Chemical Deuteration of α-Amino Acids and Optical Resolution: Overview of Research Developments
Abstract
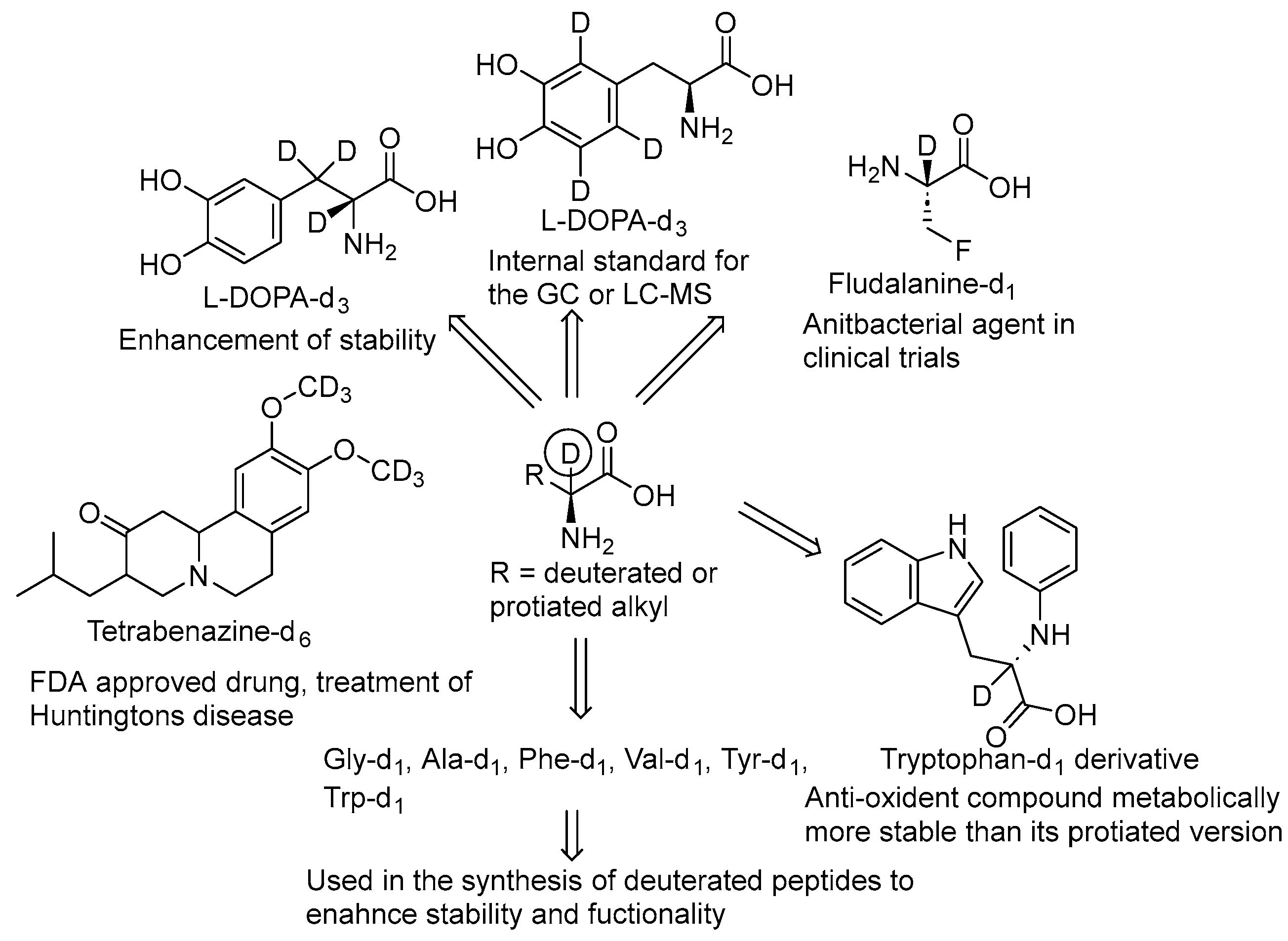
1. Introduction
2. Methods
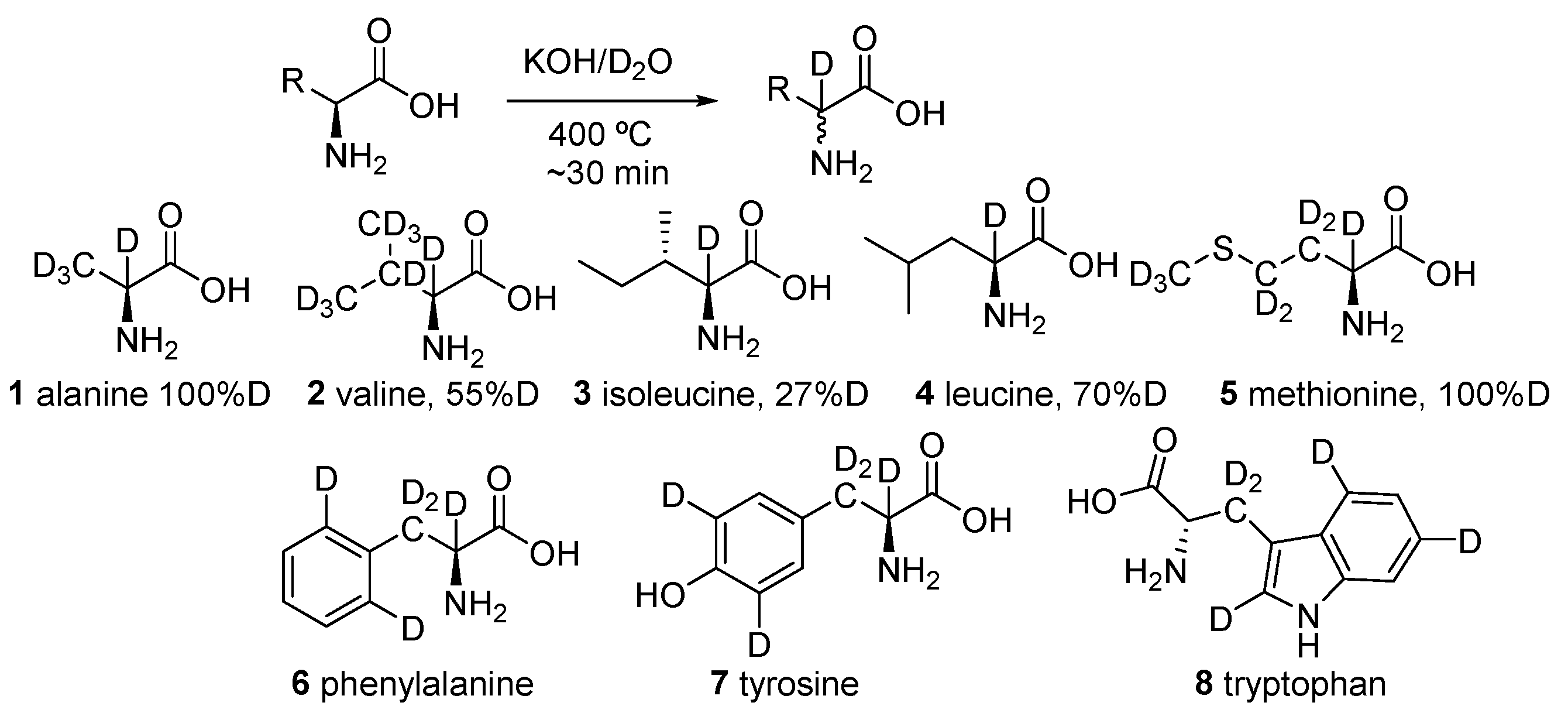
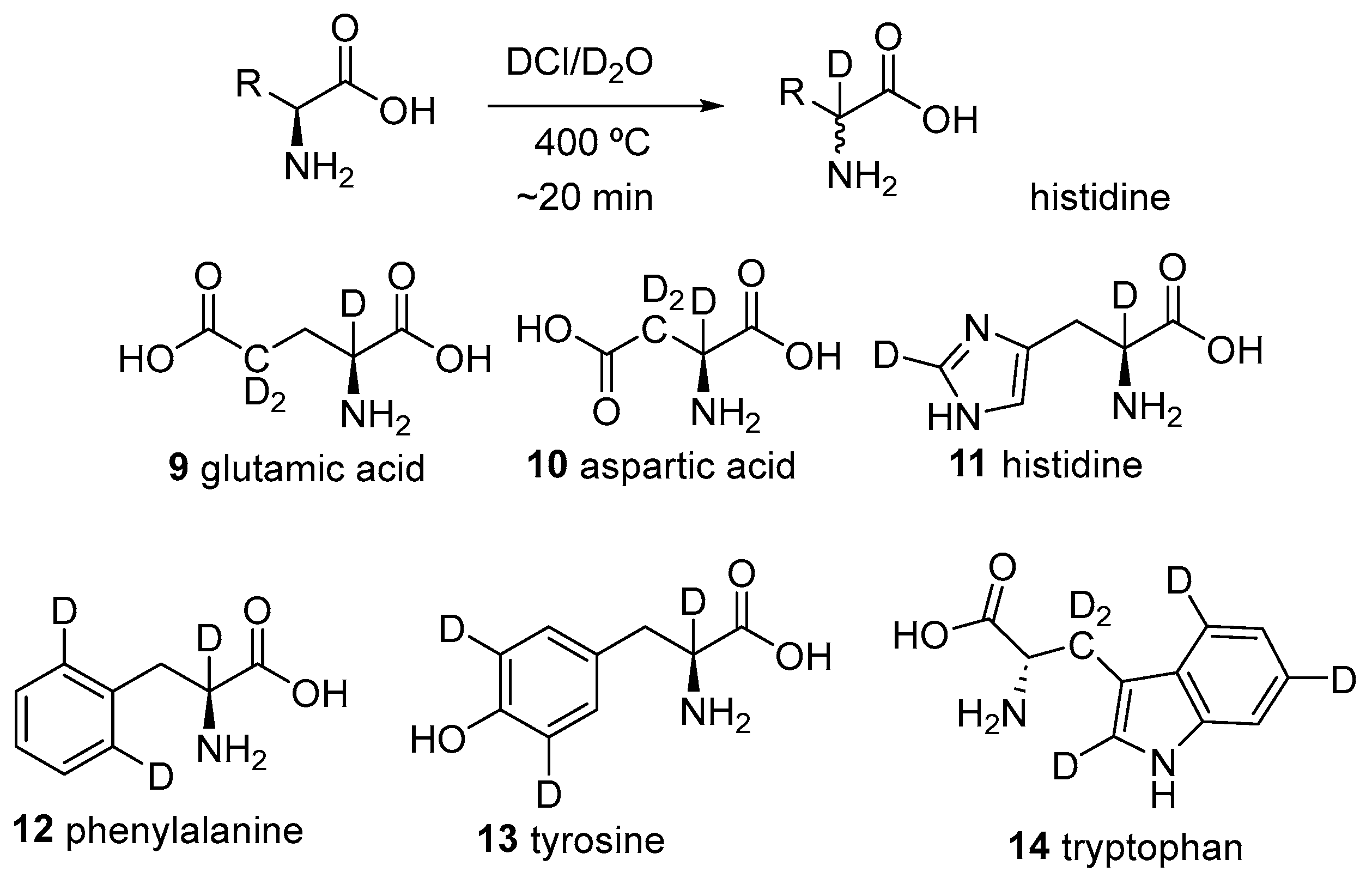
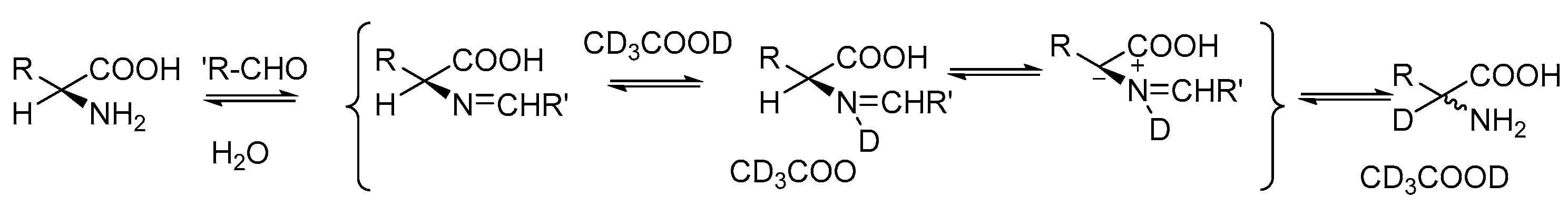
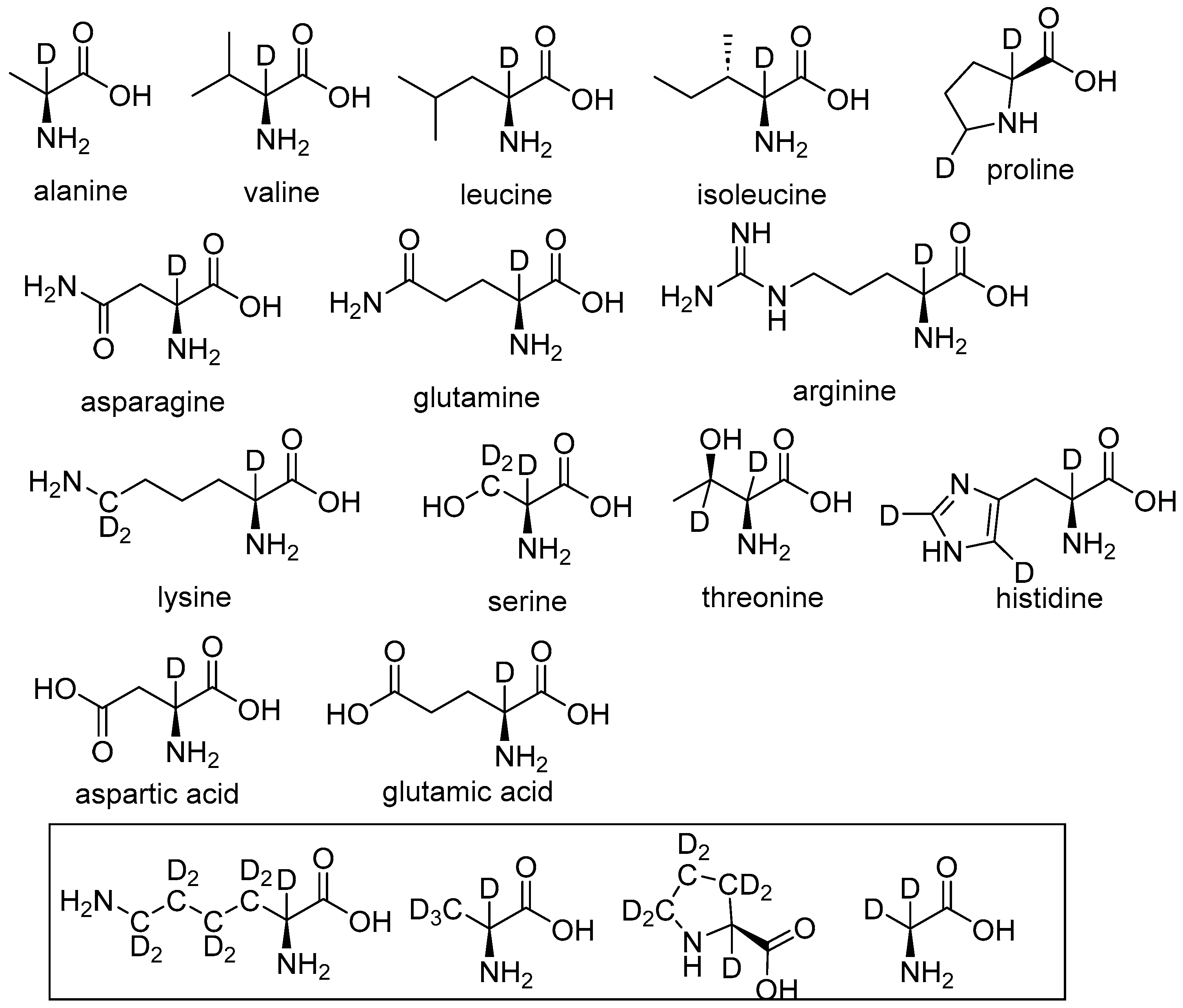
2.1. Specific Deuteration
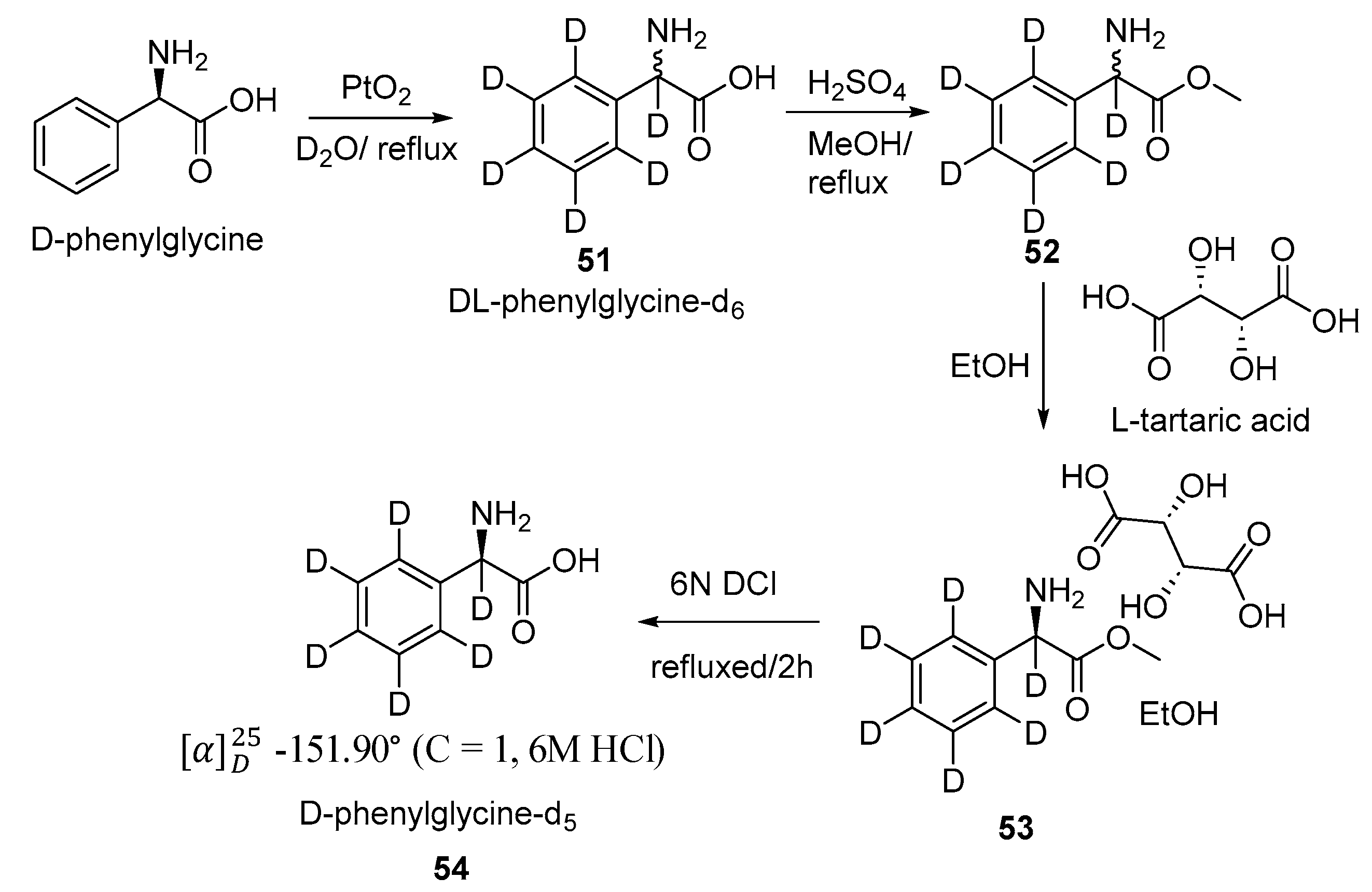
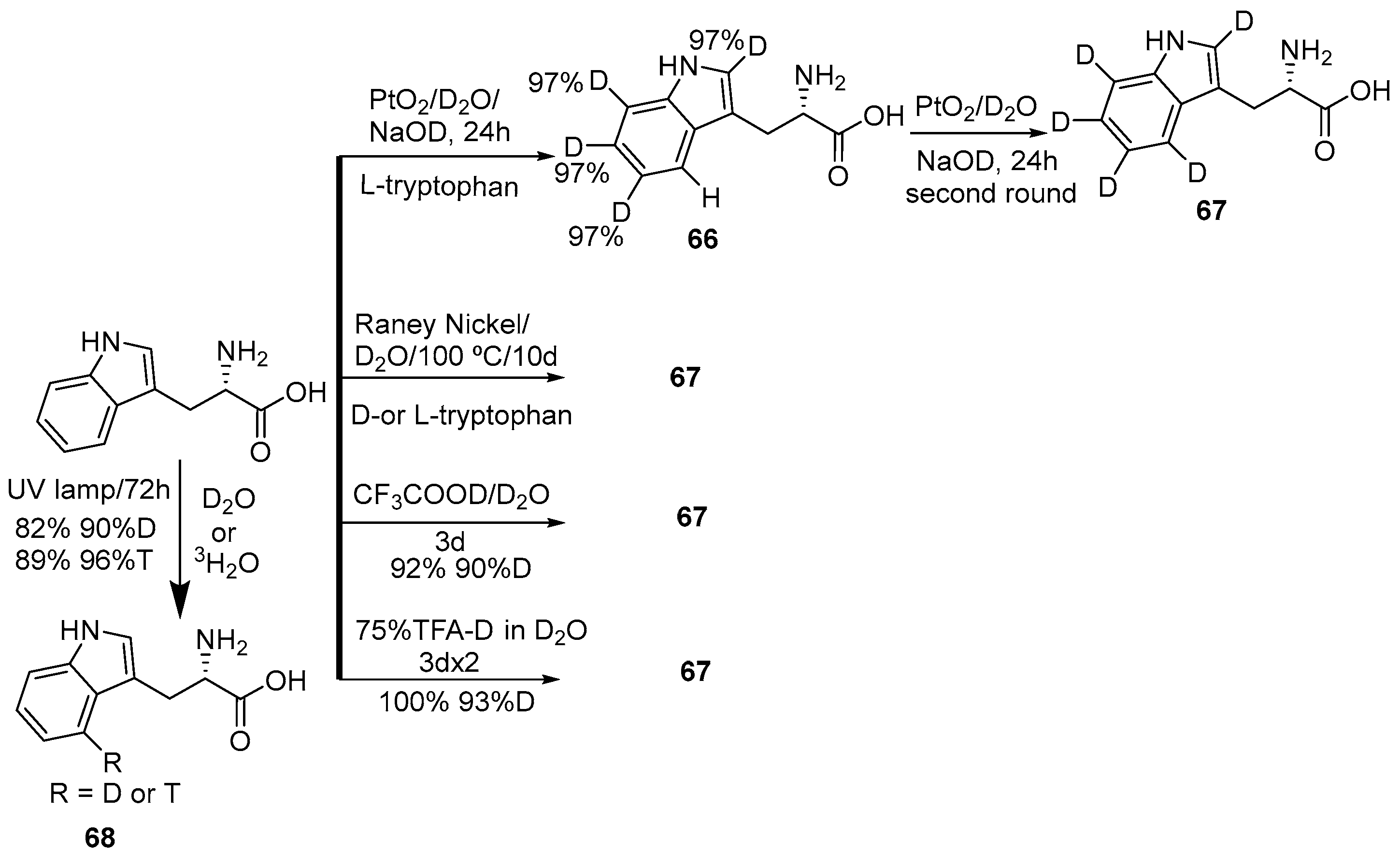
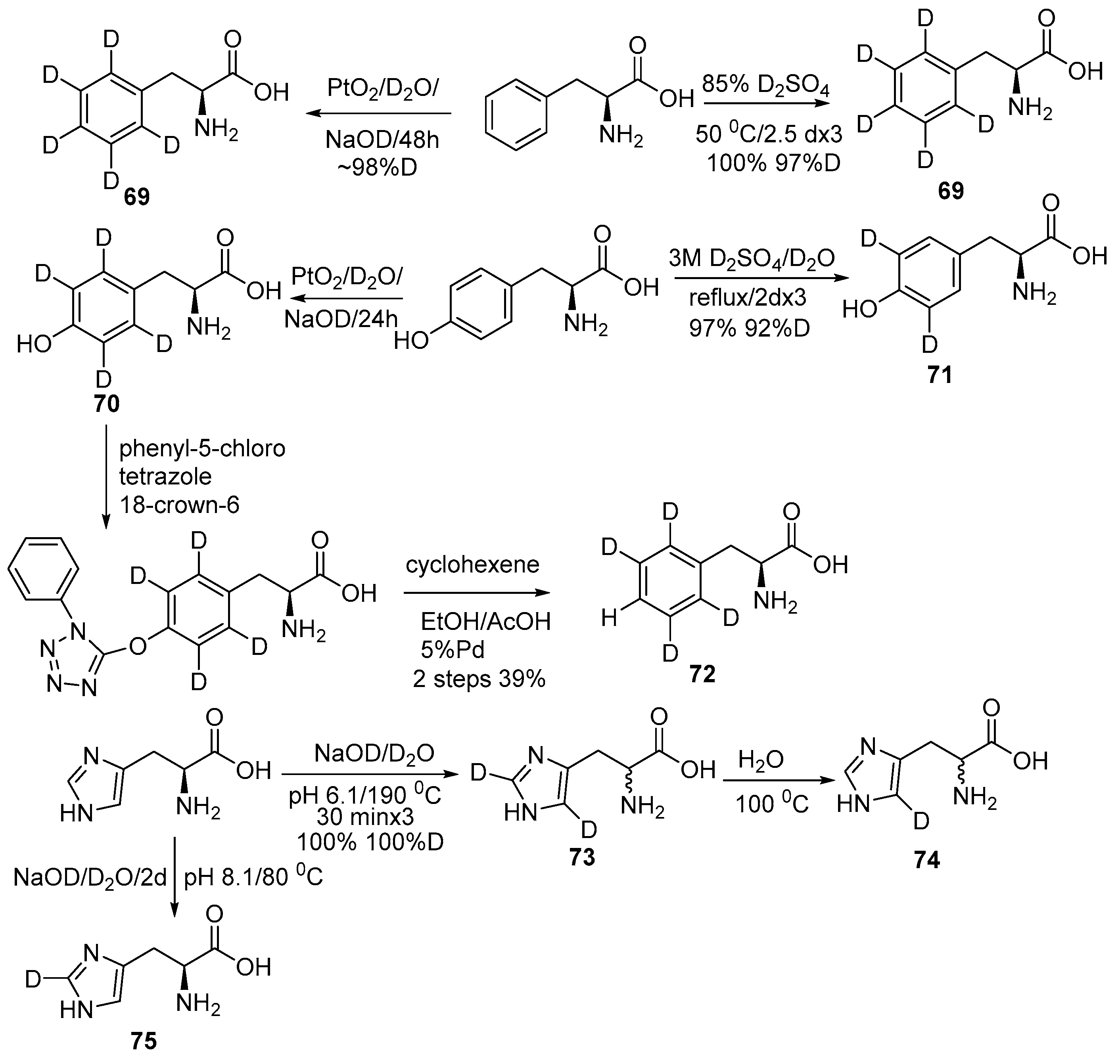
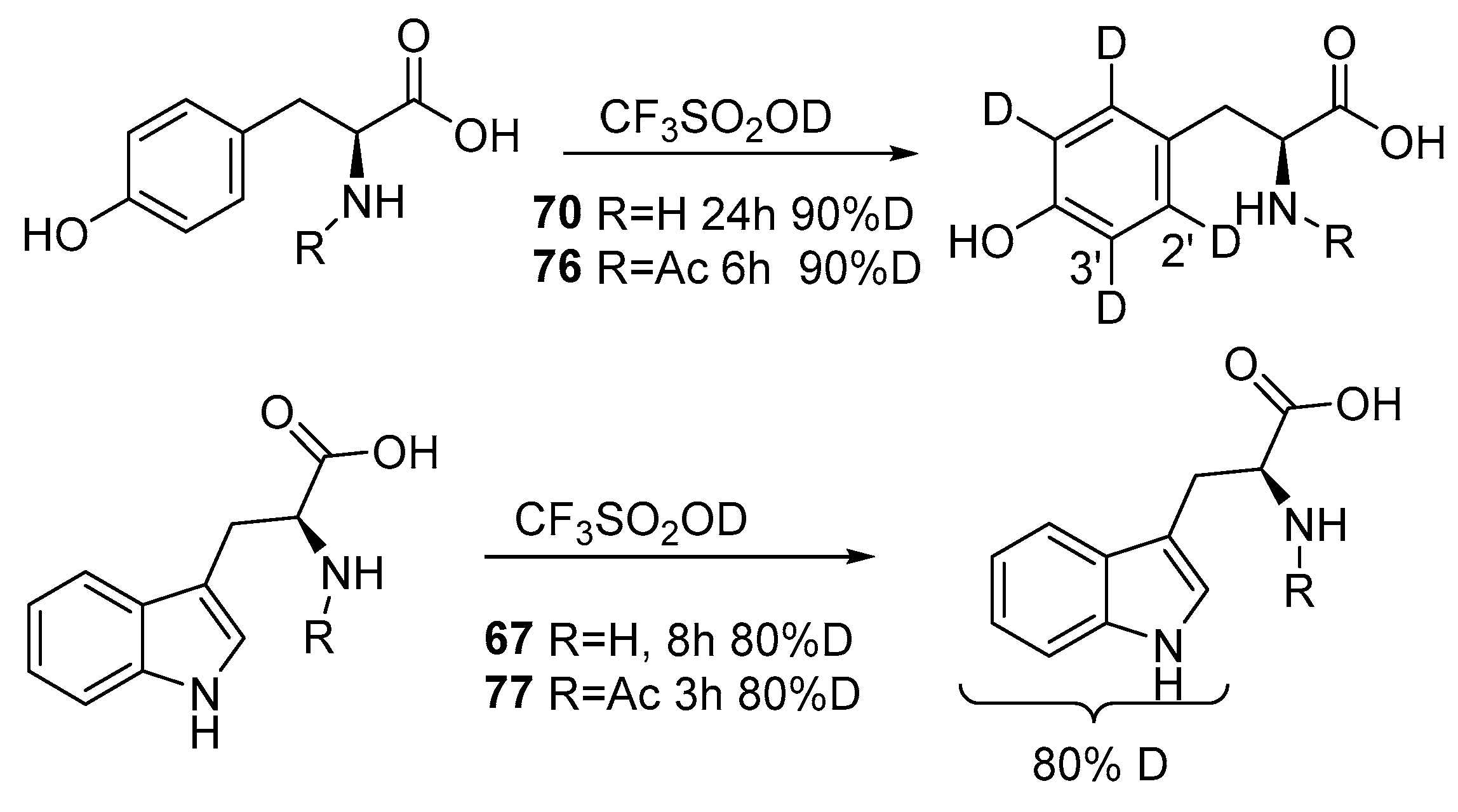
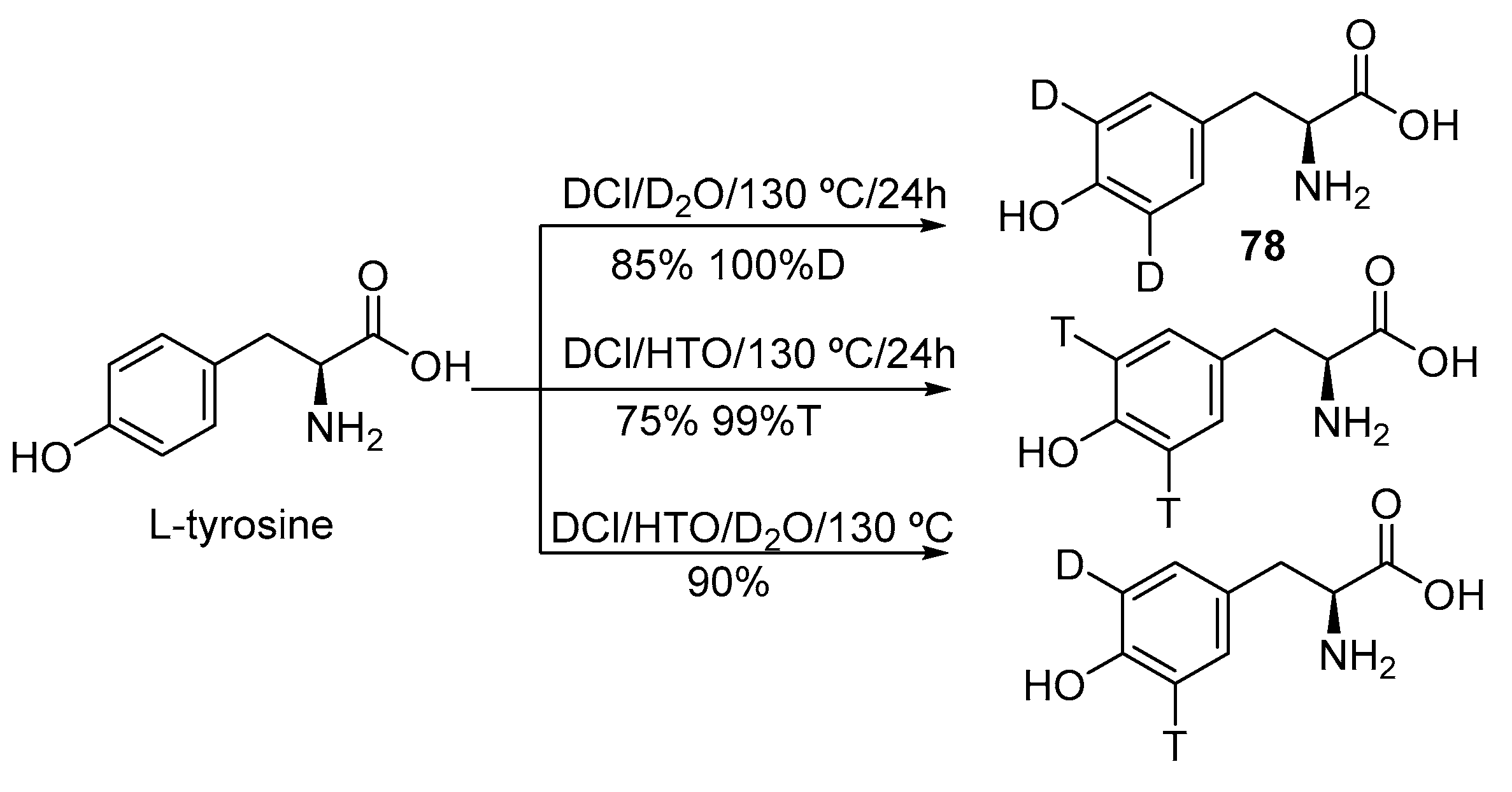
2.2. Selective and Perdeuteration of Aromatic or Heterocyclic Amino Acids
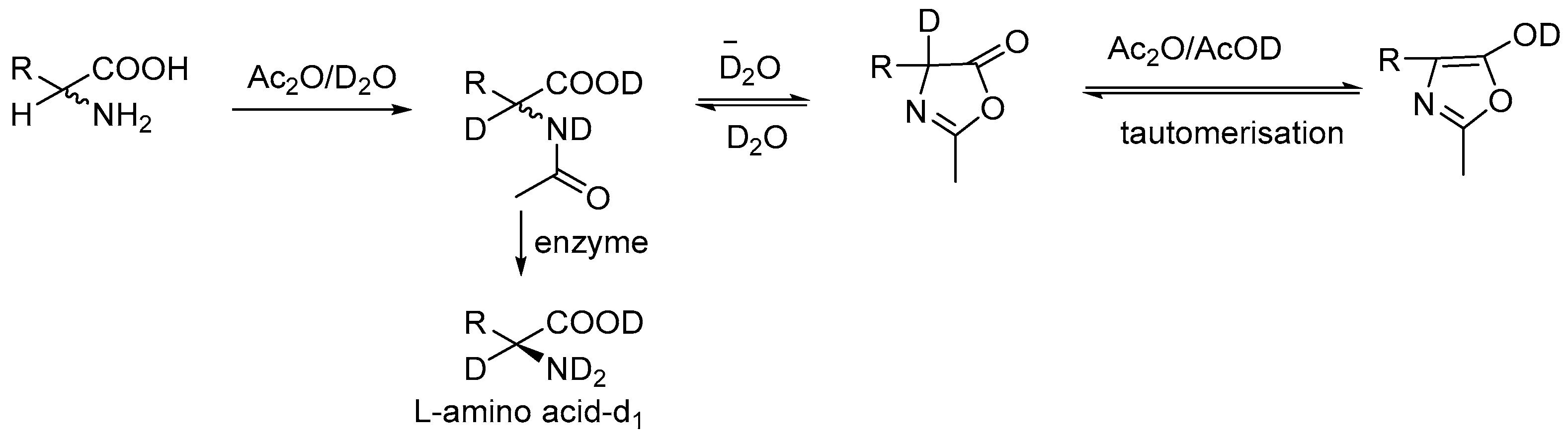
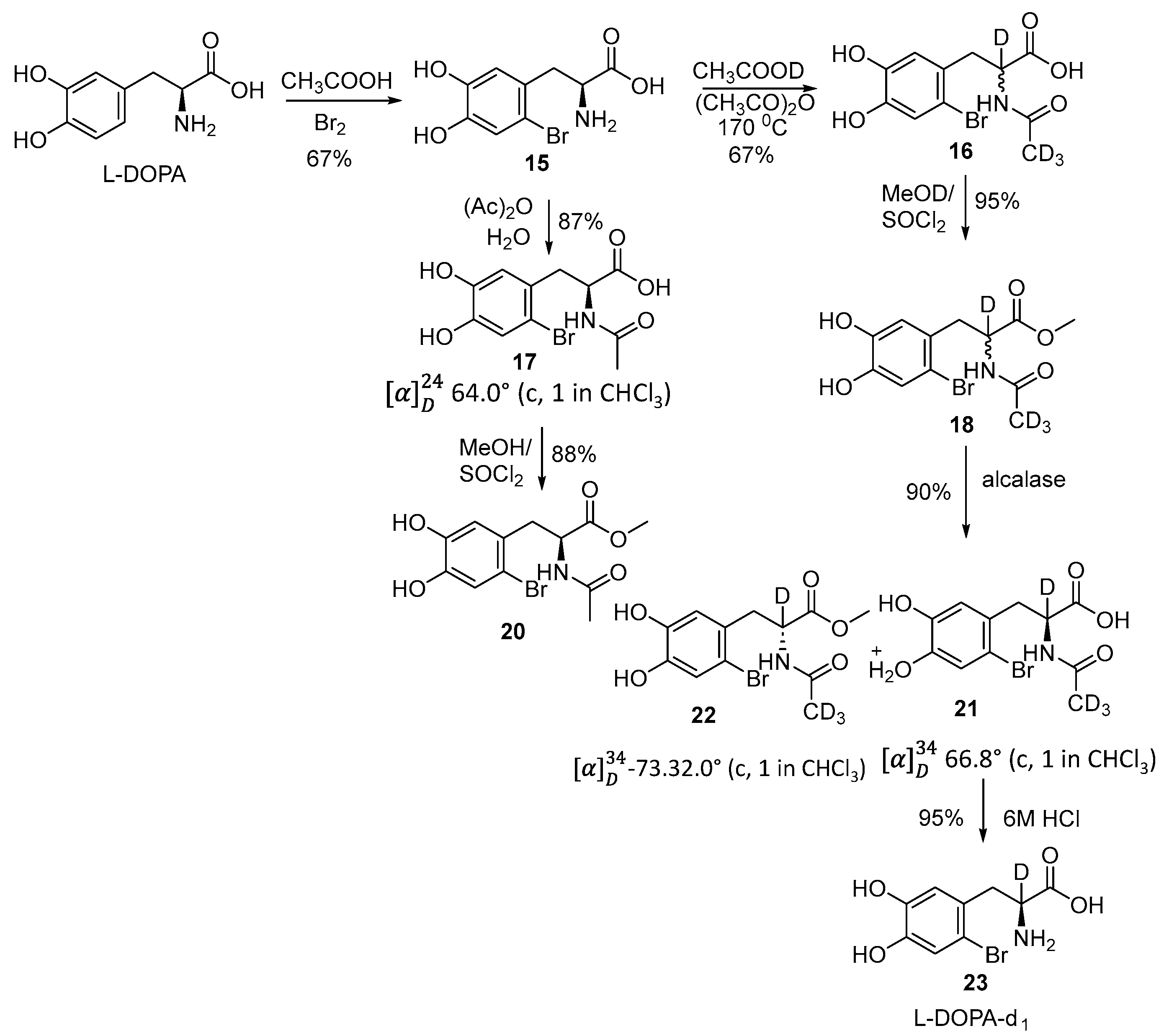
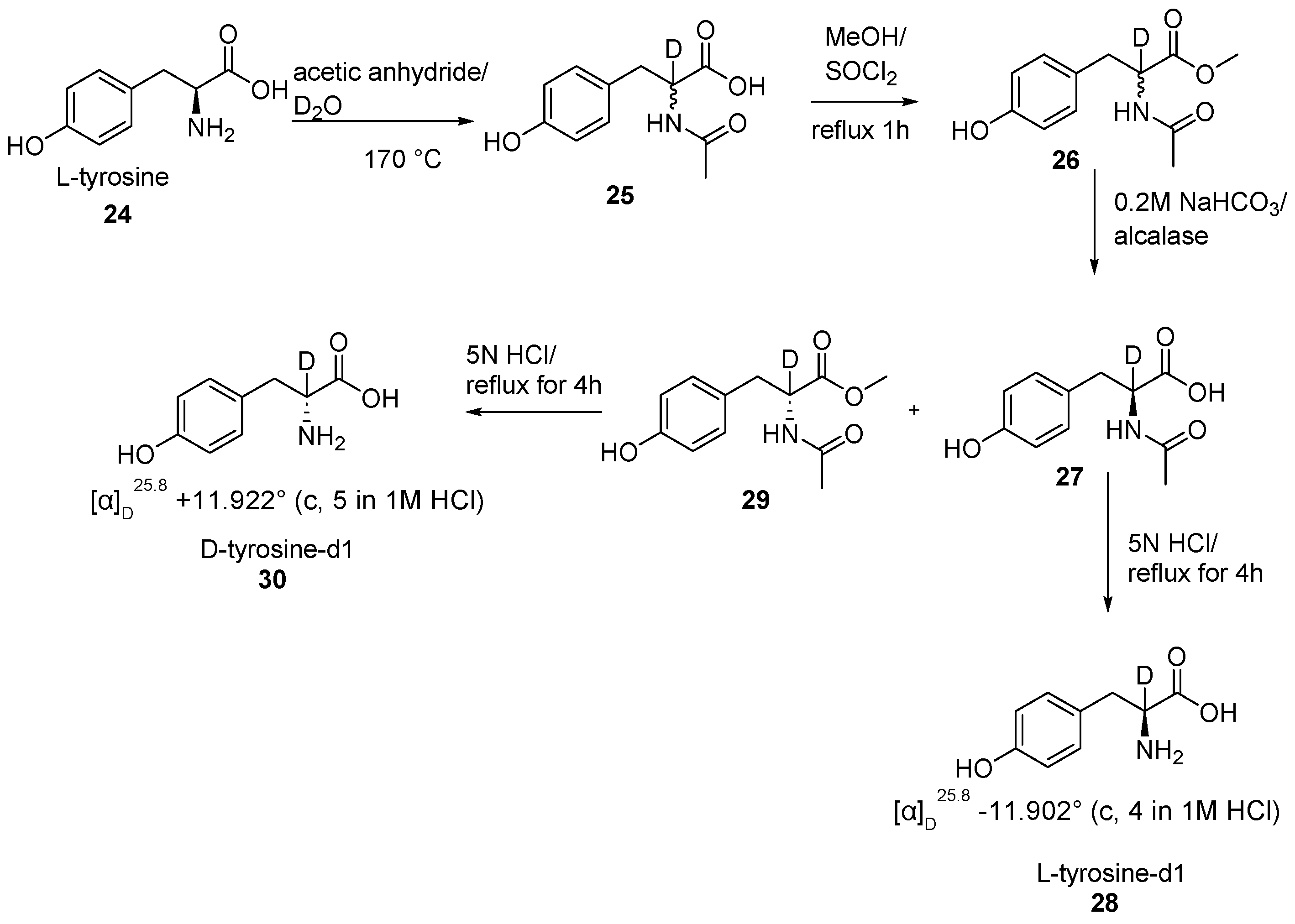
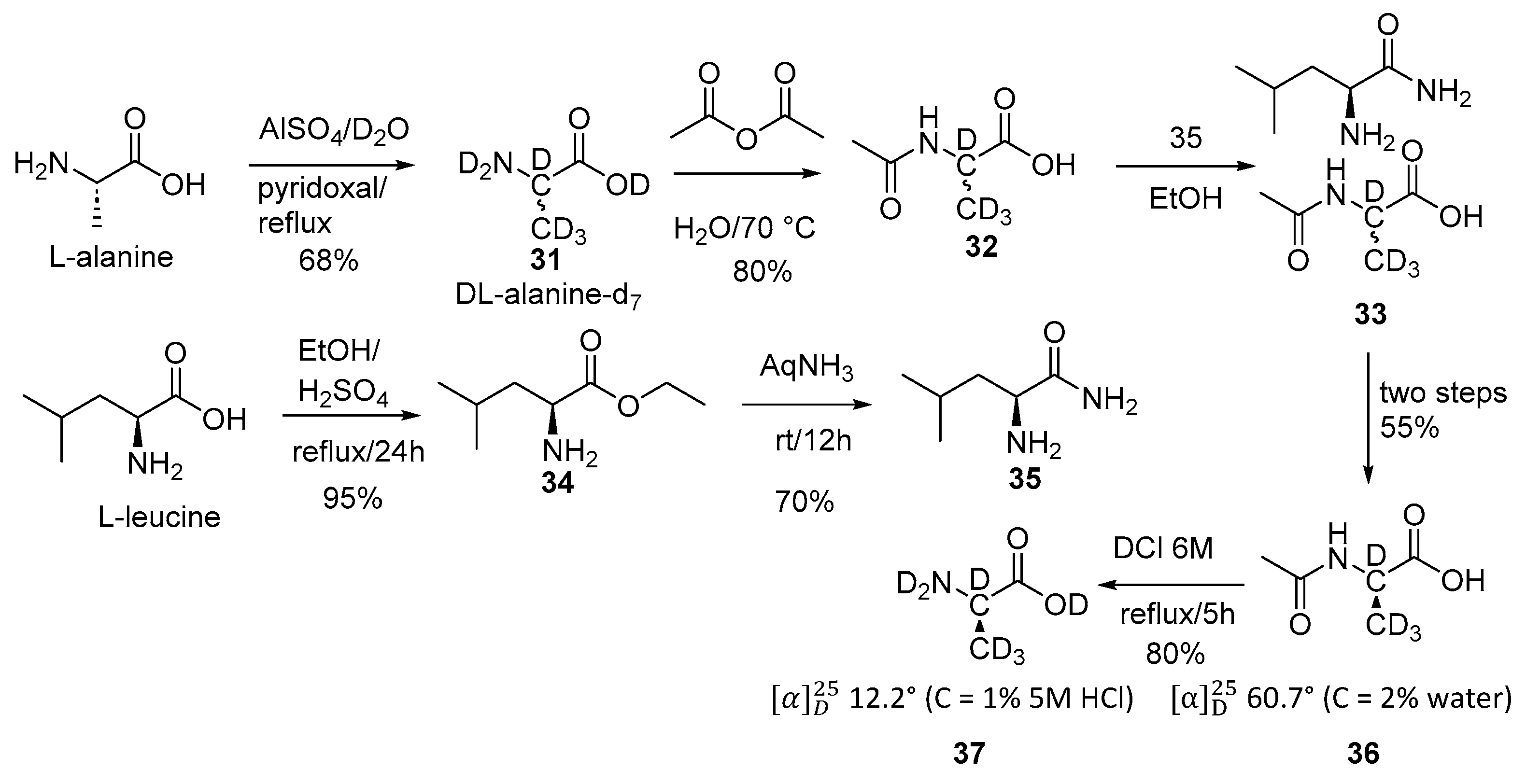
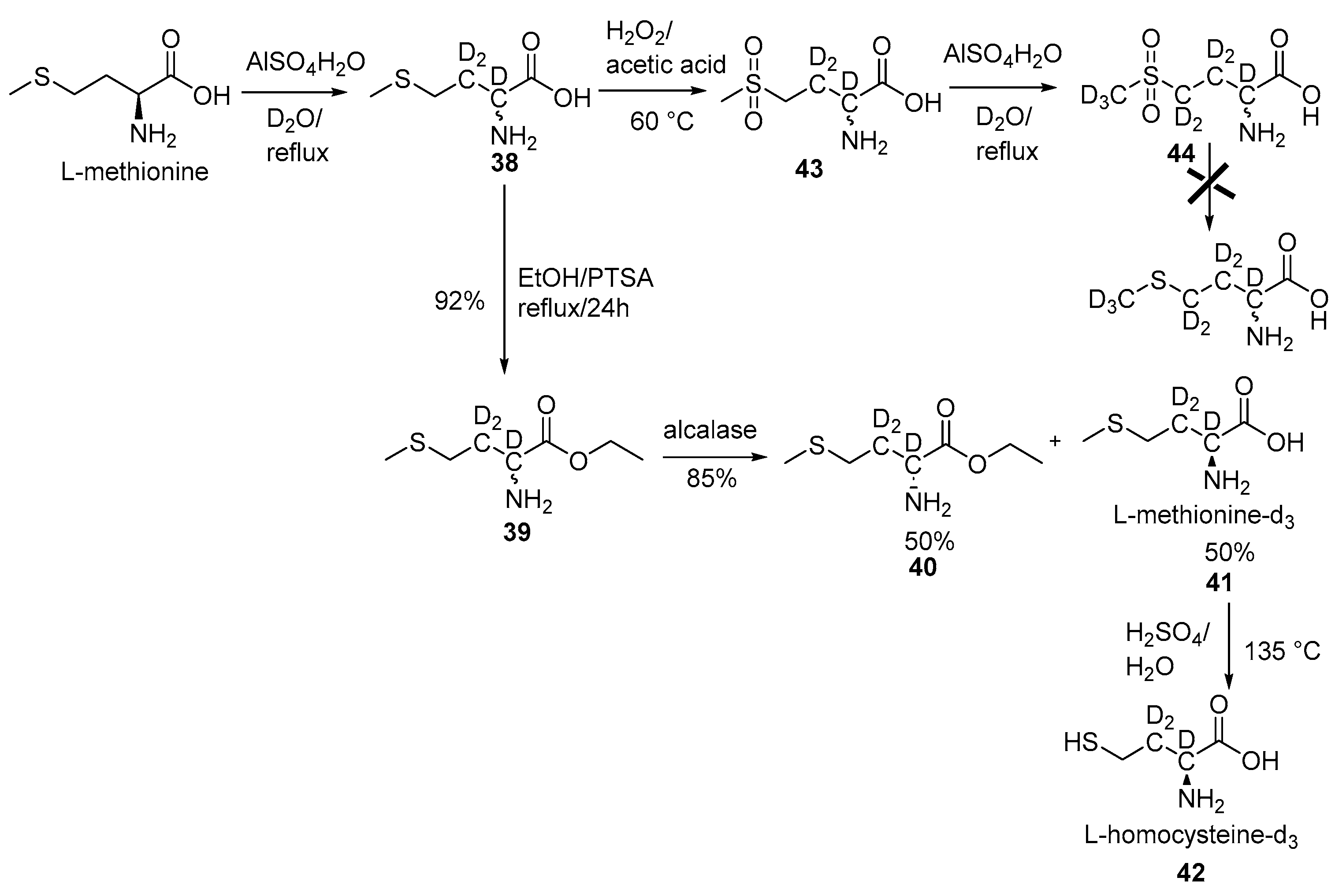
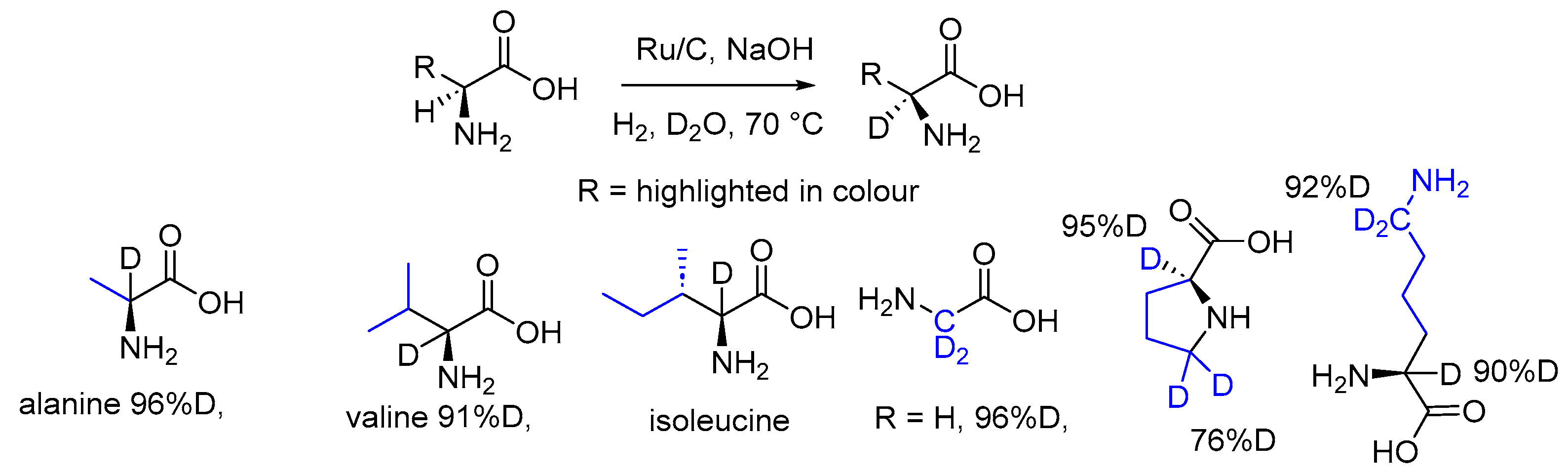
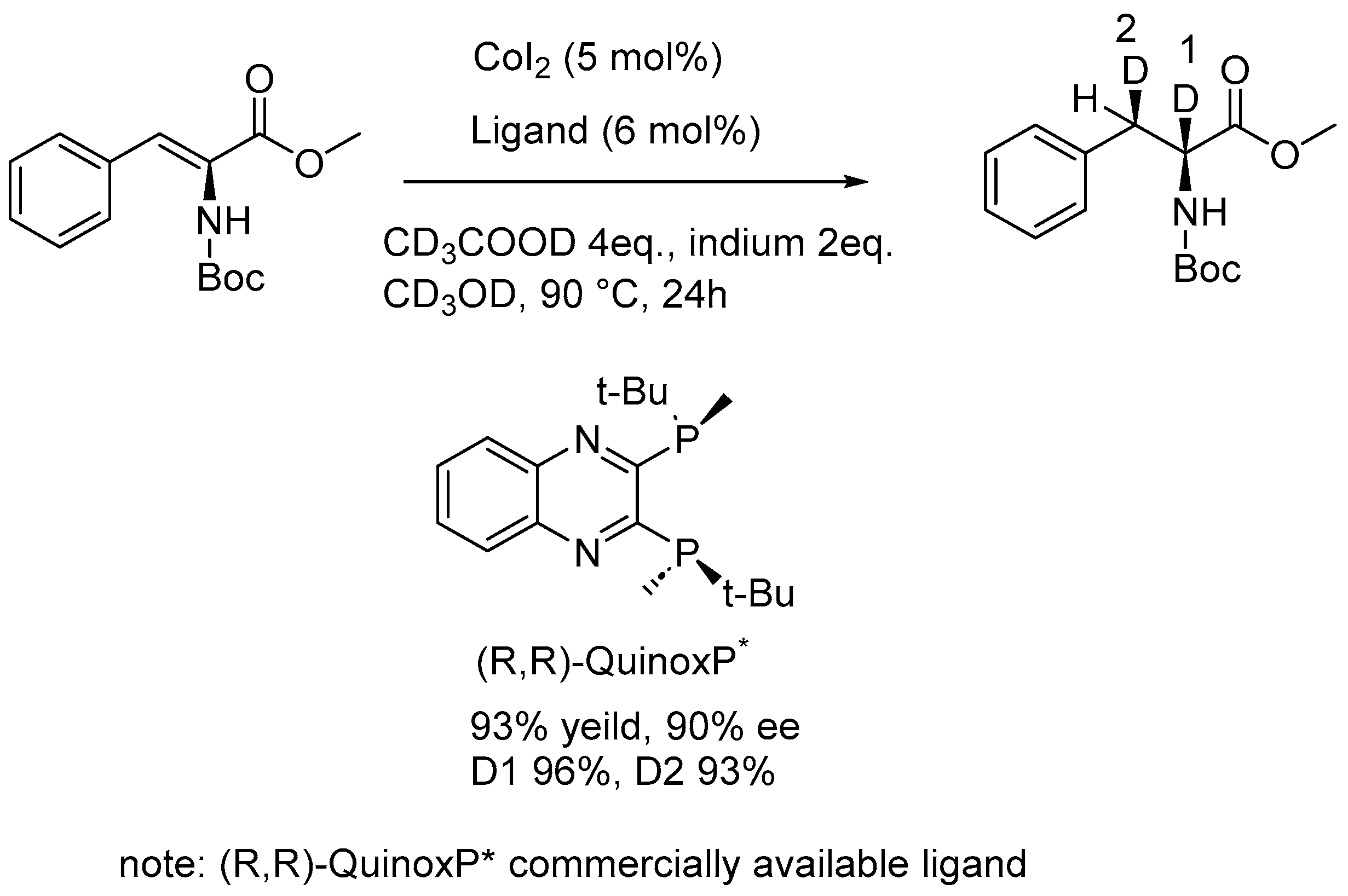
2.3. Specific Deuteration with Retention of Stereochemistry
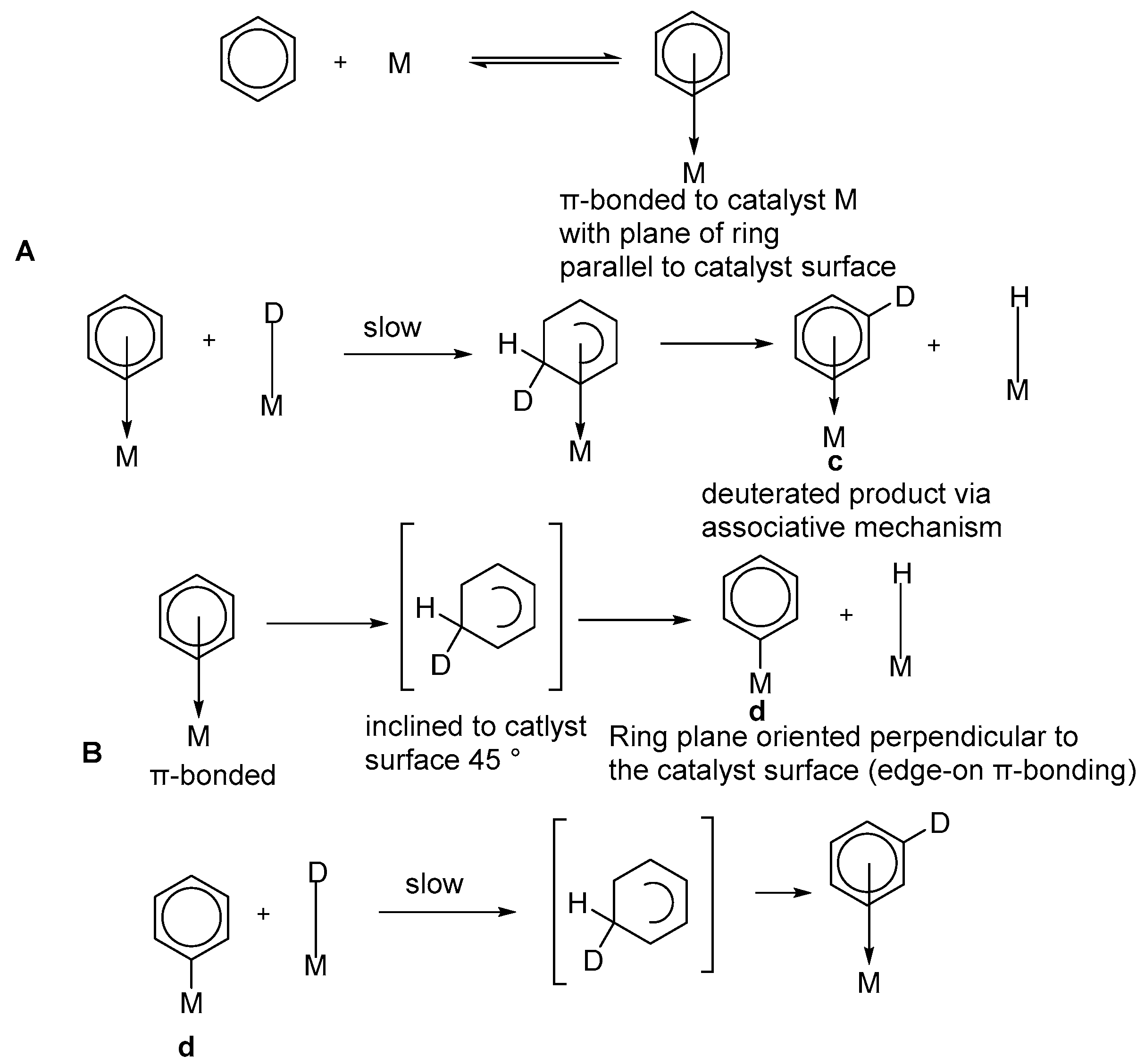
2.4. Metal Mediated Heterogenous Conditions Selective Direct Deuteration
3. Deuteration by Chemical Synthesis
Deuteration by Stereoselective Chemical Synthesis
| Method | Cost | Scalability | Stereochemistry/Site Specific | Applicability |
|---|---|---|---|---|
| Direct Deuteration (Acid/Base Catalyzed) | Low | High (with D2O) | Typically loses stereochemistry; challenges in site-selectivity without directing groups. | Broad applicability for many functional groups; effective for introducing multiple deuterium atoms. |
| Specific Deuteration (Acetic Anhydride/Acid/Aldehyde) | Low | High (with D2O) | Loss of stereochemistry but high regiospecificity. | Broad applicability for many functional groups. |
| Specific Deuteration (Pyridoxal/AlSO4) | Low | High (with D2O) | Loss of stereochemistry but high regiospecificity. | Not applicable to cysteine, serine, threonine, histidine, and tryptophan. |
