Analysis of Conventional and Enhanced-Biocompatibility ZnO/Ag Heterojunction Nanorod-Based Advanced Root Canal Sealers
Abstract
1. Introduction
2. Materials and Methods
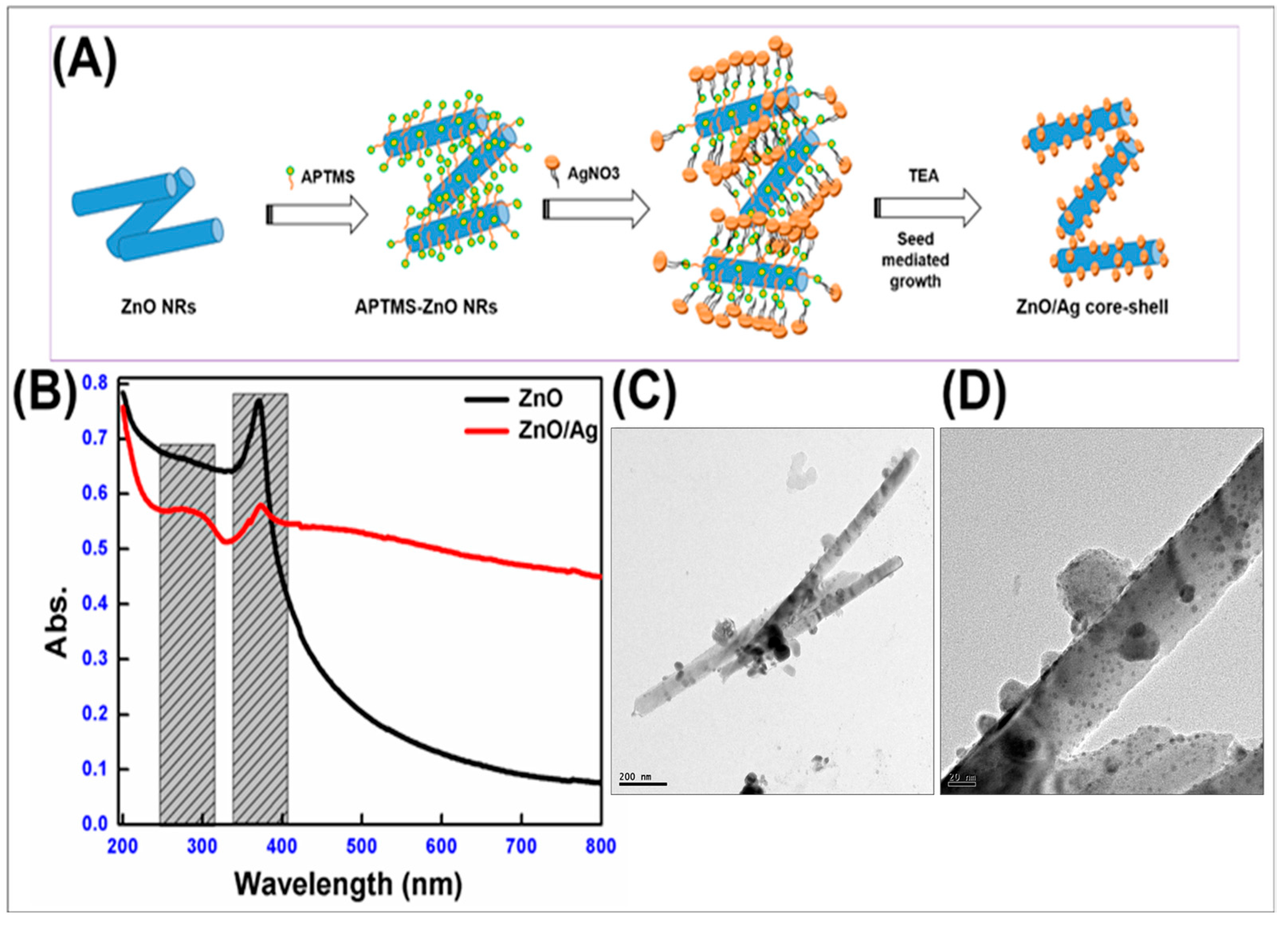
2.1. Synthesis of ZnO/Ag Heterojunction Nanorods
2.2. Biocompatibility Testing
2.3. Antimicrobial Studies
3. Results
3.1. Synthesis and Characterization of Nano ZnO and ZnO/Ag Heterojunction Nanorods
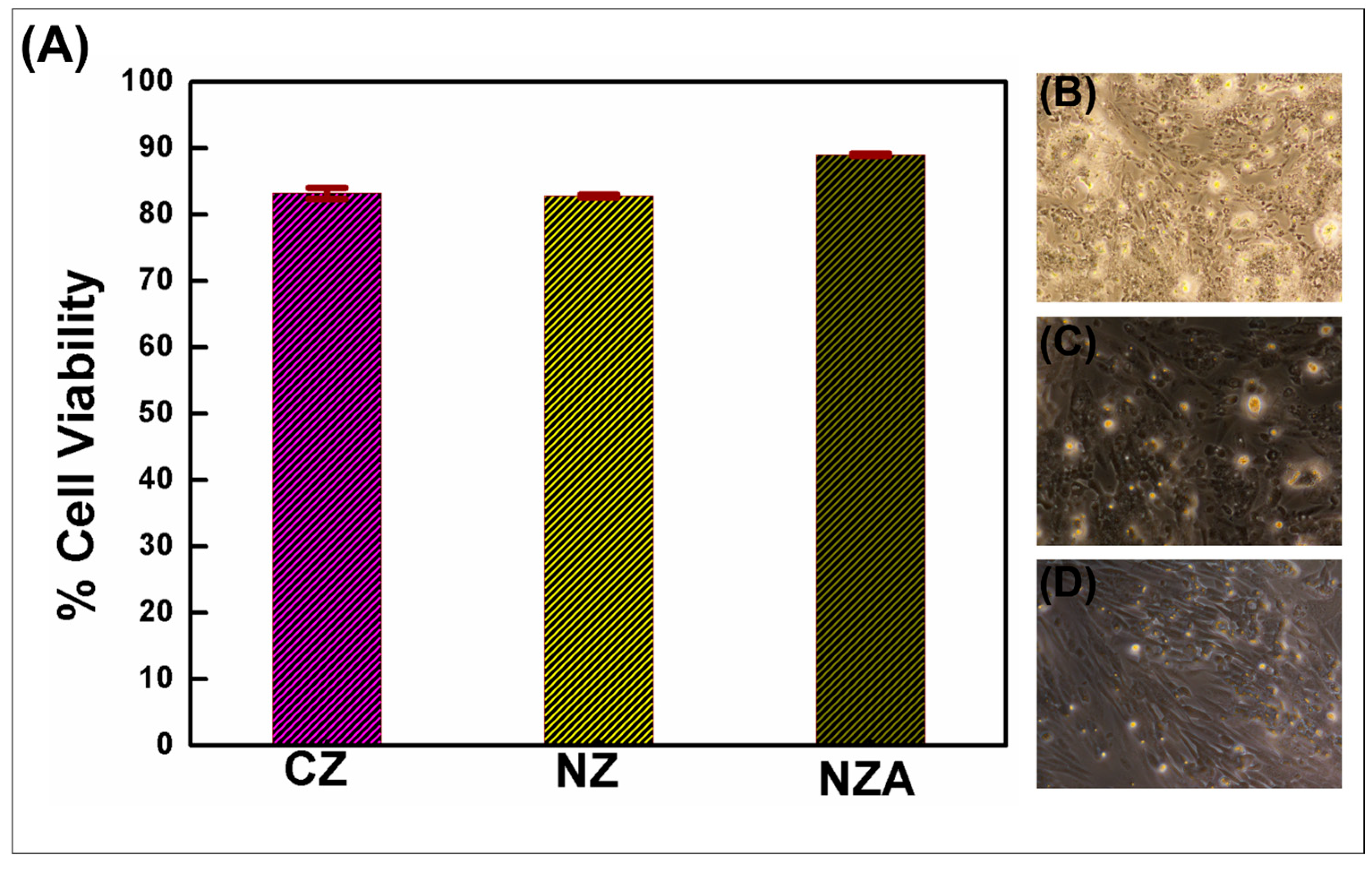
3.2. Assessment of MG63 Cell Proliferation in Response to Material Eluates via MTT Assay
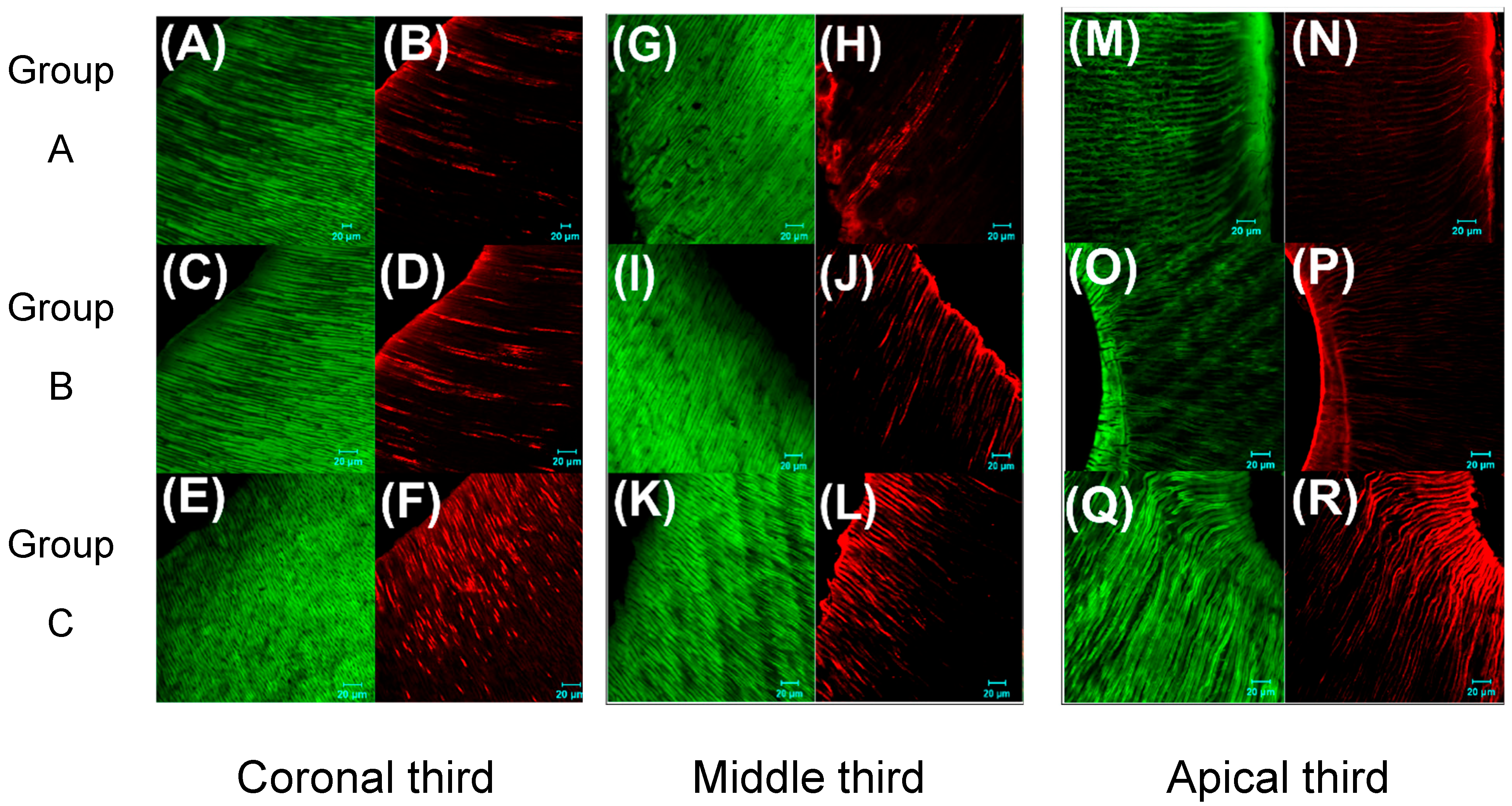
3.3. Effects of Eluates on Antimicrobial Efficcacy Against E. faecalis in an Ex Vivo Tooth Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Shen, Y.; Ruse, N.D.; Haapasalo, M. Antibacterial activity of endodontic sealers by modified direct contact test against Enterococcus faecalis. J. Endod. 2009, 35, 1051–1055. [Google Scholar] [CrossRef]
- Desai, S.; Chandler, N. Calcium hydroxide-based root canal sealers: A review. J. Endod. 2009, 35, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.M.; Lee, S.S.; Yang, S.F.; Chang, Y.C. Up-regulation of receptor activator nuclear factor-kappaB ligand expression by root canal sealers in human osteoblastic cells. J. Endod. 2009, 35, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.M.; Yang, S.F.; Chang, Y.C. Up-regulation of gelatinases and tissue type plasminogen activator by root canal sealers in human osteoblastic cells. J. Endod. 2008, 34, 291–294. [Google Scholar] [CrossRef]
- Grossman, L.I. An improved root canal cement. J. Am Dent. Assoc. 1958, 56, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Gröger, S.; Wu, Z.; Ruf, S.; Ye, Y.; Chen, X. Photobiomodulation Therapy and Pulp-Regenerative Endodontics: A Narrative Review. Bioengineering 2023, 10, 371. [Google Scholar] [CrossRef]
