Superior Capsule Reconstruction Graft Selection: The Influence of Biological Properties of Grafts on Healing and Re-Tearing
Abstract
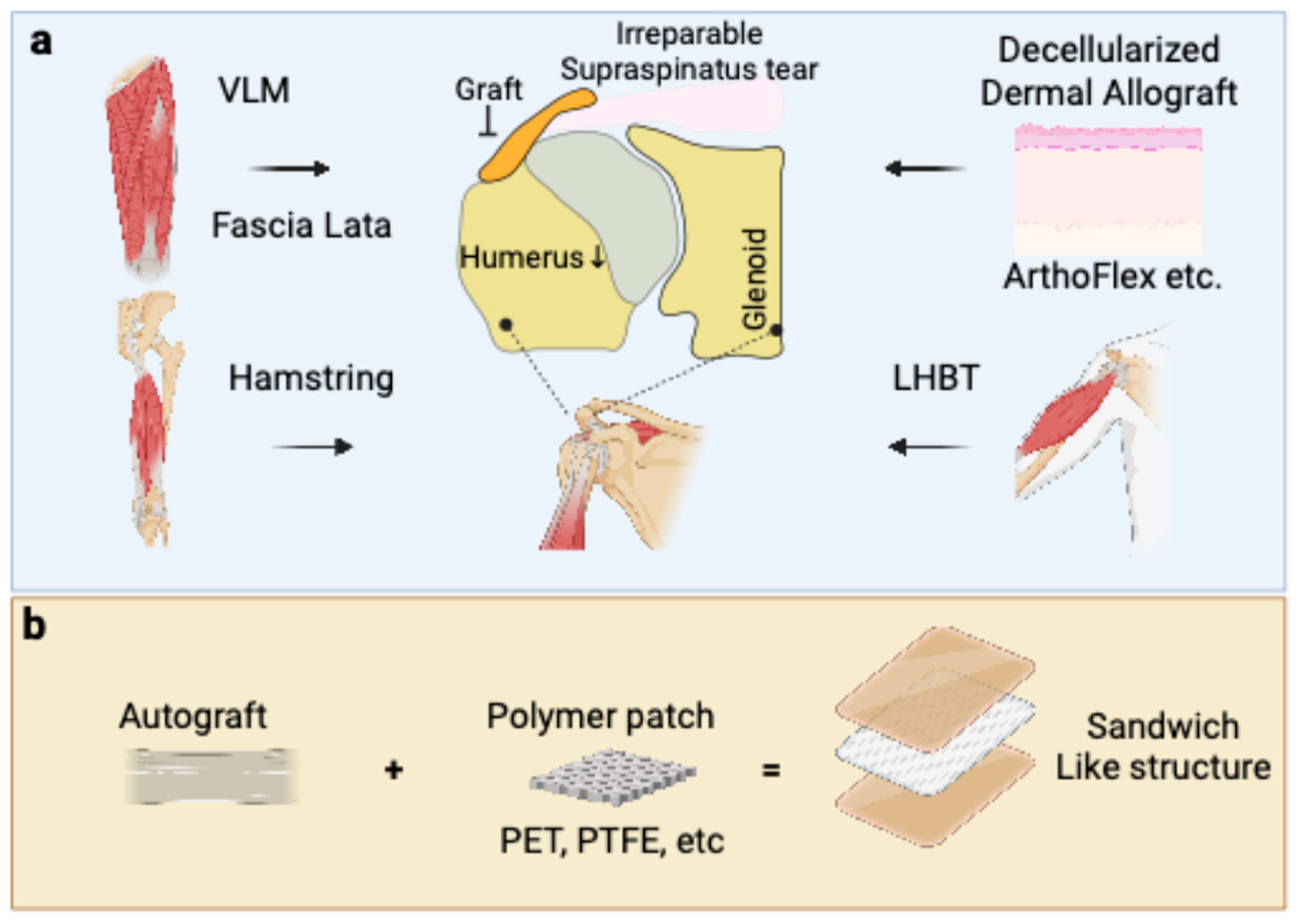
1. Introduction
2. The Biological Imperative in Graft Healing for ASCR
3. Autografts: Leveraging Native Biology for Enhanced Healing
3.1. Fascia Lata Autograft
3.2. Long Head of the Biceps Tendon (LHBT) Autograft
3.3. Hamstring Tendon Autograft
4. Allografts: Balancing Availability with Biological Considerations
4.1. Acellular Dermal Allograft (ADA)
4.2. Fascia Lata Allograft
4.3. General Considerations for Allografts
5. Xenografts: Navigating Significant Biological Hurdles
Porcine Dermal Xenograft
6. Synthetic Grafts: Off-the-Shelf Scaffolds and Their Biological Interactions
6.1. Non-Resorbable Synthetic Grafts (e.g., PTFE, PET–LARS)
6.2. Sandwich-Like Structure: Hybrid Grafts for Augmentation
7. The Role of Graft Thickness and Fixation in Supporting Biological Healing
7.1. Graft Thickness
7.2. Fixation Methods
8. Conclusions
Funding
Conflicts of Interest
References
- Bedi, A.; Bishop, J.; Keener, J.; Lansdown, D.A.; Levy, O.; MacDonald, P.; Maffulli, N.; Oh, J.H.; Sabesan, V.J.; Sanchez-Sotelo, J.; et al. Rotator cuff tears. Nat. Rev. Dis. Primers 2024, 10, 8. [Google Scholar] [CrossRef]
- Kooistra, B.; Gurnani, N.; Weening, A.; van den Bekerom, M.; van Deurzen, D. Low level of evidence for all treatment modalities for irreparable posterosuperior rotator cuff tears. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 4038–4048. [Google Scholar] [CrossRef]
- Mercurio, M.; Castricini, R.; Castioni, D.; Cofano, E.; Familiari, F.; Gasparini, G.; Galasso, O. Better functional outcomes and a lower infection rate can be expected after superior capsular reconstruction in comparison with latissimus dorsi tendon transfer for massive, irreparable posterosuperior rotator cuff tears: A systematic review. J. Shoulder Elb. Surg. 2023, 32, 892–906. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, W.; Xu, J.; Ruan, D.; Heng, B.C.; Ding, Q.; Shen, L.; Ding, S.; Shen, W. Arthroscopic Superior Capsular Reconstruction for Massive Irreparable Rotator Cuff Tears Results in Significant Improvements in Patient Reported Outcomes and Range of Motion: A Systematic Review. Arthrosc. Sports Med. Rehabil. 2022, 4, e1523–e1537. [Google Scholar] [CrossRef]
- de Campos Azevedo, C.I.; Andrade, R.; Leiria Pires Gago Angelo, A.C.; Espregueira-Mendes, J.; Ferreira, N.; Sevivas, N. Fascia Lata Autograft Versus Human Dermal Allograft in Arthroscopic Superior Capsular Reconstruction for Irreparable Rotator Cuff Tears: A Systematic Review of Clinical Outcomes. Arthroscopy 2020, 36, 579–591.e572. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.; Singh, P.; Reilly, P.; Sabharwal, S.; Malhas, A. Superior capsule reconstruction, partial cuff repair, graft interposition, arthroscopic debridement or balloon spacers for large and massive irreparable rotator cuff tears: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2022, 17, 552. [Google Scholar] [CrossRef] [PubMed]
