Abstract
(1) Background: Chronic pulmonary diseases (CPDs), such as COPD, asthma, and interstitial lung disease, are often accompanied by psychological distress and reduced quality of life. Mindfulness-Based Interventions (MBIs), including digital and virtual reality (VR) formats, have emerged as promising non-pharmacological approaches to improve symptom management and well-being. This systematic review aimed to evaluate the effectiveness of MBIs—delivered in-person or digitally—on anxiety, depression, disease-related stress, dyspnea, and health-related quality of life in individuals with CPDs. (2) Methods: A systematic review was conducted following PRISMA guidelines across PubMed, Scopus, and Web of Science (2005–2025). Thirteen studies (8 randomized controlled trials, 5 non-randomized) met inclusion criteria. Outcomes assessed included psychological distress, physical symptoms, and health-related quality of life (HRQoL). Risk of bias was evaluated using RoB 2 and ROBINS-I tools. (3) Results: MBIs were associated with improvements in anxiety, depression, stress, and HRQoL in several studies. Interventions were generally well-tolerated and effective across various delivery methods, including digital and VR-based formats. Improvements were especially notable when interventions were tailored and sustained. (4) Conclusions: MBIs, including digital and VR-delivered formats, show promise in supporting psychological and physical outcomes in CPD populations. They represent a feasible and complementary tool in modern respiratory care.
1. Introduction
Chronic pulmonary diseases (CPDs)—including chronic obstructive pulmonary disease (COPD), interstitial lung disease (ILD), and chronic asthma—are progressive, debilitating conditions that affect millions of people worldwide and are associated with a significant physical and psychological burden [1]. In addition to core respiratory symptoms such as dyspnea, fatigue, and reduced exercise tolerance, many people with CPDs suffer from high levels of anxiety, depression, and health-related distress [2,3]. Psychological symptoms are not only secondary to physical illness but can exacerbate symptom perception, reduce self-management, and negatively affect clinical outcomes [4]. Therefore, there is growing recognition of the need for integrative, biopsychosocial approaches to the treatment of chronic respiratory diseases.
Among non-pharmacological strategies, mindfulness-based interventions (MBIs) have proven to be promising adjuncts in the management of chronic diseases. MBIs are rooted in contemplative traditions and have been operationalized in Western clinical psychology through interventions such as Mindfulness-Based Stress Reduction (MBSR) and Mindfulness-Based Cognitive Therapy (MBCT). MBIs are structured programs that teach individuals to develop non-judgmental, present-moment awareness [5]. Theoretical models suggest that mindfulness promotes psychological well-being through mechanisms such as improved attentional control, emotional regulation, interoceptive awareness, and decentering, allowing individuals to observe thoughts, emotions, and sensations without reactivity or avoidance [6,7].
In patients with CPDs, MBIs may be particularly relevant as they can help sufferers develop a more accepting relationship with unpleasant sensations such as breathlessness, reduce catastrophic thinking, and improve mood and quality of life (QoL) [8,9]. Several studies have shown that traditional, face-to-face MBIs can lead to a reduction in anxiety and depression, an improvement in the perception of breathlessness, and improved coping. However, these programs typically require weekly attendance, physical travel, and sustained effort over several weeks—barriers that may limit access or participation for individuals with limited mobility, fatigue, or rural residence.
In response to these limitations, technology-enabled methods, particularly virtual reality (VR), have been explored as a way to broaden access and increase engagement. VR-based MBIs use immersive, computer-generated environments—often replicating calming natural environments—to guide mindfulness practice with rich sensory input and spatial presence [10]. Preliminary research suggests that VR can improve attentional focus, reduce distraction, and increase emotional engagement, potentially enhancing the effects of mindfulness practice [11,12].
Beyond accessibility, VR may be uniquely suited for CPD patients because of its potential for physiological modulation (e.g., immersive calming environments that can influence autonomic activity and alter the perception of breathlessness), enhanced attentional focus (reducing external distractions and supporting mindfulness practice in the presence of fatigue or dyspnea), multisensory engagement (reinforcing interoceptive awareness and breathing regulation), and improved adherence (increasing motivation and reducing dropout, particularly among those with mobility limitations) [13,14,15,16,17].
Nevertheless, the current evidence on MBIs in CPDs is heterogeneous and fragmented: most studies focus on COPD, with limited data available for asthma and ILD; comparability is poor due to variability in study design, outcomes, and patient populations; and no systematic synthesis has examined different delivery formats (in-person vs. digital) in a single review. While VR is discussed in the broader literature as an innovative delivery mode, none of the studies included in this review actually used VR, leaving its clinical impact in CPDs unexplored. We acknowledge that COPD, ILD, and asthma differ in symptom profile and disease course, but they share persistent symptoms such as dyspnea and fatigue, high prevalence of psychological distress, and the need for long-term self-management. MBIs target transdiagnostic mechanisms—such as attentional control, emotion regulation, and acceptance of bodily sensations—that are relevant across these conditions, making pooled analysis valuable for identifying cross-cutting benefits while noting phenotype-specific differences.
To the best of our knowledge, previous systematic reviews on psychological interventions in COPD or MBIs in other chronic illnesses have not included asthma and ILD in the same synthesis, have not compared delivery formats, have not discussed the potential role of VR in CPDs, and in some cases have referred only generically to “relaxation techniques” without specifying structured mindfulness-based approaches [9,18,19,20,21,22]. Our review is the first to integrate these elements into a single, comprehensive analysis.
This systematic review therefore aims to summarize the current evidence on MBIs for people with chronic lung disease, examining their effects on psychological outcomes (e.g., anxiety, depression, stress), physical symptoms (e.g., dyspnea, fatigue), and health-related quality of life (HRQoL). In addition, feasibility, acceptability, and engagement in different forms of delivery are explored, and the potential role of VR is discussed as a promising future direction for research and practice in respiratory medicine.
2. Materials and Methods
2.1. Study Protocol
The study protocol was designed in accordance with the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews) [23], a rigorous methodological approach prior to the literature search and data synthesis with a specific focus on the research questions and the clinical effectiveness of mindfulness-based interventions (MBIs), delivered with or without VR. The analysis focuses on the effects of these interventions and their role in improving psychological distress, reducing anxiety, depression, low mood, disease-related stress, and other health-related outcomes, evaluating their impact on self-management of symptoms and HRQoL. Before starting the june and data synthesis, the related study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database of systematic reviews (https://www.crd.york.ac.uk/PROSPERO/view/CRD420251072411, accessed on 29 May 2025). The formulation of the research question and the strategy and criteria for study selection were developed using the PICO model [24]. The study question focused on the following:
- P (Population): Patients with chronic respiratory diseases (e.g., COPD, pulmonary fibrosis, asthma, etc.)
- I (Intervention): Psychological intervention combined with mindfulness, where mindfulness is delivered with or without VR
- C (Comparison): Psychological intervention alone or no intervention
- O (Outcomes): QoL, anxiety, disease-related stress, any healthcare outcome
2.2. Search Strategy and Study Selection
The literature search was conducted electronically across three databases: PubMed, Scopus, and WOS. The database screening was carried out independently by three reviewers (MG, CP, AB) covering studies published from January 2005 to 1 June 2025, which represents the time frame considered for this review, using the following keywords combined with Boolean operators: Chronic respiratory disease, COPD, chronic obstructive pulmonary disease, mindfulness OR mindfulness meditation, virtual reality, VR, avatar, psychological intervention, psychosocial intervention. Complete search strategies are provided in Appendix A. The collected citations were recorded, duplicates were eliminated using EndNote, and the titles and abstracts obtained were independently screened by two reviewers (MG, CP). The full texts of potentially relevant papers and ambiguous abstracts were reviewed independently by the same authors (MG, CP), who resolved disagreements through discussion and consensus and, if necessary, with the involvement of a third reviewer (AB). The inclusion criteria were as follows:
- Source: studies published in the English language from 2005 to 1 June 2025;
- Study design: randomized controlled trial (RCT), observational studies, feasibility studies, and qualitative studies;
- Study population: Adults (>18) with CPDs
- Study intervention: MBIs delivered with or without VR;
- Study outcomes: MBIs are useful for clinicians to reduce stress, disease-related psychological distress and suffering, improving QoL or other healthcare outcomes in the sample of patients with chronic pulmonary diseases.