- Figueiredo, J.A.; Pesce, H.F.; Gioso, M.A.; Figueiredo, M.A. The histological effects of four endodontic sealers implanted in the oral mucosa: Submucous injection versus implant in polyethylene tubes. Int. Endod. J. 2001, 34, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Xu, X. Bioceramics in Endodontics: Updates and Future Perspectives. Bioengineering 2023, 10, 354. [Google Scholar] [CrossRef]
- Alhujaily, M.; Albukhaty, S.; Yusuf, M.; Mohammed, M.K.A.; Sulaiman, G.M.; Al-Karagoly, H.; Alyamani, A.A.; Albaqami, J.; AlMalki, F.A. Recent Advances in Plant-Mediated Zinc Oxide Nanoparticles with Their Significant Biomedical Properties. Bioengineering 2022, 9, 541. [Google Scholar] [CrossRef]
- Huang, Q.; Li, Z.; Lyu, P.; Zhou, X.; Fan, Y. Current Applications and Future Directions of Lasers in Endodontics: A Narrative Review. Bioengineering 2023, 10, 296. [Google Scholar] [CrossRef]
- Akram, W.; Zahid, R.; Usama, R.M.; AlQahtani, S.A.; Dahshan, M.; Basit, M.A.; Yasir, M. Enhancement of Antibacterial Properties, Surface Morphology and In Vitro Bioactivity of Hydroxyapatite-Zinc Oxide Nanocomposite Coating by Electrophoretic Deposition Technique. Bioengineering 2023, 10, 693. [Google Scholar] [CrossRef] [PubMed]
- Shayani Rad, M.; Kompany, A.; Khorsand Zak, A.; Javidi, M.; Mortazavi, S.M. Microleakage and antibacterial properties of ZnO and ZnO:Ag nanopowders prepared via a sol–gel method for endodontic sealer application. J. Nanopart. Res. 2013, 15, 1925. [Google Scholar] [CrossRef]
- Ali, S.; Sudha, K.G.; Thirumalaivasan, N.; Ahamed, M.; Pandiaraj, S.; Rajeswari, V.D.; Vinayagam, Y.; Thiruvengadam, M.; Govindasamy, R. Green Synthesis of Magnesium Oxide Nanoparticles by Using Abrus precatorius Bark Extract and Their Photocatalytic, Antioxidant, Antibacterial, and Cytotoxicity Activities. Bioengineering 2023, 10, 302. [Google Scholar] [CrossRef]
