Extracellular Vesicle Secretion from 3D Culture of Human Adipose-Derived Mesenchymal Stem Cells in Scalable Bioreactors
Abstract
1. Introduction
2. Methods and Materials
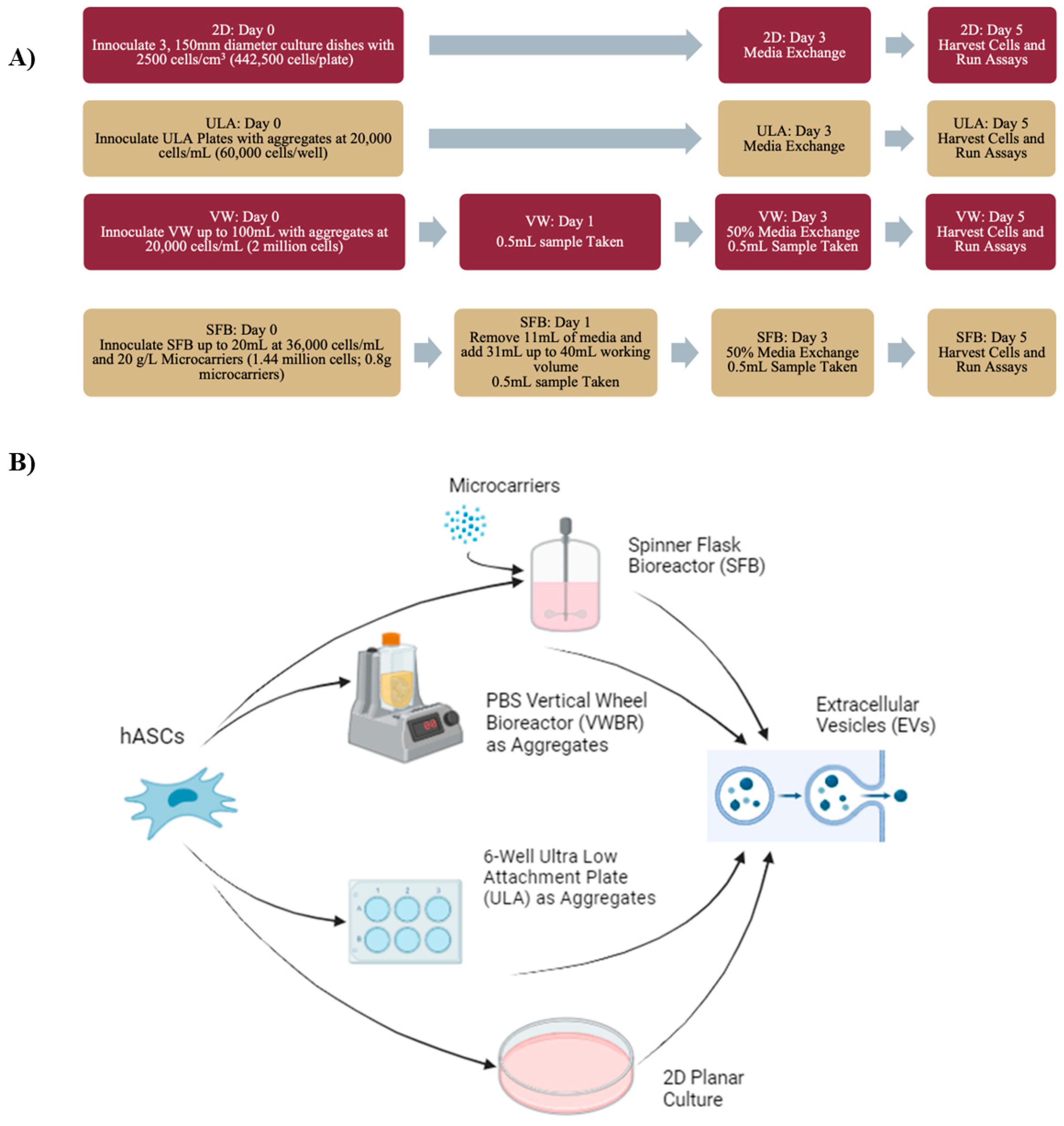
2.1. Human Mesenchymal Stem Cell Culture
2.2. 2D Culture
2.3. Spinner Flask Bioreactor Culture
2.4. Vertical Wheel Bioreactor Culture
2.5. Flow Cytometry
2.6. Extracellular Vesicle Isolation
2.7. Nanoparticle Tracking Analysis (NTA)
2.8. Transmission Electron Microscopy (TEM)
2.9. Western Blot
2.10. Reverse-Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
2.11. In Vitro Functional Assay Under Aβ42 Oligomer Treatment
2.12. Enzyme-Linked Immunosorbent Assay (ELISA)
2.13. In Vitro Functional Assay Under AD Brain Organoid-Conditioned Media Treatment
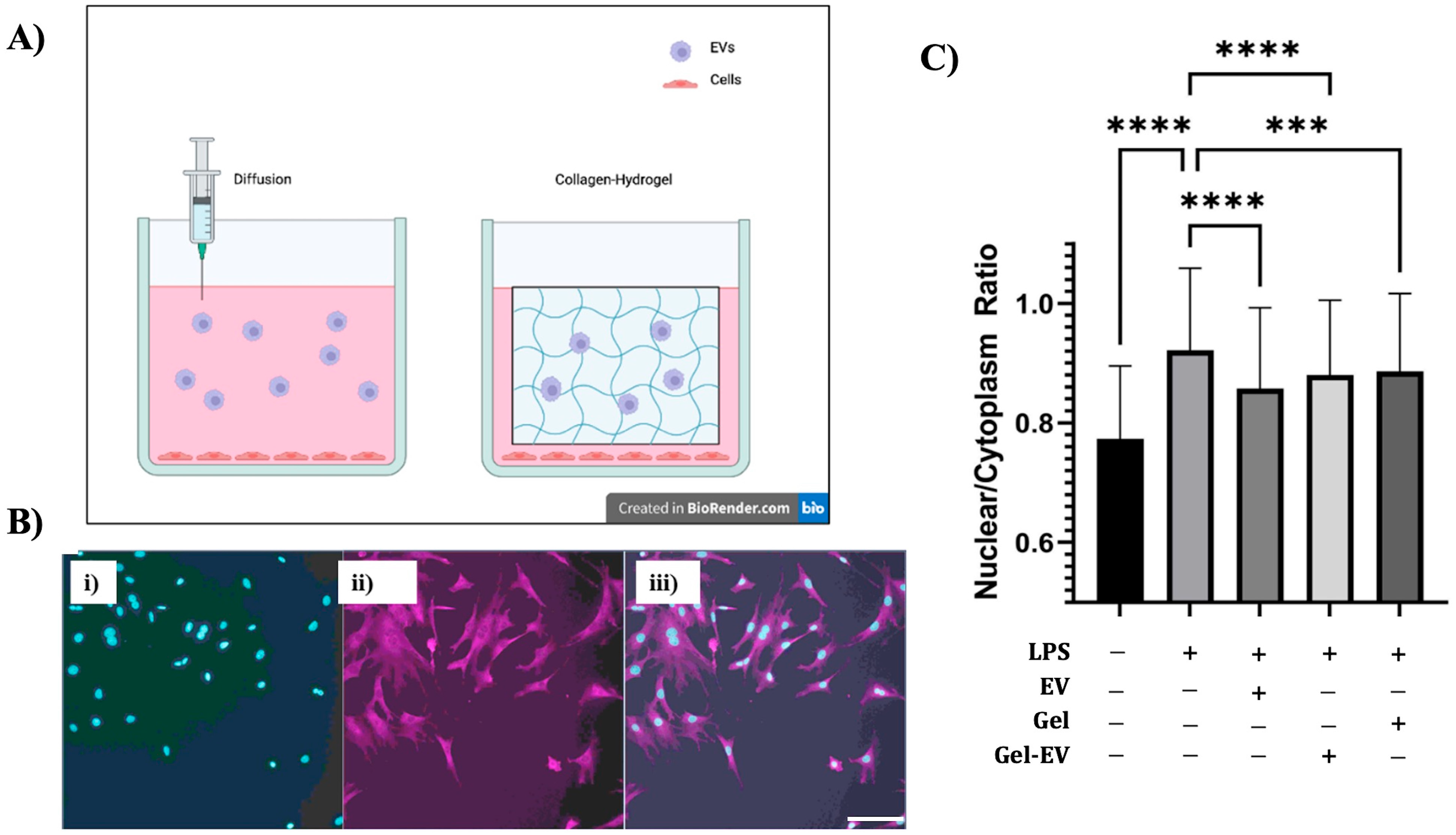
2.14. EV Delivery in Hydrogels to Reduce Inflammation
2.15. Statistical Analysis
3. Results
3.1. Expansion and Characterization of hMSCs
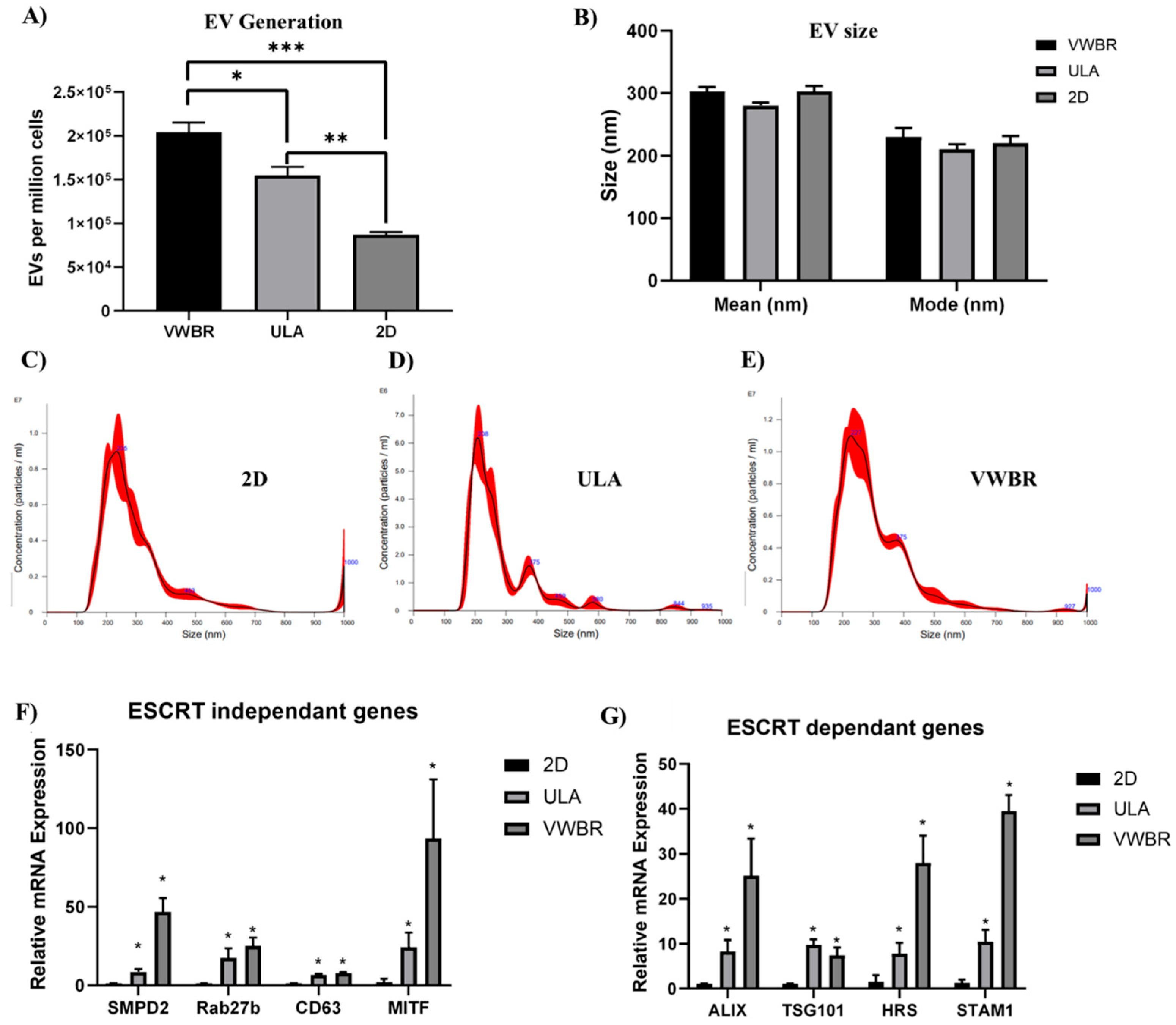
3.2. Extracellular Vesicle Secretion
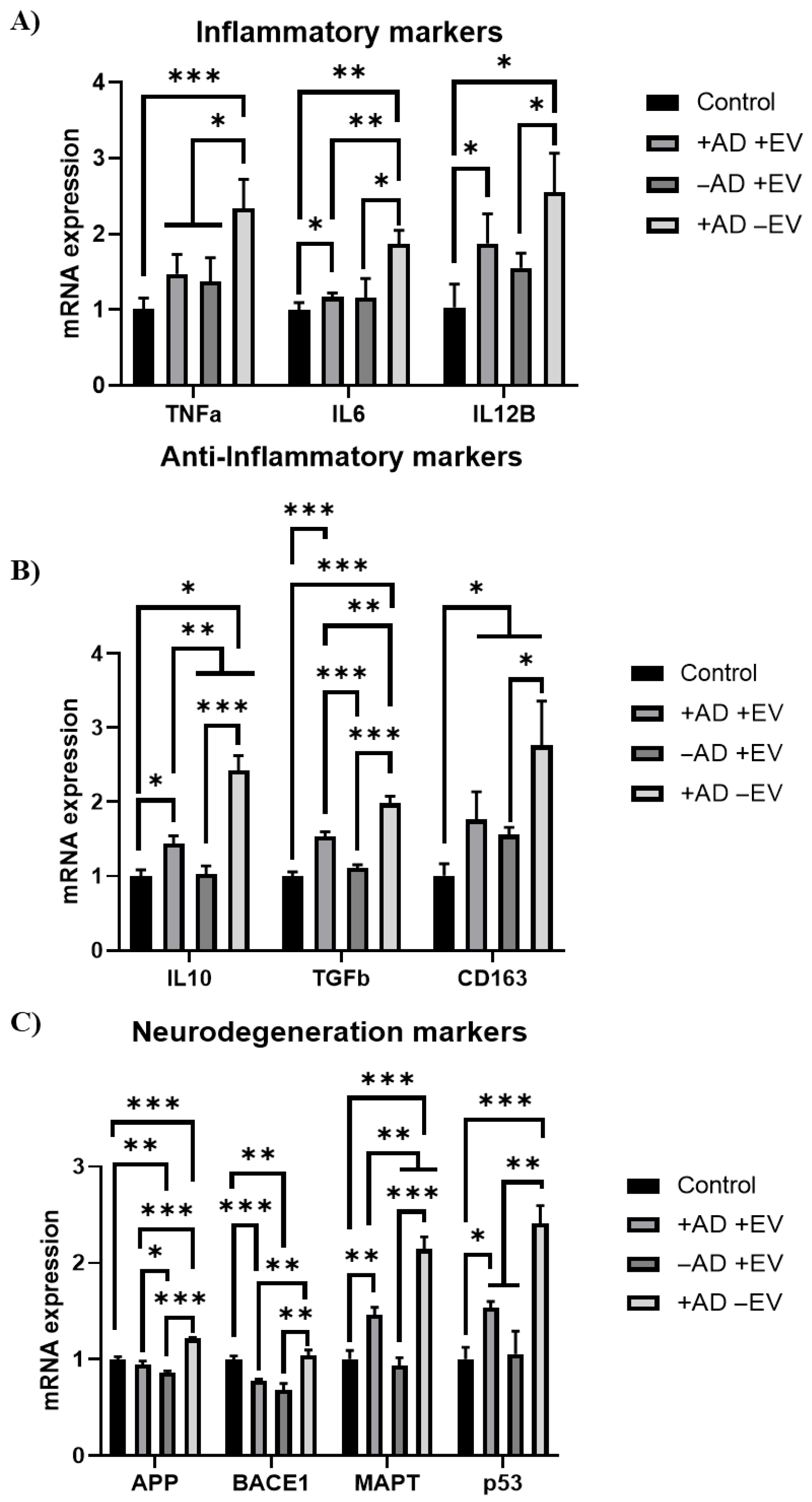
3.3. Extracellular Vesicle Functional Analysis In Vitro
4. Discussions
4.1. Influence of Dynamic Bioreactor Microenvironment on EV Secretion from hMSCs
4.2. hMSC EV Functional Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| hMSCs | human mesenchymal stem cells |
| iPSCs | induced pluripotent stem cells |
| EVs | extracellular vesicles |
| ULA | ultralow attachment |
| SFB | spinner flask bioreactor |
| VWBR | vertical wheel bioreactor |
| FBS | fetal bovine serum |
| PBS | phosphate-buffered saline |
| NTA | nanoparticle tracking analysis |
| TEM | transmission electron microscopy |
| RT-qPCR | reverse-transcription quantitative polymerase chain reaction |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated b cells |
| ROS | reactive oxygen species |
| ESCRT | endosomal sorting complex required for transport |
| TNF-α | tumor necrosis factor |
| Aβ42 | amyloid beta 1–42 |
| YAP | Yes-associated protein |
| LPS | lipopolysaccharide |
| ANOVA | analysis of variance |
References
- Jankovic, M.G.; Stojkovic, M.; Bojic, S.; Jovicic, N.; Kovacevic, M.M.; Ivosevic, Z.; Juskovic, A.; Kovacevic, V.; Ljujic, B. Scaling up human mesenchymal stem cell manufacturing using bioreactors for clinical uses. Curr. Res. Transl. Med. 2023, 71, 103393. [Google Scholar] [CrossRef]
- Vij, R.; Stebbings, K.A.; Kim, H.; Park, H.; Chang, D. Safety and efficacy of autologous, adipose-derived mesenchymal stem cells in patients with rheumatoid arthritis: A phase I/IIa, open-label, non-randomized pilot trial. Stem Cell Res. Ther. 2022, 13, 88. [Google Scholar] [CrossRef]
- Roesch, E.A.; Bonfield, T.L.; Lazarus, H.M.; Reese, J.; Hilliard, K.; Hilliard, J.; Khan, U.; Heltshe, S.; Gluvna, A.; Dasenbrook, E.; et al. A phase I study assessing the safety and tolerability of allogeneic mesenchymal stem cell infusion in adults with cystic fibrosis. J. Cyst. Fibros. 2023, 22, 407–413. [Google Scholar] [CrossRef]
