Integrated Fluidic Platform for Washing and Mechanical Processing of Lipoaspirate for Downstream Fat Grafting and Regenerative Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Subjects and Ethical Considerations
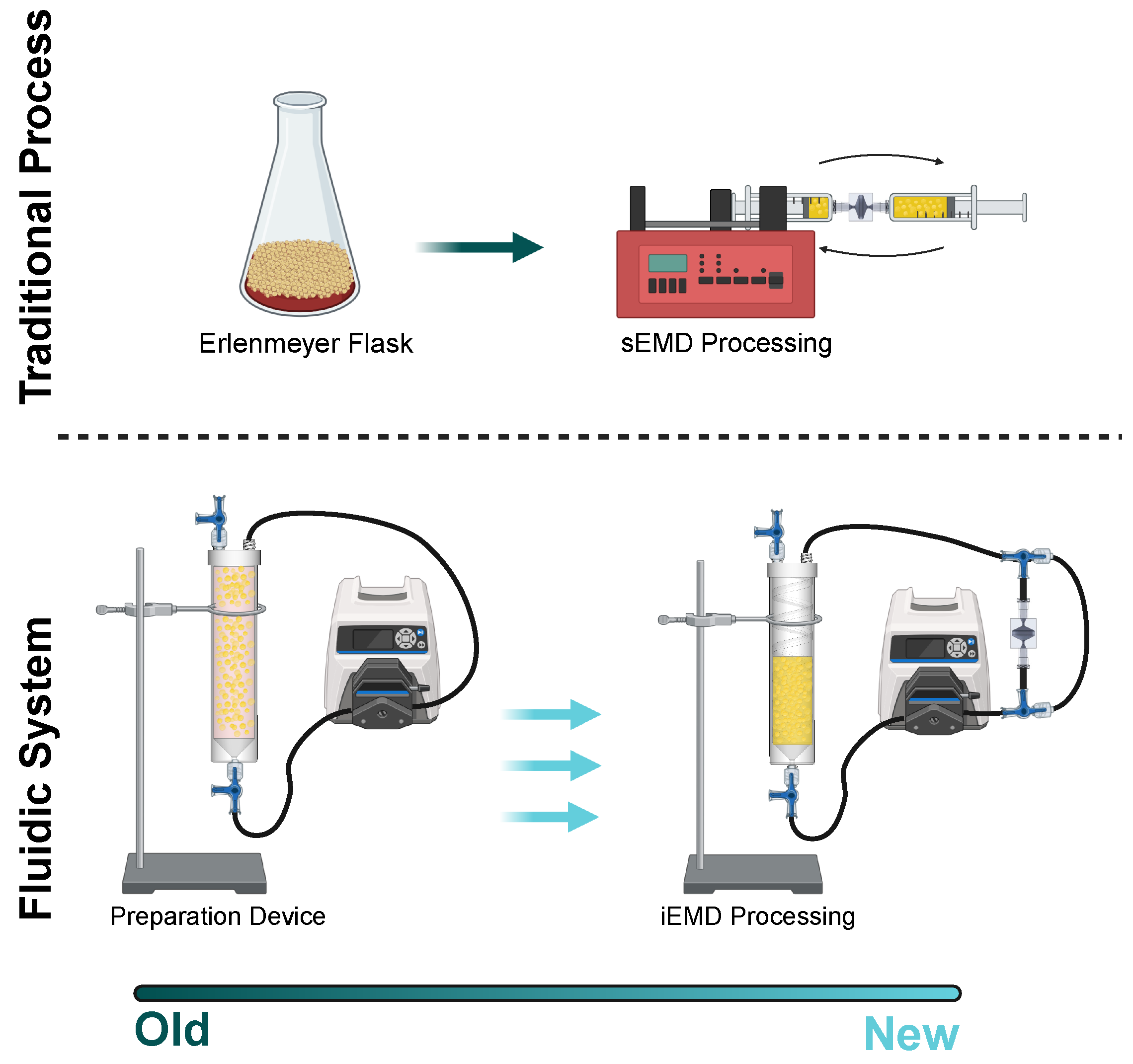
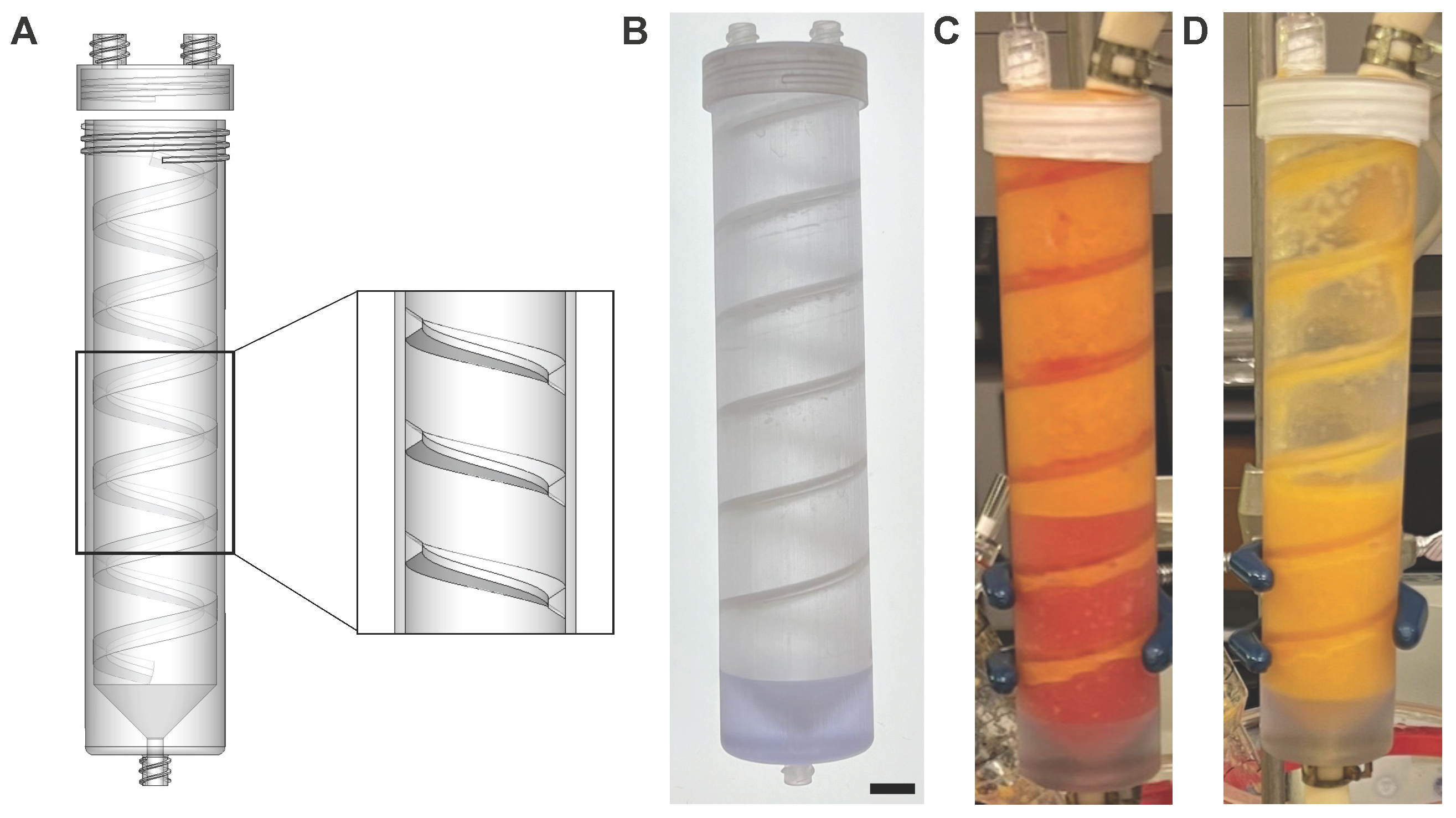
2.2. Preparation Device and Emulsification and Micronization Device
2.3. PD Device Operation
2.4. Cell Analysis
2.5. Statistics
3. Results
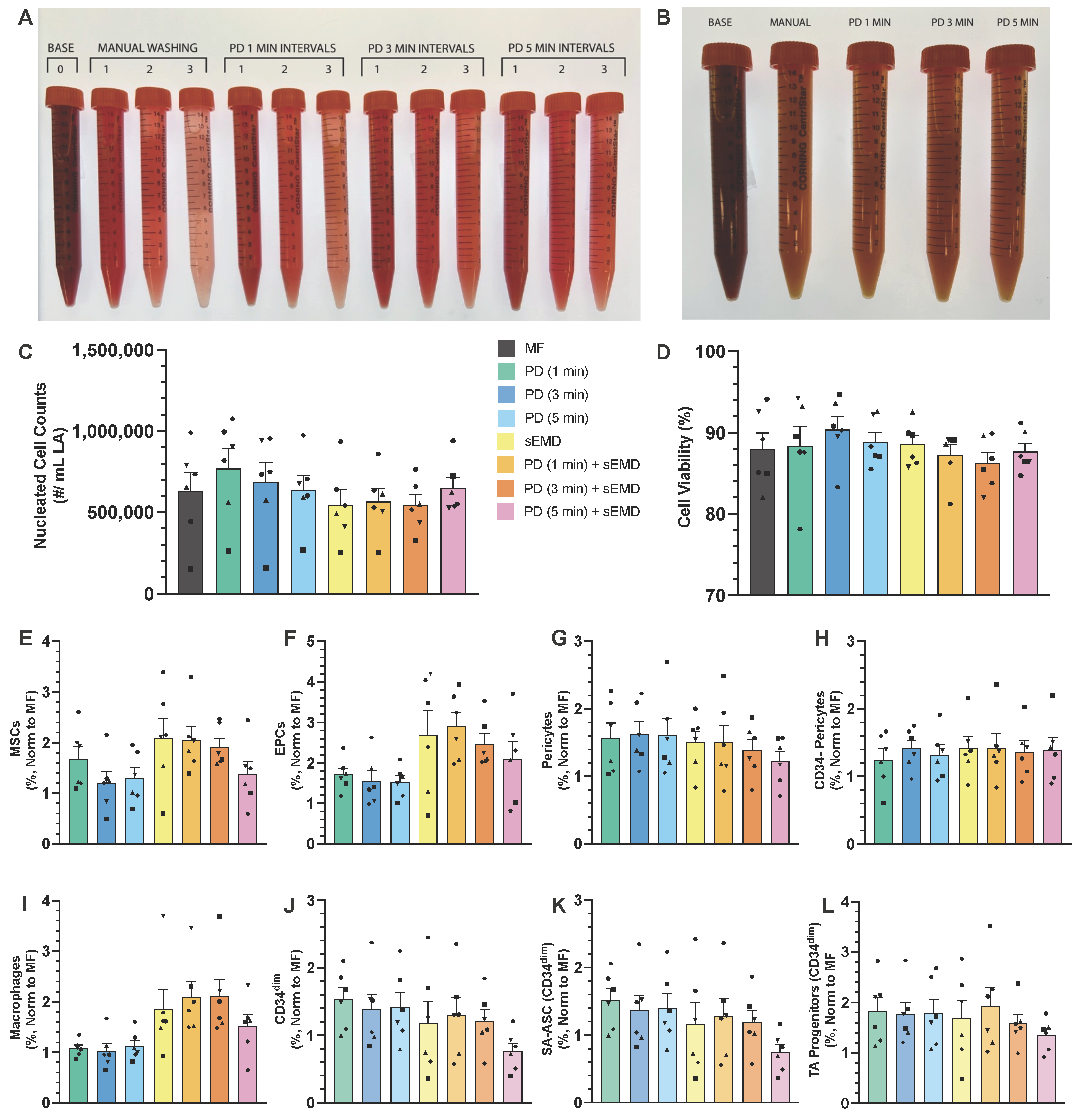
3.1. Optimization of PD Washing Performance
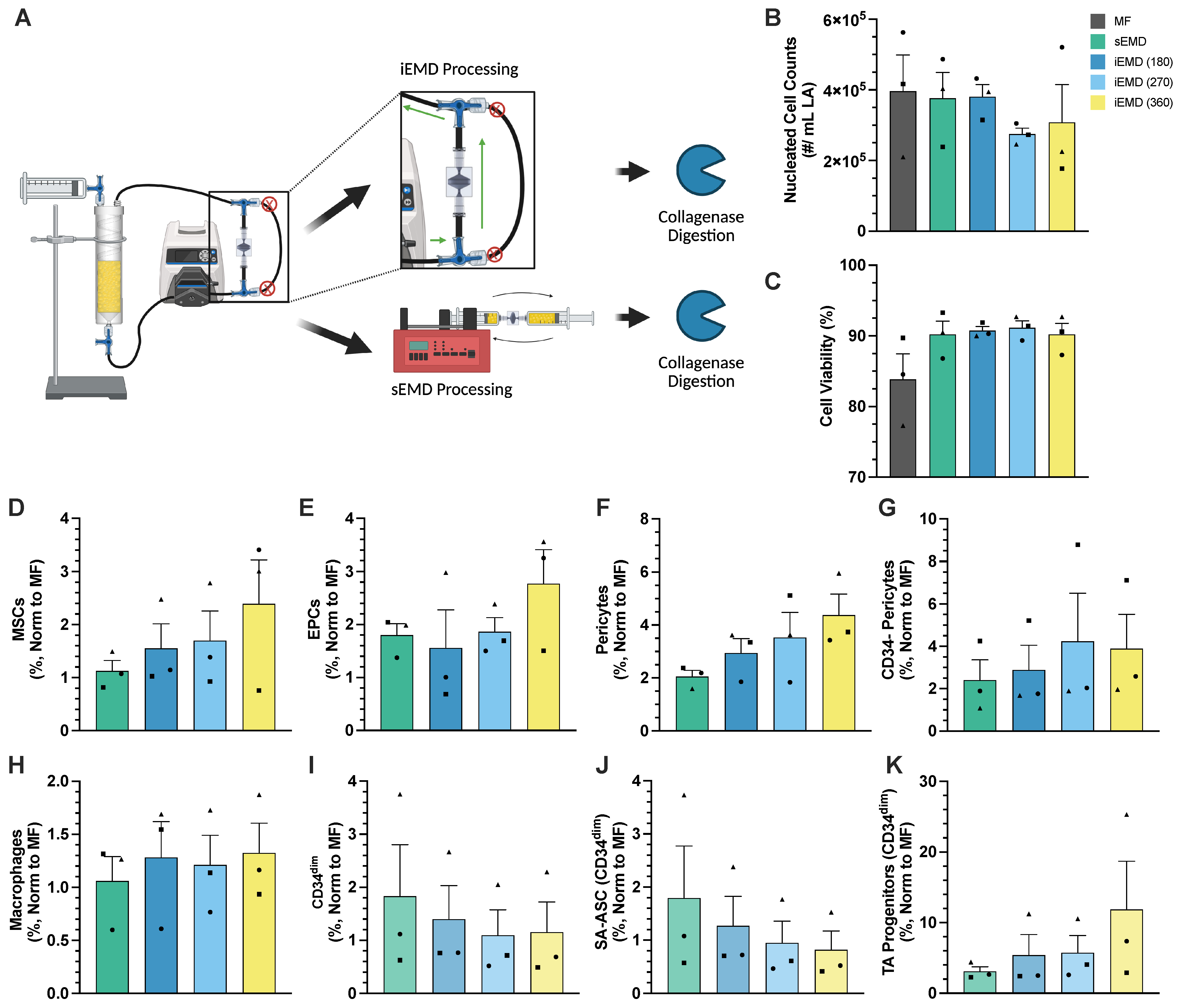
3.2. Peristaltic EMD Processing Is Equivalent to Syringe Pumping
3.3. Integrated Platform for PD Washing EMD Mechanical Processing
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 7-AAD | 7-Aminoactinomycin D |
| ANOVA | Analysis of Variance |
| AO/PI | Acridine Orange/Propidium Iodide |
| ADSCs | Adipose-derived Stem Cells |
| ASCs | Adipose Stromal Cells |
| BSA | Bovine Serum Albumin |
| CAL | Cell-Assisted Lipotransfer |
| DMEM | Dulbecco’s Modified Eagle Medium |
| EMD | Emulsification and Micronization Device |
| EPCs | Endothelial Progenitor Cells |
| FMO | Fluorescence Minus One |
| HIPAA | Health Insurance Portability and Accountability Act |
| ID | Internal Diameter |
| iEMD | Integrated Emulsification and Micronization Device |
| LA | Lipoaspirate |
| MF | Macrofat |
| MSCs | Mesenchymal Stem Cells |
| pEMD | Peristaltic Emulsification and Micronization Device |
| PBS | Phosphate-Buffered Saline |
| PD | Preparation Device |
| SA-ASCs | Supra-Adventitial Adipose Stromal Cells |
| sEMD | Syringe Emulsification and Micronization Device |
| SLA | Stereolithography |
| SVF | Stromal Vascular Fraction |
| TA | Transit Amplifying |
References
- Fraser, J.; Wulur, I.; Alfonso, Z.; Hedrick, M. Fat Tissue: An Underappreciated Source of Stem Cells for Biotechnology. Trends Biotechnol. 2006, 24, 150–154. [Google Scholar] [CrossRef]
- Bunnell, B. Adipose Tissue-Derived Mesenchymal Stem Cells. Cells 2021, 10, 3433. [Google Scholar] [CrossRef]
- Trevor, L.; Riches-Suman, K.; Mahajan, A.; Thornton, M. Adipose Tissue: A Source of Stem Cells with Potential for Regenerative Therapies for Wound Healing. J. Clin. Med. 2020, 9, 2161. [Google Scholar] [CrossRef]