- Bi, M.; Ding, W.; Zheng, M.; Peng, Z.; Li, J.; Ding, S. Arthroscopic Superior Capsule Reconstruction With Combined Fascia Lata Autograft and Synthetic Scaffold Patch Graft for the Treatment of Irreparable Rotator Cuff Tears Yields Favorable Clinical and Radiographic Outcomes at Minimum 2-Year Follow-Up. Arthroscopy 2023, 39, 1800–1810. [Google Scholar] [CrossRef]
- Zhao, X.; Jia, J.; Wen, L.; Zhang, B. Biomechanical outcomes of superior capsular reconstruction for irreparable rotator cuff tears by different graft materials-a systematic review and meta-analysis. Front. Surg. 2022, 9, 939096. [Google Scholar] [CrossRef]
- Antunes, M.; Quental, C.; Folgado, J.; de Campos Azevedo, C.; Angelo, A.C. Shoulder Positioning during Superior Capsular Reconstruction: Computational Analysis of Graft Integrity and Shoulder Stability. Biology 2021, 10, 1263. [Google Scholar] [CrossRef]
- Ben, H.; Kholinne, E.; Guo, J.; Ryu, S.M.; Ling, J.L.; Koh, K.H.; Jeon, I.H. Improved Acromiohumeral Distance Independently Predicts Better Outcomes After Arthroscopic Superior Capsular Reconstruction Graft Tears. Arthroscopy 2025, 41, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Killian, M.L.; Cavinatto, L.; Galatz, L.M.; Thomopoulos, S. The role of mechanobiology in tendon healing. J. Shoulder Elb. Surg. 2012, 21, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Ran, J.; Chen, W.; Hu, Y.; Zhu, T.; Chen, X.; Yin, Z.; Heng, B.C.; Feng, G.; Le, H.; et al. Alignment of collagen fiber in knitted silk scaffold for functional massive rotator cuff repair. Acta Biomater. 2017, 51, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.J.; Hanson, J.A.; Millett, P.J. Editorial Commentary: Shoulder Superior Capsular Reconstruction Graft Tensioning Between 30 degrees and 40 degrees of Glenohumeral Abduction Is Recommended: The Balance Beam of Superior Capsular Reconstruction. Arthroscopy 2022, 38, 1408–1410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bogdanowicz, D.; Erisken, C.; Lee, N.M.; Lu, H.H. Biomimetic scaffold design for functional and integrative tendon repair. J. Shoulder Elb. Surg. 2012, 21, 266–277. [Google Scholar] [CrossRef]
- Khan, M. Editorial Commentary: Superior Capsular Reconstruction: Indications and Proper Technique Results in Good Outcomes but Reports of Complications. Arthroscopy 2021, 37, 2973–2974. [Google Scholar] [CrossRef]
- Jeon, I.H.; Kholinne, E. Editorial Commentary: Collagen Bridging of Massive Rotator Cuff Tears Using Fascia Lata Autograft Could Provide a Lasting Solution. Arthroscopy 2024, 40, 2667–2668. [Google Scholar] [CrossRef]
- Mihata, T.; McGarry, M.H.; Kahn, T.; Goldberg, I.; Neo, M.; Lee, T.Q. Biomechanical Role of Capsular Continuity in Superior Capsule Reconstruction for Irreparable Tears of the Supraspinatus Tendon. Am. J. Sports Med. 2016, 44, 1423–1430. [Google Scholar] [CrossRef]
