Mechanical Properties and Microstructure of Decellularized Brown Seaweed Scaffold for Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Decellularization
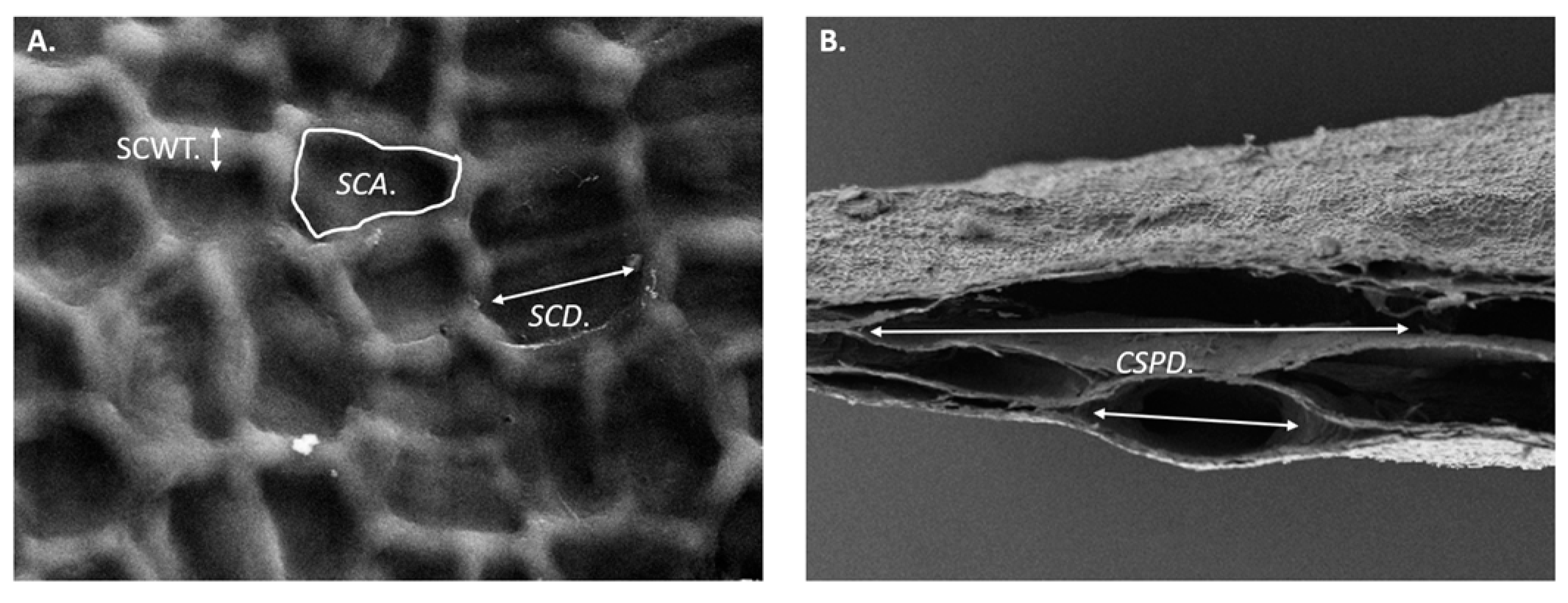
2.3. Microstructural Analysis by Scanning Electron Microscopy
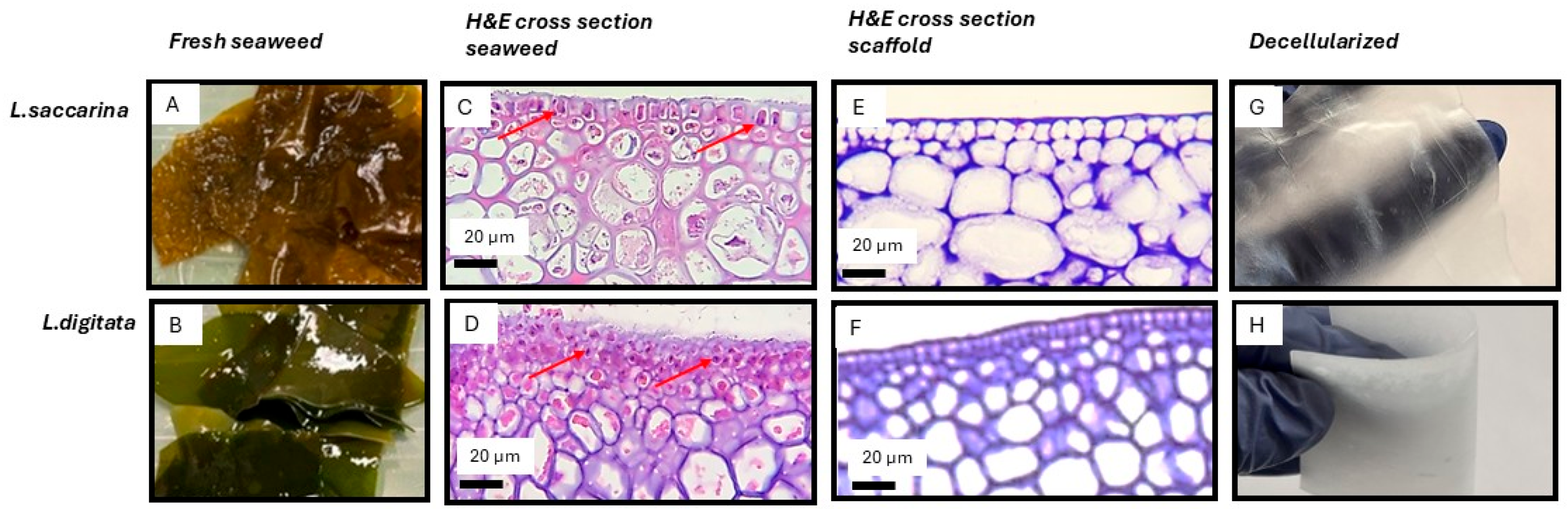
2.4. Histology (H&E) for Assessment of Decellularization
2.5. Chemical Composition
2.6. Swelling Ratio
2.7. Absorption
2.8. Porosity
2.9. Micro-CT Analysis
2.10. Mechanical Testing
2.11. Statistical Analysis
3. Results
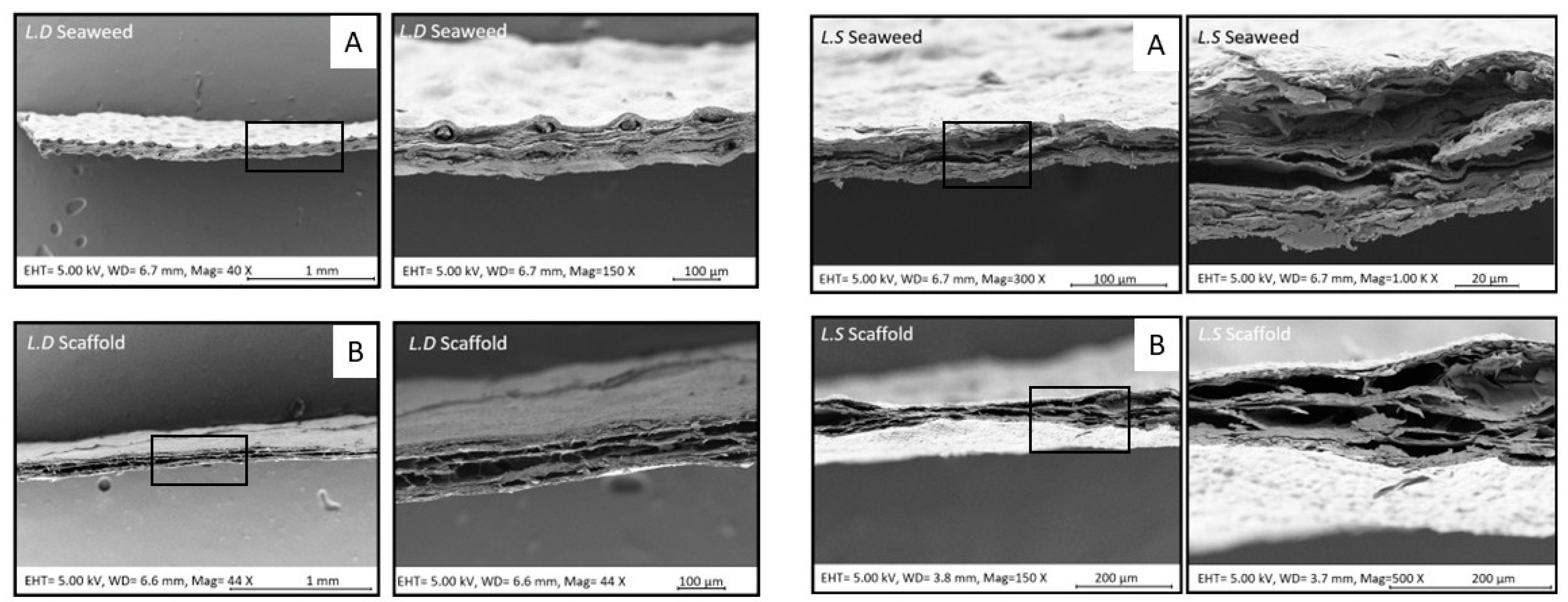
3.1. Visible-Light Decellularization Produces Acellular Seaweed Scaffolds with Preserved Architecture and Selective Biomatrix Retention