| Metal-Mediated Hydrothermal Deuteration (Pt/C, Pd/C, PtO2) | Low | High (with D2O) | Loss of stereochemistry and less regiospecific. | Applicable to a limited number of amino acids like phenylalanine, tyrosine, and phenylglycine. |
| Ruthenium-Catalyzed Deuteration | High | Low to Medium | May or may not be stereoselective; high regiospecificity. | Broad applicability for many amino acids; not suitable for perdeuteration. |
| Metal or nonmetal ligand-Based Deuteration | High | Low | Highly stereoselective and highly regiospecific. | Broad applicability for many amino acids; not suitable for perdeuteration. |
| Chemical Synthesis | High | Low | May be stereoselective. | Broad applicability for many functional groups; good for introducing multiple deuterium atoms. |
| Stereoselective Chemical Synthesis (Chiral Auxiliary) | High | Low | Highly stereoselective and highly regiospecific. | Broad applicability for many amino acids, enabling both perdeuteration and site-specific labelling. |
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urey, H.C.; Brickwedde, F.G.; Murphy, G.M. A Hydrogen Isotope of Mass 2. Phys. Rev. 1932, 39, 164–165. [Google Scholar] [CrossRef]
- Nolin, B.; Jones, R.N. The Infrared Absorption Spectra of Diethyl Ketone and its Deuterium Substitution Products. J. Am. Chem. Soc. 1953, 75, 5626–5628. [Google Scholar] [CrossRef]
- Kindness, A.; McKean, D.C.; Stewart, D. Infrared intensities of CH stretching bands in partially deuterated compounds. and effects of conformation and substitution. J. Mol. Struct. 1990, 224, 363–384. [Google Scholar] [CrossRef]