- Mintun, M.A.; Lo, A.C.; Duggan Evans, C.; Wessels, A.M.; Ardayfio, P.A.; Andersen, S.W.; Shcherbinin, S.; Sparks, J.; Sims, J.R.; Brys, M.; et al. Donanemab in Early Alzheimer's Disease. N. Engl. J. Med. 2021, 384, 1691–1704. [Google Scholar] [CrossRef]
- Baak, L.M.; Wagenaar, N.; van der Aa, N.E.; Groenendaal, F.; Dudink, J.; Tataranno, M.L.; Mahamuud, U.; Verhage, C.H.; Eijsermans, R.; Smit, L.S.; et al. Feasibility and safety of intranasally administered mesenchymal stromal cells after perinatal arterial ischaemic stroke in the Netherlands (PASSIoN): A first-in-human, open-label intervention study. Lancet Neurol. 2022, 21, 528–536. [Google Scholar] [CrossRef]
- Kebriaei, P.; Hayes, J.; Daly, A.; Uberti, J.; Marks, D.I.; Soiffer, R.; Waller, E.K.; Burke, E.; Skerrett, D.; Shpall, E.; et al. A Phase 3 Randomized Study of Remestemcel-L versus Placebo Added to Second-Line Therapy in Patients with Steroid-Refractory Acute Graft-versus-Host Disease. Biol. Blood Marrow Transpl. 2020, 26, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Wu, X.; Zhou, J.; Lin, Y.; He, Y.; Fan, C.; Zeng, Z.; Xiong, W. Mitochondrial transfer in tunneling nanotubes-a new target for cancer therapy. J. Exp. Clin. Cancer Res. 2024, 43, 147. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, L.; Liu, Q. miR-18a-5p derived from mesenchymal stem cells-extracellular vesicles inhibits ovarian cancer cell proliferation, migration, invasion, and chemotherapy resistance. J. Transl. Med. 2022, 20, 258. (In English) [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Sun, L.A.-O.; Jeske, R.; Nkosi, D.; York, S.B.; Liu, Y.; Grant, S.A.-O.X.; Meckes, D.G.J.A.-O.; Li, Y. Engineering extracellular vesicles by three-dimensional dynamic culture of human mesenchymal stem cells. J. Extracell. Vesicles 2022, 11, e12235. (In English) [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay Am Fau-Beck, S.C.; Beck Sc Fau-Jaiswal, R.K.; Jaiswal Rk Fau-Douglas, R.; Douglas R Fau-Mosca, J.D.; Mosca Jd Fau-Moorman, M.A.; Moorman Ma Fau-Simonetti, D.W.; Simonetti Dw Fau-Craig, S.; Craig S Fau-Marshak, D.R.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. (In English) [Google Scholar] [CrossRef]
- Klingenstein, S.; Kleger, A.; Liebau, S.; Klingenstein, M. State-of-the-Art: Somatic Cell Sources Used for the Generation of Human Induced Pluripotent Stem Cells. Stem Cells Dev. 2025, 34, 317–332. [Google Scholar] [CrossRef]
- Gangoda, L.; Boukouris S Fau-Liem, M.; Liem M Fau-Kalra, H.; Kalra H Fau-Mathivanan, S.; Mathivanan, S. Extracellular vesicles including exosomes are mediators of signal transduction: Are they protective or pathogenic? Proteomics 2015, 15, 260–271. (In English) [Google Scholar] [CrossRef]
- Patel, D.B.; Santoro, M.; Born, L.J.; Fisher, J.P.; Jay, S.M. Towards rationally designed biomanufacturing of therapeutic extracellular vesicles: Impact of the bioproduction microenvironment. Biotechnol. Adv. 2018, 36, 2051–2059. (In English) [Google Scholar] [CrossRef]
