The Influence of a Predegenerated Autological Nerve Graft on the Results of Peripheral Nerve Repair in the Upper Extremities After Injuries
Abstract
1. Introduction
2. Materials and Methods
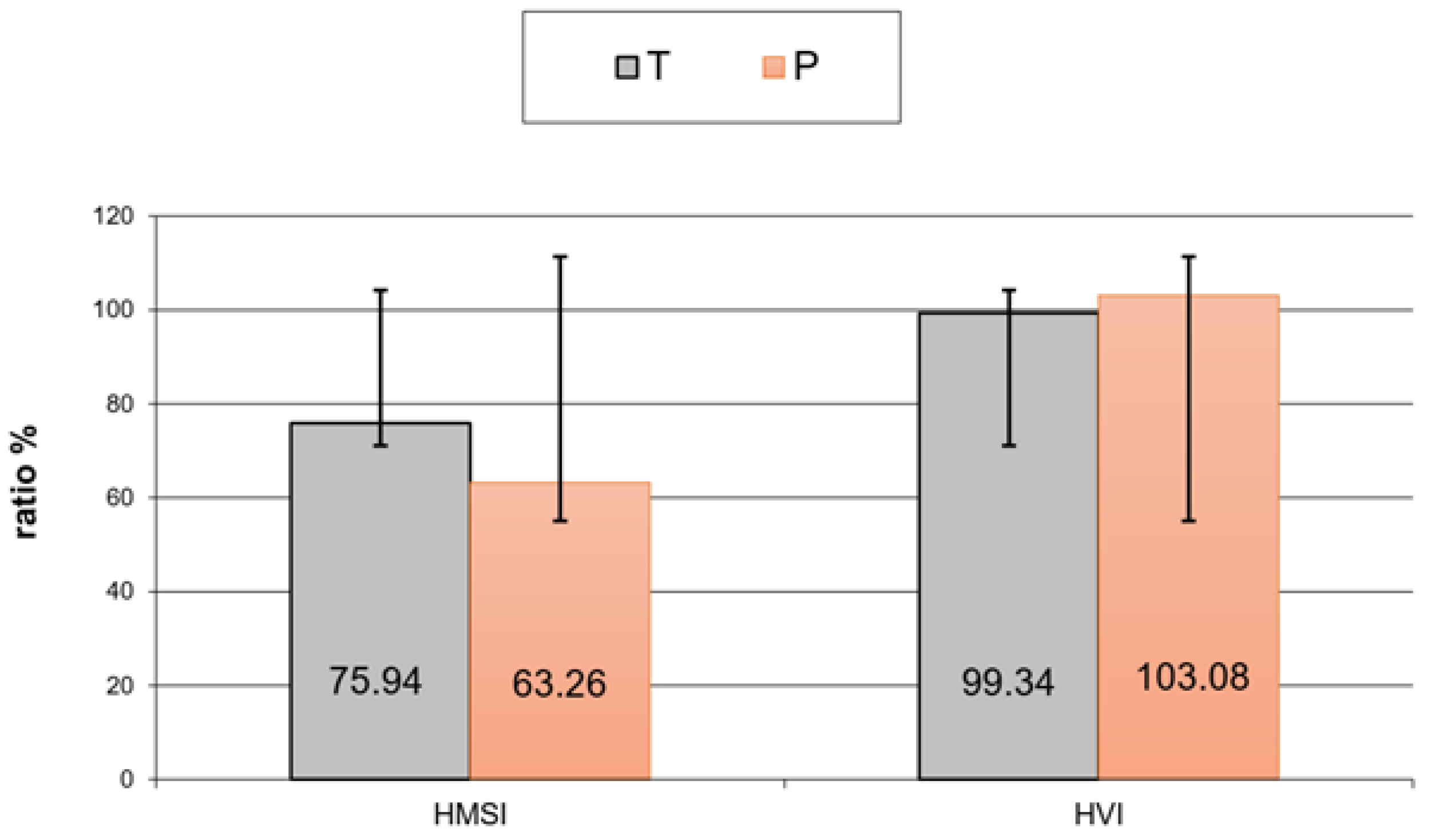
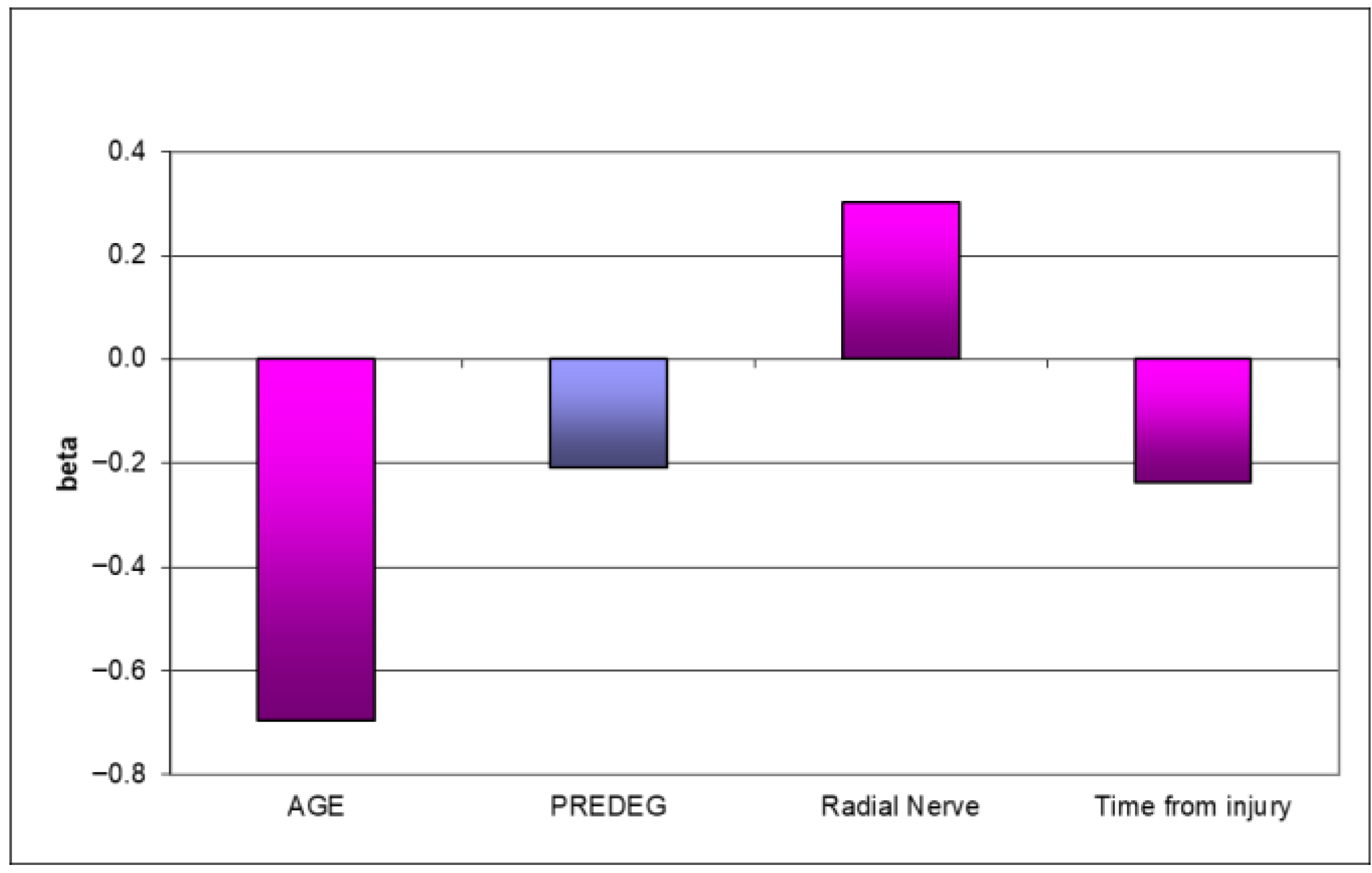
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HVI | Hand volume index |
| HMSI | Hand muscle strength index |
| NCV | Nerve conduction velocity |
| ENG | Electroneurography |
| MNCS_APA | Motor Nerve Conduction Study—Action Potential Amplitude |
| MNCS_APAA | Motor Nerve Conduction Study—Action Potential Amplitude Assessment |
| SUM | Medical University of Silesia |
| kDa | kilo Daltons |
| NGF | Nerve growth factor |
| BDNF | Brain-derived neurotropic factor |
| CNTF | Ciliary neurotrophic factor |
| IGF-I | Insulin-like growth factor 1 |
| IGF-II | Insulin-like growth factor 2 |
| S-100 | Family of multigenic group of nonubiquitous cytoplasmic homologous intracellular Ca2+ binding proteins with EF hand motifs and significant structural similarities at both protein and genomic levels |
| PLP | Proteolipid proteins |
References
- Sulaiman, W.; Gordon, T. Neurobiology of Peripheral Nerve Injury, Regeneration, and Functional Recovery: From Bench Top Research to Bedside Application. Ochsner J. 2013, 13, 100–108. [Google Scholar]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Qasim, M.; Zafar, S.; Aziz, N.; Razzaq, A.; Hussain, R.; de Aguilar, J.-L.G.; et al. Current status of therapeutic approache against peripheral nerve injuries: A detailed story from injury to recovery. Int. J. Biol. Sci. 2020, 16, 116–134. [Google Scholar] [CrossRef]
- Pigońska, J.; Bogucki, A. Pourazowe uszkodzenia nerwów obwodowych—Diagnostyka elektrofizjologiczna. Fam. Med. Prim. Care Rev. 2015, 17, 334–337. [Google Scholar] [CrossRef]
- Sinis, N.; Kraus, A.; Werdin, F.; Manoli, T.; Jaminet, P.; Haerle, M.; Schaller, H.E. Nerve reconstruction and nerve grafting. Die Chir. 2009, 80, 875–881, quiz 882. [Google Scholar] [CrossRef]
- Mahar, M.; Cavalli, V. Intrinsic mechanisms of neuronal axon regeneration. Nat. Rev. Neurosci. 2018, 19, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Yang, Y.; Deng, J.; Saif Ur Rahman, M.; Sun, C.; Xu, S. Physical Stimulation Combined with Biomaterials Promotes Peripheral Nerve Injury Repair. Bioengineering 2022, 9, 292. [Google Scholar] [CrossRef]