- Wishart, D.S.; Sykes, B.D.; Richards, F.M. Improved synthetic methods for the selective deuteration of aromatic amino acids: Applications of selective protonation towards the identification of protein folding intermediates through nuclear magnetic resonance. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1993, 1164, 36–46. [Google Scholar] [CrossRef]
- Feeney, J.; Birdsall, B.; Ostler, G.; Carr, M.D.; Kairi, M. A novel method of preparing totally α-deuterated amino acids for selective incorporation into proteins: Application to assignment of 1H resonances of valine residues in dihydrofolate reductase. FEBS Lett. 1990, 272, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Remijnse, J.D.; van Bekkum, H.; Wepster, B.M. Proton magnetic resonance studies of specifically deuterated cyclohexane compounds. Part I.: Cyclohexane Methylcyclohexane. Recl. Trav. Chim. Pays-Bas 1970, 89, 658–666. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Zhang, S.; Ma, L.; Zeng, Z.; Zhou, Z.; Gandon, V.; Xu, H.; Yi, W.; Wang, S. Access to N-α-deuterated amino acids and DNA conjugates via Ca(II)-HFIP-mediated reductive deutero-amination of α-oxo-carbonyl compounds. Nat. Commun. 2025, 16, 1816. [Google Scholar] [CrossRef]
- MacLeod, J.K.; Djerassi, C. Mass spectrometry in structural and stereochemical problems CVIII. Deuterium isotope effects in site-specific mass spectrometric rearrangement processess. Tetrahedron Lett. 1966, 7, 2183–2187. [Google Scholar]
- Murphy, R.B.; Wyatt, N.A.; Fraser, B.H.; Yepuri, N.R.; Holden, P.J.; Wotherspoon, A.T.L.; Darwish, T.A. A rapid MS/MS method to assess the deuterium kinetic isotope effect and associated improvement in the metabolic stability of deuterated biological and pharmacological molecules as applied to an imaging agent. Anal. Chim. Acta 2019, 1064, 65–70. [Google Scholar] [CrossRef]