- Ângelo, A.C.L.P.G.; de Campos Azevedo, C.I. Minimally invasive fascia lata harvesting in ASCR does not produce significant donor site morbidity. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 245–250. [Google Scholar] [CrossRef]
- Ângelo, A.C.L.P.G.; de Campos Azevedo, C.I. Donor-site morbidity after autologous fascia lata harvest for arthroscopic superior capsular reconstruction: A midterm follow-up evaluation. Orthop. J. Sports Med. 2022, 10, 23259671211073133. [Google Scholar] [CrossRef]
- Sethi, P.; Fares, M.Y.; Murthi, A.; Tokish, J.M.; Abboud, J.A. The long head of the biceps tendon: A valuable tool in shoulder surgery. J. Shoulder Elb. Surg. 2023, 32, 1801–1811. [Google Scholar] [CrossRef]
- Aicale, R.; Poeta, N.; Savarese, E.; Bernardini, G.; Oliva, F.; Maffulli, N. The use of long head biceps tendon autograft for massive rotator cuff tears: A PRISMA compliant systematic review. Br. Med. Bull. 2022, 144, 76–89. [Google Scholar] [CrossRef]
- Voloshin, I. Editorial Commentary: Long Head Biceps Tendon Autograft Is an Ideal and Cost-effective Graft Choice in Superior Capsular Reconstruction of the Glenohumeral Joint. Arthroscopy 2021, 37, 2768. [Google Scholar] [CrossRef]
- Perry, N.P.J.; Farina, E.M.; Wang, C.; Price, M.D.; Mazzocca, A.D. Editorial Commentary: Fascia Lata Allograft for Shoulder Superior Capsular Reconstruction: In the Fight Over Optimal Graft Choice for Irreparable Rotator Cuff Tears, Superior Capsular Reconstruction Proponents May Be Changing Their Gloves. Arthroscopy 2023, 39, 29–31. [Google Scholar] [CrossRef]
- Berthold, D.P.; Bell, R.; Muench, L.N.; Jimenez, A.E.; Cote, M.P.; Obopilwe, E.; Edgar, C.M. A new approach to superior capsular reconstruction with hamstring allograft for irreparable posterosuperior rotator cuff tears: A dynamic biomechanical evaluation. J. Shoulder Elb. Surg. 2021, 30, S38–S47. [Google Scholar] [CrossRef] [PubMed]
- Modi, A.; Haque, A.; Deore, V.; Singh, H.P.; Pandey, R. Interposition GraftJacket allografts for irreparable rotator cuff tears. Bone Joint J. 2022, 104-B, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Knapp, T.P. Editorial Commentary: Dermal Allografts Are Indicated for Repair of Irreparable Rotator Cuff Tears and for Revision Surgery, and May Be Cost-Effective for Primary Repair. Arthroscopy 2022, 38, 2175–2177. [Google Scholar] [CrossRef]
- Lubowitz, J.H.; Brand, J.C.; Rossi, M.J. Shoulder Superior Capsular Reconstruction Using Acellular Human Dermal Allograft. Arthroscopy 2019, 35, 2769–2770. [Google Scholar] [CrossRef]
- Denard, P.J.; Brady, P.C.; Adams, C.R.; Tokish, J.M.; Burkhart, S.S. Preliminary Results of Arthroscopic Superior Capsule Reconstruction with Dermal Allograft. Arthroscopy 2018, 34, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Scheiderer, B.; Kia, C.; Obopilwe, E.; Johnson, J.D.; Cote, M.P.; Imhoff, F.B.; Dyrna, F.; Beitzel, K.; Imhoff, A.B.; Adams, C.R.; et al. Biomechanical Effect of Superior Capsule Reconstruction Using a 3-mm and 6-mm Thick Acellular Dermal Allograft in a Dynamic Shoulder Model. Arthroscopy 2020, 36, 355–364. [Google Scholar] [CrossRef]
