmol. 2025, 263, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, S.J.; Kang, S.W.; Son, K.Y.; Hwang, S. Macular hole with epiretinal proliferation, Diagnostic value of en-face optical coherence tomography and clinical characteristics. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 2461–2470. [Google Scholar] [CrossRef]
- Abousy, M.; Drew-Bear, L.E.; Gibbons, A.; Pan-Doh, N.; Li, X.; Handa, J.T. In-Depth Analysis of Preoperative OCT Markers as Prognostic Factors for Lamellar Macular Holes and Epiretinal Membrane Foveoschisis. Ophthalmol. Retin. 2024, 8, 465–472. [Google Scholar] [CrossRef]
- Ye, X.; Xu, J.; He, S.; Wang, J.; Yang, J.; Tao, J.; Chen, Y.; Shen, L. Quantitative evaluation of dissociated optic nerve fibre layer (DONFL) following idiopathic macular hole surgery. Eye 2023, 37, 1451–1457. [Google Scholar] [CrossRef]
- Pecaku, A.; Melo, I.M.; Cao, J.A.; Sabour, S.; Naidu, S.C.; Demian, S.; Popovic, M.M.; Wykoff, C.C.; Govetto, A.; Muni, R.H. Morphologic Stages of Full-Thickness Macular Hole on Spectral-Domain OCT. Ophthalmol. Retin. 2025, 9, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Cai, C.; Chen, X. Comparing the inverted internal limiting membrane flap technique with internal limiting membrane insertion technique for treatment of large macular holes. Retina 2025. [Google Scholar] [CrossRef]
- Tian, T.; Jiao, D.; Zhang, X.; Wang, M.; Guo, S.; Lyu, J.; Zhao, P. Non-inverted and single-layer “plastic bag” ILM flap novel technique to treat large macular holes. Asia Pac. J. Ophthalmol. 2025, 21, 100164. [Google Scholar] [CrossRef]
- Kanai, A.; Takeuchi, J.; Koto, T.; Inoue, M. Combined Treatment with Inverted Internal Limiting Membrane Flap and Subretinal Injection of Balanced Salt Solution to Repair Chronic Macular Holes. J. Vitreoretin. Dis. 2024, 9, 88–91. [Google Scholar] [CrossRef]
- Peng, K.L.; Kung, Y.H.; Wu, T.T. Surgical outcomes of inverted internal limiting membrane flap technique for primary rhegmatogenous retinal detachment coexisting with a macular hole. Medicine 2024, 103, e40237. [Google Scholar] [CrossRef]
- Feng, J.; Shao, Q.; Xie, J.; Yu, J.; Li, M.; Liu, C.; Zhou, S.; Zhou, H.; Wang, W.; Fan, Y. Comparison of Three Internal Limiting Membrane Peeling Techniques for Myopic Traction Maculopathy with High Risk Of Postoperative Macular Hole Development. Retina 2023, 43, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Keyal, K.; Li, B.; Liu, C.; Tian, Z.; Li, H.; Bi, Y. Petaloid technique and prognostic significance of macular hole shapes by optical coherence tomography for full thickness macular hole. Front. Med. 2024, 11, 1424580. [Google Scholar] [CrossRef]
- Baltă, G.; Tofolean, I.T.; Tiu, T.; Dinu, V.; Alexandrescu, C.M.; Baltă, F.; Voinea, L.M. Sequential Pars Plana Vitrectomy And Inverted Internal Limiting Membrane Flap Technique For Rhegmatogenous Retinal Detachments With Peripheral Breaks And Concomitant Noncausative Macular Hole In Nonhighly Myopic Patients. Retina 2024, 44, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.T.; Hou, T.Y.; Peng, K.L.; Kung, Y.H. Inverted flap technique versus internal limiting membrane insertion for macular hole in eyes with extremely high myopia. BMC Ophthalmol. 2024, 24, 286. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Okamoto, F.; Sugiura, Y.; Izumi, I.; Iioka, A.; Morikawa, S.; Hiraoka, T.; Oshika, T. Internal Limiting Membrane Peeling and Inverted Flap Technique in Macular Hole, Postoperative Metamorphopsia and Optical Coherence Tomography. Ophthalmologica 2024, 247, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Z.; Yu, Y.; Yang, X.; Qi, B.; Zhang, K.; Liu, W. Microstructural and microperimetric comparison of internal limiting membrane peeling and insertion in large idiopathic macular hole. BMC Ophthalmol. 2023, 23, 274. [Google Scholar] [CrossRef]
- Tian, T.; Tan, H.; Zhu, X.; Zhang, X.; Zhao, P. Peeled Internal Limiting Membrane Reposition For Idiopathic Macular Holes, A Pilot Randomized Controlled Trial. Retina 2023, 43, 191–199. [Google Scholar] [CrossRef]