- Barfod, K.W.; Blønd, L. Treatment of osteoarthritis with autologous and microfragmented adipose tissue. Dan. Med J. 2019, 66, A5565. [Google Scholar]
- Ude, C.; Shah, S.; Ogueri, K.; Nair, L.; Laurencin, C. Stromal Vascular Fraction for Osteoarthritis of the Knee Regenerative Engineering. Regen. Eng. Transl. Med. 2022, 8, 210–224. [Google Scholar] [CrossRef]
- Boada-Pladellorens, A.; Avellanet, M.; Pages-Bolibar, E.; Veiga, A. Stromal Vascular Fraction Therapy for Knee Osteoarthritis: A Systematic Review. Ther. Adv. Musculoskelet. Dis 2022, 14, 1759720X221117879. [Google Scholar] [CrossRef]
- Garza, J.; Campbell, R.; Tjoumakaris, F.; Freedman, K.; Miller, L.; Santa Maria, D.; Tucker, B. Clinical Efficacy of Intra-Articular Mesenchymal Stromal Cells for the Treatment of Knee Osteoarthritis: A Double-Blinded Prospective Randomized Controlled Clinical Trial. Am. J. Sport. Med. 2020, 48, 588–598. [Google Scholar] [CrossRef]
- Baria, M.; Barker, T.; Durgam, S.; Pedroza, A.; Flanigan, D.; Jia, L.; Kaeding, C.; Magnussen, R. Microfragmented Adipose Tissue Is Equivalent to Platelet-Rich Plasma for Knee Osteoarthritis at 12 Months Posttreatment: A Randomized Controlled Trial. Orthop. J. Sports Med. 2024, 12, 23259671241233916. [Google Scholar] [CrossRef]
- Álvaro Afonso, F.; Sanz-Corbalán, I.; Lázaro-Martínez, J.; Kakagia, D.; Papanas, N. Adipose-Derived Mesenchymal Stem Cells in the Treatment of Diabetic Foot Ulcers: A Review of Preclinical and Clinical Studies. Angiology 2020, 71, 853–863. [Google Scholar] [CrossRef]
- Carstens, M.; Quintana, F.; Calderwood, S.; Sevilla, J.; Ríos, A.; Rivera, C.; Calero, D.; Zelaya, M.; Garcia, N.; Bertram, K. Treatment of Chronic Diabetic Foot Ulcers with Adipose-Derived Stromal Vascular Fraction Cell Injections: Safety and Evidence of Efficacy at 1 Year. Stem. Cells Transl. Med. 2021, 10, 1138–1147. [Google Scholar] [CrossRef]