The exclusion criteria were as follows:
- Source: studies published before 2005 or after 1 June 2025;
- Study intervention: Studies not involving MBIs, other systematic reviews, studies utilizing other psychological interventions, or interventions focusing on pharmacological or surgical treatments.
- Study outcomes: studies not reporting psychological outcomes; studies without clinical outcomes related to stress reduction, healthcare impact, or QoL.
2.3. Data Extraction and Collection
Eligible studies were included or excluded upon mutual agreement. In cases of disagreement regarding a manuscript’s inclusion based on abstract evaluation, discrepancies were resolved through discussion and consensus. If consensus was not reached, a third reviewer (AB) was consulted for the final decision. The data extraction process followed established methodologies and was structured to align with the research questions of this review. Extracted data included: (a) author, year and country; (b) study design; (c) sample size (population, mean age, % male); (d) intervention group; (e) control group; (f) main outcomes; (g) secondary outcomes; (h) key findings (Table 1). This systematic approach ensured a rigorous and consistent collection of relevant data, facilitating a comprehensive synthesis of evidence to address the research questions.

Table 1.
Descriptive characteristics of the included studies.
Quality Assessment
The risk of bias for the studies included in this systematic review was independently evaluated by two reviewers (MG, AB). In cases of disagreement, a third reviewer (CP) was consulted to facilitate discussion and reach a consensus. For RCTs, the Cochrane Risk of Bias tool (RoB 2) [37] was applied, assessing five key domains: randomization process, deviations from intended interventions, missing outcome data, outcome measurement, and selection of the reported results. Each domain was rated as having a “low”, “high”, or “unclear” risk of bias (Table 2).

Table 2.
Cochrane risk of bias tool for the risk of bias in individual studies.
For non-randomized studies, the ROBINS-I tool [38] was employed to assess potential bias across several domains: confounding, participant selection, intervention classification, deviations from intended interventions, missing data, outcome measurement, and selection of reported results. Each study was then categorized as having a “low”, “moderate”, or “high” risk of bias (Table 3).

Table 3.
ROBINS-I Tool for non-RCTs; abbreviations: PY (probably yes), P (possibly), PN (probably no).
For qualitative and mixed-methods studies, the CASP Qualitative Checklist [39] was used to critically appraise methodological quality, focusing on clarity of aims, appropriateness of design, recruitment strategy, data collection, reflexivity, ethical considerations, rigor of analysis, transparency of findings, and overall value (Table 4).

Table 4.
Critical appraisal of qualitative and mixed-methods studies using the CASP Qualitative Checklist.
2.4. Data Analysis
All extracted data were first synthesized qualitatively to describe the characteristics, interventions, and outcomes of the included studies. Whenever possible, standardized mean differences (Hedges’ g) and corresponding 95% confidence intervals (CIs) were calculated using the available statistical information (means and standard deviations, p-values, or reported Cohen’s d). When measures of variability were not reported directly, they were derived from other statistics (e.g., standard errors, confidence intervals) following Cochrane Handbook guidance. Since only randomized controlled trials (RCTs) reported sufficient quantitative data, we restricted the calculation of standardized effect sizes to this study design (Table 5). For feasibility trials, pilot RCTs, or studies reporting only medians, change scores, or p-values, effect sizes could not be computed and were therefore not included in the quantitative synthesis.

Table 5.
Summary of extracted quantitative data from included studies.
For outcomes with sufficient data from at least two randomized controlled trials, a quantitative synthesis was conducted using random-effects meta-analysis (inverse-variance method) to account for potential heterogeneity in study populations, interventions, and measurement tools. Statistical heterogeneity was assessed using the I2 statistic, with values >50% indicating substantial heterogeneity. Analyses were performed using Review Manager (RevMan) version 5.4 (The Cochrane Collaboration, 2020).
Given that anxiety and depression were the only outcomes assessed in four RCTs, separate meta-analyses were conducted for each of these variables to provide pooled effect estimates and evaluate the consistency of findings across studies.
3. Results
3.1. Study Selection and Characteristics
A total of 555 records were identified through database searches (PubMed: 63; Scopus: 256; Web of Science: 236). After removing 121 duplicate records, 434 records remained for title and abstract screening. Of these, 409 records were excluded. The full texts of the remaining 25 articles were retrieved and assessed for eligibility. Following this, 12 articles were excluded for the following reasons: not MBIs (n = 5), not reporting psychological outcomes (n = 3), not being scientific publications (n = 2), being another review (n = 1), or falling outside the inclusion dates (n = 1). Ultimately, the review included 13 studies (Figure 1), 8 of which were RCTs, 3 of which were qualitative studies, 1 pre-post observational study, and 1 cross-sectional survey. There were around 1000 people in the total sample who had COPD, asthma, or ILD as their illness. MBIs encompassed MBSR, MBCT, mindful breathing, body scan meditation, and yoga-based mindfulness. The delivery of these was done through various formats, including in-person sessions, digital platforms, audio guides, and mobile applications. It has been possible to search for different variables, which ones were included in our research question and examined through the different empirical studies, as follows. Given the heterogeneity of study designs, results were organized by outcome domain to provide clinically relevant insights. Effect sizes and 95% confidence intervals were calculated exclusively for RCTs, and a meta-analysis was conducted for anxiety and depression outcomes. Qualitative and observational findings were synthesized narratively to complement the quantitative evidence.
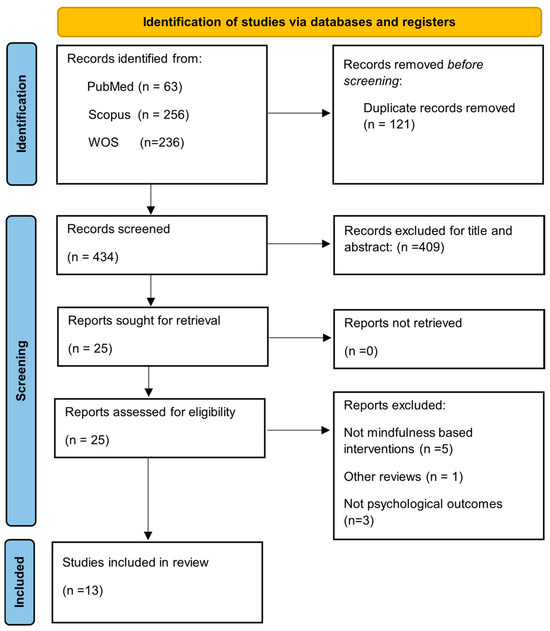
Figure 1.
Flow diagram of study selection.
3.2. Outcomes
Health-Related Quality of Life
A total of nine studies explored the impact of MBIs on HRQoL among individuals with chronic respiratory diseases, primarily COPD and asthma. These studies used a mix of RCTs, qualitative methods, and observational designs. The most commonly employed instruments were the St. George’s Respiratory Questionnaire (SGRQ) and the Chronic Respiratory Questionnaire (CRQ), both validated measures of HRQoL in pulmonary populations. Among the studies, three reported statistically significant improvements in HRQoL in the intervention group compared to controls. For example, Ugli et al. [31] demonstrated that an 8-week MBSR program produced a clinically relevant reduction in SGRQ scores, maintained at 3-month follow-up (p < 0.001). Similarly, an RCT by Hiles et al. [28] reported substantial HRQoL improvements following a 16-week yoga and mindfulness program for patients with severe asthma. Chan et al. [8] in their RCT found that participants in a mindful meditation group experienced significantly enhanced respiratory function and QoL compared to a waitlist control group. Qualitative studies provided additional insights into the subjective experience of improved HRQoL. Benzo and Malpass et al. [25,29] described themes such as increased life appreciation, self-compassion, and reorientation to illness, which were reported by patients as beneficial for their day-to-day well-being. Furthermore, recent studies utilizing remote or self-guided MBIs [27,33] revealed that digital delivery methods are both feasible and effective in enhancing HRQoL, increasing accessibility for patients with mobility limitations or during lockdown conditions. However, one study by Miranda et al. [30], conducted in a population with ILD, did not find statistically significant improvements in disease-specific QoL outcomes measured via the King’s Brief Interstitial Lung Disease questionnaire—K-BILD and the Leicester Cough Questionnaire—LCQ, underscoring potential differences in responsiveness based on disease phenotype. Overall, the evidence suggests that MBIs can lead to clinically meaningful improvements in dyspnea (e.g., mMRC, Borg), symptom burden (CAT, SGRQ) [34], and patient-reported HRQoL, particularly when delivered over an extended period and adapted to the patient’s condition (Table 6).