- Wang, L.; Murai, Y.; Yoshida, T.; Okamoto, M.; Masuda, K.; Sakihama, Y.; Hashidoko, Y.; Hatanaka, Y.; Hashimoto, M. Hydrogen/deuterium exchange of cross-linkable α-amino acid derivatives in deuterated triflic acid. Biosci. Biotechnol. Biochem. 2014, 78, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Petit-Haertlein, I.; Blakeley, M.P.; Howard, E.; Hazemann, I.; Mitschler, A.; Podjarny, A.; Haertlein, M. Incorporation of methyl-protonated valine and leucine residues into deuterated ocean pout type III antifreeze protein: Expression. crystallization and preliminary neutron diffraction studies. Acta Crystallogr. Sect. F 2010, 66, 665–669. [Google Scholar] [CrossRef]
- De Souza, J.M.; Freire, P.T.C.; Bordallo, H.N.; Argyriou, D.N. Structural isotopic effects in the smallest chiral amino acid: Observation of a structural phase transition in fully deuterated alanine. J. Phys. Chem. B 2007, 111, 5034–5039. [Google Scholar] [CrossRef] [PubMed]
- Arsene, C.G.; Ohlendorf, R.; Burkitt, W.; Pritchard, C.; Henrion, A.; O’Connor, G.; Bunk, D.M.; Guettler, B. Protein Quantification by Isotope Dilution Mass Spectrometry of Proteolytic Fragments: Cleavage Rate and Accuracy. Anal. Chem. 2008, 80, 4154–4160. [Google Scholar] [CrossRef]
- Bachor, R.; Setner, B.; Kluczyk, A.; Stefanowicz, P.; Szewczuk, Z. The unusual hydrogen-deuterium exchange of α-carbon protons in N-substituted glycine-containing peptides. J. Mass. Spectrom. 2014, 49, 43–49. [Google Scholar] [CrossRef]
- Hazen, S.L.; D’Avignon, A.; Anderson, M.M.; Hsu, F.F.; Heinecke, J.W. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to oxidize α-amino acids to a family of reactive aldehydes. Mechanistic studies identifying labile intermediates along the reaction pathway. J. Biol. Chem. 1998, 273, 4997–5005. [Google Scholar] [CrossRef]
- Blake, M.I.; Crespi, H.L.; Katz, J.J. Studies with deuterated drugs. J. Pharm. Sci. 1975, 64, 367–391. [Google Scholar] [CrossRef]