- Debnath, K.; Heras, K.L.; Rivera, A.; Lenzini, S.; Shin, J.W. Extracellular vesicle-matrix interactions. Nat. Rev. Mater. 2023, 8, 390–402. [Google Scholar] [CrossRef]
- Wiklander, O.A.-O.; Brennan, M.; Lötvall, J.A.-O.; Breakefield, X.A.-O.; El Andaloussi, S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11, eaav8521. (In English) [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Liu, Y.; Ji, W.; Zhuang, J.; Chen, X.; Gong, B.; Chu, J.; Liang, W.; Gao, J.; Yin, Y. Engineered mesenchymal stem cell-derived extracellular vesicles: A state-of-the-art multifunctional weapon against Alzheimer’s disease. Theranostics 2023, 13, 1264–1285. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, D.; Ye, Z.; Xu, J. Engineering Extracellular Vesicles as Delivery Systems in Therapeutic Applications. Adv. Sci. 2023, 10, e2300552. [Google Scholar] [CrossRef]
- Costa, M.H.G.; Costa, M.S.; Painho, B.; Sousa, C.D.; Carrondo, I.; Oltra, E.; Pelacho, B.; Prosper, F.; Isidro, I.A.; Alves, P.; et al. Enhanced bioprocess control to advance the manufacture of mesenchymal stromal cell-derived extracellular vesicles in stirred-tank bioreactors. Biotechnol. Bioeng. 2023, 120, 2725–2741. [Google Scholar] [CrossRef]
- Borys, B.S.; Dang, T.; Worden, H.; Larijani, L.; Corpuz, J.M.; Abraham, B.D.; Gysel, E.J.; Malinovska, J.; Krawetz, R.; Revay, T.; et al. Robust bioprocess design and evaluation of commercial media for the serial expansion of human induced pluripotent stem cell aggregate cultures in vertical-wheel bioreactors. Stem Cell Res. Ther. 2024, 15, 232. [Google Scholar] [CrossRef]
- Li, X.; Jin, S.; Wang, D.; Wu, Y.; Tang, X.; Liu, Y.; Yao, T.; Han, S.; Sun, L.; Wang, Y.; et al. Accumulation of Damaging Lipids in the Arf1-Ablated Neurons Promotes Neurodegeneration through Releasing mtDNA and Activating Inflammatory Pathways in Microglia. Adv. Sci. 2025, 12, 2414260. [Google Scholar] [CrossRef]
- Jalilian, E.; Massoumi, H.; Bigit, B.; Amin, S.; Katz, E.; Guaiquil, V.; Anwar, K.; Hematti, P.; Rosenblatt, M.; Djalilian, A. Bone marrow mesenchymal stromal cells in a 3D system produce higher concentration of extracellular vesicles (EVs) with increased complexity and enhanced neuronal growth properties. Stem Cell Res. Ther. 2022, 13, 425. [Google Scholar] [CrossRef]
- Rong, Y.; Zhang, J.; Jiang, D.; Ji, C.; Liu, W.; Wang, J.; Ge, X.; Tang, P.; Yu, S.; Cui, W.; et al. Hypoxic pretreatment of small extracellular vesicles mediates cartilage repair in osteoarthritis by delivering miR-216a-5p. Acta Biomater. 2020, 122, 325–342. [Google Scholar] [CrossRef]
- Sung, D.; Chang, Y.S.; Sung, S.; Ahn, S.Y.; Park, W.S. Thrombin Preconditioning of Extracellular Vesicles Derived from Mesenchymal Stem Cells Accelerates Cutaneous Wound Healing by Boosting Their Biogenesis and Enriching Cargo Content. J. Clin. Med. 2019, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, Z.; Gao, W.; Gao, W.; He, R.; Li, Y.; Crawford, R.; Zhou, Y.; Xiao, L.; Xiao, Y. Current Advances in 3D Dynamic Cell Culture Systems. Gels 2022, 8, 829. [Google Scholar] [CrossRef]