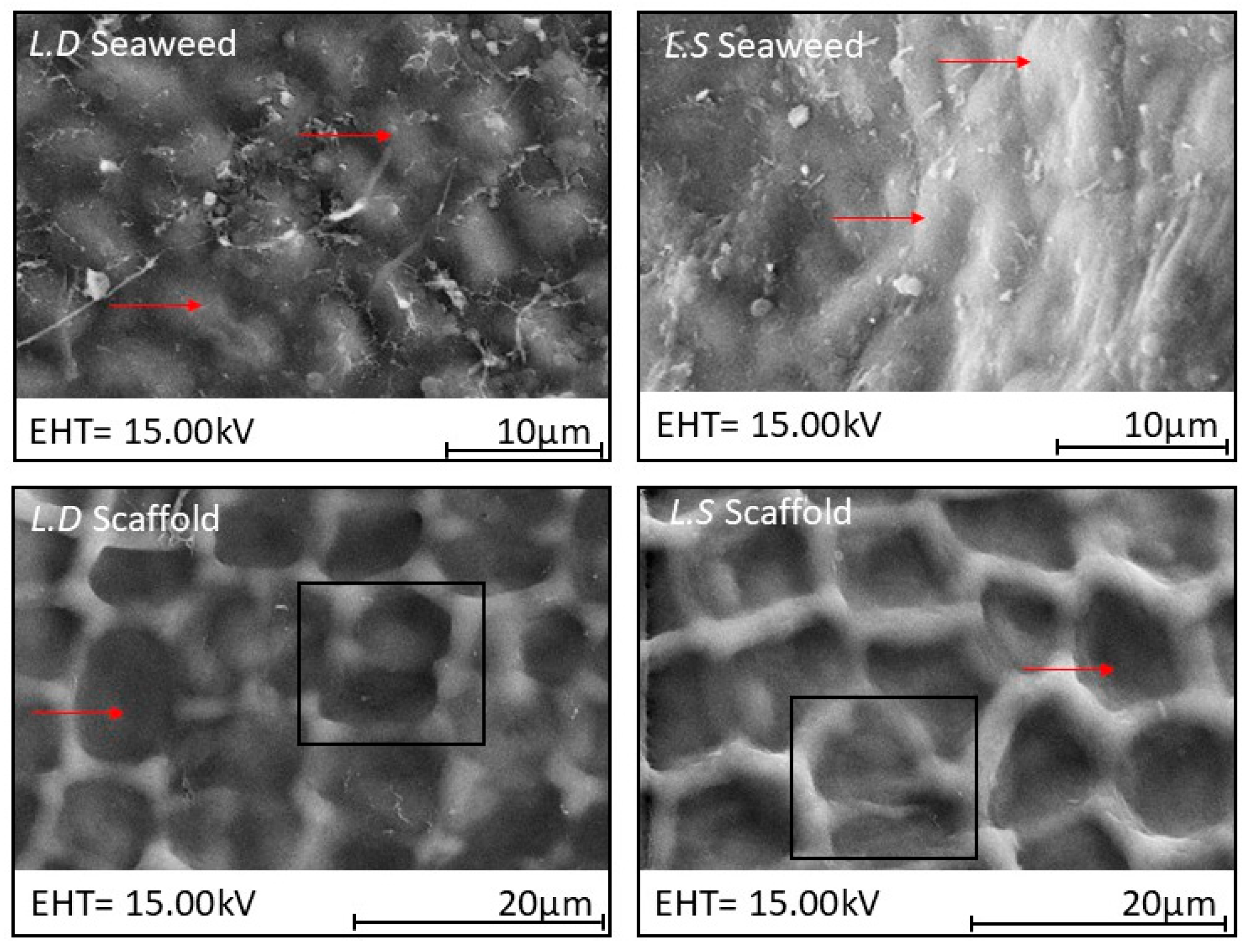
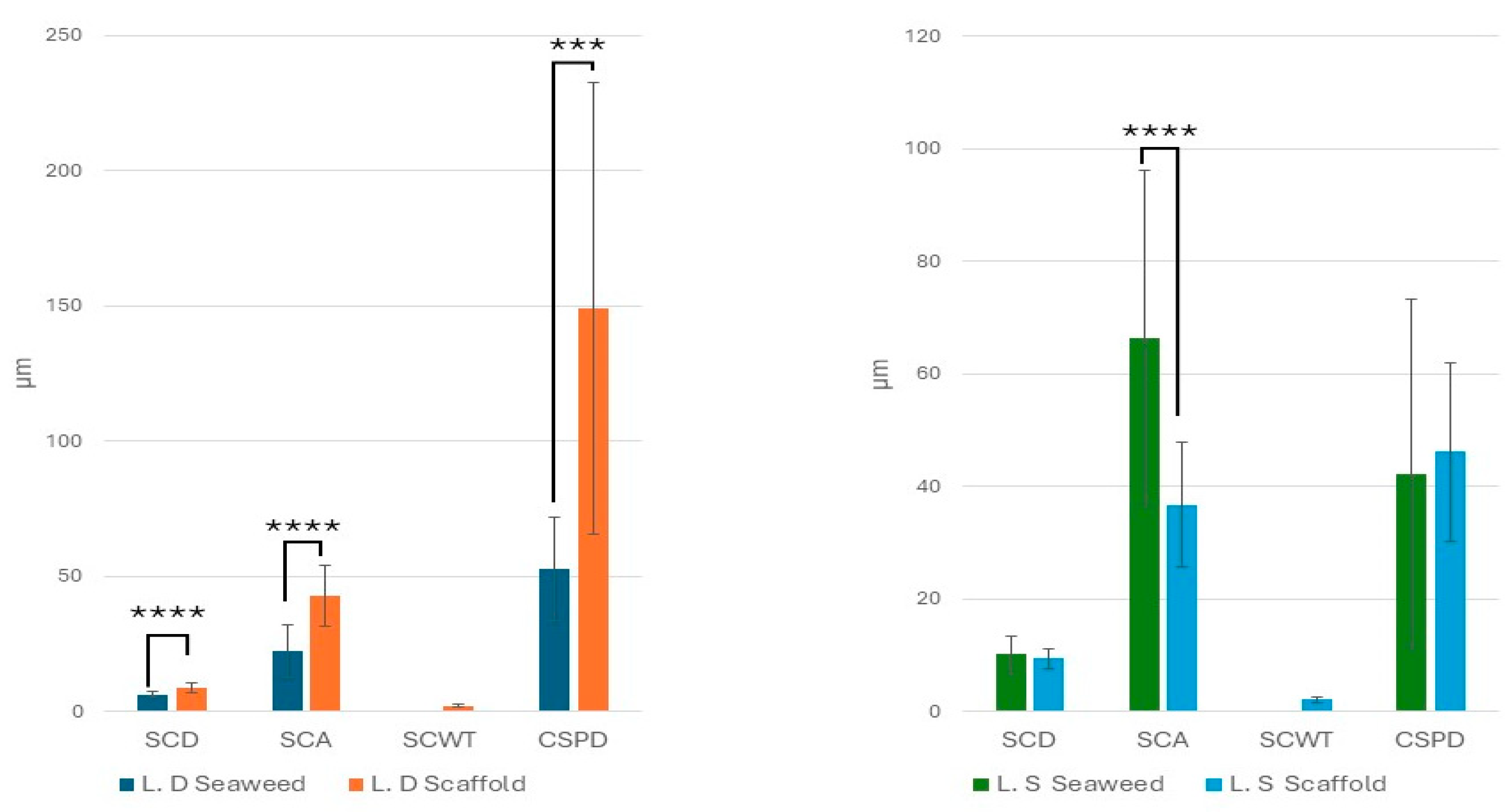
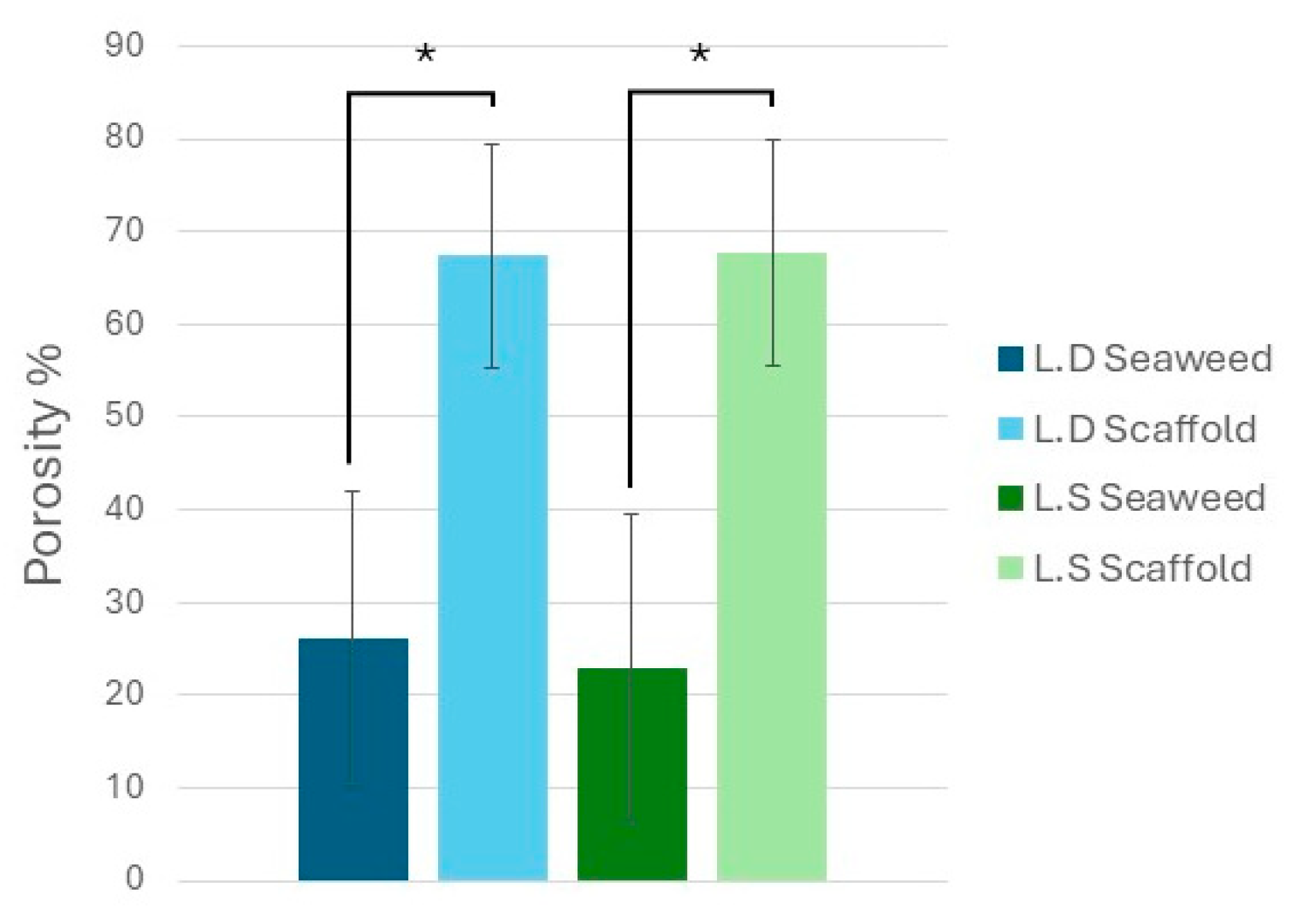
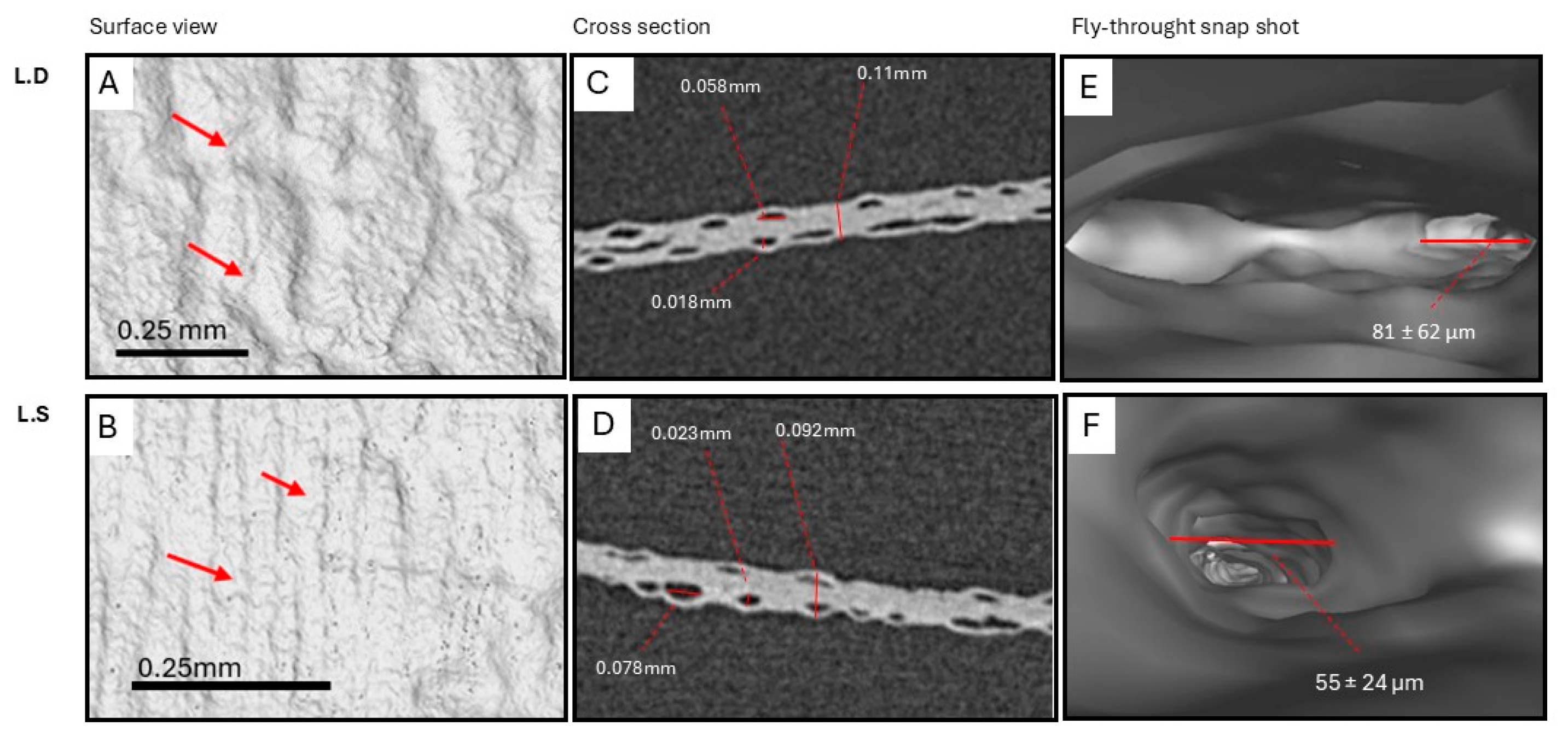
3.2. Biological Seaweed Scaffolds Retain Their Microstructural Features with Increased Porosity
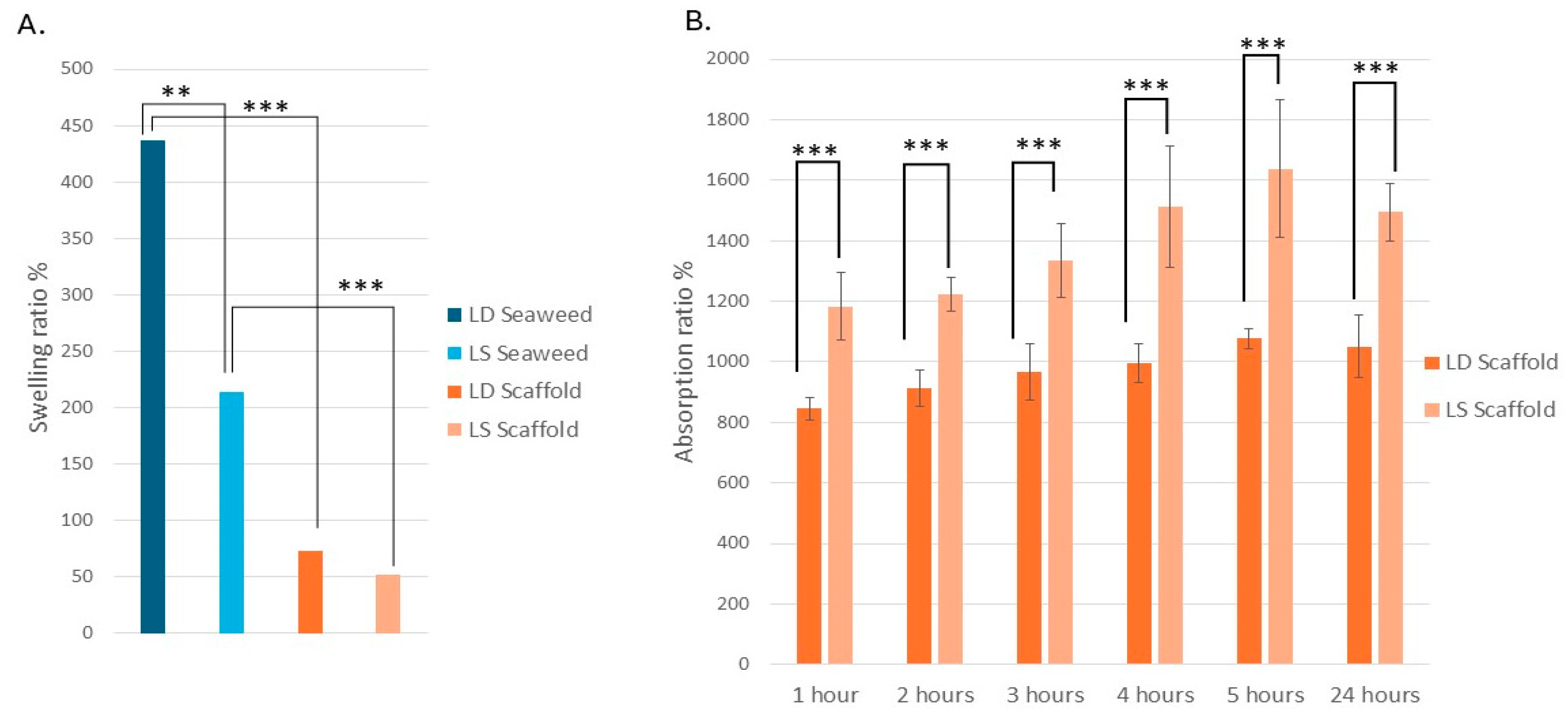
3.3. The L.D. and L.S. Biological Scaffolds Have Similar Swelling Properties but Different Fluid Absorption Properties
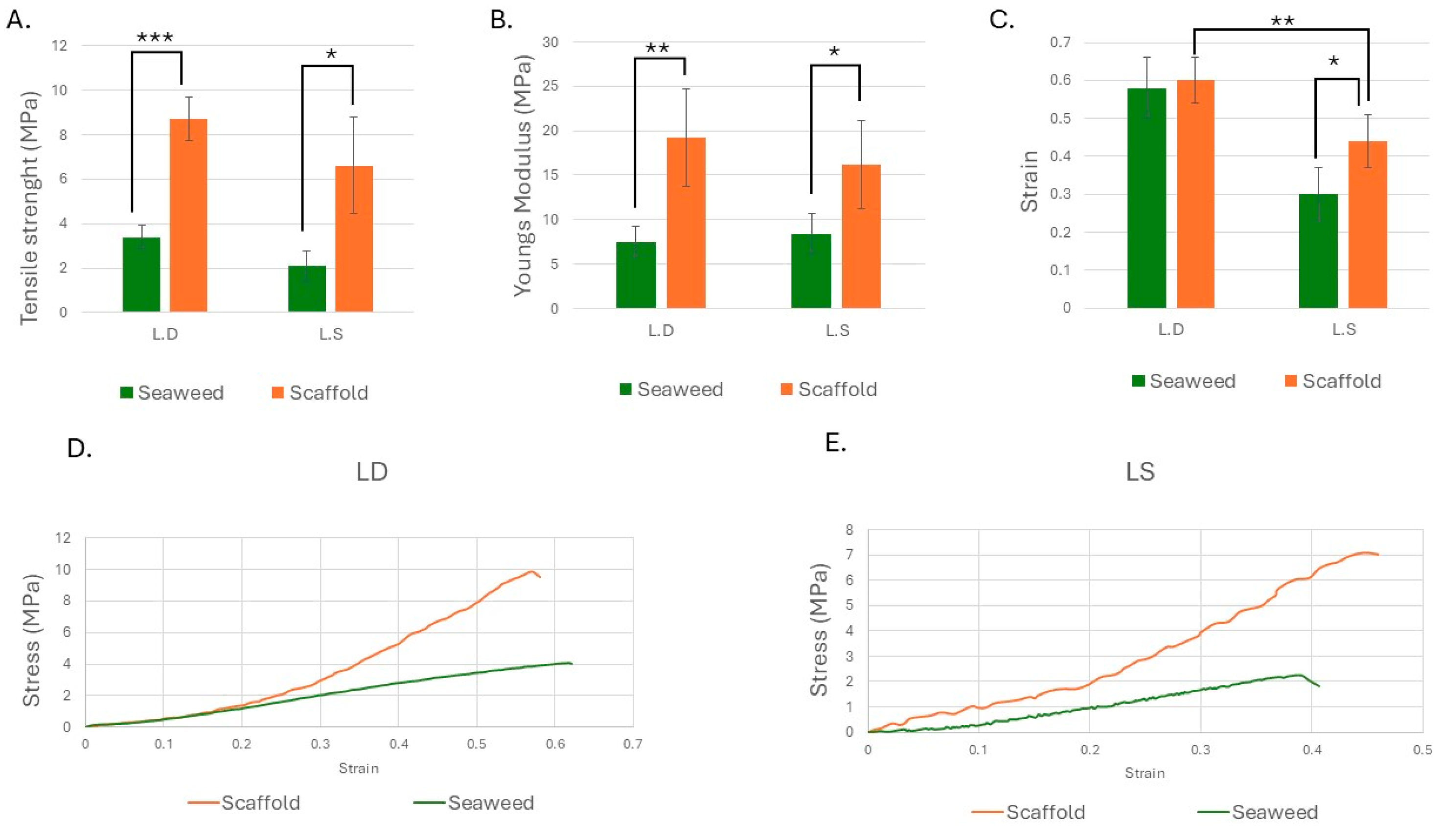
3.4. Strength and Stiffness Increased in Both Scaffolds, with Strain Enhancement Observed in L.S.
4. Discussion
4.1. Sequence and Species-Dependent Visible-Light Decellularization
4.2. Choosing the Default Decellularization Method of Scaffold Production