- Namgoong, S.; Yoon, I.J.; Han, S.K.; Son, J.W.; Kim, J. A Pilot Study Comparing a Micronized Adipose Tissue Niche versus Standard Wound Care for Treatment of Neuropathic Diabetic Foot Ulcers. J. Clin. Med. 2022, 11, 5887. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.; Sisakht, M.; Amirkhani, M.; Seifalian, A.; Banafshe, H.; Verdi, J.; Nouradini, M. Engineered Skin Graft with Stromal Vascular Fraction Cells Encapsulated in Fibrin–Collagen Hydrogel: A Clinical Study for Diabetic Wound Healing. J. Tissue Eng. Regen. Med. 2020, 14, 424–440. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.C.; Ma, J.J.; Sun, W.J.; Wang, S.W.; Gu, Z.C.; Yang, X. Autologous Nanofat Transplantation Accelerates Foot Wound Healing in Diabetic Rats. Regen. Med. 2019, 14, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Gareev, I.; Beylerli, O.; Ilyasova, T.; Ahmad, A.; Shi, H.; Chekhonin, V. Therapeutic Application of Adipose-Derived Stromal Vascular Fraction in Myocardial Infarction. Iscience 2024, 27, 109791. [Google Scholar] [CrossRef] [PubMed]
- Houtgraaf, J.; Den Dekker, W.; Dalen, B.; Springeling, T.; Jong, R.; Geuns, R.; Geleijnse, M.; Fernandez-Aviles, F.; Zijlsta, F.; Serruys, P. First Experience in Humans Using Adipose Tissue–Derived Regenerative Cells in the Treatment of Patients With ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2012, 59, 539–540. [Google Scholar] [CrossRef]
- Hutchings, G.; Janowicz, K.; Moncrieff, L.; Dompe, C.; Strauss, E.; Kocherova, I.; Nawrocki, M.; Kruszyna, L.; Wąsiatycz, G.; Antosik, P. The Proliferation and Differentiation of Adipose-Derived Stem Cells in Neovascularization and Angiogenesis. Int. J. Mol. Sci. 2020, 21, 3790. [Google Scholar] [CrossRef]
- Laschke, M.; Menger, M. Microvascular Fragments in Microcirculation Research and Regenerative Medicine. Tissue Eng. Part B Rev. 2022, 28, 1109–1120. [Google Scholar] [CrossRef]
- Parsons, A.; Ahsan, N.; Darling, E. Identifying Immunomodulatory Subpopulations of Adipose Stromal Vascular Fraction and Stem/Stromal Cells Through Single-Cell Transcriptomics and Bulk Proteomics. Stem Cell Rev. Rep. 2025, 21, 1484–1500. [Google Scholar] [CrossRef]
- Ramakrishnan, V.; Boyd, N. The Adipose Stromal Vascular Fraction as a Complex Cellular Source for Tissue Engineering Applications. Tissue Eng. Part B Rev. 2018, 24, 289–299. [Google Scholar] [CrossRef]
- Zimmerlin, L.; Donnenberg, V.; Rubin, J.; Donnenberg, A. Mesenchymal Markers on Human Adipose Stem/Progenitor Cells. Cytom. A 2013, 83A, 134–140. [Google Scholar] [CrossRef]