Table 6.
Health-related Quality of Life (HRQoL).
3.3. Psychological Distress (Anxiety and Depression)
Eight studies assessed the effects of MBIs on psychological distress, with a focus on symptoms of anxiety and depression. The majority were RCTs, with validated psychometric tools such as the Hospital Anxiety and Depression Scale (HADS), the Depression Anxiety Stress Scales (DASS-21), and the Patient Health Questionnaire (PHQ-8) used for outcome assessment. In total, three studies reported statistically significant reductions in either anxiety, depression, or both. Mukhiddin Ugli et al. [31] demonstrated significant decreases in HADS anxiety and depression scores after an 8-week MBSR program, with sustained improvements at follow-up. Similarly, Tschenett et al. [35] found significant reductions in anxiety using HADS-A and emotional functioning scores, though effects on depression were not statistically significant. Farver-Vestergaard et al. [26], who combined MBCT with pulmonary rehabilitation, reported a significant time-by-group interaction for depressive symptoms, suggesting additive benefits from integrated delivery models. Observational and qualitative data offered further support. Von Visger et al. [36] found that participants with higher dispositional mindfulness, measured via CAMS-R, reported lower levels of depressive symptoms and better self-management. Chan et al. [8] reported a significant reduction in anxiety sensitivity assessed with the Anxiety Sensitivity Index-3 (ASI-3) in their mindfulness group, suggesting improvements in physiological and emotional reactivity. Conversely, two studies [30,32] did not report significant changes in anxiety or depression scores, which the authors attributed to low baseline symptom levels or limited intervention intensity. Additionally, the study by Sun et al. [33] conducted during the COVID-19 lockdown found significant reductions in psychological distress through online MBCT delivery via WeChat, demonstrating the adaptability and utility of remote mindfulness formats in reducing anxiety and panic in older COPD patients.
Taken together, these findings indicate that MBIs may serve as a non-pharmacological tool to mitigate psychological comorbidities in respiratory disease. However, response patterns varied: digital and online delivery formats appeared particularly effective for reducing anxiety [33,35], while effects on depression were more heterogeneous [9,26,31]. Face-to-face interventions tended to yield broader but less consistent benefits across both anxiety and depression [8,9]. Patients with higher baseline distress showed the most pronounced improvements [31,36], whereas trials including participants with low initial symptom burden often reported null effects [30,32].
Qualitative findings further supported these results, with patients describing a greater sense of control over their breathing difficulties and reduced illness-related anxiety [40]. Participants also reported moving away from catastrophic interpretations of symptoms towards more accepting and compassionate attitudes, sometimes reframing their illness identity in a less oppressive way [29]. Similarly, mindfulness practices were described as alleviating anticipatory anxiety and fostering a calmer, more resilient engagement with bodily sensations, particularly breathlessness [32]. Overall, these subjective accounts are consistent with quantitative outcomes, suggesting that delivery mode, baseline severity, and patient characteristics may moderate treatment response (Table 7).

Table 7.
Psychological distress (anxiety and depression).
3.4. Quantitative Synthesis—Anxiety and Depression
Four randomized controlled trials provided sufficient quantitative data to allow pooled analysis of anxiety and depression outcomes following MBIs. Because the included studies used different validated instruments to assess these psychological outcomes (e.g., HADS, BDI, GAD-7), we calculated standardized mean differences (SMD, Hedges’ g) to enable comparison across trials using a common metric. Given the expected variability in outcome measures, intervention formats, and patient populations, a random-effects model (inverse-variance method) was selected to provide more conservative pooled estimates and account for between-study heterogeneity.
For anxiety, the meta-analysis showed a significant reduction in symptoms in the MBI groups compared with controls (SMD = −0.66, 95% CI [−0.91, −0.41], p < 0.00001; I2 = 33%), indicating a moderate effect size with low-to-moderate heterogeneity (Figure 2).
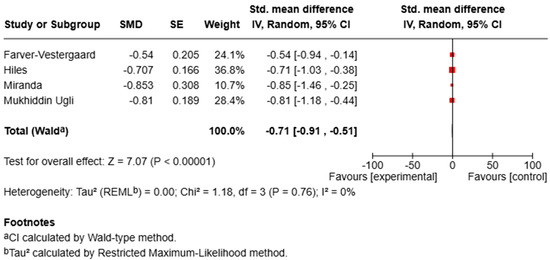
Figure 2.
Forest plot of RCTs assessing the effect of MBIs on anxiety.
For depression, pooled analysis demonstrated a significant improvement in symptoms (SMD = −0.71, 95% CI [−0.91, −0.51], p < 0.00001; I2 = 0%), reflecting a moderate-to-large effect size with no observed heterogeneity (Figure 3).
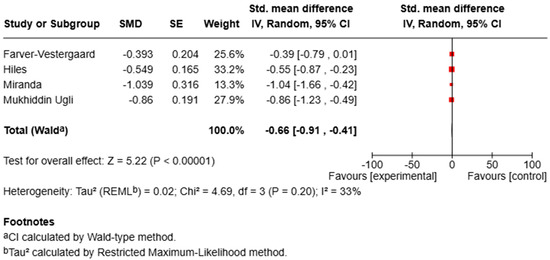
Figure 3.
Forest plot of RCTs assessing the effect of MBIs on depression.
These results indicate that, despite differences in measurement tools, MBIs consistently improved anxiety and depression across studies. The low levels of statistical heterogeneity suggest that the effect estimates are robust and generalizable to populations with chronic respiratory diseases.
3.5. Disease-Related Stress
A total of six studies investigated the impact of MBIs on disease-related stress in individuals with chronic respiratory diseases, primarily COPD. Disease-related stress was conceptualized as the subjective experience of psychological strain associated with dyspnea, symptom exacerbation, and illness uncertainty. Measurement tools included the Perceived Stress Scale (PSS-10), the Chronic Respiratory Questionnaire—Self-Administered Standardized Format (CRQ-SAS; emotional functioning domain), the Self-Compassion Scale (SCS), and qualitative interviews. Of the included studies, five reported significant reductions in disease-related or momentary stress following mindfulness training. For example, Tschenett et al. [35] conducted an RCT of a digital MBI and observed a large reduction in momentary stress (η2 = 0.75, p < 0.001) and dyspnea immediately following guided meditation sessions. Similarly, Mukhiddin Ugli et al. [31] reported that increases in self-compassion and mindfulness—developed through MBSR—were associated with patients’ improved capacity to cope with illness-related stress, including fatigue, symptom flare-ups, and psychological burden. Hiles et al. [28] found that a combined yoga and mindfulness intervention significantly improved participants’ ability to regulate stress and anxiety associated with symptom unpredictability. Chan et al. [8] reported reduced emotional reactivity and anxiety sensitivity, both of which are psychological constructs closely tied to disease-related stress and breathlessness. In a study by Perkins-Porras et al. [32], participants described an emotional buffering effect from brief audio-guided mindfulness exercises, even in the absence of significant changes in HADS scores, suggesting an experiential reduction in stress during acute recovery periods. These qualitative accounts can be interpreted as complementary to the quantitative findings of other trials, where significant reductions in perceived stress and dyspnea were observed, and collectively they support the view that mindfulness promotes adaptive emotion regulation and a decoupling from symptom-related distress [41,42]. Only one study failed to find statistically significant changes in stress as measured by the DASS-21, although it did report a notable reduction in dyspnea [30]. Although the construct of disease-related stress was not operationalized uniformly across studies, with some trials using direct stress measures (e.g., PSS-10, ASI-3, DASS-21) and others assessing related domains such as emotional functioning, dyspnea-related distress, or self-compassion, all outcomes were conceptually aligned with the overarching construct of disease-related stress, understood as the psychological burden of chronic respiratory diseases. Collectively, these findings suggest that MBIs may help individuals with chronic respiratory conditions manage stress arising from symptoms and disease impact by fostering acceptance, self-regulation, and emotional resilience (Table 8).