- Merino-Casallo, F.; Gomez-Benito, M.J.; Hervas-Raluy, S.; Garcia-Aznar, J.M. Unravelling cell migration: Defining movement from the cell surface. Cell Adh. Migr. 2022, 16, 25–64. [Google Scholar] [CrossRef]
- Panchalingam, K.; Wang, T.; Jung, S.; Ahmadian Baghbaderani, B. Development of bioreactor protocols for stem cell-based therapies. Can. J. Chem. Eng. 2021, 99, 2505–2516. [Google Scholar] [CrossRef]
- Gong, S.; Li, N.; Peng, Q.; Wang, F.; Du, R.; Zhang, B.; Wang, J.; Han, L.; Zhang, Y.; Ning, Z.; et al. A scalable platform for EPSC-Induced MSC extracellular vesicles with therapeutic potential. Stem Cell Res. Ther. 2025, 16, 426. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.; Papoutsakis, E.T. The role of biomechanical stress in extracellular vesicle formation, composition and activity. Biotechnol. Adv. 2023, 66, 108158. [Google Scholar] [CrossRef]
- Park, H.; Seo, Y.K.; Arai, Y.; Lee, S.H. Physicochemical Modulation Strategies for Mass Production of Extracellular Vesicle. Tissue Eng. Regen. Med. 2025, 22, 569–591. [Google Scholar] [CrossRef]
- Kang, H.; Bae, Y.-h.; Kwon, Y.; Kim, S.; Park, J. Extracellular Vesicles Generated Using Bioreactors and their Therapeutic Effect on the Acute Kidney Injury Model. Adv. Healthc. Mater. 2022, 11, 2101606. [Google Scholar] [CrossRef]
- Xie, M.; Cao, H.; Qiao, W.; Yan, G.; Qian, X.; Zhang, Y.; Xu, L.; Wen, S.; Shi, J.; Cheng, M.; et al. Shear stress activates the Piezo1 channel to facilitate valvular endothelium-oriented differentiation and maturation of human induced pluripotent stem cells. Acta Biomater. 2024, 178, 181–195. [Google Scholar] [CrossRef]
- Sangha, G.S.; Weber, C.M.; Sapp, R.M.; Setua, S.; Thangaraju, K.; Pettebone, M.; Rogers, S.C.; Doctor, A.; Buehler, P.W.; Clyne, A.M. Mechanical stimuli such as shear stress and piezo1 stimulation generate red blood cell extracellular vesicles. Front. Physiol. 2023, 14, 1246910. [Google Scholar] [CrossRef]
- Koh, B.; Sulaiman, N.; Fauzi, M.B.; Law, J.X.; Ng, M.H.; Idrus, R.B.H.; Yazid, M.A.-O. Three dimensional microcarrier system in mesenchymal stem cell culture: A systematic review. Cell Biosci. 2020, 10, 75. (In English) [Google Scholar] [CrossRef]
- Lembong, J.A.-O.X.; Kirian, R.; Takacs, J.D.; Olsen, T.R.; Lock, L.T.; Rowley, J.A.; Ahsan, T.A.-O. Bioreactor Parameters for Microcarrier-Based Human MSC Expansion under Xeno-Free Conditions in a Vertical-Wheel System. Bioengineering 2020, 7, 73. (In English) [Google Scholar] [CrossRef]
- Maillot, C.; De Isla, N.; Loubiere, C.; Toye, D.; Olmos, E.A.-O. Impact of microcarrier concentration on mesenchymal stem cell growth and death: Experiments and modeling. Biotechnol. Bioeng. 2022, 119, 3537–3548. (In English) [Google Scholar] [CrossRef]