- Lopes, B.; Sousa, P.; Alvites, R.; Branquinho, M.; Sousa, A.C.; Mendonça, C.; Atayde, L.M.; Luís, A.L.; Varejão, A.S.P.; Maurício, A.C. Peripheral Nerve Injury Treatments and Advances: One Health Perspective. Int. J. Mol. Sci. 2022, 23, 918. [Google Scholar] [CrossRef] [PubMed]
- Rochkind, S.; Sirota, S.; Kushnir, A. Nerve Reconstruction Using ActiGraft Blood Clot in Rabbit Acute Peripheral Injury Model: Preliminary Study. Bioengineering 2024, 11, 298. [Google Scholar] [CrossRef]
- Karabeg, R.; Jakirlic, M.; Dujso, V. Sensory recovery after forearm median and ulnar nerve grafting. Med. Arch. 2009, 63, 97–99. [Google Scholar]
- Guy, R.; Grynspan, F.; Ben-Zur, T.; Panski, A.; Lamdan, R.; Danon, U.; Yaffe, D.; Offen, D. Human Muscle Progenitor Cells Overexpressing Neurotrophic Factors Improve Neuronal Regeneration in a Sciatic Nerve Injury Mouse Model. Front. Neurosci. 2019, 13, 151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zou, X.F.; Zhang, B.Z.; Qian, W.W.; Cheng, F.M. Bone marrow mesenchymal stem cells in treatment of peripheral nerve injury. World J. Stem Cells 2024, 16, 799–810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crabtree, J.R.; Mulenga, C.M.; Tran, K.; Feinberg, K.; Santerre, J.P.; Borschel, G.H. Biohacking Nerve Repair: Novel Biomaterials, Local Drug Delivery, Electrical Stimulation, and Allografts to Aid Surgical Repair. Bioengineering 2024, 11, 776. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Li, C.; Guan, Y.; Liu, Y.; Zhang, T.; Meng, F.; Cheng, H.; Song, X.; Jia, Z.; et al. Biological characteristics of tissue engineered-nerve grafts enhancing peripheral nerve regeneration. Stem Cell Res. Ther. 2024, 15, 215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gordon, T. Peripheral nerve regeneration and muscle reinnervation. Int. J. Mol. Sci. 2020, 21, 8652. [Google Scholar] [CrossRef] [PubMed]
- Pietrucha-Dutczak, M.; Marcol, W.; Gorka, D.; Golka, B.; Kotulska, K.; Lewin-Kowalik, J. Quantitative and qualitative analysis of proteins in rat peripheral nerves predegenerated for 7 days. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2006, 150, 249–254. [Google Scholar] [CrossRef]
- Gołka, B.; Lewin-Kowalik, J.; Święch-Sabuda, E.; Larysz-Brysz, M.; Górka, D.; Małecka-Tendera, E. Predegenerated peripheral nerve grafts rescue retinal ganglion cells from axotomy-induced death. Experim. Neurol. 2001, 167, 118. [Google Scholar] [CrossRef]