4.3. Scaffold Macro- and Microstructure Preservation and Implications for Recellularization
4.4. The Scaffolds’ Selective Retention and/or Depletion of Polysaccharides
4.5. Mineral Shifts and Alginate–Calcium Interactions in the Scaffolds
4.6. Scaffolds’ Microstructural Features and Porosity
4.7. Species -Specific Decellularization Effect on Microstructure: Proposed Mechanistic Insights
4.8. Functional Implications of the Scaffold’s Microstructure and Clinical Application
4.9. Comparisons to Plant-Derived and Clinical Scaffolds
4.10. Scaffold Swelling and Absorption Performance
4.11. Time-Dependent Kinetics: Early Burst Then Plateau
4.12. Comparison with Related Scaffolds and Dressings
4.13. Clinical Indications Suggested by the Swelling and Absorption Data
4.14. The Mechanical Properties of the Seaweed Scaffolds
4.15. Mechanical Properties and Clinical Relevance
5. Conclusions
- L.D. offered increased stiffness and strength without losing ductility; L.S. achieved more strength along with enhanced extensibility.
- Histological, SEM, and micro-CT analyses verified that the original layered microstructure and internal tunnel networks were preserved.
- Biochemical analysis showed reduced mannitol, laminarin, and sodium in seaweed, alongside a relative increase in calcium, likely due to its strong binding to the alginate matrix.