- Jeske, R.; Yuan, X.; Fu, Q.; Bunnell, B.A.; Logan, T.M.; Li, Y. In Vitro Culture Expansion Shifts the Immune Phenotype of Human Adipose-Derived Mesenchymal Stem Cells. Front. Immunol. 2021, 12, 621744. (In English) [Google Scholar] [CrossRef]
- Zhou, X.; Hong, Y.; Zhang, H.; Li, X. Mesenchymal Stem Cell Senescence and Rejuvenation: Current Status and Challenges. Front. Cell Dev. Biol. 2020, 8, 364. (In English) [Google Scholar] [CrossRef]
- Cone, A.S.; Yuan, X.; Sun, L.; Duke, L.C.; Vreones, M.P.; Carrier, A.N.; Kenyon, S.M.; Carver, S.R.; Benthem, S.D.; Stimmell, A.C.; et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer’s disease-like phenotypes in a preclinical mouse model. Theranostics 2021, 11, 8129–8142. (In English) [Google Scholar] [CrossRef]
- Huang, C.C.; Kang, M.; Lu, Y.; Shirazi, S.; Diaz, J.I.; Cooper, L.F.; Gajendrareddy, P.; Ravindran, S. Functionally engineered extracellular vesicles improve bone regeneration. Acta Biomater. 2020, 109, 182–194. (In English) [Google Scholar] [CrossRef]
- Udalamaththa, V.L.; Kaluarachchi, A.; Wijeratne, S.; Udagama, P.V. Therapeutic uses of post-partum tissue-derived mesenchymal stromal cell secretome. Indian J. Med. Res. 2020, 152, 541–552. (In English) [Google Scholar] [CrossRef]
- Jeske, R.; Chen, X.; Mulderrig, L.; Liu, C.; Cheng, W.A.-O.; Zeng, O.Z.; Zeng, C.A.-O.; Guan, J.; Hallinan, D.A.-O.; Yuan, X.; et al. Engineering Human Mesenchymal Bodies in a Novel 3D-Printed Microchannel Bioreactor for Extracellular Vesicle Biogenesis. Bioengineering 2022, 9, 795. (In English) [Google Scholar] [CrossRef]
- Tsai, A.C.; Liu, Y.; Yuan, X.; Chella, R.; Ma, T. Aggregation kinetics of human mesenchymal stem cells under wave motion. Biotechnol. J. 2017, 12, 1600448. (In English) [Google Scholar] [CrossRef]
- Ene, J.; Liu, C.; Syed, F.; Sun, L.; Berry, D.; Durairaj, P.; Liu, Z.L.; Zeng, C.; Jung, S.; Li, Y. Biomanufacturing and Lipidomics Analysis of Extracellular Vesicles Secreted by Human Blood Vessel Organoids in a Vertical Wheel Bioreactor. Stem Cell Res. Ther. 2025, 16, 207. [Google Scholar] [CrossRef]
- Liu, C.; Sun, L.; Worden, H.; Ene, J.; Zeng, O.Z.; Bhagu, J.; Grant, S.C.; Bao, X.; Jung, S.; Li, Y. Profiling biomanufactured extracellular vesicles of human forebrain spheroids in a Vertical Wheel bioreactor. J. Extracell. Biol. 2024, 3, e70002. [Google Scholar] [CrossRef]
- Rider, M.A.; Hurwitz, S.N.; Meckes, D.G., Jr. ExtraPEG: A Polyethylene Glycol-Based Method for Enrichment of Extracellular Vesicles. Sci. Rep. 2016, 6, 23978. (In English) [Google Scholar] [CrossRef]
- Yan, Y.; Bejoy, J.; Xia, J.; Guan, J.; Zhou, Y.; Li, Y. Neural patterning of human induced pluripotent stem cells in 3-D cultures for studying biomolecule-directed differential cellular responses. Acta Biomater. 2016, 42, 114–126. (In English) [Google Scholar] [CrossRef]