- Kanai, M.; Sakimoto, S.; Takahashi, S.; Nishida, K.; Maruyama, K.; Sato, S.; Sakaguchi, H.; Nishida, K. Embedding Technique versus Conventional Internal Limiting Membrane Peeling for Lamellar Macular Holes with Epiretinal Proliferation. Ophthalmol. Retin. 2023, 7, 44–51. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Inada, T. Amniotic Membrane Coverage for Intractable Large Macular Holes, A First Report with Japanese Patients. J. Clin. Med. 2025, 14, 3708. [Google Scholar] [CrossRef]
- Choi, M.; Lee, S.H.; Kim, S.W.; Lee, J.Y.; Lee, S.J. Foveal Configuration After Amniotic Membrane Transplantation in Complex Macular Holes. Retina 2025. [Google Scholar] [CrossRef]
- Trivedi, V.; You, Q.; Me, R.; Lee, P.S.; Le, K.; Tran, D.; Lin, X. Temporary Amniotic Membrane Graft Placement For Treatment Of Refractory Macular Holes. Retin. Cases Brief Rep. 2024, 10–1097. [Google Scholar] [CrossRef]
- Proença, H.; Antunes, M.; Ferreira, J.T.; Magro, P.; Faria, M.; Marques-Neves, C. Outcomes of amniotic membrane transplant for refractory macular hole–an optical coherence tomography and autofluorescence long-term study. Graefes Arch. Clin. Exp. Ophthalmol. 2025, 263, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Lai, C.C.; Hwang, Y.S.; Chen, K.J.; Wu, W.C. A Surgical Approach for Managing Refractory Macular Holes Using the Human Amniotic Membrane Patch Technique. Ophthalmic Surg. Lasers Imaging Retin. 2024, 55, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Medina-Medina, S.; Ramírez-Estudillo, A.; Rojas Juárez, S. OCT Features of the Donor Area in Autologous Retinal Transplant Surgery for Macular Hole. J. Vitr. Dis. 2024, 8, 593–596. [Google Scholar] [CrossRef]
- Carlà, M.M.; Mateo, C. Autologous retina transplantation for refractory highly myopic macular holes, A long-term follow-up. Jpn. J. Ophthalmol. 2025, 69, 259–267. [Google Scholar] [CrossRef]
- Parisi, G.; Ricardi, F.; Boscia, G.; Ghilardi, A.; Gelormini, F.; Marolo, P.; Fallico, M.; D’Antico, S.; Salafia, M.; Reibaldi, M. Macula-Off Retinal Detachment with Refractory Macular Hole Previously Closed with Autologous Platelet-Rich Plasma, A Case Report. Case Rep. Ophthalmol. 2023, 14, 462–468. [Google Scholar] [CrossRef]
- Ricardi, F.; Gelormini, F.; Parisi, G.; Vallino, V.; Borrelli, E.; Marolo, P.; D’Antico, S.; Salafia, M.; Reibaldi, M. The no-retina-touch technique, Vitrectomy and platelet-rich plasma in the treatment of lamellar macular hole. New insights into pathogenesis. Eye 2025, 39, 300–306. [Google Scholar] [CrossRef]
- Jujo, T.; Shiono, A.; Sato, K.; Sekine, R.; Uchiyama, N.; Kakehashi, K.; Endo, A.; Arakawa, A.; Shinkai, Y.; Kitaoka, Y. Retinal Migration and Surgical Outcome After Hemi-Temporal Internal Limiting Membrane Peeling Versus Conventional Peeling for Macular Hole, A Multicenter, Randomized, Controlled Trial. Retina 2024, 44, 1793–1799. [Google Scholar] [CrossRef]
- Ehrhardt, A.; Delpuech, M.; Luc, A.; Zessler, A.; Pastor, G.; Angioi-Duprez, K.; Berrod, J.P.; Thilly, N.; Conart, J.B. Dissociated Optic Nerve Fiber Layer Appearance after Macular Hole Surgery, A Randomized Controlled Trial Comparing the Temporal Inverted Internal Limiting Membrane Flap Technique with Conventional Peeling. Ophthalmol. Retin. 2023, 7, 227–235. [Google Scholar] [CrossRef]
- Marolo, P.; Caselgrandi, P.; Fallico, M.; Parisi, G.; Borrelli, E.; Ricardi, F.; Gelormini, F.; Ceroni, L.; Reibaldi, M. SMALL Study Group. Vitrectomy in Small idiopathic MAcuLar hoLe (SMALL) study, Internal limiting membrane peeling versus no peeling. Acta Ophthalmol. 2025, 103, e156–e164. [Google Scholar] [CrossRef]
- Fallico, M.; Caselgrandi, P.; Marolo, P.; Parisi, G.; Borrelli, E.; Ricardi, F.; Gelormini, F.; Ceroni, L.; Reibaldi, M. SMALL Study Group. Vitrectomy in Small idiopathic MAcuLar hoLe (SMALL) study, Conventional internal limiting membrane peeling versus inverted flap. Eye 2024, 38, 3334–3340. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, F.; Banderas-García, S.; Patton, N.; Dhawahir-Scala, F. Intraoperative Closure of Large Full-Thickness Macular Holes with Perfluorocarbon Liquid. Retina 2025, 45, 1012–1015. [Google Scholar] [CrossRef]