- Hirahara, A.M.; Andersen, W.J.; Panero, A.J. Ultrasound Assessment of the Superior Capsular Reconstruction With Dermal Allograft: An Evaluation of Graft Thickness and Vascularity. Arthroscopy 2019, 35, 3194–3202. [Google Scholar] [CrossRef]
- Lee, A.; Farooqi, A.S.; Novikov, D.; Li, X.; Kelly, J.D.t.; Parisien, R.L. Clinical and Functional Outcomes by Graft Type in Superior Capsular Reconstruction: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2022, 50, 3998–4007. [Google Scholar] [CrossRef]
- Hinz, M.; Fritsch, L.; Degenhardt, H.; Rupp, M.C.; Lacheta, L.; Muench, L.N.; Achtnich, A.; Siebenlist, S.; Scheiderer, B. Superior Capsular Reconstruction Using an Acellular Dermal Xenograft or Allograft Improves Shoulder Function but Is Associated with a High Graft Failure Rate. J. Clin. Med. 2024, 13, 4646. [Google Scholar] [CrossRef]
- Hasan, S.S. Editorial Commentary: Superior Capsular Reconstruction Employing Allograft Heals and Functions Well if the Graft Is Sufficiently Thick and Stiff. Arthroscopy 2023, 39, 1425–1428. [Google Scholar] [CrossRef]
- Beraldo, R.A.; Gracitelli, M.E.C.; Malavolta, E.A.; Assuncao, J.H.; Silva, F.; Neto, A.A.F. Treatment of Irreparable Rotator Cuff Tears: Superior Capsular Reconstruction with Fascia Lata Allograft. Rev. Bras. Ortop. 2022, 57, 876–883. [Google Scholar] [CrossRef]
- Nguyen, H.; Morgan, D.A.; Forwood, M.R. Sterilization of allograft bone: Effects of gamma irradiation on allograft biology and biomechanics. Cell Tissue Bank. 2007, 8, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.E.; Orgill, D.P. Simultaneous in vivo regeneration of neodermis, epidermis, and basement membrane. Adv. Biochem. Eng. Biotechnol. 2005, 94, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Davalos Herrera, D.A.; Woodmass, J.M.; Boorman, R.S.; Thornton, G.M.; Lo, I.K. Can Grafts Provide Superior Tendon Healing and Clinical Outcomes After Rotator Cuff Repairs?: A Meta-analysis. Orthop. J. Sports Med. 2016, 4, 2325967116674191. [Google Scholar] [CrossRef]
- Flury, M.; Rickenbacher, D.; Jung, C.; Schneider, M.M.; Endell, D.; Audige, L. Porcine Dermis Patch Augmentation of Supraspinatus Tendon Repairs: A Pilot Study Assessing Tendon Integrity and Shoulder Function 2 Years After Arthroscopic Repair in Patients Aged 60 Years or Older. Arthroscopy 2018, 34, 24–37. [Google Scholar] [CrossRef]
- Okamura, K.; Abe, M.; Yamada, Y.; Makihara, T.; Yoshimizu, T.; Sakaki, Y.; Suzumori, Y.; Mihata, T. Arthroscopic superior capsule reconstruction with Teflon felt synthetic graft for irreparable massive rotator cuff tears: Clinical and radiographic results at minimum 2-year follow-up. J. Shoulder Elb. Surg. 2021, 30, 625–634. [Google Scholar] [CrossRef]