- Berteli, J.A.; Taleb, M.; Mira, J.C.; Ghizoni, M.F. Functional recovery improvement is related to aberrant reinnervation trimming. A comperative study using fresh or predegenerated nerve grafts. Acta Neuropathol. 2006, 111, 601–609. [Google Scholar] [CrossRef]
- Lewin-Kowalik, J.; Koksanowicz, E.; Barski, J.J.; Krause, M.; Górka, D.; Gołka, B.; Kwiek, S. Experimental hyperthyroidism enhances the regeneration of central neurites promoted by peripheral nerve grafts in the hippocampus. Restor. Neurol. Neurosci. 1993, 6, 57–63. [Google Scholar] [CrossRef]
- Kerns, J.M.; Danielsen, N.; Holmquist, B.; Kanje, M.; Lundborg, G. The influence of predegeneration on regeneration through peripheral nerve grafts in the rat. Exp. Neurol. 1993, 122, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.F.; Jones, S.; Jia, X.F. Stem cell transplantation for peripheral nerve regeneration: Current options and opportunities. Int. J. Mol. Sci. 2017, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Aberg, M.; Ljungberg, C.; Edin, E.; Millqvist, H.; Nordh, E.; Theorin, A.; Terenghi, G.; Wiberg, M. Clinical evaluation of a resorbable wrap-around implant as an alternative to nerve repair: A prospective, assessor-blinded, randomised clinical study of sensory, motor and functional recovery after peripheral nerve repair. J. Plast. Reconstr. Aesthetic Surg. 2008, 62, 1503–1509. [Google Scholar] [CrossRef]
- Connolly, S.S.; Yoo, J.J.; Abouheba, M.; Soker, S.; McDougal, W.S.; Atala, A. Cavernous nerve regeneration using acellular nerve grafts. World J. Urol. 2008, 26, 333–339. [Google Scholar] [CrossRef]
- Shi, W.; Yao, J.; Chen, X.; Lin, W.; Gu, X.; Wang, X. The Delayed Repair of Sciatic Nerve Defects with Tissue-engineered Nerve Grafts in Rats. Artif. Cells Blood Substit. Immobil. Biotechnol. 2010, 38, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Thumble, T.E.; Kahn, U.; Vanderhooft, E.; Bach, A.W. A technique to quantitate motor recovery following nerve grafting. J. Hand Surg. 1995, 20, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Dillon, G.P.; Shidharan, A.; Ranieri, J.P.; Bellamkonda, R.V. The influence of physical structure and charge on neurite extension in a 3D hydrogel scaffold. J. Biomed. Sci. Polym. Ed. 1998, 9, 1049. [Google Scholar] [CrossRef]