- de Freitas, L.P.; Neto, J.M.; Neves, L.L.; Bastos, T.; Pires, A.C.; Casella, A.M.; Isaac, D.L.; de Ávila, M.P. Pioneering evaluation in Brazil of microscope-integrated optical coherence tomography with a three-dimensional digital visualization system during pars plana vitrectomy for the treatment of macular hole. Int. J. Retin. Vitr. 2025, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Cakir, Y.; Sassine, A.G.; Amine, R.; Matar, K.; Talcott, K.E.; Srivastava, S.K.; Reese, J.L.; Ehlers, J.P. Analysis of retinal alterations utilizing intraoperative OCT following surgical interventions with novel ILM forceps in the DISCOVER study. Sci. Rep. 2024, 14, 16959. [Google Scholar] [CrossRef]
- Górska, A.; Sirek, S.; Woszczek, D.; Leszczyński, R. Microvascular Changes in Full-Thickness Macular Hole Patients Before and After Vitrectomy, An Optical Coherence Tomography-Angiography Study. Clin. Pract. 2025, 15, 58. [Google Scholar] [CrossRef]
- Lamas-Francis, D.; Rodríguez-Fernández, C.A.; Bande, M.; Blanco-Teijeiro, M.J. Foveal microvascular features following inverted flap technique for closure of large macular holes. Eur. J. Ophthalmol. 2024, 34, 260–266. [Google Scholar] [CrossRef]
- Sahoo, N.K.; Suresh, A.; Patil, A.; Ong, J.; Kazi, E.; Tyagi, M.; Narayanan, R.; Nayak, S.; Jacob, N.; Venkatesh, R.; et al. Novel En Face OCT-Based Closure Patterns in Idiopathic Macular Holes. Ophthalmol. Retin. 2023, 7, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Tsuboi, K.; Wakabayashi, T.; Baba, K.; Kamei, M. En Face OCT Detects Preretinal Abnormal Tissues Before and After Internal Limiting Membrane Peeling in Eyes with Macular Hole. Ophthalmol. Retin. 2023, 7, 153–163. [Google Scholar] [CrossRef]
- Alkabes, M.; Rabiolo, A.; Govetto, A.; Fogagnolo, P.; Ranno, S.; Marchetti, M.; Frerio, F.; Wild, D.; Gatti, V.; Muraca, A.; et al. Choroidal hypertransmission width on optical coherence tomography, A prognostic biomarker in idiopathic macular hole surgery. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 2481–2489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Zhang, W.; Zhang, Y.; Gu, X.; Zhang, Y. Comparison of the morphological characteristics of the choroidal sublayer between idiopathic macular holes and epiretinal membranes with automatic analysis. BMC Ophthalmol. 2023, 23, 277. [Google Scholar] [CrossRef]
- Endo, H.; Kase, S.; Takahashi, M.; Ito, Y.; Sonoda, S.; Sakoguchi, T.; Sakamoto, T.; Katsuta, S.; Ishida, S.; Kase, M. Changes in choriocapillaris structure occurring in idiopathic macular hole before and after vitrectomy. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 1901–1912. [Google Scholar] [CrossRef]
- Alkabes, M.; Muraca, A.; La Franca, L.; Rabiolo, A.; Fogagnolo, P.; Ranno, S.; Frerio, F.; Marchetti, M.; Wild, D.; Gatti, V.; et al. Association between Ectopic Inner Foveal Layer on Optical Coherence Tomography and Postoperative Quantitative Metamorphopsia in Patients Undergoing Epiretinal Membrane Surgery. Ophthalmologica 2025, 3, 1–10. [Google Scholar] [CrossRef]
- Mafi, M.; Govetto, A.; Mahmoudinezhad, G.; Prasad, P.; Bousquet, E.; Voichanski, S.; Feo, A.; Sarraf, D. Pathogenesis Of Ectopic Inner Foveal Layers And Its Impact On Visual Recovery After Epiretinal Membrane Peeling. Retina 2025, 45, 1108–1116. [Google Scholar] [CrossRef]
- Leisser, C.; Schlatter, A.; Ruiss, M.; Pilwachs, C.; Findl, O. Changes of Optical Coherence Tomography Biomarkers after Peeling of Epiretinal Membranes. Ophthalmologica 2025, 248, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Besagar, S.; Chennupati, S.; Ji, X.; Chen, Q.; Thomas, A.S.; Finn, A.P. Optical Coherence Tomography Biomarkers in Epiretinal Membrane. Ophthalmic Surg. Lasers Imaging Retin. 2025, 56, 422–428. [Google Scholar] [CrossRef]