- Kakinuma, K.; Imamura, N.; Saba, Y. Facile synthesis of chiral glycine using D-glucose as a chiral template. Tetrahedron Lett. 1982, 23, 1697–1700. [Google Scholar] [CrossRef]
- Atzrodt, J.; Derdau, V.; Fey, T.; Zimmermann, J. The renaissance of H/D exchange. Angew. Chem. Int. Ed. 2007, 46, 7744–7765. [Google Scholar] [CrossRef]
- Li, H.; Shabbir, M.; Li, W.; Lei, A. Recent Advances in Deuteration Reactions. Chin. J. Chem. 2024, 42, 1145–1156. [Google Scholar] [CrossRef]
- Chanatry, J.A.; Schafer, P.H.; Kim, M.S.; Lemaster, D.M. Synthesis of α,β-Deuterated 15N Amino Acids Using a Coupled Glutamate Dehydrogenase-Branched-Chain Amino Acid Aminotransferase System. Anal. Biochem. 1993, 213, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, D.V.; Feeney, J.; Roberts, G.C.K.; Burgen, A.S.V. Preparation of selectively deuterated aromatic amino acids for use in 1H NMR studies of proteins. Biochim. Biophys. Acta (BBA) Protein Struct. 1976, 446, 479–485. [Google Scholar] [CrossRef]
- Stark, W.; Glucksman, M.J.; Makowski, L. Conformation of the coat protein of filamentous bacteriophage Pf1 determined by neutron diffraction from magnetically oriented gels of specifically deuterated virions. J. Mol. Biol. 1988, 199, 171–182. [Google Scholar] [CrossRef]
- Morgan, A.J.; Nguyen, S.; Uttamsingh, V.; Bridson, G.; Harbeson, S.; Tung, R.; Masse, C.E. Design and synthesis of deuterated boceprevir analogs with enhanced pharmacokinetic properties. J. Label. Compd. Radiopharm. 2011, 54, 613–624. [Google Scholar] [CrossRef]
- Nand, D.; Cukkemane, A.; Becker, S.; Baldus, M. Fractional deuteration applied to biomolecular solid-state NMR spectroscopy. J. Biomol. Nmr 2012, 52, 91–101. [Google Scholar] [CrossRef]
- Oba, M.; Iwasaki, A.; Hitokawa, H.; Ikegame, T.; Banba, H.; Ura, K.; Takamura, T.; Nishiyama, K. Preparation of L-serine and L-cystine stereospecifically labeled with deuterium at the β-position. Tetrahedron: Asymmetry 2006, 17, 1890–1894. [Google Scholar] [CrossRef]