- Borrelli, M.; Patel, R.; Blackshear, C.; Vistnes, S.; Diaz Deleon, N.; Adem, S.; Shen, A.; Sokol, J.; Momeni, A.; Nguyen, D. CD34+CD146+ Adipose-Derived Stromal Cells Enhance Engraftment of Transplanted Fat. Stem Cells Transl. Med. 2020, 9, 1389–1400. [Google Scholar] [CrossRef]
- Deleon, N.; Adem, S.; Lavin, C.; Abbas, D.; Griffin, M.; King, M.; Borrelli, M.; Patel, R.; Fahy, E.; Lee, D. Angiogenic CD34+CD146+ Adipose-derived Stromal Cells Augment Recovery of Soft Tissue after Radiotherapy. J. Tissue Eng. Regen. Med. 2021, 15, 1105–1117. [Google Scholar] [CrossRef]
- Vuerich, R.; Groppa, E.; Vodret, S.; Ring, N.; Stocco, C.; Bossi, F.; Agostinis, C.; Cauteruccio, M.; Colliva, A.; Ramadan, M. Ischemic Wound Revascularization by the Stromal Vascular Fraction Relies on Host-Donor Hybrid Vessels. Npj Regen. Med. 2023, 8, 8. [Google Scholar] [CrossRef]
- Gandolfi, S.; Sanouj, A.; Chaput, B.; Coste, A.; Sallerin, B.; Varin, A. The Role of Adipose Tissue-Derived Stromal Cells, Macrophages and Bioscaffolds in Cutaneous Wound Repair. Biol. Direct 2024, 19, 85. [Google Scholar] [CrossRef]
- Gu, W.; Nowak, W.; Xie, Y.; Le Bras, A.; Hu, Y.; Deng, J.; Issa Bhaloo, S.; Lu, Y.; Yuan, H.; Fidanis, E. Single-Cell RNA-Sequencing and Metabolomics Analyses Reveal the Contribution of Perivascular Adipose Tissue Stem Cells to Vascular Remodeling. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2049–2066. [Google Scholar] [CrossRef]
- Hu, N.W.; Shang, H.; Kogan, S.; Llull, R.; Traktuev, D.; Katz, A.; Murfee, W. Stromal Vascular Fraction–Derived Vasculogenesis Is Associated with the Formation of Lymphatic Endothelial Cell Structures. Stem Cells Dev. 2025, 34, 2024.0210. [Google Scholar] [CrossRef]
- Tonnard, P.; Verpaele, A.; Peeters, G.; Hamdi, M.; Cornelissen, M.; Declercq, H. Nanofat Grafting: Basic Research and Clinical Applications. Plast. Reconstr. Surg. 2013, 132, 1017–1026. [Google Scholar] [CrossRef]
- FDA Document: Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use. In Guidance for Industry and Food and Drug Administration Staff; Food and Drug Administration: Rockville, MD, USA, 2017.