- Hussain, S.; Khakwani, N.; Faiz, Y.; Zulfqar, S.; Shafq, Z.; Faiz, F.; Elhakem, A.; Sami, R.; Aljuraide, N.I.; Farid, T. Green Production and Interaction of Carboxylated CNTs/Biogenic ZnO Composite for Antibacterial Activity. Bioengineering 2022, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Waltimo, T.; Brunner, T.J.; Vollenweider, M.; Stark, W.J.; Zehnder, M. Antimicrobial effect of nanometric bioactive glass 45S5. J. Dent. Res. 2007, 86, 754–757. [Google Scholar] [CrossRef]
- Sanvicens, N.; Marco, M.P. Multifunctional nanoparticles–properties and prospects for their use in human medicine. Trends Biotechnol. 2008, 26, 425–433. [Google Scholar] [CrossRef]
- Busscher, H.J.; Engels, E.; Dijkstra, R.J.; Van der Mei, H.C. Influence of a chitosan on oral bacterial adhesion and growth in vitro. Eur. J. Oral Sci. 2008, 116, 493–495. [Google Scholar] [CrossRef]
- Xu, P.; Liang, J.; Dong, G.; Zheng, L.; Ye, L. Cytotoxicity of RealSeal on Human Osteoblast-like MG63 Cells. J. Endod. 2010, 36, 40–44. [Google Scholar] [CrossRef]
- Attik, G.N.; Villat, C.; Hallay, F.; Pradelle-Plasse, N.; Bonnet, H.; Moreau, K. In vitro biocompatibility of a dentine substitute cement on human MG63 osteoblasts cells: BiodentineTM versus MTA®. Int. Endod. J. 2014, 47, 1133–1141. [Google Scholar] [CrossRef]
- Dinesh, V.P.; Biji, P.; Ashok, A.; Dhara, S.K.; Kamruddin, M.; Tyagi, A.K. Plasmon-mediated, highly enhanced photocatalytic degradation of industrial textile dyes using hybrid ZnO@Ag core-shell nanorods. RSC Adv. 2014, 4, 58930–58940. [Google Scholar] [CrossRef]
- Fageria, P.; Gangopadhyay, S.; Pande, S. Synthesis of ZnO/Au and ZnO/Ag nanoparticles and their photocatalytic application using UV and visible light. RSC Adv. 2014, 4, 24962–24972. [Google Scholar] [CrossRef]