- Malmlöf, T.; Rylander, D.; Alken, R.-G.; Schneider, F.; Svensson, T.H.; Cenci, M.A.; Schilström, B. Deuterium substitutions in the L-DOPA molecule improve its anti-akinetic potency without increasing dyskinesias. Exp. Neurol. 2010, 225, 408–415. [Google Scholar] [CrossRef]
- Kržan, M.; Keuschler, J.; Mavri, J.; Vianello, R. Relevance of Hydrogen Bonds for the Histamine H2 Receptor-Ligand Interactions: A Lesson from Deuteration. Biomolecules 2020, 10, 196. [Google Scholar] [CrossRef]
- Hok, L.; Mavri, J.; Vianello, R. The Effect of Deuteration on the H2 Receptor Histamine Binding Profile: A Computational Insight into Modified Hydrogen Bonding Interactions. Molecules 2020, 25, 6017. [Google Scholar] [CrossRef]
- Oksanen, E.; Chen, J.C.-H.; Fisher, S.Z. Neutron Crystallography for the Study of Hydrogen Bonds in Macromolecules. Molecules 2017, 22, 596. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Adachi, M.; Sugimoto, Y. A simple protocol for controlling protein deuteration for neutron scattering. Protein Expr. Purif. 2025, 233, 106749. [Google Scholar] [CrossRef]
- Kainosho, M.; Torizawa, T.; Iwashita, Y.; Terauchi, T.; Mei Ono, A.; Güntert, P. Optimal isotope labelling for NMR protein structure determinations. Nature 2006, 440, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Takeda, R.; Abe, H.; Shibata, N.; Moriwaki, H.; Izawa, K.; Soloshonok, V.A. Asymmetric synthesis of α-deuterated α-amino acids. Org. Biomol. Chem. 2017, 15, 6978–6983. [Google Scholar] [CrossRef] [PubMed]
- Kokel, A.; Kadish, D.; Török, B. Preparation of Deuterium Labeled Compounds by Pd/C-Al-D2O Facilitated Selective H-D Exchange Reactions. Molecules 2022, 27, 614. [Google Scholar] [CrossRef]