- Lundborg, G. Nerve injury and repair-a challenge to the plastic brain. J. Peripher. Nerv. Syst. 2003, 8, 209–226. [Google Scholar] [CrossRef]
- Danielsen, N.; Kerns, J.; Holmquist, B.; Zhao, Q.; Lundborg, G.; Kanje, M. Pre-degenerated grafts enhance regeneration by shortening the initial delay period. Brain Res. 1994, 666, 250–254. [Google Scholar] [CrossRef]
- Danielsen, N.; Varon, S. Characterization of neurotrophic activity in the silicone chamber model for nerve regeneration. J. Reconstr. Microsurg. 1995, 11, 231–235. [Google Scholar] [CrossRef]
- Liu, J.; Lin, Z.; Wu, H.; Zhang, J.; Wang, F.; Wang, L.; Lu, S.; Gao, J. Dual-regulation biomimetic composite nerve scaffold with oriented structure and conductive function for skin peripheral nerve injury repair. Colloids Surf. B Biointerfaces 2025, 253, 114768. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.D.; Naghieh, S.; McInnes, A.D.; Schreyer, D.J.; Chen, X. Regeneration of peripheral nerves by nerve guidance conduits: Influence of design, biopolymers, cells, growth factors, and physical stimuli. Prog. Neurobiol. 2018, 171, 125–150. [Google Scholar] [CrossRef]
- Volkov, A.V.; Muraev, A.A.; Zharkova, I.I.; Voinova, V.V.; Akoulina, E.A.; Zhuikov, V.A.; Khaydapova, D.D.; Chesnokova, D.V.; Menshikh, K.A.; Dudun, A.A.; et al. Poly(3-hydroxybutyrate)/hydroxyapatite/alginate scaffolds seeded with mesenchymal stem cells enhance the regeneration of critical-sized bone defect. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 110991. [Google Scholar] [CrossRef] [PubMed]
| MNCS_CV | MNCS_CV | MNCS_CV | MNCS_CV | |
|---|---|---|---|---|
| Group | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| T (n) | 3 | 2 | 0 | 7 |
| % | 25.00% | 16.67% | 0.00% | 58.33% |
| P (n) | 1 | 8 | 3 | 7 |
| % | 5.26% | 42.11% | 15.79% | 36.84% |
| MNCS_OL | MNCS_OL | MNCS_OL | MNCS_OL | |
| Group | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| T (n) | 3 | 3 | 1 | 5 |
| % | 25.00% | 25.00% | 8.33% | 41.67% |
| P (n) | 1 | 7 | 3 | 8 |
| % | 5.26% | 36.84% | 15.79% | 42.11% |
| MNCS_APA | MNCS_APA | MNCS_APA | MNCS_APA | |
| Group | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| T (n) | 3 | 0 | 7 | 2 |
| % | 25.00% | 0.00% | 58.33% | 16.67% |
| P (n) | 5 | 2 | 7 | 5 |