- Ting, R.S.; Rosenthal, R.; Law, T.K.; Al-Housni, H.S.A.; Hackett, L.; Lam, P.H.; Murrell, G.A.C. Reliability of a Novel Preoperative Protocol for Determining Graft Sizes for Superior Capsular Reconstruction Using Plain Film Radiography. J. Clin. Med. 2023, 12, 2707. [Google Scholar] [CrossRef] [PubMed]
- Bi, M.; Sun, Z.; Wei, L.; Ding, W.; Zheng, M.; Li, J.; Ding, S. Arthroscopic Superior Capsule Reconstruction With Combined Fascia Lata Autograft Augmented With Either LARS Ligament or Polypropylene Mesh Synthetic Scaffold Patch Graft: A Comparison of Techniques With a Minimum 2-Year Follow-up for the Treatment of Irreparable Rotator Cuff Tears. Am. J. Sports Med. 2025, 53, 1721–1730. [Google Scholar] [CrossRef]
- Longo, U.G.; Rizzello, G.; Berton, A.; Fumo, C.; Maltese, L.; Khan, W.S.; Denaro, V. Synthetic grafts for anterior cruciate ligament reconstruction. Curr. Stem Cell Res. Ther. 2013, 8, 429–437. [Google Scholar] [CrossRef]
- Berman, M.; Pearce, W.J.; Tinnin, M. The use of Gore-Tex E-PTFE bonded to silicone rubber as an alloplastic implant material. Laryngoscope 1986, 96, 480–483. [Google Scholar] [CrossRef]
- Yu, C.; Feng, S.; Li, Y.; Chen, J. Application of Nondegradable Synthetic Materials for Tendon and Ligament Injury. Macromol. Biosci. 2023, 23, e2300259. [Google Scholar] [CrossRef]
- Polacek, M.; Nyegaard, C.P. Superior capsular reconstruction using 3-layered fascia lata autograft reinforced with a nonresorbable suture mesh. Arthrosc. Sports Med. Rehabil. 2020, 2, e489–e497. [Google Scholar] [CrossRef] [PubMed]
- Mihata, T.; McGarry, M.H.; Kahn, T.; Goldberg, I.; Neo, M.; Lee, T.Q. Biomechanical Effect of Thickness and Tension of Fascia Lata Graft on Glenohumeral Stability for Superior Capsule Reconstruction in Irreparable Supraspinatus Tears. Arthroscopy 2016, 32, 418–426. [Google Scholar] [CrossRef]
- Shin, S.J.; Lee, S.; Hwang, J.Y.; Lee, W.; Koh, K.H. Superior Capsular Reconstruction Using Acellular Dermal Allograft Combined With Remaining Rotator Cuff Augmentation Improved Shoulder Pain and Function at 1 Year After The Surgery. Arthroscopy 2022, 38, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Weiler, A.; Hoffmann, R.F.; Bail, H.J.; Rehm, O.; Sudkamp, N.P. Tendon healing in a bone tunnel. Part II: Histologic analysis after biodegradable interference fit fixation in a model of anterior cruciate ligament reconstruction in sheep. Arthroscopy 2002, 18, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.W.; Meier, J.D. Rotator cuff repair: The effect of double-row fixation on three-dimensional repair site. J. Shoulder Elb. Surg. 2006, 15, 691–696. [Google Scholar] [CrossRef]
- Ng, S.H.A.; Tan, C.H.J. Double-row repair of rotator cuff tears: Comparing tendon contact area between techniques. World J. Orthop. 2020, 11, 10–17. [Google Scholar] [CrossRef]