- Zhang, D.; Pekkanen-Mattila, M.; Shahsavani, M.; Falk, A.; Teixeira, A.I.; Herland, A. A 3D Alzheimer’s disease culture model and the induction of P21-activated kinase mediated sensing in iPSC derived neurons. Biomaterials 2014, 35, 1420–1428. (In English) [Google Scholar] [CrossRef]
- Zhao, J.; Fu, Y.; Yamazaki, Y.; Ren, Y.; Davis, M.D.; Liu, C.C.; Lu, W.; Wang, X.; Chen, K.; Cherukuri, Y.; et al. APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer's disease patient iPSC-derived cerebral organoids. Nat. Commun. 2020, 11, 5540. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, C.; Li, R.; Zhong, W.A.-O.; Xu, G.; Zhang, W. Role of Yes-associated protein (YAP) in regulation of mesenchymal stem cell tenogenic differentiation. J. Mol. Histol. 2022, 53, 273–283. (In English) [Google Scholar] [CrossRef]
- Xie, W.; Xiao, W.; Tang, K.; Zhang, L.; Li, Y. Yes-Associated Protein 1: Role and Treatment Prospects in Orthopedic Degenerative Diseases. Front. Cell Dev. Biol. 2020, 8, 573455. (In English) [Google Scholar] [CrossRef]
- Yuan, Z.; Ye, L.; Feng, X.; Zhou, T.; Zhou, Y.; Zhu, S.; Jia, C.; Li, H.; Qiu, D.; Li, K.; et al. YAP-Dependent Induction of CD47-Enriched Extracellular Vesicles Inhibits Dendritic Cell Activation and Ameliorates Hepatic Ischemia-Reperfusion Injury. Oxid. Med. Cell Longev. 2021, 2021, 6617345. [Google Scholar] [CrossRef]
- Patwardhan, S.; Mahadik, P.; Shetty, O.; Sen, S. ECM stiffness-tuned exosomes drive breast cancer motility through thrombospondin-1. Biomaterials 2021, 279, 121185. (In English) [Google Scholar] [CrossRef]
- Yuan, X.; Liu, Y.; Bijonowski, B.; Tsai, A.C.; Fu, Q.; Logan, T.M.; Ma, T.; Li, Y. NAD+/NADH Redox Alterations Reconfigure Metabolism and Rejuvenate Senescent Human Mesenchymal Stem Cells In Vitro. Commun. Biol. 2020, 3, 774. [Google Scholar] [CrossRef]
- Detela, G.; Bain, O.W.; Kim, H.W.; Williams, D.J.; Mason, C.; Mathur, A.; Wall, I.B. Donor Variability in Growth Kinetics of Healthy hMSCs Using Manual Processing: Considerations for Manufacture of Cell Therapies. Biotechnol. J. 2018, 13, 1700085. (In English) [Google Scholar] [CrossRef]
- Jeske, R.; Chen, X.; Ma, S.; Zeng, E.Z.; Driscoll, T.; Li, Y.A.-O. Bioreactor Expansion Reconfigures Metabolism and Extracellular Vesicle Biogenesis of Human Adipose-derived Stem Cells In Vitro. LID Biochem. Eng. J. 2022, 188, 108711. (In English) [Google Scholar] [CrossRef]
- Jeske, R.; Liu, C.; Duke, L.; Canonicco Castro, M.L.; Muok, L.; Arthur, P.; Singh, M.; Jung, S.; Sun, L.; Li, Y. Upscaling human mesenchymal stromal cell production in a novel vertical-wheel bioreactor enhances extracellular vesicle secretion and cargo profile. Bioact. Mater. 2022, 25, 732–747. (In English) [Google Scholar] [CrossRef]
- Tsai, H.H.; Yang, K.C.; Wu, M.H.; Chen, J.C.; Tseng, C.L. The Effects of Different Dynamic Culture Systems on Cell Proliferation and Osteogenic Differentiation in Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 4024. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Wang, T. How the mechanical microenvironment of stem cell growth affects their differentiation: A review. Stem Cell Res. Ther. 2022, 13, 415. [Google Scholar] [CrossRef]