Table 8.
Disease-related stress.
3.6. Healthcare Outcomes
Evidence from the seven studies included in this review suggests that MBIs have the potential to improve clinical outcomes in people with chronic respiratory conditions. In this section, the term “healthcare outcomes” refers specifically to physical and symptom-related endpoints (e.g., dyspnea, fatigue, respiratory function, and health-related quality of life). In the RCT by Mukhiddin Ugli et al. [31], MBSR led to a significant and sustained improvement in QoL as measured by the SGRQ (−8.4 points; 95% CI −11.9 to −4.8), alongside better symptom management including dyspnea and fatigue. Similarly, Tschenett et al. [35] reported that a digital MBI significantly reduced momentary dyspnea (ηp2 = 0.70, p < 0.001) in COPD patients. Harris et al. [27] observed improved therapy adherence and better QoL following home-based mindful breathing, with a mean SGRQ improvement of −6.1 points (95% CI −10.4 to −1.8). Hiles et al. [28] found that a 16-week yoga and mindfulness intervention enhanced health-related QoL (SGRQ −7.2 points, p = 0.01) and improved breathing control (Dyspnea-12 −4.5 points, p = 0.03) in patients with severe asthma. Chan et al. [8] highlighted improved respiratory patterns, with a reduction in respiratory rate (−1.2 breaths/min) and increased inspiratory time (p = 0.03), as well as improved dyspnea scores. Perkins-Porras et al. [32] noted a clinically meaningful reduction in dyspnea following a brief body scan intervention (Borg Dyspnea −1.2 units, p = 0.02). Finally, Tan et al. [34] reported a rapid and significant reduction in dyspnea at 5 and 20 min post-intervention (p < 0.001) in patients with COPD, asthma, or lung cancer, suggesting immediate physiological benefits of mindful breathing techniques. Overall, in-person interventions such as MBSR and yoga-based programs tended to produce more sustained improvements in QoL and symptom control, whereas digital or home-based approaches showed promising but more targeted effects, particularly on momentary dyspnea and self-management. Collectively, these findings suggest the feasibility and potential clinical relevance of MBIs in improving physical symptoms and health management in chronic respiratory disease populations (Table 9).

Table 9.
Healthcare outcomes.
3.7. Follow-Up Outcomes
Two studies reported follow-up data. Benzo et al. (2013) [25] conducted a 12-month follow-up and found that 70% of participants maintained engagement with the mindfulness program, continuing to report benefits such as sustained use of breathing strategies, enhanced emotional resilience, and greater connectedness. Similarly, Mukhiddin Ugli et al. (2024) [31] reported that the improvements observed immediately post-intervention in quality of life, anxiety, depression, mindfulness, and self-compassion were preserved at 3-month follow-up, suggesting good short-term sustainability of the effects. Harris et al. (2025) [27] also planned follow-up assessments; however, quantitative data are not yet available, as this was a feasibility study in progress.
4. Discussion
Through this systematic review, we summarized findings from thirteen studies investigating MBIs in patients with chronic respiratory diseases. Most studies focused on COPD, but several included other chronic conditions such as asthma, lung cancer, and ILD. We also explored the different delivery formats of MBIs, from in-person group sessions to digital tools such as brief audio practices or virtual reality-based interventions. Our analysis examined MBSR and MBCT programs, focusing on their potential benefits for psychological, functional, and symptom-related outcomes, including low mood, anxiety, QoL, and symptom self-management. Previous systematic reviews on chronic illnesses—particularly cardiovascular disease—reported improvements in anxiety, depression, distress, and perceived stress [43]. Compared to previous reviews in chronic illnesses, this is, to our knowledge, the first systematic review to provide a quantitative synthesis of RCT evidence on MBIs specifically in chronic respiratory diseases, while also integrating observational and qualitative findings and addressing the emerging field of digital delivery modalities. We acknowledge the heterogeneity of study designs included in this review. While we chose to present the results by outcome domain to provide clinically relevant insights, we strengthened the methodological rigor by calculating effect sizes and confidence intervals exclusively for RCTs and conducting a meta-analysis for anxiety and depression outcomes. In contrast, qualitative and observational findings were synthesized narratively. This combined approach ensured both adherence to the evidence hierarchy and the preservation of a clinically meaningful structure.
4.1. Psychological Outcomes
Psychological factors such as stress, depression, and anxiety can negatively influence disease course, underlining the importance of addressing these aspects in clinical care [44]. Our review evaluated how the literature has addressed MBIs in chronic pulmonary patients. A consistent theme across studies is the positive effect of MBIs on psychological well-being, particularly anxiety and depression, two common comorbidities in chronic respiratory diseases that contribute to functional decline and reduced QoL [45]. The quantitative synthesis conducted for anxiety and depression provides further support for the potential psychological benefits of MBIs in individuals with chronic respiratory diseases. The meta-analysis, which included four randomized controlled trials with sufficient data for these outcomes, revealed moderate-to-large and statistically significant effects for both anxiety (SMD = –0.66, 95% CI [–0.91, –0.41], p < 0.00001; I2 = 33%) and depression (SMD = –0.71, 95% CI [–0.91, –0.51], p < 0.00001; I2 = 0%). These results indicate that MBIs are associated with consistent improvements across studies and relatively low heterogeneity, particularly for depression, despite variations in delivery format and outcome assessment.
These findings are consistent with previous meta-analyses in other chronic illness populations, such as cardiovascular disease, which similarly reported reductions in psychological distress and improvements in emotional well-being. In the context of chronic respiratory disease, where psychological comorbidities such as anxiety and depression are prevalent and can exacerbate symptom perception, these results reinforce the value of integrating MBIs as adjunctive interventions to standard care.
Nonetheless, the small number of trials available for quantitative pooling, as well as the variability in measurement tools (e.g., HADS, GAD-7, PHQ-8, BDI-II), warrants caution in generalizing these findings. Future high-quality, adequately powered RCTs with standardized outcome measures would help confirm these effects and clarify their durability over time.
4.2. Symptom Perception and Management
In line with previous research, MBIs appear effective in improving health-related outcomes [46,47,48]. Several RCTs [8,9,31] reported significant improvements in depression and anxiety scores, measured by validated tools such as the HADS [49]. Some studies maintained these improvements for three to six months after intervention [50], suggesting a potential for lasting benefits. Symptom burden is closely linked to psychological distress [51], and both can be modified through non-pharmacological interventions [52]. For COPD and ILD patients, where pharmacological treatment may not fully address psychological symptoms, MBIs offer an important patient-centered care option [53].
In our review, we investigated the possibility of improving QoL and symptom management through mindfulness-based digital tools. Improvements in HRQoL were reported in several studies using the SGRQ [54], COPD Assessment Test (CAT), and K-BILD questionnaire [27,28,30]. Participants frequently described increased energy and better symptom control, particularly for dyspnea and fatigue. Emotional and cognitive factors strongly influence these symptoms, and practices such as mindful breathing [34] or brief body scan audio [32] led to rapid reductions in perceived breathlessness. Miranda et al. [30] found that online MBIs significantly reduced dyspnea in ILD patients, with nearly half reporting meaningful improvements. However, this finding should be interpreted with caution, as other outcomes did not show significant changes. Possible explanations include the greater disease severity and symptom burden typically observed in ILD compared to COPD, the characteristics of the online MBSR program applied, and the potential limited sensitivity of the chosen outcome measures to capture subtle psychological or functional benefits. This highlights the heterogeneity in response to MBIs across different chronic pulmonary disease phenotypes and underlines the need for further condition-specific trials.