- Mariotti, C.; Mangoni, L.; Muzi, A.; Fella, M.; Mogetta, V.; Bongiovanni, G.; Rizzo, C.; Chhablani, J.; Midena, E.; Lupidi, M. Artificial intelligence-based assessment of imaging biomarkers in epiretinal membrane surgery. Eur. J. Ophthalmol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.H.; Li, M.S.; Tseng, C.L.; Tu, H.P.; Sheu, S.J. Analysis of retinal microstructure and electrophysiology in eyes following pars plana vitrectomy and membrane peeling for vitreomacular interface disorders. BMC Ophthalmol. 2025, 25, 235. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, N.; Nagai, N.; Ui, R.; Takano, S.I.; Kawakita, T.; Imamura, Y. Correlation between internal limiting membrane preservation and sub-epiretinal membrane space during epiretinal membrane surgery. Jpn. J. Ophthalmol. 2025, 69, 253–258. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, S.W.; Byon, I. Topographic changes in macula and its association with visual outcomes in idiopathic epiretinal membrane surgery. PLoS ONE 2025, 20, e0316847. [Google Scholar] [CrossRef]
- Matos, A.M.F.; Defina, R.L.S.; Costa-Cunha, L.V.F.; Zacharias, L.C.; Preti, R.C.; Monteiro, M.L.R.; Cunha, L.P. Correlation between retinal sensitivity assessed by microperimetry and structural abnormalities on optical coherence tomography after successful epiretinal membrane surgery. Int. J. Retin. Vitr. 2024, 10, 24. [Google Scholar] [CrossRef]
- Li, H.; Zhang, C.; Li, H.; Yang, S.; Liu, Y.; Wang, F. Effects of disorganization of retinal inner layers for Idiopathic epiretinal membrane surgery, The surgical status and prognosis. BMC Ophthalmol. 2023, 23, 108. [Google Scholar] [CrossRef]
- Uzel, M.M.; Gelisken, F.; Konrad, E.; Neubauer, J. Clinical and morphological characteristics of patients with idiopathic epiretinal membrane with foveal herniation. Eye 2023, 37, 1357–1360. [Google Scholar] [CrossRef]
- Yang, X.; Wu, X.; Qi, B.; Zhang, K.; Yu, Y.; Wang, X.; Feng, X.; Jia, Q.; Jin, Z.B.; Liu, W. Foveal Microstructure and Visual Outcomes after Pars Plana Vitrectomy in Patients with Different Types of Epiretinal Membrane Foveoschisis. Ophthalmic Res. 2024, 67, 137–144. [Google Scholar] [CrossRef]
- Hetzel, A.; Neubauer, J.; Gelisken, F. Clinical characteristics of patients with epiretinal membrane-Foveoschisis. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 1579–1585. [Google Scholar] [CrossRef]
- Caretti, L.; Pillon, G.; Verzola, G.; Angelini, E.; Monterosso, C.; Bonfiglio, V.; Longo, A.; Formisano, M. Idiopathic epiretinal membrane surgery with internal limiting membrane peeling, An optical coherence tomography angiography analysis of macular capillary plexus changes. Eur. J. Ophthalmol. 2025, 35, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiuseppe, E.; Visioli, G.; Albanese, G.M.; Iannetti, L.; Romano, E.; Guillot, A.; Lucchino, L.; Gharbiya, M. Peripapillary and Macular Optical Coherence Tomography Angiography Predictors of Visual Improvement in Patients Treated with Vitrectomy for Idiopathic Epiretinal Membrane. Ophthalmologica 2025, 248, 54–66. [Google Scholar] [CrossRef]
- Zhang, Z.; Mao, J.; Lao, J.; Deng, X.; Fang, Y.; Chen, N.; Liu, C.; Chen, Y.; Shen, L. A classification of idiopathic epiretinal membrane based on foveal avascular zone area using optical coherence tomography angiography. Ann Med. 2024, 56, 2316008. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.; Park, J.B.; Kang, M.S.; Kim, E.S.; Yu, S.Y.; Kim, K. Peripapillary microvasculature changes after vitrectomy in epiretinal membrane via swept-source OCT angiography. BMC Ophthalmol. 2023, 23, 50. [Google Scholar] [CrossRef] [PubMed]
- Luan, R.; Liu, B.; Cai, B.; Gong, Y.; Li, X. Application of Subretinal Balanced Salt Solution Injection, A Novel technique in treating Severe idiopathic Epiretinal Membrane. Retina 2024. [Google Scholar] [CrossRef]
- Mehta, N.N.; Le, A.D.; Nagel, I.D.; Agnihotri, A.; Heinke, A.; Cheng, L.; Bartsch, D.U.; Tran, M.; Truong, N.; Cheolhong, A.; et al. AI-Enhanced OCT Analysis for Detecting ILM Removal in ERM Surgery. Retina 2025. [Google Scholar] [CrossRef]