| % | 26.32% | 10.53% | 36.84% | 26.32% |
| SNCS_CV | SNCS_CV | SNCS_CV | SNCS_CV | |
| Group | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| T (n) | 5 | 2 | 0 | 5 |
| % | 41.67% | 16.67% | 0.00% | 41.67% |
| P (n) | 4 | 5 | 1 | 9 |
| % | 21.05% | 26.32% | 5.26% | 47.37% |
| SNCS_APA | SNCS_APA | SNCS_APA | ||
| Group | Grade 1 | Grade 3 | Grade 4 | |
| T (n) | 4 | 2 | 6 | |
| % | 33.33% | 16.67% | 50.00% | |
| P (n) | 5 | 8 | 6 | |
| % | 26.32% | 42.11% | 31.58% |
| MNCS_CV (Ulnar Nerve) | Group P | Group T |
|---|---|---|
| mean | 37.59 m/s | 49.9 m/s |
| MNCS_OL (ulnar nerve) | P | T |
| mean | 3.12 ms | 3.24 ms |
| MNCS_APA (ulnar nerve) | P | T |
| mean | 3.45 mV | 3.32 mV |
| SNCS_CV (ulnar nerve) | P | T |
| mean | 37.86 m/s | 40.6 m/s |
| SNCS_APA (ulnar nerve) | P | T |
| mean | 3.85 mV | 13.43 mV |
| MNCS_CV (median nerve) | P | T |
| mean | 50.32 m/s | 31.51 m/s |
| MNCS_OL (median nerve) | P | T |
| mean | 4.9 ms | 3.4 ms |
| MNCS_APA (median nerve) | P | T |
| mean | 5 mV | 4.54 mV |
| SNCS_CV (median nerve) | P | T |
| mean | 35.24 m/s | 20.08 m/s |
| SNCS_APA (median nerve) | P | T |
| mean | 7.52 mV | 9.82 mV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suszyński, K.; Błaszczyk, B.; Górka, D.; Kwiek, S. The Influence of a Predegenerated Autological Nerve Graft on the Results of Peripheral Nerve Repair in the Upper Extremities After Injuries. Bioengineering 2025, 12, 945. https://doi.org/10.3390/bioengineering12090945
Suszyński K, Błaszczyk B, Górka D, Kwiek S. The Influence of a Predegenerated Autological Nerve Graft on the Results of Peripheral Nerve Repair in the Upper Extremities After Injuries. Bioengineering. 2025; 12(9):945. https://doi.org/10.3390/bioengineering12090945
Chicago/Turabian StyleSuszyński, Krzysztof, Bartłomiej Błaszczyk, Dariusz Górka, and Stanisław Kwiek. 2025. "The Influence of a Predegenerated Autological Nerve Graft on the Results of Peripheral Nerve Repair in the Upper Extremities After Injuries" Bioengineering 12, no. 9: 945. https://doi.org/10.3390/bioengineering12090945
APA StyleSuszyński, K., Błaszczyk, B., Górka, D., & Kwiek, S. (2025). The Influence of a Predegenerated Autological Nerve Graft on the Results of Peripheral Nerve Repair in the Upper Extremities After Injuries. Bioengineering, 12(9), 945. https://doi.org/10.3390/bioengineering12090945