- Li, X.; Zhou, X.; Guo, H.; Wang, C.; Liu, J.; Sun, P. Design of Au@ZnO Yolk–Shell Nanospheres with Enhanced Gas Sensing Properties. ACS Appl. Mater. Interfaces 2014, 6, 18661–18667. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.R.; Shao, G.X.; Zhao, J.F.; Zhang, Z.X.; Zhang, Y.; Liang, J. Worm-Like Ag/ZnO Core–Shell Heterostructural Composites: Fabrication, Characterization, and Photocatalysis. J. Phys. Chem. C 2012, 116, 16182–16190. [Google Scholar] [CrossRef]
- ISO 7405:2025; Dentistry—Valuation of Biocompatibility of Medical Devices Used in Dentistry. ISO: Geneva, Switzerland, 2025. Available online: https://www.iso.org/standard/7405 (accessed on 20 August 2025).
- ISO 10993-5:2009; Biological Evaluation of Medical Devices Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2025. Available online: https://www.iso.org/standard/36406.html (accessed on 20 August 2025).
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramirez, J.T. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Clover, J.; Gowen, M. Are MG-63 and HOS TE85 human osteosarcoma cell lines representative models of the osteoblastic phenotype? Bone 1994, 15, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Annie, S.; Shi, Z.; Neoh, K.G.; Anil, K. Nanoparticulates for Antibiofilm Treatment and Effect of Aging on Its Antibacterial Activity. J. Endod. 2010, 36, 1030–1035. [Google Scholar] [CrossRef]
- Jose, J.; Teja, K.V.; Janani, K.; Alam, M.K.; Khattak, O.; Salloum, M.G.; Magar, S.S.; Magar, S.P.; Rajeshkumar, S.; Palanivelu, A.; et al. Preparation of a Novel Nanocomposite and Its Antibacterial Effectiveness against Enterococcus faecalis—An In Vitro Evaluation. Polymers 2022, 14, 1499. [Google Scholar] [CrossRef]