- Fucoidan content was maintained or even relatively increased in L.S. scaffolds, potentially supporting future bioactivity.
- Both scaffolds were highly hydrophilic: L.S. absorbed 1494% fluid in 24 h while L.D. showed greater swelling, a key property for wound healing and exudate management.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| L.D. | Laminaria digitata |
| L.S. | Laminaria saccharina |
| SCD | Surface Chamber Diameter |
| SCA | Surface Chamber Area |
| CSPD | Cross-Sectional Pore Diameter |
| SCWT | Surface Chamber Wall Thickness |
| SEM | Scanning electron microscopy |
| Micro-CT | Micro-computed tomography |
| H&E | Hematoxylin and eosin staining |
| W0 | Initial weight |
| W1 | Test weight |
| Wi | Weight at time i |
| σuts | Tensile strenght |
| E | Elastic modulus or Young’s modulus |
| ε | Strain |
References
- Fan, S.; Chen, K.; Yuan, W.; Zhang, D.; Yang, S.; Lan, P.; Song, L.; Shao, H.; Zhang, Y. BiomaterialBased Scaffolds as Antibacterial Suture Materials. ACS Biomater. Sci. Eng. ACS Biomater. Sci. Eng. 2020, 6, 3154–3161. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Liu, X.; Lu, S.; Wang, T.; Wang, X.; Yang, K.; Yang, H. Advances and prospects of 3D printed antibacterial bone implants: A systematic review. J. Mater. Sci. Technol. 2024, 200, 227–242. [Google Scholar] [CrossRef]
- Shen, H.Y.; Xing, F.; Shang, S.Y.; Jiang, K.; Kuzmanović, M.; Huang, F.W.; Liu, Y.; Luo, E.; Edeleva, M.; Cardon, L.; et al. Biomimetic Mineralized 3DPrinted Polycaprolactone Scaffold Induced by SelfAdaptive Nanotopology to Accelerate Bone Regeneration. ACS Appl. Mater. Interfaces 2024, 16, 18658–18670. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, W.; Chen, Q.; Ren, T.; Yang, J.; Li, G.; Qi, Y.; Yuan, C.; Wang, P. Bioprinted PDLSCs with highconcentration GelMA hydrogels exhibit enhanced osteogenic differentiation in vitro and promote bone regeneration in vivo. Clin. Oral Investig. 2023, 27, 5153–5170. [Google Scholar] [CrossRef] [PubMed]
- Decellularization—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/decellularization (accessed on 22 August 2025).
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Azhim, A.; Syazwani, N.; Morimoto, Y.; Furukawa, K.; Ushida, T. The use of sonication treatment to decellularize aortic tissues for preparation of bioscaffolds. J. Biomater. Appl. 2014, 29, 130–141. [Google Scholar] [CrossRef]
- Adamski, M.; Fontana, G.; Gershlak, J.R.; Gaudette, G.R.; Le, H.D.; Murphy, W.L. Two Methods for Decellularization of Plant Tissues for Tissue Engineering Applications. J. Vis. Exp. JoVE 2018, 135, 57586. [Google Scholar] [CrossRef]
- BerryKilgour, C.; Oey, I.; Cabral, J.; Dowd, G.; Wise, L. Decellularized Green and Brown Macroalgae as Cellulose Matrices for Tissue Engineering. J. Funct. Biomater. 2024, 15, 390. [Google Scholar] [CrossRef] [PubMed]
- Gershlak, J.R.; Burgess, C.K.; Fontana, G.; Perreault, L.R.; Thyden, R.; Shrem, L.A.; dos Santos, A.C.F.; Weathers, P.J.; Dominko, T.; Murphy, W.L.; et al. Biocompatibility of decellularized spinach leaves. npj Biomed. Innov. 2025, 2, 23. [Google Scholar] [CrossRef]
- Chun, Y.S.; Verma, K.; Rosen, H.; Lipsitz, S.; Morris, D.; Kenney, P.; Eriksson, E. ImplantBased Breast Reconstruction Using Acellular Dermal Matrix and the Risk of Postoperative Complications. Plast. Reconstr. Surg. 2010, 125, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Deeken, C.R.; Lake, S.P. Mechanical properties of the abdominal wall and biomaterials utilized for hernia repair. J. Mech. Behav. Biomed. Mater. 2017, 74, 411–427. [Google Scholar] [CrossRef]
- Yoon, J.; Yoon, D.; Lee, H.; Lee, J.; Jo, S.; Kym, D.; Yim, H.; Hur, J.; Chun, W.; Kim, G.; et al. Wound healing ability of acellular fish skin and bovine collagen grafts for splitthickness donor sites in burn patients: Characterization of acellular grafts and clinical application. Int. J. Biol. Macromol. 2022, 205, 452–461. [Google Scholar] [CrossRef]
- Faulkner, A.; Kent, J.; Geesink, I.; FitzPatrick, D. Purity and the dangers of regenerative medicine: Regulatory innovation of human tissueengineered technology. Soc. Sci. Med. 2006, 63, 2277–2288. [Google Scholar] [CrossRef]
- Collagen—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/materials-science/collagen (accessed on 13 October 2021).
- Hickey, R.J.; Pelling, A.E. Cellulose Biomaterials for Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 45. Available online: https://www.frontiersin.org/article/10.3389/fbioe.2019.00045 (accessed on 27 January 2022). [CrossRef]
- Gershlak, J.R.; Hernandez, S.; Fontana, G.; Perreault, L.R.; Hansen, K.J.; Larson, S.A.; Binder, B.Y.; Dolivo, D.M.; Yang, T.; Dominko, T.; et al. Crossing kingdoms: Using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials 2017, 125, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dominko, T.; Weathers, P.J. Using decellularized grafted leaves as tissue engineering scaffolds for mammalian cells. Vitr. Cell. Dev. Biol. Plant 2020, 56, 765–774. [Google Scholar] [CrossRef]
- Madub, K.; Goonoo, N.; Gimié, F.; Arsa, I.A.; Schönherr, H.; BhawLuximon, A. Green seaweeds ulvancellulose scaffolds enhance in vitro cell growth and in vivo angiogenesis for skin tissue engineering. Carbohydr. Polym. 2021, 251, 117025. [Google Scholar] [CrossRef] [PubMed]
- Biancarosa, I.; Espe, M.; Bruckner, C.G.; Heesch, S.; Liland, N.; Waagbø, R.; Torstensen, B.; Lock, E.J. Amino acid composition, protein content, and nitrogen-to-protein conversion factors of 21 seaweed species from Norwegian waters. J. Appl. Phycol. 2017, 29, 1001–1009. [Google Scholar] [CrossRef]
- Adams, J.; Ross, A.; Anastasakis, K.; Hodgson, E.; Gallagher, J.; Jones, J.; Donnison, I. Seasonal variation in the chemical composition of the bioenergy feedstock Laminaria digitata for thermochemical conversion. Bioresour. Technol. 2011, 102, 226–234. [Google Scholar] [CrossRef]