- Lin, H.L.; Tseng, P.C.; Hsu, M.H.; Peng, S.J. Using a Deep Learning Model to Predict Postoperative Visual Outcomes of Idiopathic Epiretinal Membrane Surgery. Am. J. Ophthalmol. 2025, 272, 67–78. [Google Scholar] [CrossRef]
- Kim, G.H.; Lee, J.; Park, Y.H. Exploratory analysis of choriocapillaris vasculature as a biomarker of idiopathic epiretinal membrane. PLoS ONE 2024, 19, e0306735. [Google Scholar] [CrossRef]
- Ruan, K.; Zhang, Y.; Cheng, D.; Qiao, Y.; Yu, Y.; Wu, M.; Zhu, X.; Tao, J.; Shen, M.; Shen, L. Short-term postoperative changes in the choroidal vascularity index in patients with a unilateral epiretinal membrane. BMC Ophthalmol. 2023, 23, 64. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Li, Z.; Hou, Q.; Wang, C.; Li, X. Insights into the underlying choroid in different stages of idiopathic epiretinal membranes after Viteromacular surgery. Acta Ophthalmol. 2023, 101, 403–412. [Google Scholar] [CrossRef]
- Nespolo, R.G.; Yi, D.; Cole, E.; Wang, D.; Warren, A.; Leiderman, Y.I. Feature Tracking and Segmentation in Real Time via Deep Learning in Vitreoretinal Surgery, A Platform for Artificial Intelligence-Mediated Surgical Guidance. Ophthalmol. Retin. 2023, 7, 236–242. [Google Scholar] [CrossRef]
- Kanzaki, Y.; Matoba, R.; Kimura, S.; Hosokawa, M.M.; Shiode, Y.; Doi, S.; Morita, T.; Kanzaki, S.; Takasu, I.; Tanikawa, A.; et al. Epiretinal Membrane Impairs the Inner Retinal Layer in a Traction Force-Dependent Manner. Ophthalmol. Sci. 2023, 3, 100312. [Google Scholar] [CrossRef]
- Ishikura, M.; Muraoka, Y.; Nishigori, N.; Kogo, T.; Akiyama, Y.; Numa, S.; Hata, M.; Ishihara, K.; Ooto, S.; Tsujikawa, A. Cellular Determinants of Visual Outcomes in Eyes with Epiretinal Membrane, Insights from Adaptive Optics OCT. Ophthalmol. Sci. 2024, 4, 100536. [Google Scholar] [CrossRef]
- Govetto, A.; Francone, A.; Lucchini, S.; Garavaglia, S.; Carini, E.; Virgili, G.; Radice, P.; Vogt, D.; Edwards, M.; Spaide, R.F.; et al. Microcystoid Macular Edema in Epiretinal Membrane, Not a Retrograde Maculopathy. Am. J. Ophthalmol. 2025, 272, 48–57. [Google Scholar] [CrossRef]
- Fan, Y.; Jiang, Y.; Mu, Z.; Xu, Y.; Xie, P.; Liu, Q.; Pu, L.; Hu, Z. Optical Coherence Tomography Characteristics Between Idiopathic Epiretinal Membranes and Secondary Epiretinal Membranes due to Peripheral Retinal Hole. J. Ophthalmol. 2025, 2025, 9299651. [Google Scholar] [CrossRef] [PubMed]
- Reichel, F.F.; Labbe, E.; Gelisken, F.; Seitz, I.P.; Hagazy, S.; Dimopoulos, S. Persistence and recurrence after removal of idiopathic epiretinal membrane. Eye 2025, 39, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Jin, S.; Li, F.; Zhao, J. Optical coherence tomography parameters as prognostic factors for stereopsis after vitrectomy for unilateral epiretinal membrane, A cohort study. Sci. Rep. 2024, 14, 6715. [Google Scholar] [CrossRef] [PubMed]
- Martins Melo, I.; Bansal, A.; Naidu, S.; Oquendo, P.L.; Hamli, H.; Lee, W.W.; Muni, R.H. Morphologic Stages of Rhegmatogenous Retinal Detachment Assessed Using Swept-Source OCT. Ophthalmol. Retin. 2023, 7, 398–405. [Google Scholar] [CrossRef]
- El-Sehemy, A.; Martins Melo, I.; Pecaku, A.; Zajner, C.; Naidu, S.; Motekalem, Y.; Muni, R.H. Postoperative Photoreceptor Integrity and Anatomical Outcomes Based On Presenting Morphologic Stage Of Rhegmatogenous Retinal Detachment. Retina 2024, 44, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Martins Melo, I.; Naidu, S.; Pecaku, A.; Zajner, C.; Bansal, A.; Oquendo, P.L.; Lee, W.W.; Muni, R.H. Impact of Baseline Morphologic Stage of Rhegmatogenous Retinal Detachment on Postoperative Visual Acuity. Ophthalmol. Retin. 2024, 8, 624–632. [Google Scholar] [CrossRef]
- Lee, W.W.; Francisconi, C.L.M.; Marafon, S.B.; Juncal, V.R.; Chaudhary, V.; Hillier, R.J.; Muni, R.H. Imaging Predictors Of Functional Outcomes After Rhegmatogenous Retinal Detachment Repair. Retina 2024, 44, 1758–1765. [Google Scholar] [CrossRef]