- Chen, M.; Xu, W.; Dong, Q.; Huang, Q.; Xie, Z.; Mao, Y. Outcomes of single-row versus double-row arthroscopic rotator cuff repair: A systematic review and meta-analysis of current evidence. Arthroscopy 2013, 29, 1437–1449. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Tang, Z.H.; Hu, J.Z.; Zou, G.Y.; Xiao, R.C. Incidence of retear with double-row versus single-row rotator cuff repair. Orthopedics 2014, 37, e1006–e1013. [Google Scholar] [CrossRef]
- Wylie, J.D.; Baran, S.; Granger, E.K.; Tashjian, R.Z. A Comprehensive Evaluation of Factors Affecting Healing, Range of Motion, Strength, and Patient-Reported Outcomes After Arthroscopic Rotator Cuff Repair. Orthop. J. Sports Med. 2018, 6, 2325967117750104. [Google Scholar] [CrossRef] [PubMed]
- Yang, J., Jr.; Robbins, M.; Reilly, J.; Maerz, T.; Anderson, K. The Clinical Effect of a Rotator Cuff Retear: A Meta-analysis of Arthroscopic Single-Row and Double-Row Repairs. Am. J. Sports Med. 2017, 45, 733–741. [Google Scholar] [CrossRef] [PubMed]

| Graft Category | Specific Graft Type | Key Biological Properties and Healing Mechanism | Reported Clinical Outcomes (Healing/Re-tear Rates) | Advantages | Disadvantages and Challenges |
|---|---|---|---|---|---|
| Autografts | Fascia Lata Autograft (FLA) | Contains viable fibroblasts and a native collagen matrix; non-immunogenic. Heals via robust biological incorporation. | High healing rates reported: 91.7% at 1 year, 90% at 5 years. A systematic review found a pooled tear rate of only 9%. | Considered a gold standard; excellent biocompatibility and durability. fold to a desired thickness (6–10 mm) | Donor site morbidity (pain, scarring, muscle herniation at the harvest site). Potential for the graft to stretch over time (“graft creep”). |
| Long Head of Biceps Tendon (LHBT) | Contains viable tenocytes and an organized collagen matrix. May preserve some native blood supply, aiding initial healing. | Functional outcomes and relapse rates are comparable to FLA. | Avoids a second incision site (e.g., the thigh). Uses available local tissue. | Only an option if the tendon is present and of sufficient quality and size. | |
| Hamstring Tendon | Contains viable cells and a native collagen structure. Promotes biological healing without an immune response. | Expected to integrate well, similar to other autografts. (Specific rates not provided in the text.) | Provides the inherent biological benefits of autologous tissue. | Donor site morbidity at the harvest location. | |
| Allografts | Acellular Dermal Allograft (ADA) | Healing occurs via “creeping substitution”, a slow process of host cell repopulation. | Highly variable. Pooled tear rate of 7% was found in one review. Thicker grafts (>3 mm) perform better. | Readily available “off-the-shelf,” avoids donor site morbidity, and reduces operative time. | Slower, less robust healing compared to autografts. Risk of inflammatory response and highly variable outcomes. |