- Millar, R.; Houghton, J.D.R.; Kregting, L. The stress and strain of life—How differences in the mechanical properties and cellular composition enable the kelp Laminaria digitata to thrive in different hydrodynamic environments. Environ. Res. 2021, 169, 105330. [Google Scholar] [CrossRef] [PubMed]
- Zargarzadeh, M.; Amaral, A.J.R.; Custódio, C.A.; Mano, J.F. Biomedical applications of laminarin. Carbohydr. Polym. 2020, 232, 115774. [Google Scholar] [CrossRef]
- Ashton, R.S.; Banerjee, A.; Punyani, S.; Schaffer, D.V.; Kane, R.S. Scaffolds based on degradable alginate hydrogels and poly(lactidecoglycolide) microspheres for stem cell culture. Carbohydr. Polym. Biomaterials 2007, 28, 5518–5525. [Google Scholar] [CrossRef]
- Machado, B.; Costa, S.M.; Costa, I.; Fangueiro, R.; Ferreira, D.P. The potential of algae as a source of cellulose and its derivatives for biomedical applications. Cellulose 2024, 31, 3353–3376. [Google Scholar] [CrossRef]
- Manns, D.; Nielsen, M.M.; Bruhn, A.; Saake, B.; Meyer, A.S. Compositional variations of brown seaweeds Laminaria digitata and Saccharina latissima in Danish waters. J. Appl. Phycol. 2017, 29, 1493–1506. [Google Scholar] [CrossRef]
- Olsson, J.; Toth, G.B.; Albers, E. Biochemical composition of red, green and brown seaweeds on the Swedish west coast. J. Appl. Phycol. 2020, 32, 3305–3317. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef]
- Jeong, J.W.; Park, D.J.; Kim, S.C.; Kang, H.W.; Lee, B.; Kim, H.W.; Kim, Y.M.; Linh, N.V.; Jung, W.K. Wound healing effect of fucoidanloaded gelatin/oxidized carboxymethyl cellulose hydrogel. Int. J. Biol. Macromol. 2024, 286, 138254. [Google Scholar] [CrossRef]
- Qureshi, A.T.; Afrin, S.; Asim, S.; Rizwan, M. Imine Crosslinked, Injectable, and SelfHealing Fucoidan Hydrogel with Immunomodulatory Properties. Adv. Healthc. Mater. 2025, 14, e2405260. [Google Scholar] [CrossRef]
- Terauchi, M.; Nagasato, C.; Motomura, T. Plasmodesmata of brown algae. J. Plant Res. 2014, 128, 7–15. [Google Scholar] [CrossRef]
- Ronowicz, M.; Kukliński, P.; WłodarskaKowalczuk, M. Morphological variation of kelps (Alaria esculenta, cf. Laminaria digitata, and Saccharina latissima) in an Arctic glacial fjord. Estuar. Shelf Sci. 2022, 268, 107802. [Google Scholar] [CrossRef]
- Hasan, M.M.; Swapon, A.R.; Dipti, T.I.; Choi, Y.J.; Yi, H.G. PlantBased Decellularization: A Novel Approach for PerfusionCompatible Tissue Engineering Structures. J. Microbiol. Biotechnol. 2024, 34, 1003–1016. [Google Scholar] [CrossRef]
- Ratri, M.C.; Brilian, A.I.; Setiawati, A.; Nguyen, H.T.; Soum, V.; Shin, K. Recent Advances in Regenerative Tissue Fabrication: Tools, Materials, and Microenvironment in Hierarchical Aspects. Adv. NanoBiomed Res. 2021, 1, 2000088. [Google Scholar] [CrossRef]
- He, Z.; Lv, J.C.; Zheng, Z.L.; Gao, C.T.; Xing, J.W.; Li, B.L.; Liu, H.H.; Liu, Y.; Xu, J.Z.; Li, Z.M.; et al. Hierarchically structured nanofibrous scaffolds spatiotemporally mediate the osteoimmune microenvironment and promote osteogenesis for periodontitisrelated alveolar bone regeneration. Acta Biomater. 2024, 189, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Sofian, A.D.A.B.A.; Lim, H.R.; Chew, K.W.; Show, P.L. Advancing 3D Printing through Integration of Machine Learning with AlgaeBased Biopolymers. ChemBioEng Rev. 2024, 11, 406–425. [Google Scholar] [CrossRef]
- Mazurek, Ł.; Kuś, M.; Jurak, J.; Rybka, M.; Kuczeriszka, M.; StradczukMazurek, M.; Konop, M. Biomedical potential of alginate wound dressings—From preclinical studies to clinical applications: A review. Int. J. Biol. Macromol. 2025, 309, 142908. [Google Scholar] [CrossRef]
- BarShai, N.; SharabaniYosef, O.; Zollmann, M.; Lesman, A.; Golberg, A. Seaweed cellulose scaffolds derived from green macroalgae for tissue engineering. Sci. Rep. 2021, 11, 11843. [Google Scholar] [CrossRef]
- Sæther, M.; Diehl, N.; Monteiro, C.; Li, H.; Niedzwiedz, S.; BurgunterDelamare, B.; Scheschonk, L.; Bischof, K.; Forbord, S. The sugar kelp Saccharina latissima II: Recent advances in farming and applications. J. Appl. Phycol. 2024, 36, 1953–1985. [Google Scholar] [CrossRef]
- Krumhansl, K.A.; Okamoto, D.K.; Rassweiler, A.; Novak, M.; Bolton, J.J.; Cavanaugh, K.C.; Connell, S.D.; Johnson, C.R.; Konar, B.; Ling, S.D.; et al. Global patterns of kelp forest change over the past halfcentury. Proc. Natl. Acad. Sci. USA 2016, 113, 13785–13790. [Google Scholar] [CrossRef]
- ASTM D638-14; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2014.
- Annaidh, A.N.; Bruyère, K.; Destrade, M.; Gilchrist, M.D.; Otténio, M. Characterization of the anisotropic mechanical properties of excised human skin. J. Mech. Behav. Biomed. Mater. 2012, 5, 139–148. [Google Scholar] [CrossRef]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef] [PubMed]
- dos Anjos, C.; Leanse, L.G.; Ribeiro, M.S.; Sellera, F.P.; Dropa, M.; AranaChavez, V.E.; Lincopan, N.; Baptista, M.S.; Pogliani, F.C.; Dai, T.; et al. New Insights into the Bacterial Targets of Antimicrobial Blue Light. Microbiol. Spectr. 2023, 11, e0283322. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, Q.; Wang, S.; Zhang, J.; Fan, S.; Lin, X. Current Advances in the Development of Decellularized Plant Extracellular Matrix. Front. Bioeng. Biotechnol. 2021, 9, 712262. [Google Scholar] [CrossRef]
- Nielsen, C.W.; Holdt, S.L.; Sloth, J.J.; Marinho, G.S.; Sæther, M.; Funderud, J.; Rustad, T. Reducing the High Iodine Content of Saccharina latissima and Improving the Profile of Other Valuable Compounds by Water Blanching. Foods 2020, 9, 569. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Eggbox modelbased gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- HassanzadehTabrizi, S.A. Alginate based hemostatic materials for bleeding management: A review. Int. J. Biol. Macromol. 2024, 274, 133218. [Google Scholar] [CrossRef]
- Note on the Use of Different Approaches to Determine the Pore Sizes of Tissue Engineering Scaffolds: What Do We Measure?|BioMedical Engineering OnLine|Full Text. Available online: https://biomedical-engineering-online.biomedcentral.com/articles/10.1186/s12938-018-0543-z (accessed on 18 August 2025).