- Mahmoudzadeh, R.; Swaminathan, S.; Salabati, M.; Wakabayashi, T.; Patel, D.; Mehta, S.; Kuriyan, A.E.; Khan, M.A.; Klufas, M.A.; Garg, S.J.; et al. Retinal Displacement Following Rhegmatogenous Retinal Detachment Repair. Ophthalmic Surg. Lasers Imaging Retin. 2024, 55, 560–566. [Google Scholar] [CrossRef]
- dell’Omo, R.; Cucciniello, P.; Affatato, M.; Rapino, G.; D’Albenzio, A.; Venturi, F.; Campagna, G. Influence of Vitreous Cortex Remnants on Normal Retinal Anatomy in Eyes with Primary Rhegmatogenous Retinal Detachment. Ophthalmol. Retin. 2024, 8, 1002–1012. [Google Scholar] [CrossRef]
- Sassen, S.H.; Sassen, J.; Sassmannshausen, M.; Goerdt, L.; Liermann, Y.; Liegl, R.G.; Herrmann, P.; Finger, R.P.; Holz, F.G.; Thiele, S. Early Photoreceptor Alterations After Retinal Detachment Repair. Investig. Ophthalmol. Vis. Sci. 2025, 66, 32. [Google Scholar] [CrossRef]
- Kolli, A.; Wong, J.; Duret, S.; Stewart, J.M.; Connor TBJr Roorda, A.; Carroll, J.; Duncan, J.L. Outer retinal reflectivity and visual function loss after anatomically successful macula-off rhegmatogenous retinal detachment repair. Am. J. Ophthalmol. Case Rep. 2025, 38, 102294. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Abdel-Radi, M.; Aly, M.O.M.; Alattar, S. Correlation between multifocal electroretinogram and optical coherence tomography findings with visual acuity after vitrectomy surgery for retinal detachment, An observational study. Int. J. Retin. Vitreous. 2024, 10, 10. [Google Scholar] [CrossRef]
- Jhaveri, A.; Martins Melo, I.; Pecaku, A.; Zajner, C.; Naidu, S.; Batawi, H.; Muni, R.H. Outer Retinal Hyperreflective Dots, A Potential Imaging Biomarker in Rhegmatogenous Retinal Detachment. Ophthalmol. Retin. 2023, 7, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Savastano, M.C.; Carlà, M.M.; Giannuzzi, F.; Fossataro, C.; Cestrone, V.; Boselli, F.; Biagini, I.; Beccia, F.; Raffaele, Q.; Gravina, G.; et al. OCT analysis of preoperative foveal microstructure in recent-onset macula-off rhegmatogenous retinal detachment, Visual acuity prognostic factors. Br. J. Ophthalmol. 2024, 108, 1743–1748. [Google Scholar] [CrossRef]
- Mhibik, B.; Bernabei, F.; Pellegrini, M.; Constant, A.; Azri, C.; Eymard, P.; Romeo, M.A.; Azan, F.; Giannaccare, G.; Rothschild, P.R. Preretinal Hyperreflective Dots, A novel OCT Sign Associated with The Development of Epiretinal Membrane After Vitrectomy for Rhegmatogenous Retinal Detachment. Retina 2025. [Google Scholar] [CrossRef]
- dell’Omo, R.; Carosielli, M.; Rapino, G.; Affatato, M.; Cucciniello, P.; Virgili, G.; Filippelli, M.; Costagliola, C.; Campagna, G. Biomarkers of Vitreous Cortex Remnants in Eyes with Primary Rhegmatogenous Retinal Detachment. Transl. Vis. Sci. Technol. 2023, 12, 24. [Google Scholar] [CrossRef]
- Noji, S.; Mizuno, M.; Inoue, M.; Koto, T.; Hirakata, A. Characteristics of subretinal particles detected after pars plana vitrectomy for rhegmatogenous retinal detachment. BMC Ophthalmol. 2023, 23, 115. [Google Scholar] [CrossRef]
- Eton, E.A.; Pan, W.W.; Besirli, C.G.; Zacks, D.N.; Wubben, T.J. Incidence and Impact of Outer Retinal Folds After Pars Plana Vitrectomy for Primary Rhegmatogenous Retinal Detachment Repair. J Vitreoretin Dis. 2025. [Google Scholar] [CrossRef] [PubMed]
- Crincoli, E.; Savastano, A.; Savastano, M.C.; Rizzo, C.; Kilian, R.; De Vico, U.; Biagini, I.; Carlà, M.M.; Giannuzzi, F.; Rizzo, S. Diameter of cystoid spaces and choroidal hypertransmission as novel prognostic biomarkers in myopic foveoschisis. Eye 2025, 39, 1781–1786. [Google Scholar] [CrossRef]
- Ferro Desideri, L.; Danilovska, T.; Bernardi, E.; Artemiev, D.; Paschon, K.; Hayoz, M.; Jungo, A.; Sznitman, R.; Zinkernagel, M.S.; Anguita, R. Artificial Intelligence-Enhanced OCT Biomarkers Analysis in Macula-off Rhegmatogenous Retinal Detachment Patients. Transl. Vis. Sci. Technol. 2024, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ou, C.; Wang, G.; Lu, B.; Li, X.; Yang, T.; Zhang, J. Prediction of Visual Outcome After Rhegmatogenous Retinal Detachment Surgery Using Artificial Intelligence Techniques. Transl. Vis. Sci. Technol. 2024, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.H.M.; John, N.C.R.A.; Guillemaut, J.Y.; Yorston, D.; Frohlich, D.; Steel, D.H.W.; Williamson, T.H. BEAVRS Retinal Detachment Outcomes Group. Artificial intelligence using deep learning to predict the anatomical outcome of rhegmatogenous retinal detachment surgery, A pilot study. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 715–721. [Google Scholar] [CrossRef]