4.3. Digital Delivery Modalities
A notable strength of the current literature is the exploration of different delivery modalities, including face-to-face, online platforms, and app-based guided audio. Digital MBIs—delivered via specific platforms [25], WeChat [22], or audio recordings [27]—were generally feasible, well tolerated, and effective for patients with psychological symptoms. This is particularly relevant in post-pandemic healthcare systems [42] and in resource-limited settings, as digital delivery can overcome barriers such as mobility issues, geographical distance, and stigma [43]. Utilization rates and satisfaction scores were high, and dropout rates were generally low (<25%), supporting the feasibility of remote MBIs for chronic disease management.
According to the literature [55], self-efficacy is a key factor in improving adherence and self-management. Several studies show that MBIs can enhance this capacity. Harris et al. [27] and Benzo et al. [25] found that mindfulness practice increased patient compliance, improved motivation to adopt health-related behaviors, encouraged positive habit changes in symptom management, and promoted adherence to pulmonary rehabilitation or home-based therapy plans. These effects may arise from greater introspective awareness and reduced emotional reactivity—central mechanisms cultivated through mindfulness.
Remote monitoring and motion-tracking technology can further enhance compliance [56]. A notable strength of the current literature is the exploration of diverse delivery methods, reflecting the shift toward more personalized and accessible care. Digital MBIs—delivered through specific online platforms [35], WeChat-based delivery [33], or guided audio sessions [32]—proved feasible, well tolerated, and effective, even in patients with psychological symptoms. These formats are especially valuable in post-pandemic healthcare systems [57] and in resource-limited contexts. They provide scalable interventions that overcome barriers such as mobility limitations, geographic distance, and stigma [58]. Studies reported high utilization and satisfaction rates and generally low dropout rates (<25%), supporting the uptake of remote MBIs in chronic disease populations. Nevertheless, the implementation of digital MBIs may also face challenges, including variability in digital literacy, unequal access to devices or reliable internet connections, and concerns about privacy and data security, which could limit uptake despite their demonstrated feasibility and benefits [59,60].
4.4. Mechanistic Insights
Theoretically, these findings align with a mechanistic framework suggesting that MBIs exert benefits through interacting psychological and neurobiological processes [6]. Attentional regulation—including sustained attention, attentional switching, and reduced distractibility—enables patients to direct awareness toward present-moment experience, diminishing maladaptive attentional biases and catastrophic interpretations of dyspnea and other symptoms [61]. Body awareness (interoceptive awareness) is enhanced, fostering a more accurate and accepting perception of internal sensations rather than reactive avoidance [62,63]. Emotion regulation mechanisms, such as reappraisal and exposure, are supported by decentering, which helps patients observe thoughts and feelings as transient events rather than fixed truths, thereby reducing emotional reactivity and rumination [64]. Changes in self-perspective, including reduced self-referential processing and an increased sense of self-as-observer, contribute to greater psychological flexibility [65,66]. Self-efficacy and sense of agency are strengthened, promoting adherence to symptom management strategies and treatment plans [67]. Finally, processes of acceptance and meaning-making facilitate resilience, psychological adjustment, and improved HRQoL, even in the context of progressive disease [68]. Based on these mechanisms, we propose a conceptual model in which MBIs influence key mediators—attentional control, interoceptive awareness, emotion regulation, and changes in self-perspective—that collectively lead to reductions in anxiety and depression, improvements in symptom perception (including dyspnea), and enhanced HRQoL. The qualitative findings further support and illustrate these mechanistic pathways, providing patient-centered narratives that reinforce and contextualize the quantitative improvements observed in anxiety, depression, and symptom perception.
4.5. Qualitative Perspectives
Another key finding from our review concerns patients’ personal perceptions of living with a chronic condition. Qualitative studies [25,29] described experiences of illness acceptance, reduced stigma, improved emotional awareness and greater engagement in daily life. These changes often translated into greater enjoyment, acceptance, and satisfaction. Patients reported developing a more compassionate relationship with their bodies and symptoms, illustrating the transformative potential of mindfulness to enhance self-awareness, identity, and meaning making. The meaning-making process has been identified as a crucial factor in increasing resilience [69] and in helping individuals reinterpret their illness in ways that support well-being and HRQoL [70]. Empirical studies highlight that meaning making influences both psychological well-being and HRQoL [71,72,73,74]. Such perspectives are particularly relevant in progressive conditions like COPD and ILD, where clinical deterioration is likely and psychological adjustment is essential. Beyond symptom reduction, mindfulness may thus foster resilience, agency, and self-determination [75]. Importantly, these insights suggest that clinicians can tailor MBI delivery by integrating reflective and compassion-based practices that promote meaning-making and resilience, thereby supporting illness acceptance, self-efficacy, and adherence in patients with chronic respiratory diseases.
5. Conclusions
The studies reviewed indicate that MBIs are safe, well-received, and may serve as effective complements to standard respiratory care. Their affordability, scalability, and adaptability make them especially valuable in integrated care settings, particularly for patients with significant psychological comorbidities or challenges in adhering to treatment plans. Digital mindfulness solutions show particular potential in supporting remote care and telehealth initiatives.
From a clinical standpoint, MBIs are best viewed not as substitutes for medical treatment but as complementary strategies that promote self-management, relieve distress, and contribute to overall patient wellness. Integrating mindfulness into personalized care plans can be particularly valuable in areas such as pulmonary rehabilitation, geriatric care, and palliative support, providing an additional layer of holistic care.
5.1. Limitations
Only thirteen studies met the inclusion criteria, which is relatively low for a systematic review. This limited number, combined with significant heterogeneity in study design, sample size, population characteristics, intervention content, control conditions, and outcome measures, despite all the positive findings, may reduce the generalizability of our findings. A number of studies used small sample sizes or did not include control groups, which limited the conclusions’ robustness and generalizability.
Furthermore, few studies used active controls that were matched for time and attention, and blinding was frequently impractical. The methods used to standardize mindfulness protocols varied; some employed self-guided or brief techniques, while others used formal MBSR or MBCT structures. The identification of essential therapeutic components is made more difficult by this variability.
Since most results were self-reported, bias may have been introduced. Objective physiological measures, such as oxygen saturation, spirometry, and biomarkers, were rarely reported. Additionally, there is a lack of long-term follow-up data, which makes evaluating the sustainability of benefits challenging. Furthermore, very few studies looked at change mechanisms like autonomic regulation, interoceptive accuracy, or cognitive reappraisal.
5.2. Future Directions
The evidence emerging from this review highlights several priorities for future research. First, larger multicenter randomized controlled trials are needed to increase statistical power and enhance the external validity of findings. The adoption of standardized outcome measures for anxiety, depression, dyspnea, and HRQoL in patients with chronic pulmonary diseases is strongly recommended to improve comparability across studies and enable more robust meta-analyses. Another priority is to compare different delivery formats of MBIs (in-person vs. digital) to identify patient subgroups that may benefit most from each modality. Future studies should also investigate the mechanisms through which MBIs influence symptom perception and psychological well-being, as well as assess the long-term sustainability of effects beyond six months post-intervention. Finally, research should address the cost-effectiveness and scalability of digital MBIs in diverse healthcare contexts, including resource-limited settings.
Author Contributions
A.B. coordinated the study, supervised methodological design, and led manuscript drafting; C.P. and F.C. defined psychological outcomes, contributed to study design, and interpreted mental health findings; M.G. and C.P. developed the methodological framework, conducted the systematic review, and co-led data analysis; A.M. (Angelantonio Maglio) and A.V. assessed respiratory outcomes and ensured clinical relevance for chronic lung disease; M.C. (Michele Ciccarelli), F.L. and C.V. contributed to the interpretation of cardiopulmonary comorbidity and patient burden; P.B. contributed to the neuropsychological framing of the interventions; M.C. (Mariaconsiglia Calabrese), A.M. (Andrea Marino) and L.B. evaluated functional applicability and feasibility of interventions in rehabilitation settings. All authors have read and agreed to the published version of the manuscript.