- Wang, X.; Lou, T.; Zhao, W.; Song, G.; Li, C.; Cui, G. The effect of fiber size and pore size on cell proliferation and infiltration in PLLA scaffolds on bone tissue engineering. J. Biomater. Appl. 2016, 30, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. ThreeDimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagenglycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Negrini, N.C.; Toffoletto, N.; Farè, S.; Altomare, L. Plant Tissues as 3D Natural Scaffolds for Adipose, Bone and Tendon Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 723. [Google Scholar] [CrossRef]
- Modulevsky, D.J.; Lefebvre, C.; Haase, K.; AlRekabi, Z.; Pelling, A.E. Apple Derived Cellulose Scaffolds for 3D Mammalian Cell Culture. PLoS ONE 2014, 9, e97835. [Google Scholar] [CrossRef]
- Stefanelli, V.L.; Mintz, B.; Gandhi, A.; Smith, J. Design matters: A comparison of natural versus synthetic skin substitutes across benchtop and porcine wound healing metrics: An experimental study. Health Sci. Rep. 2023, 6, e1462. [Google Scholar] [CrossRef]
- Yang, L.; Jin, S.; Shi, L.; Ullah, I.; Yu, K.; Zhang, W.; Bo, L.; Zhang, X.; Guo, X. Cryogenically 3D printed biomimetic scaffolds containing decellularized small intestinal submucosa and Sr2+/Fe3+ cosubstituted hydroxyapatite for bone tissue engineering. Chem. Eng. J. 2022, 431, 133459. [Google Scholar] [CrossRef]
- Adamiak, K.; Sionkowska, A. State of Innovation in AlginateBased Materialss. Mar. Drugs 2023, 21, 353. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Sun, H.; Cao, L. Water absorption dynamics in medical foam: Empirical validation of the Lucas–Washburn model. Eur. Phys. J. E 2025, 48, 13. [Google Scholar] [CrossRef] [PubMed]
- Dikici, S. Enhancing wound regeneration potential of fibroblasts using ascorbic acidloaded decellularized baby spinach leaves. Polym. Bull. 2024, 81, 9995–10016. [Google Scholar] [CrossRef]
- Kus, K.J.B.; Ruiz, E.S. Wound Dressings—A Practical Review. Curr. Dermatol. Rep. 2020, 9, 298–308. [Google Scholar] [CrossRef]
- Liew, C.V.; Chan, L.W.; Ching, A.L.; Heng, P.W.S. Evaluation of sodium alginate as drug release modifier in matrix tablets. Int. J. Pharm. 2006, 309, 25–37. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginatebased hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.G.; Yousaf, A.M.; Kim, K.S.; Kim, D.S.; Kim, J.K.; Yong, C.S.; Youn, Y.S.; Kim, J.O.; Choi, H.-G. Influence of hydrophilic polymers on functional properties and wound healing efficacy of hydrocolloid based wound dressings. Int. J. Pharm. 2016, 501, 160–166. [Google Scholar] [CrossRef]
- Mitsuhashi, K.; Ghosh, S.; Koibuchi, H. Mathematical Modeling and Simulations for LargeStrain JShaped Diagrams of Soft Biological Materials. Polymers 2018, 10, 715. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Feng, X.; Rogers, J.A.; Huang, Y.; Zhang, Y. Design and application of ‘Jshaped’ stress–strain behavior in stretchable electronics: A review. Lab Chip 2017, 17, 1689–1704. [Google Scholar] [CrossRef] [PubMed]
- Biewener, A.A. Tendons and Ligaments: Structure, Mechanical Behavior and Biological Function. In Collagen: Structure and Mechanics; Fratzl, P., Ed.; Springer: Boston, MA, USA, 2008; pp. 269–284. [Google Scholar] [CrossRef]
- Sun, G.; He, X.; Feng, M.; Xu, X.; Chen, J.; Wang, Y. Flavin mononucleotide in visible light photoinitiating systems for multiplephotocrosslinking and photoencapsulation strategies. Acta Biomater. 2023, 172, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Holmdahl, V.; Backman, O.; Gunnarsson, U.; Strigård, K. The Tensile Strength of FullThickness Skin: A Laboratory Study Prior to Its Use as Reinforcement in Parastomal Hernia Repair. Front. Surg. 2019, 6, 69. [Google Scholar] [CrossRef]
- Karimi, A.; Navidbakhsh, M.; Shojaei, A.; Faghihi, S. Measurement of the uniaxial mechanical properties of healthy and atherosclerotic human coronary arteries. Mater. Sci. Eng. C 2013, 33, 2550–2554. [Google Scholar] [CrossRef]