- García-Vásquez, Á.; Rojas-Juárez, S.; Rios-Nequis, G.; Ramirez-Estudillo, A. Lyophilised amniotic membrane patches are a safe and effective treatment for rhegmatogenous lesions in combined tractional and rhegmatogenous retinal detachment, A prospective interventional study. Eye 2025, 39, 307–313. [Google Scholar] [CrossRef]
- Machairoudia, G.; Kazantzis, D.; Chatziralli, I.; Theodossiadis, G.; Georgalas, I.; Theodossiadis, P. Microvascular changes after pars plana vitrectomy for rhegmatogenous retinal detachment repair, A comparative study based on gas tamponade agent. Eur. J. Ophthalmol. 2024, 34, 1247–1254. [Google Scholar] [CrossRef]
- Barequet, D.; Shemesh, R.; Zvi, D.; Cohen, R.; Trivizki, O.; Schwartz, S.; Barak, A.; Loewenstein, A.; Rabina, G. Functional and anatomical outcomes of fovea on, fovea off and fovea-splitting rhegmatogenous retinal detachment. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 3187–3192. [Google Scholar] [CrossRef]
- Hsieh, T.H.; Wang, J.K.; Chen, F.T.; Chen, Y.J.; Wang, L.U.; Huang, T.L.; Chang, P.Y.; Hsu, Y.R. Three-Dimensional Quantitative Analysis of Internal Limiting Membrane Peeling Related Structural Changes in Retinal Detachment Repair. Am. J. Ophthalmol. 2025, 269, 94–104. [Google Scholar] [CrossRef]
- Skeiseid, L.; Thomseth, V.M.; Achour, H.; Forsaa, V.A. Choroidal thickness and vascular density after rhegmatogenous retinal detachment. Acta Ophthalmol. 2025, 103, 432–438. [Google Scholar] [CrossRef]
- Ozal, E.; Ermis, S.; Karapapak, M.; Canli, O.F.; Ozal, S.A. Comparison of choroidal vascularity index in rhegmatogenous retinal detachment, Impact of silicone-oil vs. gas tamponade following pars plana vitrectomy. BMC Ophthalmol. 2025, 25, 261. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Ribarich, N.; Querques, G.; Park, D.H.; Kim, Y.J.; Choi, E.Y.; Byeon, S.H.; Kim, S.S.; Lee, C.S. Optic disc pit maculopathy-like retinoschisis without a clinically visible optic disc pit. Retina 2025. [Google Scholar] [CrossRef]
- Gklavas, K.; Athanasiou, A.; Neubauer, J.; Lilou, E.; Pohl, L.; Bartz-Schmidt, K.U.; Dimopoulos, S. Long-term outcomes of autologous platelet treatment for optic disc pit maculopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 3177–3185. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Jia, H.; Wang, H.; Li, T.; Sun, J.; Sun, X. Preoperative OCT-Derived Nasal Perifoveal Retinal Nerve Fiber Layer Thickness Predictively Correlates Long-Term Visual Acuity Post Oil Removal Surgery. Transl. Vis. Sci. Technol. 2024, 13, 16. [Google Scholar] [CrossRef]
- Bai, J.X.; Zheng, X.; Zhu, X.Q.; Peng, X.Y. Clinical and spectral-domain optical coherence tomography findings and changes in new-onset macular edema after silicone oil tamponade. BMC Ophthalmol. 2025, 25, 153. [Google Scholar] [CrossRef] [PubMed]
- Desideri, L.F.; Aissani, M.; Bernardi, E.; Zinkernagel, M.; Anguita, R. Macula Edema And Silicone Oil Tamponade, Clinical Features, Predictive Factors, And Treatment. Retina 2025. [Google Scholar] [CrossRef]
- Al-Shehri, A.M.; Aljohani, S.; Aldihan, K.A.; Alrashedi, M.J.; Alrasheed, S.; Schatz, P. Effect of silicone oil versus gas tamponade on macular layer microstructure after pars plana vitrectomy for macula on rhegmatogenous retinal detachment. BMC Ophthalmol. 2024, 24, 119. [Google Scholar] [CrossRef]
- Lin, Z.; Gao, K.; Tuxun, R.; Tsai, C.L.; Xu, Z.; Jiang, L.; Liu, Y.; Chen, Z.; Chen, Z.; Liu, B.; et al. Preoperative Widefield Swept-Source Optical Coherence Tomography Versus Intraoperative Findings in Detecting Posterior Vitreous Detachment. Transl. Vis. Sci. Technol. 2024, 13, 39. [Google Scholar] [CrossRef]