- Lavagnolo, U.; Veronese, S.; Negri, S.; Magnan, B.; Sbarbati, A. Lipoaspirate Processing for the Treatment of Knee Osteoarthritis: A Review of Clinical Evidences. Biomed. Pharmacother. 2021, 142, 111997. [Google Scholar] [CrossRef]
- Sesé, B.; Sanmartín, J.; Ortega, B.; Matas-Palau, A.; Llull, R. Nanofat Cell Aggregates: A Nearly Constitutive Stromal Cell Inoculum for Regenerative Site-Specific Therapies. Plast. Reconstr. Surg. 2019, 144, 1079–1088. [Google Scholar] [CrossRef]
- Veronese, S.; Dai Prè, E.; Conti, G.; Busato, A.; Mannucci, S.; Sbarbati, A. Comparative Technical Analysis of Lipoaspirate Mechanical Processing Devices. J. Tissue Eng. Regen. Med. 2020, 14, 1213–1226. [Google Scholar] [CrossRef]
- Tremolada, C.; Colombo, V.; Ventura, C. Adipose Tissue and Mesenchymal Stem Cells: State of the Art and Lipogems® Technology Development. Curr. Stem Cell Rep. 2016, 2, 304–312. [Google Scholar] [CrossRef]
- Krześniak, A.; Radzimowski, K.; Stolarczyk, A. Comparison of the Treatment Results of Knee Osteoarthritis Using Adipose Tissue Mesenchymal Stromal Cells Derived through Enzymatic Digestion and Mechanically Fragmented Adipose Tissue. Medicine 2021, 100, e24777. [Google Scholar] [CrossRef]
- Cohen, S.; Tiryaki, T.; Womack, H.; Canikyan, S.; Schlaudraff, K.; Scheflan, M. Cellular Optimization of Nanofat: Comparison of Two Nanofat Processing Devices in Terms of Cell Count and Viability. Aesthetic Surg. J. Open Forum 2019, 1, ojz028. [Google Scholar] [CrossRef]
- You, X.; Gao, J.; Yao, Y. Advanced Methods to Mechanically Isolate Stromal Vascular Fraction: A Concise Review. Regen. Ther. 2024, 27, 120–125. [Google Scholar] [CrossRef]
- Lombardo, J.; Banyard, D.; Widgerow, A.; Haun, J. Fluidic Device System for Mechanical Processing and Filtering of Human Lipoaspirate Enhances Recovery of Mesenchymal Stem Cells. Plast. Reconstr. Surg. 2023, 151, 72–84. [Google Scholar] [CrossRef]
- Lombardo, J.; Banyard, D.; Zalazar, D.; Ziegler, M.; Sorensen, A.; Phummirat, P.; Widgerow, A.; Haun, J. Mechanical Processing of Lipoaspirate With a Fluidic Device Platform Promotes Wound Healing Transcriptional Programs and Angiogenesis In Vitro. Aesthetic Surg. J. 2025, 45, 850–859. [Google Scholar] [CrossRef]
- Francis, M.; Sachs, P.; Elmore, L.; Holt, S. Isolating Adipose-Derived Mesenchymal Stem Cells from Lipoaspirate Blood and Saline Fraction. Organogenesis 2010, 6, 11–14. [Google Scholar] [CrossRef]
- Yu, G.; Floyd, Z.E.; Wu, X.; Halvorsen, Y.D.C.; Gimble, J.M. Isolation of Human Adipose-Derived Stem Cells from Lipoaspirates. In Adipose-Derived Stem Cells; Methods in Molecular Biology Series; Gimble, J.M., Bunnell, B.A., Eds.; Humana Press: Totowa, NJ, USA, 2011; Volume 702, pp. 17–27. [Google Scholar] [CrossRef]
- Fruscella, P. Morphodynamics and Surgical Correction of the Body?S Creases, Folds, and Wrinkles. Aesthetic Plast. Surg. 2004, 28, 37–44. [Google Scholar] [CrossRef]
- Cleveland, E.C.; Albano, N.J.; Hazen, A. Roll, Spin, Wash, or Filter? Processing of Lipoaspirate for Autologous Fat Grafting: An Updated, Evidence-Based Review of the Literature. Plast. Reconstr. Surg. 2015, 136, 706–713. [Google Scholar] [CrossRef]
- Mecott, G.; Cueto-Ramos, R.; González-Martínez, A.; Perez-Trujillo, J.; Chacon-Martinez, H.; Oca, R.; Garcia-Perez, M. Determination of the Ratio of the Decantation Time and the Separation of Components in Lipoaspirate. Ann. Plast. Surg. 2020, 85, 7–11. [Google Scholar] [CrossRef]
- Condé-Green, A.; Cansanção, A.L. Fat Processing Methods. In Plastic and Aesthetic Regenerative Surgery and Fat Grafting; Kalaaji, A., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 197–205. [Google Scholar] [CrossRef]
- Girard, A.C.; Mirbeau, S.; Gence, L.; Hivernaud, V.; Delarue, P.; Hulard, O.; Festy, F.; Roche, R. Effect of Washes and Centrifugation on the Efficacy of Lipofilling With or Without Local Anesthetic: Plast. Reconstr. Surg. Glob. Open 2015, 3, e496. [Google Scholar] [CrossRef]
- Harats, M.; Millet, E.; Jaeger, M.; Orenstein, A.; Haik, J.; Hajdu, S.; Markel, G.; Winkler, E.; Tessone, A. Adipocytes Viability After Suction-Assisted Lipoplasty: Does the Technique Matter? Aesthetic Plast. Aesthetic Plast. Surg. 2016, 40, 578–583. [Google Scholar] [CrossRef]
- An, Y.; Panayi, A.; Mi, B.; Fu, S.; Orgill, D. Comparative Analysis of Two Automated Fat-Processing Systems. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2587. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.A., III; Nguyen, J.T.; Kirkham, J.C.; Lee, J.H.; McCormack, M.C.; Randolph, M.A.; Austen, W.G., Jr. Polymer Therapy: A Novel Treatment to Improve Fat Graft Viability. Plast. Reconstr. Surg. 2011, 127, 2270–2282. [Google Scholar] [CrossRef]