- Aguiar, A.S.; Guerreiro-Tanomaru, J.M.; Faria, G.; Leonardo, R.T.; Tanomaru-Filho, M. Antimicrobial Activity and pH of Calcium Hydroxide and Zinc Oxide Nanoparticles Intracanal Medication and Association with Chlorhexidine. J. Contemp. Dent. Pract. 2015, 16, 624–629. [Google Scholar] [PubMed]
- Ritika, C.; Arpita, R.; Jayanthi, A. Biosynthesis of silver and zinc oxide nanoparticles using Pichia fermentans JA2 and their antimicrobial property. Appl. Nanosci. 2015, 5, 63–71. [Google Scholar]
- Kishen, A.; Shi, Z.; Shrestha, A.; Gee, K. An Investigation on the Antibacterial and Antibiofilm Efficacy of Cationic Nanoparticulates for Root Canal Disinfection. J. Endod. 2008, 34, 1515–1520. [Google Scholar] [CrossRef]
- Lok, C.; Ho, C.; Chen, R.; He, Q.; Yu, W.; Sun, H. Silver nanoparticles: Partial oxidation and antibacterial activities. J. Biol. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef]
- Doty, C.; Tshikhudo, R.; Brust, M.; Fernig, D. Extremely stable water-soluble Ag nanoparticles. Chem. Mater. 2005, 17, 4630–4635. [Google Scholar] [CrossRef]
- Ghandour, W.; Hubbard, J.A.; Deistrung, J.; Hughes, M.N.; Poole, R.K. The uptake of silver ions by Escherichia coli: Toxic effects and interactions with copper ions. Appl. Microbiol. Biotechnol. 1988, 28, 559–565. [Google Scholar] [CrossRef]
- Schreurs, W.J.; Rosenberg, H. Effect of silver ions on transport and retention of phosphate by Escherichia coli. J. Bacteriol. 1982, 152, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Sameer, M.; Anurag, A.; Shenam, P.; Ishita, K. To evaluate the antibacterial properties of silver nano particle based irrigant as endodontic root canal irrigant. Int. J. Dent. Health Sci. 2014, 1, 485–492. [Google Scholar]