- Sucholeiki, I. Preparation of Substituted Tryptophan Derivatives as Inhibitors of Matrix Metalloproteinases for the Treatment of Pain and Other Diseases. WO2010075287A2, 1 July 2010. [Google Scholar]
- Putter, I. Selective Deuteration of Tyrosine and Aspartic and Glutamic Acids; US3699158A; Merck and Co., Inc.: Rahway, NJ, USA, 1972; 7p. [Google Scholar]
- Grabowski, E.J.J.; Reider, P.J. 2-Deutero-D-Serine. U.S. Patent US4582931A, 24 December 1984. [Google Scholar]
- Etezady-Esfarjani, T.; Wuethrich, K. Cell-Free Protein Synthesis of Deuterated Proteins and Peptides for Structural and Functional Studies by NMR Spectroscopy. EP1995324A1, 26 November 2008. [Google Scholar]
- Brown, J.M.; Homans, S.W.; Chaykovsky, M.; Murray, J.H. Synthesis of Sidechain-Deuterated Amino Acids for NMR Studies of Peptides and Proteins WO2005037853A2, 10 December 2009.
- Czarnik, A.W. Deuterium-Enriched Clopidogrel for Treating Coronary Artery Disease, Peripheral Vascular Disease, and Cerebrovascular Disease. Chemical Indexing Equivalent to 150:64024 (WO); Protia LLC: Reno, NV, USA, 2008; 11p. [Google Scholar]
- Thanassi, J.W. General procedure for the preparation of deuterated and tritiated amino acids by incorporation of solvent isotope during synthesis. J. Org. Chem. 1971, 36, 3019–3021. [Google Scholar] [CrossRef] [PubMed]
- Upson, D.A.; Hruby, V.J. A general method for the preparation of α-labeled amino acids. J. Org. Chem. 1977, 42, 2329–2330. [Google Scholar] [CrossRef]
- Tikhonov, V.E.; Yamskov, I.A.; Bakhmutov, V.I.; Tsyryapkin, V.A.; Davankov, V.A. Catalytic method for preparing deuterated amino acids. Bioorganicheskaia Khimiia 1985, 11, 149–152. [Google Scholar]
- LeMaster, D.M.; Richards, F.M. A general procedure for the preparation of α,β-labeled amino acids. J. Label. Compd. Radiopharm. 1982, 19, 639–646. [Google Scholar] [CrossRef]