- Selden, C.; Fuller, B. Role of Bioreactor Technology in Tissue Engineering for Clinical Use and Therapeutic Target Design. Bioengineering 2018, 5, 32. (In English) [Google Scholar] [CrossRef]
- Ma, T.; Grayson Wl Fau-Fröhlich, M.; Fröhlich M Fau-Vunjak-Novakovic, G.; Vunjak-Novakovic, G. Hypoxia and stem cell-based engineering of mesenchymal tissues. Biotechnol. Prog. 2009, 25, 32–42. (In English) [Google Scholar] [CrossRef]
- Martin, I.; Wendt D Fau-Heberer, M.; Heberer, M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004, 22, 80–86. (In English) [Google Scholar] [CrossRef]
- Rafiq, Q.A.; Coopman, K.; Nienow, A.W.; Hewitt, C.J. Systematic microcarrier screening and agitated culture conditions improves human mesenchymal stem cell yield in bioreactors. Biotechnol. J. 2016, 11, 473–486. (In English) [Google Scholar] [CrossRef]
- Tsai, A.C.; Liu Y Fau-Yuan, X.; Yuan X Fau-Ma, T.; Ma, T. Compaction, fusion, and functional activation of three-dimensional human mesenchymal stem cell aggregate. Tissue engineering. Part A 2015, 21, 1705–1719. (In English) [Google Scholar] [CrossRef]
- Yuan, X.; Tsai, A.C.; Farrance, I.; Rowley, J.; Ma, T. Aggregation of Culture Expanded Human Mesenchymal Stem Cells in Microcarrier-based Bioreactor. Biochem. Eng. J. 2018, 131, 39–46. (In English) [Google Scholar] [CrossRef]
- de Almeida Fuzeta, M.; Bernardes, N.; Oliveira, F.D.; Costa, A.C.; Fernandes-Platzgummer, A.; Farinha, J.P.; Rodrigues, C.A.V.; Jung, S.; Tseng, R.J.; Milligan, W.; et al. Scalable Production of Human Mesenchymal Stromal Cell-Derived Extracellular Vesicles Under Serum-/Xeno-Free Conditions in a Microcarrier-Based Bioreactor Culture System. Front. Cell Dev. Biol. 2020, 8, 553444. (In English) [Google Scholar] [CrossRef]
- McCoy, R.J.; O'Brien, F.J. Influence of shear stress in perfusion bioreactor cultures for the development of three-dimensional bone tissue constructs: A review. Tissue Engineering. Part B Rev. 2010, 16, 587–601. (In English) [Google Scholar] [CrossRef]
- Zhao, F.; Chella R Fau-Ma, T.; Ma, T. Effects of shear stress on 3-D human mesenchymal stem cell construct development in a perfusion bioreactor system: Experiments and hydrodynamic modeling. Biotechnol. Bioeng. 2007, 96, 584–595. (In English) [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Cook, K.; Li, H. Advancing extracellular vesicle production: Improving physiological relevance and yield with 3D cell culture. Nanoscale 2025, 17, 15110–15131. [Google Scholar] [CrossRef]