Funding
Research project titled “RespirAction”, identified by CUP B83C22003920001, conducted under the scientific responsibility of Prof. Alessia Bramanti as part of the cascading call for Universities, Institutions, and Research Organizations within the “THE—Tuscany Health Ecosystem” project, issued with D.D. 2004/2023—Prot. 315887 on 22 December 2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are included in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
- PUBMED
- “Respiration Disorders”[Mesh] OR “Pulmonary Disease, Chronic Obstructive”[Mesh] OR “Chronic Obstructive Pulmonary Diseases” OR “COPD” OR “Chronic Obstructive Lung Disease” OR “Chronic Airflow Obstruction” AND “Mindfulness”[Mesh] OR “Mindfulness Meditation” OR “Meditation, Mindfulness” OR “Mindfulness Meditations” OR meditation OR MBCT OR MBSR OR MBCR OR “Virtual Reality”[MeSH Terms] OR “Avatar”[Mesh] OR “virtual reality” OR “VR” OR “Humanoid Avatar” OR “Psychosocial Intervention”[Mesh] OR “Intervention, Psychosocial” OR “Psychological Intervention” OR “Psychological Interventions”
- Scopus
- “Respiration Disorders” OR “Pulmonary Disease, Chronic Obstructive” OR “Chronic Obstructive Pulmonary Diseases” OR “COPD” OR “Chronic Obstructive Lung Disease” OR “Chronic Airflow Obstruction” AND “Mindfulness” OR “Mindfulness Meditation” OR “Meditation, Mindfulness” OR “Mindfulness Meditations” OR meditation OR MBCT OR MBSR OR MBCR OR “Virtual Reality” OR “Avatar” OR “virtual reality” OR “VR” OR “Humanoid Avatar” OR “Psychosocial Intervention” OR “Intervention, Psychosocial” OR “Psychological Intervention” OR “Psychological Interventions”
- WOS
- “Respiration Disorders” OR “Pulmonary Disease, Chronic Obstructive” OR “Chronic Obstructive Pulmonary Diseases” OR “COPD” OR “Chronic Obstructive Lung Disease” OR “Chronic Airflow Obstruction” AND “Mindfulness” OR “Mindfulness Meditation” OR “Meditation, Mindfulness” OR “Mindfulness Meditations” OR meditation OR MBCT OR MBSR OR MBCR OR “Virtual Reality” OR “Avatar” OR “virtual reality” OR “VR” OR “Humanoid Avatar” OR “Psychosocial Intervention” OR “Intervention, Psychosocial” OR “Psychological Intervention” OR “Psychological Interventions”
References
- Gould, G.S.; Hurst, J.R.; Trofor, A.; Alison, J.A.; Fox, G.; Kulkarni, M.M.; Wheelock, C.E.; Clarke, M.; Kumar, R. Recognising the importance of chronic lung disease: A consensus statement from the Global Alliance for Chronic Diseases (Lung Diseases group). Respir. Res. 2023, 24, 15. [Google Scholar] [CrossRef]
- Yohannes, A.M.; Willgoss, T.G.; Baldwin, R.C.; Connolly, M.J. Depression and anxiety in chronic heart failure and chronic obstructive pulmonary disease: Prevalence, relevance, clinical implications and management principles. Int. J. Geriatr. Psychiatry 2010, 25, 1209–1221. [Google Scholar] [CrossRef]
- Panagioti, M.; Scott, C.; Blakemore, A.; Coventry, P.A. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int. J. Chronic Obs. Pulm. Dis. 2014, 9, 1289–1306. [Google Scholar] [CrossRef]
- Livermore, N.; Sharpe, L.; McKenzie, D. Catastrophic interpretations and anxiety sensitivity as predictors of panic-spectrum psychopathology in chronic obstructive pulmonary disease. J. Psychosom. Res. 2012, 72, 388–392. [Google Scholar] [CrossRef]
- Kuyken, W.; Byford, S.; Taylor, R.S.; Watkins, E.; Holden, E.; White, K.; Barrett, B.; Byng, R.; Evans, A.; Mullan, E.; et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. J. Consult. Clin. Psychol. 2008, 76, 966–978. [Google Scholar] [CrossRef]
- Hölzel, B.K.; Lazar, S.W.; Gard, T.; Schuman-Olivier, Z.; Vago, D.R.; Ott, U. How Does Mindfulness Meditation Work? Proposing Mechanisms of Action From a Conceptual and Neural Perspective. Perspect. Psychol. Sci. 2011, 6, 537–559. [Google Scholar] [CrossRef]
- Bishop, S.R.; Lau, M.; Shapiro, S.; Carlson, L.; Anderson, N.D.; Carmody, J.; Segal, Z.V.; Abbey, S.; Speca, M.; Velting, D.; et al. Mindfulness: A proposed operational definition. Clin. Psychol. Sci. Pract. 2004, 11, 230–241. [Google Scholar] [CrossRef]
- Chan, R.R.; Giardino, N.; Larson, J.L. A pilot study: Mindfulness meditation intervention in COPD. Int. J. Chronic Obs. Pulm. Dis. 2015, 10, 445–454. [Google Scholar] [CrossRef]
- Farver-Vestergaard, I.; Jacobsen, D.; Zachariae, R. Efficacy of psychosocial interventions on psychological and physical health outcomes in chronic obstructive pulmonary disease: A systematic review and meta-analysis. Psychother. Psychosom. 2015, 84, 37–50. [Google Scholar] [CrossRef]
- Navarro-Haro, M.V.; López-Del-Hoyo, Y.; Campos, D.; Linehan, M.M.; Hoffman, H.G.; García-Palacios, A.; Modrego-Alarcón, M.; Borao, L.; García-Campayo, J. Meditation experts try Virtual Reality Mindfulness: A pilot study evaluation of the feasibility and acceptability of Virtual Reality to facilitate mindfulness practice in people attending a Mindfulness conference. PLoS ONE 2017, 12, e0187777. [Google Scholar] [CrossRef] [PubMed]
- Tarrant, J.; Viczko, J.; Cope, H. Virtual Reality for Anxiety Reduction Demonstrated by Quantitative EEG: A Pilot Study. Front. Psychol. 2018, 9, 1280. [Google Scholar] [CrossRef]
- Seabrook, E.; Kelly, R.; Foley, F.; Theiler, S.; Thomas, N.; Wadley, G.; Nedeljkovic, M. Understanding How Virtual Reality Can Support Mindfulness Practice: Mixed Methods Study. J. Med. Internet Res. 2020, 22, e16106. [Google Scholar] [CrossRef]
- Riches, S.; Azevedo, L.; Bird, L.; Pisani, S.; Valmaggia, L. Virtual reality relaxation for the general population: A systematic review. Soc. Psychiatry Psychiatr. Epidemiol. 2021, 56, 1707–1727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Hua, H.; Jin, J.; Zhu, L.; Shu, L.; Xu, X.; Kuang, F.; Liu, Y. Relaxation degree analysis using frontal electroencephalogram under virtual reality relaxation scenes. Front. Neurosci. 2021, 15, 719869. [Google Scholar] [CrossRef]
- Li, G.; Anguera, J.A.; Javed, S.V.; Khan, M.A.; Wang, G.; Gazzaley, A. Enhanced Attention Using Head-mounted Virtual Reality. J. Cogn. Neurosci. 2020, 32, 1438–1454. [Google Scholar] [CrossRef]
- Nie, K.; Guo, M.; Gao, Z. Enhancing Emotional Engagement in Virtual Reality (VR) Cinematic Experiences through multi-sensory Interaction Design. In Proceedings of the 2023 Asia Conference on Cognitive Engineering and Intelligent Interaction (CEII), Hong Kong, China, 15–16 December 2023; pp. 47–53. [Google Scholar]