- Menkes, S.; Luca, M.; Soldati, G.; Polla, L. Subcutaneous Injections of Nanofat Adipose-Derived Stem Cell Grafting in Facial Rejuvenation. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2550. [Google Scholar] [CrossRef]
- Ansorge, H.; Garza, J.; McCormack, M.; Leamy, P.; Roesch, S.; Barere, A.; Connor, J. Autologous Fat Processing Via the Revolve System: Quality and Quantity of Fat Retention Evaluated in an Animal Model. Aesthetic Surg. J. 2014, 34, 438–447. [Google Scholar] [CrossRef]
- Gerth, D.; King, B.; Rabach, L.; Glasgold, R.; Glasgold, M. Long-Term Volumetric Retention of Autologous Fat Grafting Processed With Closed-Membrane Filtration. Aesthetic Surg. J. 2014, 34, 985–994. [Google Scholar] [CrossRef]
- Gabriel, A.; Kabaria, N.; Fang, C.; Lombardi, J.; Stec, E.; Huang, L.; Li, H.; Sandor, M. In Vitro Characterization of Fat Grafts Processed Using the REVOLVE ENVI System versus Decantation. Plast. Reconstr. Surg. Glob. Open 2024, 12, e5615. [Google Scholar] [CrossRef]
- Gabriel, A.; Champaneria, M.; Maxwell, G. Fat Grafting and Breast Reconstruction: Tips for Ensuring Predictability. Gland Surg. 2015, 4, 232–243. [Google Scholar] [CrossRef]
- Ferguson, R.; Cui, X.; Fink, B.; Vasconez, H.; Pu, L. The Viability of Autologous Fat Grafts Harvested With the LipiVage System: A Comparative Study. Ann. Plast. Surg. 2008, 60, 594–597. [Google Scholar] [CrossRef]
- Hanson, S.; Garvey, P.; Chang, E.; Reece, G.; Liu, J.; Baumann, D.; Butler, C. A Randomized Prospective Time and Motion Comparison of Techniques to Process Autologous Fat Grafts. Plast. Reconstr. Surg. 2021, 147, 1035–1044. [Google Scholar] [CrossRef]
- Langridge, B.; Jasionowska, S.; Khan, H.; Awad, L.; Turner, B.; Varghese, J.; Butler, P. Achieving Optimal Clinical Outcomes in Autologous Fat Grafting: A Systematic Review of Processing Techniques. J. Plast. Reconstr. Aesthetic Surg. 2023, 81, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Kølle, S.F.T.; Fischer-Nielsen, A.; Mathiasen, A.B.; Elberg, J.J.; Oliveri, R.S.; Glovinski, P.V.; Kastrup, J.; Kirchhoff, M.; Rasmussen, B.S.; Talman, M.L.M.; et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: A randomised placebo-controlled trial. Lancet 2013, 382, 1113–1120. [Google Scholar] [CrossRef]
- Kølle, S.F.T.; Duscher, D.; Taudorf, M.; Fischer-Nielsen, A.; Svalgaard, J.D.; Munthe-Fog, L.; Jønsson, B.; Selvig, P.B.; Mamsen, F.P.; Katz, A.J. Ex vivo-expanded autologous adipose tissue-derived stromal cells ensure enhanced fat graft retention in breast augmentation: A randomized controlled clinical trial. Stem Cells Transl. Med. 2020, 9, 1277–1286. [Google Scholar] [CrossRef]
- Yoshimura, K.; Sato, K.; Aoi, N.; Kurita, M.; Inoue, K.; Suga, H.; Eto, H.; Kato, H.; Hirohi, T.; Harii, K. Cell-Assisted Lipotransfer for Facial Lipoatrophy: Efficacy of Clinical Use of Adipose-Derived Stem Cells. Dermatol. Surg. 2008, 34, 1178–1185. [Google Scholar] [CrossRef]
- Chiu, C.H. Does Stromal Vascular Fraction Ensure a Higher Survival in Autologous Fat Grafting for Breast Augmentation? A Volumetric Study Using 3-Dimensional Laser Scanning. Aesthetic Surg. J. 2019, 39, 41–52. [Google Scholar] [CrossRef]
- Yoshimura, K.; Sato, K.; Aoi, N.; Kurita, M.; Hirohi, T.; Harii, K. Cell-Assisted Lipotransfer for Cosmetic Breast Augmentation: Supportive Use of Adipose-Derived Stem/Stromal Cells. Aesthetic Plast. Surg. 2020, 44, 1258–1265. [Google Scholar] [CrossRef]
- Paik, K.J.; Zielins, E.R.; Atashroo, D.A.; Maan, Z.N.; Duscher, D.; Luan, A.; Walmsley, G.G.; Momeni, A.; Vistnes, S.; Gurtner, G.C.; et al. Studies in Fat Grafting: Part V. Cell-Assisted Lipotransfer to Enhance Fat Graft Retention Is Dose Dependent. Plast. Reconstr. Surg. 2015, 136, 67–75. [Google Scholar] [CrossRef]
- Yu, Q.; Cai, Y.; Huang, H.; Wang, Z.; Xu, P.; Wang, X.; Zhang, L.; Zhang, W.; Li, W. Co-Transplantation of Nanofat Enhances Neovascularization and Fat Graft Survival in Nude Mice. Aesthetic Surg. J. 2018, 38, 667–675. [Google Scholar] [CrossRef]
- Guo, J.; Nguyen, A.; Banyard, D.A.; Fadavi, D.; Toranto, J.D.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.; Widgerow, A.D. Stromal vascular fraction: A regenerative reality? Part 2: Mechanisms of regenerative action. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 180–188. [Google Scholar] [CrossRef]