- Kirsner, R.S.; Margolis, D.J.; Baldursson, B.T.; Petursdottir, K.; Davidsson, O.B.; Weir, D.; Lantis, J.C. Fish skin grafts compared to human amnion/chorion membrane allografts: A doubleblind, prospective, randomized clinical trial of acute wound healing. Wound Repair Regen. 2019, 28, 75–80. [Google Scholar] [CrossRef]
- Nam, S.-Y.; Youn, D.; Kim, G.H.; Chai, J.H.; Lim, H.R.; Jung, H.H.; Heo, C.Y. In Vitro Characterization of a Novel Human Acellular Dermal Matrix (BellaCell HD) for Breast Reconstruction. Bioengineering 2020, 7, 39. [Google Scholar] [CrossRef]
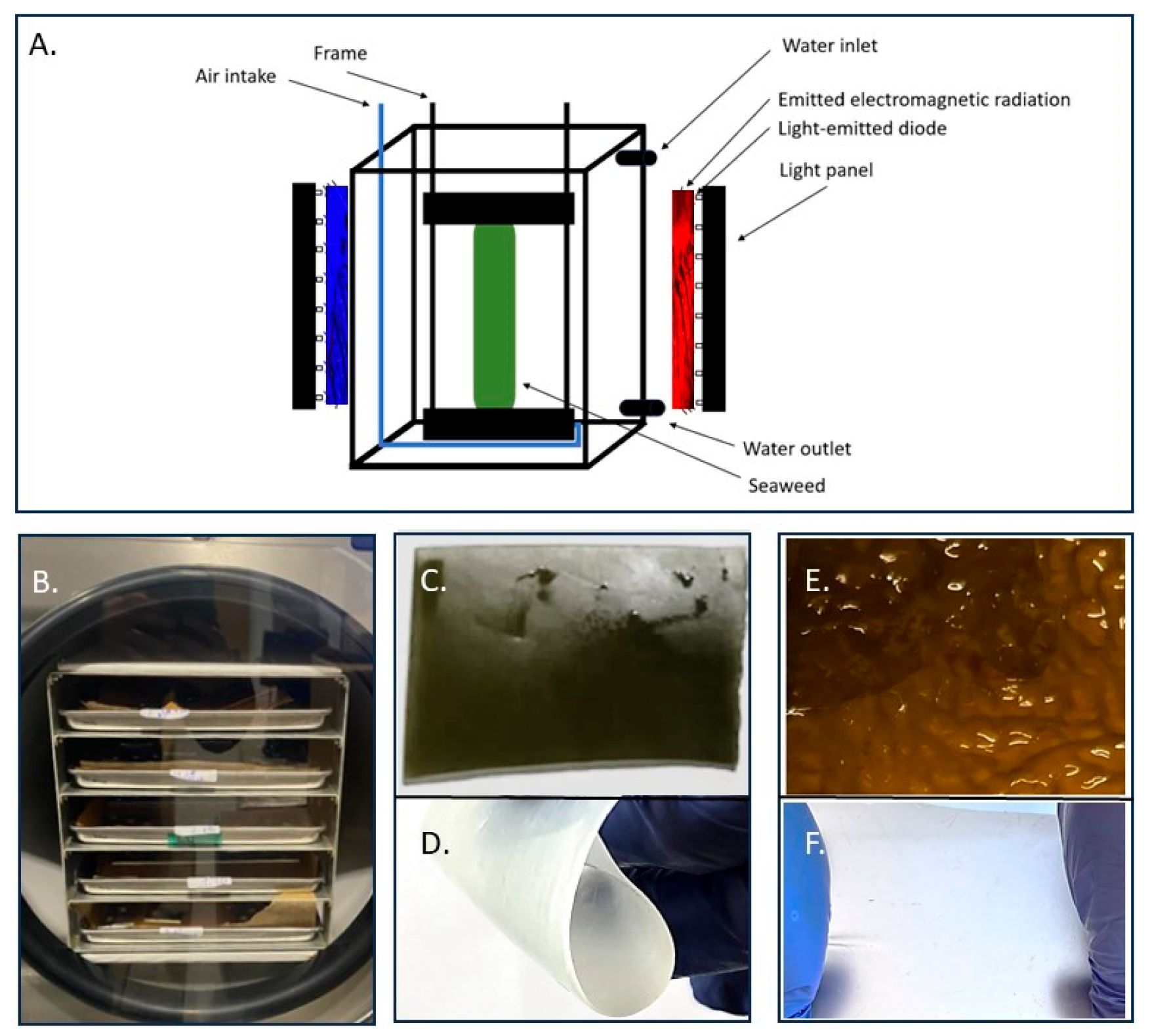
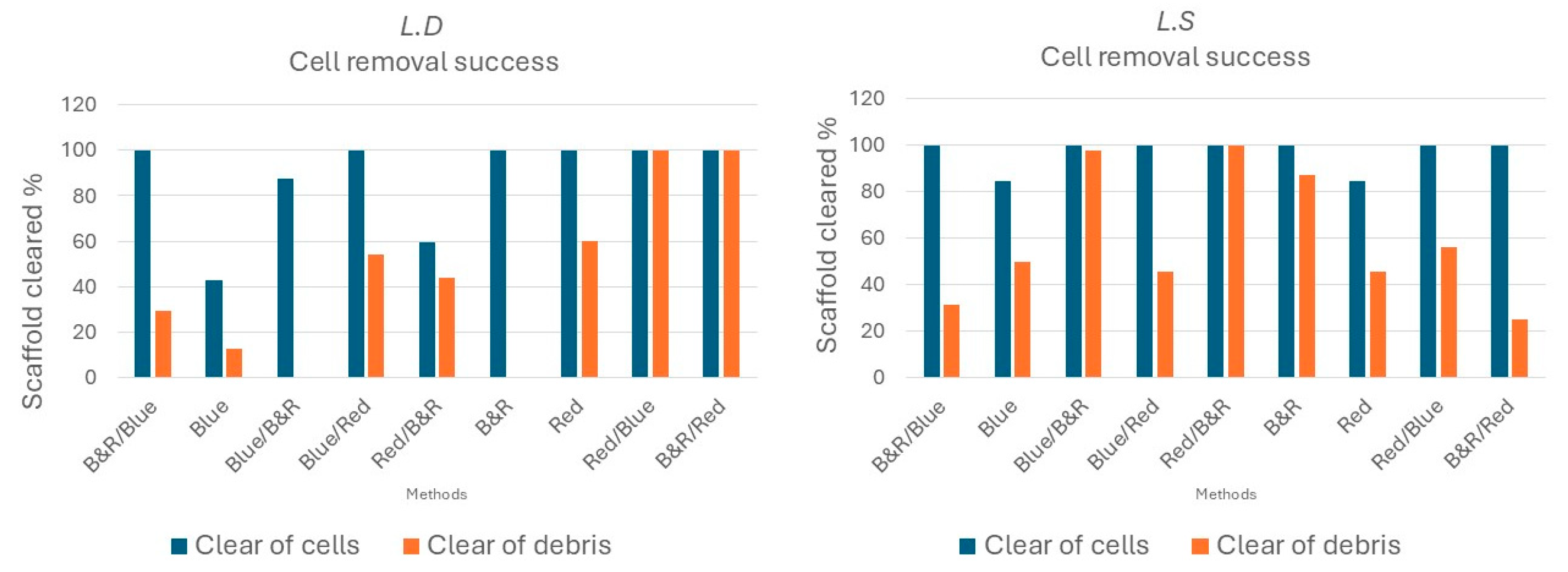
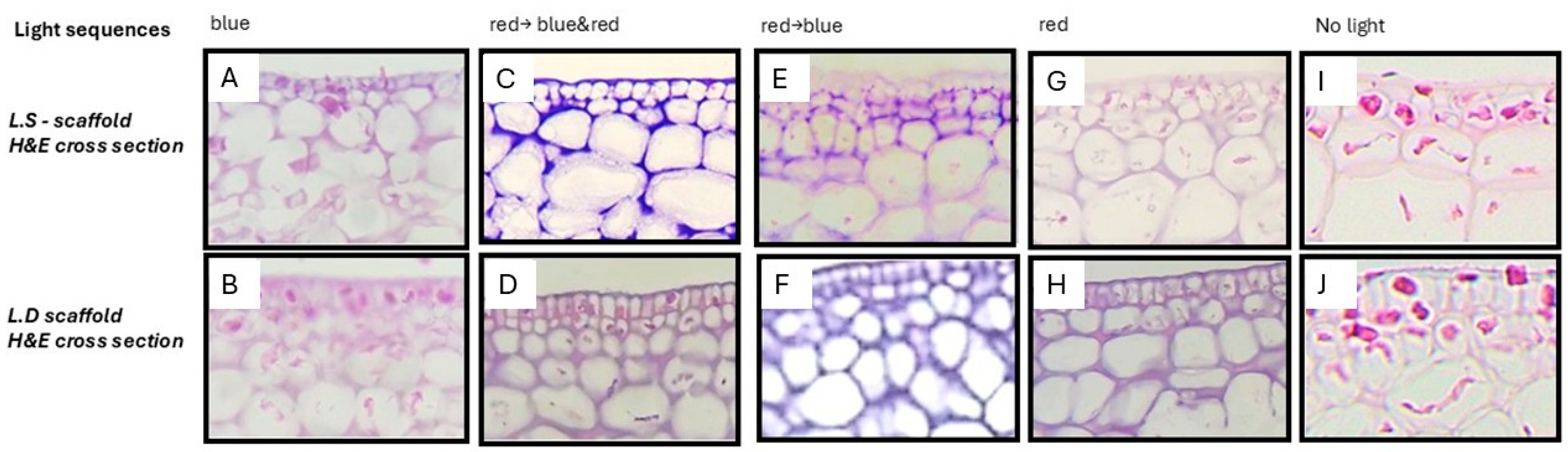
| Light Sequence | Description |
|---|---|
| blue and red → blue | Combined blue and red light for two weeks, followed by blue light for two weeks |
| blue | Blue light exposure for four weeks |
| blue → blue and red | Blue light for two weeks, followed by combined blue and red light for two weeks |
| blue → red | Blue light for two weeks, followed by with red light for two weeks |
| red → blue and red | Red light for two weeks, followed by combined blue and red light for two weeks |
| blue and red | Red and blue light exposure for four weeks |
| red | Red light exposure for four weeks |
| red → blue | Red light exposure for two weeks, followed by blue light for two weeks |
| blue and red → red | Combined blue and red light for two weeks, followed by red light for two weeks |
| Sample | Type | Protein (% dw) | Mannitol (mg/g) | Fucoidan (mg/g) | Alginate (mg/g) | Cellulose (% w/w) | Laminarin (mg/g) | Gal (mg/g) |
|---|---|---|---|---|---|---|---|---|
| L.D. | seaweed | 5.4 | 0.67 | 13 | 85 | 34 | <0.7 | 0.028 |
| scaffold | 5.1 | 0.24 | 9 | 64 | 31 | <0.7 | 0.032 | |
| L.S. | seaweed | 3.6 | 25 | 3 | 63 | 26 | 3 | 0.85 |
| scaffold | 5.5 | 0.25 | 8 | 33 | 44 | <0.7 | 0.092 |
| Sample | Type | Ca (%) | Mg (%) | Na (%) | K (%) | I (%) |
|---|---|---|---|---|---|---|
| L.D. | seaweed | 1.5 | 0.74 | 1.6 | 3.0 | 0.12 |
| scaffold | 4.3 | 0.27 | 0.097 | 0.025 | 0.04 | |
| L.S. | seaweed | 0.88 | 0.5 | 2.4 | 4.4 | 0.25 |
| scaffold | 4.4 | 0.24 | 0.082 | 0.025 | 0.04 |
| Biomaterial | Ave Pore Size (μm) |
|---|---|
| Brown seaweed L.D. | 48.9 ± 77.5 |
| Brown seaweed L.S. | 19.95 ± 18.72 |
| Green seaweed Ulva sp. | 20.2 ± 4 [39] |
| Apples | 420 ± 33 [54] |
| Celery | 125 ± 11 [54] |
| Carrots | 70 ± 12, 130 ± 26 [54] |
| Collagen–GAG, Integra® Dermal Reg. | 132 ± 91 [56] |
| Biodegradable Temporizing Matrix, BTM NovoSorb® | 589 ± 297 [56] |
| Porcine Small Intestine Submucosa (SIS) | 379.2 ± 34.8 [57] |
| Biomaterial | σuts (MPa) | Young’s Modulus (MPa) | Strain at σuts |
|---|---|---|---|
| Brown seaweed L.D | 8.73 ± 0.98 | 19.19 ± 5.51 | 48.9 ± 77.5 |
| Brown seaweed L.S | 6.61 ± 2.16 | 16.19 ± 4.94 | 19.95 ± 18.72 |
| AlloDerm® | 14.3 | NA | 20.2 ± 4 [72] |
| Kerecis Fish skin | 10.1 ± 1.8 | 0.67 ± 0.2 | 420 ± 33 [71] |
| Proheal Bovine collagen | 0.047 ± 0.003 | 0.012 ± 0.003 | 125 ± 11 [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kristinsdottir, S.; Rolfsson, O.; Sigurjonsson, O.E.; Brynjolfsson, S.; Karlsdottir, S.N. Mechanical Properties and Microstructure of Decellularized Brown Seaweed Scaffold for Tissue Engineering. Bioengineering 2025, 12, 943. https://doi.org/10.3390/bioengineering12090943
Kristinsdottir S, Rolfsson O, Sigurjonsson OE, Brynjolfsson S, Karlsdottir SN. Mechanical Properties and Microstructure of Decellularized Brown Seaweed Scaffold for Tissue Engineering. Bioengineering. 2025; 12(9):943. https://doi.org/10.3390/bioengineering12090943
Chicago/Turabian StyleKristinsdottir, Svava, Ottar Rolfsson, Olafur Eysteinn Sigurjonsson, Sigurður Brynjolfsson, and Sigrun Nanna Karlsdottir. 2025. "Mechanical Properties and Microstructure of Decellularized Brown Seaweed Scaffold for Tissue Engineering" Bioengineering 12, no. 9: 943. https://doi.org/10.3390/bioengineering12090943
APA StyleKristinsdottir, S., Rolfsson, O., Sigurjonsson, O. E., Brynjolfsson, S., & Karlsdottir, S. N. (2025). Mechanical Properties and Microstructure of Decellularized Brown Seaweed Scaffold for Tissue Engineering. Bioengineering, 12(9), 943. https://doi.org/10.3390/bioengineering12090943