- Muok, L.; Sun, L.; Esmonde, C.; Worden, H.; Vied, C.; Duke, L.; Ma, S.; Zeng, O.Z.; Driscoll, T.P.; Jung, S.; et al. Extracellular Vesicle Biogenesis of Three-dimensional Human Pluripotent Stem Cells in a Novel Vertical-Wheel Bioreactor. J. Extracell. Biol. 2024, 3, e133. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, R.; Zhang, H.; Wang, H.; Yin, H.; Tian, G.; Wang, B.; Yan, Y.; Ding, Z.; Dai, J.; et al. Amantadine against glioma via ROS-mediated apoptosis and autophagy arrest. Cell Death Dis. 2024, 15, 834. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, A.; de Oliveira, J.; da Silva Pontes, L.V.; de Souza Junior, J.F.; Goncalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and its Implications in Aging Pathways. Oxid. Med. Cell Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef] [PubMed]
- van Loo, G.; Bertrand, M.J.M. Death by TNF: A road to inflammation. Nat. Rev. Immunol. 2023, 23, 289–303. [Google Scholar] [CrossRef]
- Anuradha, U.; Kumar, A.; Singh, R.K. The clinical correlation of proinflammatory and anti-inflammatory biomarkers with Alzheimer disease: A meta-analysis. Neurol. Sci. 2022, 43, 285–298. [Google Scholar] [CrossRef]
- Sun, N.; Victor, M.B.; Park, Y.P.; Xiong, X.; Scannail, A.N.; Leary, N.; Prosper, S.; Viswanathan, S.; Luna, X.; Boix, C.A.; et al. Human microglial state dynamics in Alzheimer's disease progression. Cell 2023, 186, 4386–4403.e29. [Google Scholar] [CrossRef]
- Sobue, A.; Komine, O.; Yamanaka, K. Neuroinflammation in Alzheimer’s disease: Microglial signature and their relevance to disease. Inflamm. Regen. 2023, 43, 26. [Google Scholar] [CrossRef]
- Kronstadt, S.M.; Patel, D.B.; Born, L.J.; Levy, D.; Lerman, M.J.; Mahadik, B.; Jay, S.M. Mesenchymal Stem Cell Culture within Perfusion Bioreactors Incorporating 3D-Printed Scaffolds Enables Improved Extracellular Vesicle Yield with Preserved Bioactivity. Adv. Healthc. Mater. 2023, 12, e2300584. [Google Scholar] [CrossRef]
- Cao, J.; Wang, B.; Tang, T.; Lv, L.; Ding, Z.; Li, Z.; Liu, B. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem. Cell Res. Ther. 2020, 11, 206. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Ene, J.; McGarraugh, C.; Ma, S.; Esmonde, C.; Liu, Y.; Li, Y. Extracellular Vesicle Secretion from 3D Culture of Human Adipose-Derived Mesenchymal Stem Cells in Scalable Bioreactors. Bioengineering 2025, 12, 933. https://doi.org/10.3390/bioengineering12090933
Ma S, Ene J, McGarraugh C, Ma S, Esmonde C, Liu Y, Li Y. Extracellular Vesicle Secretion from 3D Culture of Human Adipose-Derived Mesenchymal Stem Cells in Scalable Bioreactors. Bioengineering. 2025; 12(9):933. https://doi.org/10.3390/bioengineering12090933
Chicago/Turabian StyleMa, Shaoyang, Justice Ene, Colton McGarraugh, Shaoxuan Ma, Colin Esmonde, Yuan Liu, and Yan Li. 2025. "Extracellular Vesicle Secretion from 3D Culture of Human Adipose-Derived Mesenchymal Stem Cells in Scalable Bioreactors" Bioengineering 12, no. 9: 933. https://doi.org/10.3390/bioengineering12090933
APA StyleMa, S., Ene, J., McGarraugh, C., Ma, S., Esmonde, C., Liu, Y., & Li, Y. (2025). Extracellular Vesicle Secretion from 3D Culture of Human Adipose-Derived Mesenchymal Stem Cells in Scalable Bioreactors. Bioengineering, 12(9), 933. https://doi.org/10.3390/bioengineering12090933