- Hajesmaeel Gohari, S.; Gozali, E.; Niakan Kalhori, S.R. Virtual reality applications for chronic conditions management: A review. Med. J. Islam. Repub. Iran 2019, 33, 67. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.L.; Lee, A.; Janaudis-Ferreira, T.; Goldstein, R.S.; Brooks, D. Mindfulness in people with a respiratory diagnosis: A systematic review. Patient Educ. Couns. 2016, 99, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Clari, M.; Conti, A.; Fontanella, R.; Rossi, A.; Matarese, M. Mindfulness-based programs for people with chronic obstructive pulmonary disease: A mixed methods systematic review. Mindfulness 2020, 11, 1848–1867. [Google Scholar] [CrossRef]
- Wu, L.L.; Lin, Z.K.; Weng, H.D.; Qi, Q.F.; Lu, J.; Liu, K.X. Effectiveness of meditative movement on COPD: A systematic review and meta-analysis. Int. J. Chronic. Obs. Pulm. Dis. 2018, 13, 1239–1250. [Google Scholar] [CrossRef]
- Volpato, E.; Banfi, P.; Rogers, S.M.; Pagnini, F. Relaxation Techniques for People with Chronic Obstructive Pulmonary Disease: A Systematic Review and a Meta-Analysis. Evid. Based Complement. Altern. Med. 2015, 2015, 628365. [Google Scholar] [CrossRef]
- Nolan, C.M.; Brighton, L.J.; Mo, Y.; Bayly, J.; Higginson, I.J.; Man, W.D.; Maddocks, M. Meditative movement for breathlessness in advanced COPD or cancer: A systematic review and meta-analysis. Eur. Respir. Rev. 2023, 32, 220243. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj 2021, 372, 71. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inf. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Benzo, R.P. Mindfulness and motivational interviewing: Two candidate methods for promoting self-management. Chron. Respir. Dis. 2013, 10, 175–182. [Google Scholar] [CrossRef]
- Farver-Vestergaard, I.; O'Toole, M.S.; O'Connor, M.; Løkke, A.; Bendstrup, E.; Basdeo, S.A.; Cox, D.J.; Dunne, P.J.; Ruggeri, K.; Early, F.; et al. Mindfulness-based cognitive therapy in COPD: A cluster randomised controlled trial. Eur. Respir. J. 2018, 51, 1702082. [Google Scholar] [CrossRef]
- Harris, S.; Jordan, J.; Wilkinson, A.; Seaton, P. Investigating the feasibility of an 8-week mindful breathing programme on breathlessness and self-efficacy in chronic obstructive pulmonary disease: An open-label study. Pilot. Feasibility Stud. 2025, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Hiles, S.A.; Urroz, P.D.; Gibson, P.G.; Bogdanovs, A.; McDonald, V.M. A feasibility randomised controlled trial of Novel Activity Management in severe ASthma-Tailored Exercise (NAMASTE): Yoga and mindfulness. BMC Pulm. Med. 2021, 21, 71. [Google Scholar] [CrossRef]
- Malpass, A.; Kessler, D.; Sharp, D.; Shaw, A. MBCT for Patients with Respiratory Conditions Who Experience Anxiety and Depression: A Qualitative Study. Mindfulness 2015, 6, 1181–1191. [Google Scholar] [CrossRef]
- Miranda, S.M.D.; Cunha Paiva, S.P.; Pereira, L.F.F.; de Assis Pires, G.P.A.; Leal, A.N.A.; Ribeiro-Samora, G.A.; Mancuzo, E.V. Online Mindfulness-Based Intervention (eMBI) in management of dyspnea in patients with interstitial lung disease: A randomized clinical trial. Complement. Ther. Med. 2024, 87, 103106. [Google Scholar] [CrossRef] [PubMed]
- Ugli Ak, M.; Jasim, I.K.; Ahmed, A.S.; Hani, M.M.; Soud, N.A.; Ahmed, J.K.; Shnishil, A.T.; Mohsin, R.M. Investigating the Efficacy of Stress Reduction Interventions Based on Mindfulness Principles in Improving Life Quality for Individuals with Chronic Obstructive Pulmonary Disease (COPD). Int. J. Body Mind Cult. 2024, 11, 4–15. [Google Scholar] [CrossRef]
- Perkins-Porras, L.; Riaz, M.; Okekunle, A.; Zhelezna, S.; Chakravorty, I.; Ussher, M. Feasibility study to assess the effect of a brief mindfulness intervention for patients with chronic obstructive pulmonary disease: A randomized controlled trial. Chron. Respir. Dis. 2018, 15, 400–410. [Google Scholar] [CrossRef]
- Sun, X.; Xie, L.; Feng, X.; Xia, X.; He, Y.; Tan, L.; Zhao, H. Evaluation of the application effect of online mindfulness-based cognitive therapy in the health management of elderly patients with copd during the novel coronavirus pneumonia epidemic. J. Evid. Based Psychother. 2021, 21, 57–68. [Google Scholar] [CrossRef]
- Tan, S.B.; Liam, C.K.; Pang, Y.K.; Leh-Ching Ng, D.; Wong, T.S.; Wei-Shen Khoo, K.; Ooi, C.Y.; Chai, C.S. The Effect of 20-Minute Mindful Breathing on the Rapid Reduction of Dyspnea at Rest in Patients With Lung Diseases: A Randomized Controlled Trial. J. Pain Symptom Manag. 2019, 57, 802–808. [Google Scholar] [CrossRef]
- Tschenett, H.; Vafai-Tabrizi, F.; Zwick, R.H.; Valipour, A.; Funk, G.C.; Nater, U.M. Digital mindfulness-based intervention for people with COPD—a multicentre pilot and feasibility RCT. Respir. Res. 2025, 26, 199. [Google Scholar] [CrossRef] [PubMed]
- Von Visger, T.T.; Wardlaw, K.; Li, C.S.; Chang, Y.P.; Matura, L.A. Associations between mindfulness and symptom severity among adults living with chronic obstructive pulmonary disease (COPD). Heart Lung 2025, 70, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. Bmj 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. Bmj 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- CASP, U. Critical appraisal skills programme (CASP). qualitative checklist. 2018. [Google Scholar]
- Benzo, R.P.; Ridgeway, J.; Hoult, J.P.; Novotny, P.; Thomas, B.E.; Lam, N.M.; Benzo, M.V.; Kramer, K.; Seifert, S. Feasibility of a Health Coaching and Home-Based Rehabilitation Intervention With Remote Monitoring for COPD. Respir. Care 2021, 66, 960–971. [Google Scholar] [CrossRef]
- Keng, S.L.; Smoski, M.J.; Robins, C.J. Effects of mindfulness on psychological health: A review of empirical studies. Clin. Psychol. Rev. 2011, 31, 1041–1056. [Google Scholar] [CrossRef]
- Schuman-Olivier, Z.; Trombka, M.; Lovas, D.A.; Brewer, J.A.; Vago, D.R.; Gawande, R.; Dunne, J.P.; Lazar, S.W.; Loucks, E.B.; Fulwiler, C. Mindfulness and Behavior Change. Harv. Rev. Psychiatry 2020, 28, 371–394. [Google Scholar] [CrossRef]
- Scott-Sheldon, L.A.J.; Gathright, E.C.; Donahue, M.L.; Balletto, B.; Feulner, M.M.; DeCosta, J.; Cruess, D.G.; Wing, R.R.; Carey, M.P.; Salmoirago-Blotcher, E. Mindfulness-Based Interventions for Adults with Cardiovascular Disease: A Systematic Review and Meta-Analysis. Ann. Behav. Med. 2020, 54, 67–73. [Google Scholar] [CrossRef]
- Stoney, C.M.; Kaufmann, P.G.; Czajkowski, S.M. Cardiovascular disease: Psychological, social, and behavioral influences: Introduction to the special issue. Am. Psychol. 2018, 73, 949–954. [Google Scholar] [CrossRef]