| Fascia Lata Allograft | A dense, acellular collagen scaffold from a cadaveric donor. Integration relies completely on host cell migration. | Slower and less complete healing than its autograft counterpart. One study reported a 40% complete healing rate at 6 months. | Avoids donor site morbidity. | Lacks viable cells, leading to slower biological uptake. | |
| Xenografts | Porcine Dermal Xenograft | An animal-derived collagen scaffold processed to remove cells and antigens. | Unacceptably high failure rates. Complication rates up to 30%, due to immunologic rejection (15%) or failure to heal (15%). One study showed a 64% tear rate at 2 years. | High availability and potentially lower cost. | Significant risk of a strong inflammatory and immune rejection response. Leads to poor integration, graft degradation, and frequent failure. |
| Synthetic Grafts | Non-resorbable (e.g., PTFE, PET–LARS) | Inert, biocompatible polymers that act as a mechanical scaffold. The host response is fibrous encapsulation or ingrowth, not biological remodeling. | Improved clinical outcomes with low graft rupture rates have been reported. | Provides immediate mechanical stability. Off-the-shelf, sterile, and mechanically consistent. | Lack of true biological integration. Long-term concerns include particulate wear debris and stress shielding of adjacent tissues. |
| Sandwich-like Structure (Hybrid Grafts) | A composite graft combining a synthetic scaffold (for mechanical strength) with a biological graft, often an FLA. | A LARS ligament combined with FLA showed a 91% healing rate, superior to other augmentation. A hybrid of FLA and dermal allograft showed an 83.3% healing rate. | Aims to provide both immediate mechanical stability and long-term biological healing. | A newer technique that requires more long-term clinical follow-up to confirm its benefits. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, M.; Bi, M.; Yuan, S.; Wu, Y.; Yung, P.S.-H.; Ong, M.T.-Y. Superior Capsule Reconstruction Graft Selection: The Influence of Biological Properties of Grafts on Healing and Re-Tearing. Bioengineering 2025, 12, 942. https://doi.org/10.3390/bioengineering12090942
Cao M, Bi M, Yuan S, Wu Y, Yung PS-H, Ong MT-Y. Superior Capsule Reconstruction Graft Selection: The Influence of Biological Properties of Grafts on Healing and Re-Tearing. Bioengineering. 2025; 12(9):942. https://doi.org/10.3390/bioengineering12090942
Chicago/Turabian StyleCao, Mingde, Mingguang Bi, Shuai Yuan, Yuhao Wu, Patrick Shu-Hang Yung, and Michael Tim-Yun Ong. 2025. "Superior Capsule Reconstruction Graft Selection: The Influence of Biological Properties of Grafts on Healing and Re-Tearing" Bioengineering 12, no. 9: 942. https://doi.org/10.3390/bioengineering12090942
APA StyleCao, M., Bi, M., Yuan, S., Wu, Y., Yung, P. S.-H., & Ong, M. T.-Y. (2025). Superior Capsule Reconstruction Graft Selection: The Influence of Biological Properties of Grafts on Healing and Re-Tearing. Bioengineering, 12(9), 942. https://doi.org/10.3390/bioengineering12090942