- Yuan, X.; Zhu, W.; Yang, Z.; Chen, F.; Han, X. Three-Dimensional Printing of Poly-L-Lactic Acid Composite Scaffolds with Enhanced Bioactivity and Controllable Zn Ion Release Capability by Coupling with Carbon-ZnO. Bioengineering 2023, 10, 307. [Google Scholar] [CrossRef]
| Group | Concentration (mg/mL) | Mean Count | % of Cell Viability |
|---|---|---|---|
| Control | 0.3076 | 100.00 | |
| A | 0.01 | 0.2774 | 90.19 |
| 0.05 | 0.2721 | 88.48 | |
| 0.1 | 0.2542 | 82.66 | |
| B | 0.01 | 0.3022 | 98.27 |
| 0.05 | 0.2652 | 86.23 | |
| 0.1 | 0.2544 | 82.70 | |
| C | 0.01 | 0.2936 | 95.45 |
| 0.05 | 0.2789 | 90.68 | |
| 0.1 | 0.2735 | 88.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velusamy, G.; Unnikrishnan, A.; Ponnuvelu, D.V.; Rajendran, S.; Park, S.; Pullithadathil, B. Analysis of Conventional and Enhanced-Biocompatibility ZnO/Ag Heterojunction Nanorod-Based Advanced Root Canal Sealers. Bioengineering 2025, 12, 917. https://doi.org/10.3390/bioengineering12090917
Velusamy G, Unnikrishnan A, Ponnuvelu DV, Rajendran S, Park S, Pullithadathil B. Analysis of Conventional and Enhanced-Biocompatibility ZnO/Ag Heterojunction Nanorod-Based Advanced Root Canal Sealers. Bioengineering. 2025; 12(9):917. https://doi.org/10.3390/bioengineering12090917
Chicago/Turabian StyleVelusamy, Gayathri, Aleena Unnikrishnan, Dinesh Veeran Ponnuvelu, Selvakumar Rajendran, Sungsu Park, and Biji Pullithadathil. 2025. "Analysis of Conventional and Enhanced-Biocompatibility ZnO/Ag Heterojunction Nanorod-Based Advanced Root Canal Sealers" Bioengineering 12, no. 9: 917. https://doi.org/10.3390/bioengineering12090917
APA StyleVelusamy, G., Unnikrishnan, A., Ponnuvelu, D. V., Rajendran, S., Park, S., & Pullithadathil, B. (2025). Analysis of Conventional and Enhanced-Biocompatibility ZnO/Ag Heterojunction Nanorod-Based Advanced Root Canal Sealers. Bioengineering, 12(9), 917. https://doi.org/10.3390/bioengineering12090917