- Park, S.; Sato, Y.; Takita, Y.; Tamura, N.; Ninomiya, A.; Kosugi, T.; Sado, M.; Nakagawa, A.; Takahashi, M.; Hayashida, T.; et al. Mindfulness-Based Cognitive Therapy for Psychological Distress, Fear of Cancer Recurrence, Fatigue, Spiritual Well-Being, and Quality of Life in Patients With Breast Cancer-A Randomized Controlled Trial. J. Pain Symptom Manag. 2020, 60, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Loucks, E.B.; Schuman-Olivier, Z.; Britton, W.B.; Fresco, D.M.; Desbordes, G.; Brewer, J.A.; Fulwiler, C. Mindfulness and Cardiovascular Disease Risk: State of the Evidence, Plausible Mechanisms, and Theoretical Framework. Curr. Cardiol. Rep. 2015, 17, 112. [Google Scholar] [CrossRef]
- Grossman, P.; Niemann, L.; Schmidt, S.; Walach, H. Mindfulness-based stress reduction and health benefits. A Meta Anal. J. Psychosom. Res. 2004, 57, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Jiang, X.; Dai, R.; Zhao, N.; Pan, W.; Guo, J.; Fan, J.; Bao, S. Mindfulness-based intervention for hypertension patients with depression and/or anxiety in the community: A randomized controlled trial. Trials 2024, 25, 299. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.; Booth, J.; Mercer, S.; Crawford, E. A systematic review of the benefits of mindfulness-based interventions following transient ischemic attack and stroke. Int. J. Stroke 2013, 8, 465–474. [Google Scholar] [CrossRef]
- Barnes, P.J.; Shapiro, S.D.; Pauwels, R.A. Chronic obstructive pulmonary disease: Molecular and cellular mechanisms. Eur. Respir. J. 2003, 22, 672–688. [Google Scholar] [CrossRef]
- Wang, C.; Yan, J.; Ma, C. Psychological distress and its associated factors among patients with chronic obstructive pulmonary disease in Hunan, China: A cross-sectional study. Sci. Rep. 2023, 13, 5199. [Google Scholar] [CrossRef]
- Dobkin, P.L.; Bernardi, N.F.; Bagnis, C.I. Enhancing Clinicians’ Well-Being and Patient-Centered Care Through Mindfulness. J. Contin. Educ. Health Prof. 2016, 36, 11–16. [Google Scholar] [CrossRef]
- Jones, P.W.; Quirk, F.H.; Baveystock, C.M. The St George’s Respiratory Questionnaire. Respir. Med. 1991, 85, 25–31. [Google Scholar] [CrossRef]
- Thornton, C.P.; Li, M.; Yeh, C.H.; Ruble, K. Self-efficacy in symptom management for adolescents and young adults with cancer: A systematic review. Support. Care Cancer 2021, 29, 2851–2862. [Google Scholar] [CrossRef]
- Garofano, M.; Del Sorbo, R.; Calabrese, M.; Giordano, M.; Di Palo, M.P.; Bartolomeo, M.; Ragusa, C.M.; Ungaro, G.; Fimiani, G.; Di Spirito, F.; et al. Remote Rehabilitation and Virtual Reality Interventions Using Motion Sensors for Chronic Low Back Pain: A Systematic Review of Biomechanical, Pain, Quality of Life, and Adherence Outcomes. Technologies 2025, 13, 186. [Google Scholar] [CrossRef]
- Rathnayake, D.; Clarke, M.; Jayasinghe, V.I. Health system performance and health system preparedness for the post-pandemic impact of COVID-19: A review. Int. J. Healthc. Manag. 2021, 14, 250–254. [Google Scholar] [CrossRef]
- Calandra, D.; Lanzalonga, F.; Secinaro, S.; Storti, C.C. From traditional to digital: Unravelling performance measurement systems and accounting methods in drug treatment through a systematic review and content analysis. Br. Account. Rev. 2025, 101665. [Google Scholar] [CrossRef]
- Albarqi, M.N. Exploring the Effectiveness of Technology-Assisted Interventions for Promoting Independence in Elderly Patients: A Systematic Review. Healthcare 2024, 12, 2105. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Telemedicine for healthcare: Capabilities, features, barriers, and applications. Sens. Int. 2021, 2, 100117. [Google Scholar] [CrossRef]
- Jha, A.P.; Krompinger, J.; Baime, M.J. Mindfulness training modifies subsystems of attention. Cogn. Affect. Behav. Neurosci. 2007, 7, 109–119. [Google Scholar] [CrossRef]
- Farb, N.A.; Segal, Z.V.; Mayberg, H.; Bean, J.; McKeon, D.; Fatima, Z.; Anderson, A.K. Attending to the present: Mindfulness meditation reveals distinct neural modes of self-reference. Soc. Cogn. Affect. Neurosci. 2007, 2, 313–322. [Google Scholar] [CrossRef]
- Craig, A.D. Interoception: The sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003, 13, 500–505. [Google Scholar] [CrossRef]
- Garland, E.L.; Gaylord, S.A.; Fredrickson, B.L. Positive reappraisal mediates the stress-reductive effects of mindfulness: An upward spiral process. Mindfulness 2011, 2, 59–67. [Google Scholar] [CrossRef]
- Fresco, D.M.; Moore, M.T.; van Dulmen, M.H.; Segal, Z.V.; Ma, S.H.; Teasdale, J.D.; Williams, J.M. Initial psychometric properties of the experiences questionnaire: Validation of a self-report measure of decentering. Behav. Ther. 2007, 38, 234–246. [Google Scholar] [CrossRef]
- Shapiro, S.L.; Carlson, L.E.; Astin, J.A.; Freedman, B. Mechanisms of mindfulness. J. Clin. Psychol. 2006, 62, 373–386. [Google Scholar] [CrossRef]
- Caldwell, K.; Harrison, M.; Adams, M.; Quin, R.H.; Greeson, J. Developing Mindfulness in College Students Through Movement-Based Courses: Effects on Self-Regulatory Self-Efficacy, Mood, Stress, and Sleep Quality. J. Am. Coll. Health 2010, 58, 433–442. [Google Scholar] [CrossRef]
- De Vibe, M.; Bjørndal, A.; Tipton, E.; Hammerstrøm, K.; Kowalski, K. Mindfulness based stress reduction (MBSR) for improving health, quality of life, and social functioning in adults. Campbell Syst. Rev. 2012, 8, 1–127. [Google Scholar] [CrossRef]
- Park, C.L. Meaning Making in the Context of Disasters. J. Clin. Psychol. 2016, 72, 1234–1246. [Google Scholar] [CrossRef]
- Hartog, I.; Scherer-Rath, M.; Kruizinga, R.; Netjes, J.; Henriques, J.; Nieuwkerk, P.; Sprangers, M.; van Laarhoven, H. Narrative meaning making and integration: Toward a better understanding of the way falling ill influences quality of life. J. Health Psychol. 2020, 25, 738–754. [Google Scholar] [CrossRef]
- Affleck, G.; Tennen, H.; Croog, S.; Levine, S. Causal attribution, perceived benefits, and morbidity after a heart attack: An 8-year study. J. Consult. Clin. Psychol. 1987, 55, 29–35. [Google Scholar] [CrossRef]
- Albrecht, F.; Pereira, J.B.; Mijalkov, M.; Freidle, M.; Johansson, H.; Ekman, U.; Westman, E.; Franzén, E. Effects of a Highly Challenging Balance Training Program on Motor Function and Brain Structure in Parkinson’s Disease. J. Park. Dis. 2021, 11, 2057–2071. [Google Scholar] [CrossRef] [PubMed]
- Park, C.L. Making sense of the meaning literature: An integrative review of meaning making and its effects on adjustment to stressful life events. Psychol. Bull. 2010, 136, 257–301. [Google Scholar] [CrossRef] [PubMed]
- Sprangers, M.A. How recent health-related life events affected my perspective on quality-of-life research. Qual. Life Res. 2015, 24, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.M.; Deci, E.L. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am. Psychol. 2000, 55, 68–78. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).