- Corselli, M.; Crisan, M.; Murray, I.R.; West, C.C.; Scholes, J.; Codrea, F.; Khan, N.; Péault, B. Identification of perivascular mesenchymal stromal/stem cells by flow cytometry. Cytom. Part A 2013, 83A, 714–720. [Google Scholar] [CrossRef]
- Hager, G.; Holnthoner, W.; Wolbank, S.; Husa, A.M.; Godthardt, K.; Redl, H.; Gabriel, C. Three specific antigens to isolate endothelial progenitor cells from human liposuction material. Cytotherapy 2013, 15, 1426–1435. [Google Scholar] [CrossRef]
- Vasilyev, V.S.; Borovikova, A.A.; Vasilyev, S.A.; Khramtsova, N.I.; Plaksin, S.A.; Kamyshinsky, R.A.; Presnyakov, M.Y.; Eremin, I.I. Features and Biological Properties of Different Adipose Tissue Based Products. Milli-, Micro-, Emulsified (Nano-) Fat, SVF, and AD-Multipotent Mesenchymal Stem Cells. In Plastic and Aesthetic Regenerative Surgery and Fat Grafting; Kalaaji, A., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 91–107. [Google Scholar] [CrossRef]
- Yoshimura, K.; Shigeura, T.; Matsumoto, D.; Sato, T.; Takaki, Y.; Aiba-Kojima, E.; Sato, K.; Inoue, K.; Nagase, T.; Koshima, I.; et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J. Cell. Physiol. 2006, 208, 64–76. [Google Scholar] [CrossRef]
- Lee, J.; Yoon, S.R.; Choi, I.; Jung, H. Causes and Mechanisms of Hematopoietic Stem Cell Aging. Int. J. Mol. Sci. 2019, 20, 1272. [Google Scholar] [CrossRef]
- Lew, M. Good Statistical Practice in Pharmacology Problem 2. Br. J. Pharmacol. 2007, 152, 299–303. [Google Scholar] [CrossRef]
- Zimmerlin, L.; Donnenberg, V.; Pfeifer, M.; Meyer, E.; Péault, B.; Rubin, J.; Donnenberg, A. Stromal Vascular Progenitors in Adult Human Adipose Tissue. Cytom. A 2010, 77A, 22–30. [Google Scholar] [CrossRef]
- Armulik, A.; Abramsson, A.; Betsholtz, C. Endothelial/Pericyte Interactions. Circ. Res. 2005, 97, 512–523. [Google Scholar] [CrossRef]
- Collett, G.; Canfield, A. Angiogenesis and Pericytes in the Initiation of Ectopic Calcification. Circ. Res. 2005, 96, 930–938. [Google Scholar] [CrossRef]
- Peters, D.G.; Zhang, X.C.; Benos, P.V.; Heidrich-O’Hare, E.; Ferrell, R.E. Genomic analysis of immediate/early response to shear stress in human coronary artery endothelial cells. Physiol. Genom. 2002, 12, 25–33. [Google Scholar] [CrossRef]
- Sundberg, C.; Ivarsson, M.; Gerdin, B.; Rubin, K. Pericytes as collagen-producing cells in excessive dermal scarring. Lab. Investig. A J. Tech. Methods Pathol. 1996, 74, 452–466. [Google Scholar]
- Andreeva, E.; Pugach, I.; Gordon, D.; Orekhov, A. Continuous subendothelial network formed by pericyte-like cells in human vascular bed. Tissue Cell 1998, 30, 127–135. [Google Scholar] [CrossRef]
- Dessalles, C.; Babataheri, A.; Barakat, A. Pericyte Mechanics and Mechanobiology. J. Cell Sci. 2021, 134, jcs240226. [Google Scholar] [CrossRef]
- Varma, M.; Breuls, R.; Schouten, T.; Jurgens, W.; Bontkes, H.; Schuurhuis, G.; Ham, S.; Milligen, F. Phenotypical and Functional Characterization of Freshly Isolated Adipose Tissue-Derived Stem Cells. Stem Cells Dev. 2007, 16, 91–104. [Google Scholar] [CrossRef]
- Hörl, S.; Ejaz, A.; Ernst, S.; Mattesich, M.; Kaiser, A.; Jenewein, B.; Zwierzina, M.; Hammerle, S.; Miggitsch, C.; Mitterberger-Vogt, M. CD146 (MCAM) in Human Cs-DLK1-/Cs-CD34+ Adipose Stromal/Progenitor Cells. Stem Cell Res. 2017, 22, 1–12. [Google Scholar] [CrossRef]
- Jang, Y.; Koh, Y.; Choi, Y.J.; Kim, S.H.; Yoon, D.; Lee, M.; Lee, J. Characterization of Adipose Tissue-Derived Stromal Vascular Fraction for Clinical Application to Cartilage Regeneration. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 142–150. [Google Scholar] [CrossRef]
- Hatzmann, F.; Großmann, S.; Waldegger, P.; Wiegers, G.; Mandl, M.; Rauchenwald, T.; Pierer, G.; Zwerschke, W. Dipeptidyl Peptidase-4 Cell Surface Expression Marks an Abundant Adipose Stem/Progenitor Cell Population with High Stemness in Human White Adipose Tissue. Adipocyte 2022, 11, 601–615. [Google Scholar] [CrossRef]
- Banyard, D.; Sarantopoulos, C.; Borovikova, A.; Qiu, X.; Wirth, G.; Paydar, K.; Haun, J.; Evans, G.; Widgerow, A. Phenotypic Analysis of Stromal Vascular Fraction after Mechanical Shear Reveals Stress-Induced Progenitor Populations. Plast. Reconstr. Surg. 2016, 138, 237–247. [Google Scholar] [CrossRef]
- Becker, H.; Vazquez, O.; Rosen, T. Cannula Size Effect on Stromal Vascular Fraction Content of Fat Grafts. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3471. [Google Scholar] [CrossRef]