- Valikodath, N.G.; Li, J.D.; Raynor, W.; Izatt, J.A.; Toth, C.A.; Vajzovic, L. Intraoperative OCT-Guided Volumetric Measurements of Subretinal Therapy Delivery in Humans. J. Vitr. Dis. 2024, 8, 587–592. [Google Scholar] [CrossRef]
- Sandali, O.; Tahiri Joutei Hassani, R.; Armia Balamoun, A.; Franklin, A.; Sallam, A.B.; Borderie, V. Operative Digital Enhancement of Macular Pigment during Macular Surgery. J. Clin. Med. 2023, 12, 2300. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Reyes, M.A.; Quiroz-Gonzalez, E.A.; Quiroz-Gonzalez, M.A.; Lima-Gomez, V. Choroidal Perfusion Changes After Vitrectomy for Myopic Traction Maculopathy. Semin. Ophthalmol. 2024, 39, 261–270. [Google Scholar] [CrossRef]
- Zhou, H.T.; Mei, J.H.; Lin, K.; Deng, C.Y.; Pan, A.; Lin, Z.S.; Lin, J.; Lin, W.; Lin, Z. Changes of diabetic macular edema post vitrectomy in patients with proliferative diabetic retinopathy. Int. J. Ophthalmol. 2025, 18, 868–875. [Google Scholar] [CrossRef]
- Wei-Zhang, S.; He, K.; Zhou, W.; Yu, J.; Zhao, J.; He, T.; Chen, S.; Kaysar, P.; Sun, Z.; Jia, D.; et al. Relationship between visual acuity and OCT angiography parameters in diabetic retinopathy eyes after treatment. Eur. J. Ophthalmol. 2024, 34, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Nawrocka, Z.A.; Nawrocka, Z.; Nawrocki, J. Vitrectomy With Ilm Peeling in Diabetic Macular Edema in One Eye Vs. Intravitreal Anti-Vegf Inject. Second Eye A Comp. Study. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 67–76. [Google Scholar] [CrossRef]
- Laíns, I.; Wang, J.C.; Cui, Y.; Katz, R.; Vingopoulos, F.; Staurenghi, G.; Vavvas, D.G.; Miller, J.W.; Miller, J.B. Retinal applications of swept source optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA). Prog. Retin. Eye Res. 2021, 84, 100951. [Google Scholar] [CrossRef]
- Meira, J.; Marques, M.L.; Falcão-Reis, F.; Rebelo Gomes, E.; Carneiro, Â. Immediate Reactions to Fluorescein and Indocyanine Green in Retinal Angiography, Review of Literature and Proposal for Patient’s Evaluation. Clin. Ophthalmol. 2020, 14, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, M.L.; Wollstein, G.; Ishikawa, H.; Kagemann, L.; Xu, J.; Folio, L.S.; Schuman, J.S. Optical coherence tomography, History, current status, and laboratory work. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2425–2436. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Jayaswal, R.; Madhusudhan, S.; Gibran, S.K. Macular OCT changes after vitrectomy. Ophthalmology 2007, 114, 1955. [Google Scholar] [CrossRef] [PubMed]
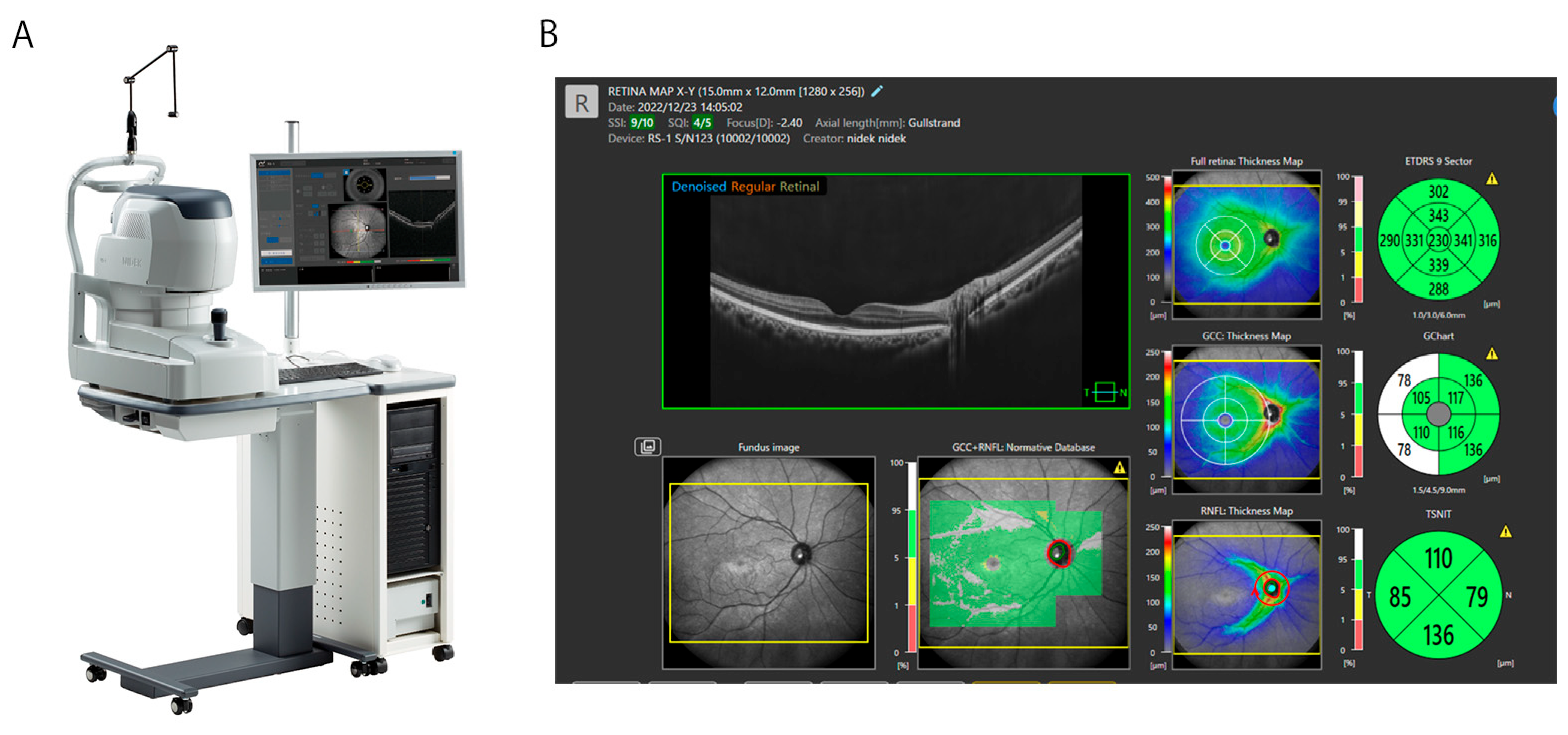
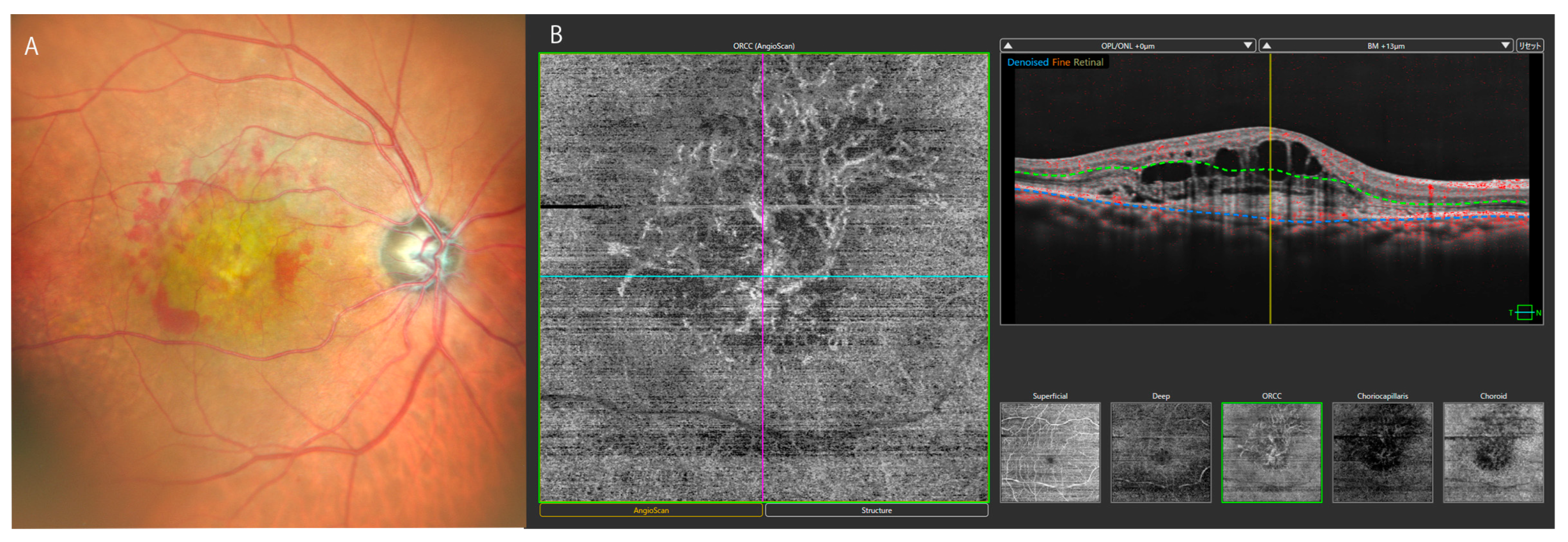
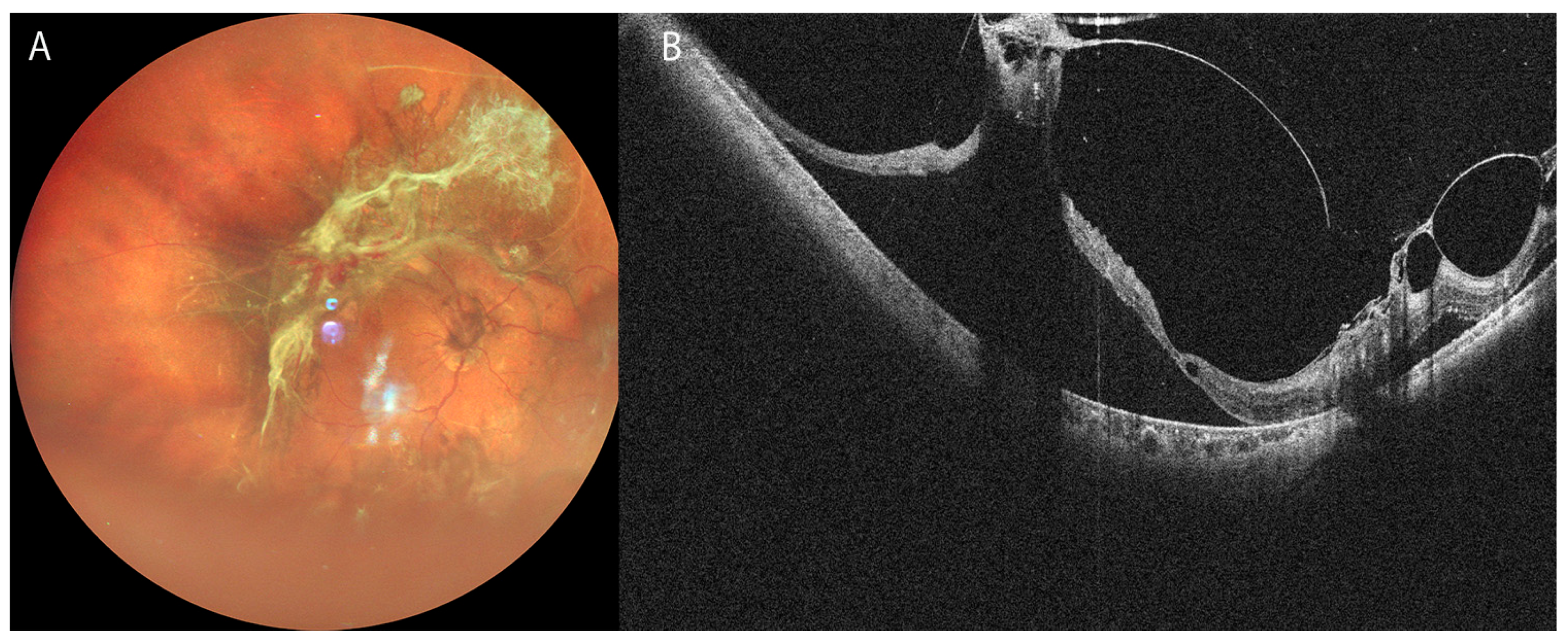
| Techniques | Major outcomes | References |
|---|---|---|
| EIFL thickness | Postoperative VA and metamorphopsia | [92,93,94] |
| EZ disruption, EZ-ELM integrity, RNFL schisis | Poor postoperative VA improvement | [95,96,97] |
| DRIL | Poor visual outcome, limited surgical outcome | [100,101] |
| Foveal herniation | Variable visual disturbances | [102] |
| RPC/SCP density, FAZ (OCTA) | Postoperative VA | [105,106,107,108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horie, S.; Yoshida, T.; Ohno-Matsui, K. Current Topics in OCT Applications in Vitreoretinal Surgery. Bioengineering 2025, 12, 962. https://doi.org/10.3390/bioengineering12090962
Horie S, Yoshida T, Ohno-Matsui K. Current Topics in OCT Applications in Vitreoretinal Surgery. Bioengineering. 2025; 12(9):962. https://doi.org/10.3390/bioengineering12090962
Chicago/Turabian StyleHorie, Shintaro, Takeshi Yoshida, and Kyoko Ohno-Matsui. 2025. "Current Topics in OCT Applications in Vitreoretinal Surgery" Bioengineering 12, no. 9: 962. https://doi.org/10.3390/bioengineering12090962
APA StyleHorie, S., Yoshida, T., & Ohno-Matsui, K. (2025). Current Topics in OCT Applications in Vitreoretinal Surgery. Bioengineering, 12(9), 962. https://doi.org/10.3390/bioengineering12090962