- Jurgens, W.; Oedayrajsingh-Varma, M.; Helder, M.; ZandiehDoulabi, B.; Schouten, T.; Kuik, D.; Ritt, M.; Milligen, F. Effect of Tissue-Harvesting Site on Yield of Stem Cells Derived from Adipose Tissue: Implications for Cell-Based Therapies. Cell Tissue Res. 2008, 332, 415–426. [Google Scholar] [CrossRef]
- Philips, B.; Grahovac, T.; Valentin, J.; Chung, C.; Bliley, J.; Pfeifer, M.; Roy, S.; Dreifuss, S.; Kelmendi-Doko, A.; Kling, R. Prevalence of Endogenous CD34+ Adipose Stem Cells Predicts Human Fat Graft Retention in a Xenograft Model: Plast. Reconstr. Surg. 2013, 132, 845–858. [Google Scholar] [CrossRef]
- Dahdah, N.; Tercero-Alcázar, C.; Malagón, M.; Garcia-Roves, P.; Guzmán-Ruiz, R. Interrelation of Adipose Tissue Macrophages and Fibrosis in Obesity. Biochem. Pharmacol. 2024, 225, 116324. [Google Scholar] [CrossRef]
- Turner, L.; Santosa, S. Putting ATM to BED: How Adipose Tissue Macrophages Are Affected by Bariatric Surgery, Exercise, and Dietary Fatty Acids. Adv. Nutr. 2021, 12, 1893–1910. [Google Scholar] [CrossRef] [PubMed]
- Eto, H.; Ishimine, H.; Kinoshita, K.; Watanabe-Susaki, K.; Kato, H.; Doi, K.; Kuno, S.; Kurisaki, A.; Yoshimura, K. Characterization of Human Adipose Tissue-Resident Hematopoietic Cell Populations Reveals a Novel Macrophage Subpopulation with CD34 Expression and Mesenchymal Multipotency. Stem Cells Dev. 2013, 22, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Guo, J.; Banyard, D.; Fadavi, D.; Toranto, J.; Wirth, G.; Paydar, K.; Evans, G.; Widgerow, A. Stromal Vascular Fraction: A Regenerative Reality? Part 1: Current concepts and review of the literature. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Alt, C.; Azares, A.; Pearce, D.; Facile, T.; Furia, J.; Maffulli, N.; Huang, C.; Alt, E. The Composition of Adipose-Derived Regenerative Cells Isolated from Lipoaspirate Using a Point of Care System Does Not Depend on the Subject’s Individual Age, Sex, Body Mass Index and Ethnicity. Cells 2022, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Frias, F.; Matos, B.; Jarnalo, M.; Freitas-Ribeiro, S.; Reis, R.; Pirraco, R.; Horta, R. Stromal Vascular Fraction Obtained From Subcutaneous Adipose Tissue: Ex-Obese and Older Population as Main Clinical Targets. J. Surg. Res. 2023, 283, 632–639. [Google Scholar] [CrossRef]


| Cell Type | Marker | Significance |
|---|---|---|
| Macrophages | CD45+, CD34−, CD11b+ | Regulate inflammation and tissue repair |
| Endothelial Progenitor Cells (EPCs) | CD45−, CD31+, CD34+ | Promote vascularization of healing tissues; localized to luminal side |
| Mesenchymal Stem Cells (MSCs) | CD45−, CD31−, CD34+ | Central to regenerative wound healing |
| Pericytes (Strategy 1) | CD45−, CD31−, CD146+ | Support angiogenesis and maintain tissue homeostasis; localized to adventitia |
| Pericytes (Strategy 2) | CD45−, CD31−, CD146+, CD34− | (Same as above) |
| Transit-Amplifying (TA) Progenitor Cells | CD45−, CD31−, CD146+, CD34+ | May give rise to EPC and SA-ASC populations |
| Supra-Adventitial Adipose Stromal Cells (SA-ASCs) | CD45−, CD31−, CD146−, CD34+ | Reside around arterioles and venules |
| Assay | Probe |
|---|---|
| CD31 | Anti-CD31 Ab (Clone WM59)–PE |
| CD34 | Anti-CD34 Ab (Clone 561)–BV421 |
| CD45 | Anti-CD45 Ab (Clone 2D1)–BV510 |
| CD11b | Anti-CD11b Ab (Clone M1/70)–FITC |
| CD146 | Anti-CD146 Ab (Clone SHM-57)–APC |
| Viability | 7-AAD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalazar, D.; Feng, J.; Banyard, D.A.; Aliaghaei, M.; Widgerow, A.D.; Haun, J.B. Integrated Fluidic Platform for Washing and Mechanical Processing of Lipoaspirate for Downstream Fat Grafting and Regenerative Applications. Bioengineering 2025, 12, 918. https://doi.org/10.3390/bioengineering12090918
Zalazar D, Feng J, Banyard DA, Aliaghaei M, Widgerow AD, Haun JB. Integrated Fluidic Platform for Washing and Mechanical Processing of Lipoaspirate for Downstream Fat Grafting and Regenerative Applications. Bioengineering. 2025; 12(9):918. https://doi.org/10.3390/bioengineering12090918
Chicago/Turabian StyleZalazar, David, Jiayi Feng, Derek A. Banyard, Marzieh Aliaghaei, Alan D. Widgerow, and Jered B. Haun. 2025. "Integrated Fluidic Platform for Washing and Mechanical Processing of Lipoaspirate for Downstream Fat Grafting and Regenerative Applications" Bioengineering 12, no. 9: 918. https://doi.org/10.3390/bioengineering12090918
APA StyleZalazar, D., Feng, J., Banyard, D. A., Aliaghaei, M., Widgerow, A. D., & Haun, J. B. (2025). Integrated Fluidic Platform for Washing and Mechanical Processing of Lipoaspirate for Downstream Fat Grafting and Regenerative Applications. Bioengineering, 12(9), 918. https://doi.org/10.3390/bioengineering12090918