- Chen, S.T.; Tu, C.C.; Wang, K.T. Facile preparation of optically pure [α-2H]-α-amino acids. Biotechnol. Lett. 1992, 14, 269–274. [Google Scholar] [CrossRef]
- Van Heyningen, W.E.; Rittenberg, D.; Schoenheimer, R. The preparation of fatty acids containing deuterium. J. Biol. Chem. 1938, 125, 495–500. [Google Scholar] [CrossRef]
- Hsiao, C.Y.Y.; Ottaway, C.A.; Wetlaufer, D.B. Preparation of fully deuterated fatty acids by simple method. Lipids 1974, 9, 913–915. [Google Scholar] [CrossRef]
- Kalpala, J.; Hartonen, K.; Huhdanpaa, M.; Riekkola, M.-L. Deuteration of 2-methylnaphthalene and eugenol in supercritical and pressurised hot deuterium oxide. Green. Chem. 2003, 5, 670–676. [Google Scholar] [CrossRef]
- Boix, C.; Poliakoff, M. Efficient H-D exchange of aromatic compounds in near-critical D2O catalysed by a polymer-supported sulphonic acid. Tetrahedron Lett. 1999, 40, 4433–4436. [Google Scholar] [CrossRef]
- Junk, T.; Catallo, W.J. Preparative supercritical deuterium exchange in arenes and heteroarenes. Tetrahedron Lett. 1996, 37, 3445–3448. [Google Scholar] [CrossRef]
- Yang, Y.; Evilia, R.F. Deuteration of amino acids in basic deuterium oxide solution at supercritical temperatures. J. Supercrit. Fluids 1996, 9, 113–117. [Google Scholar] [CrossRef]
- Matthews, H.R.; Matthews, K.S.; Opella, S.J. Selectively deuterated amino acid analogues synthesis. Incorporation into proteins and NMR properties. Biochim. Biophys. Acta (BBA) Gen. Subj. 1977, 497, 1–13. [Google Scholar] [CrossRef]
- Dalamaga, M. Clinical metabolomics: Useful insights, perspectives and challenges. Metab. Open 2024, 22, 100290. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Wang, K.T.; Wong, C.H. Chirally selective hydrolysis of D,L-amino acid esters by alkaline protease. J. Chem. Soc. Chem. Commun. 1986, 20, 1514–1516. [Google Scholar] [CrossRef]
- Mitulovi, G.; Lammerhofer, M.; Maier, N.M.; Lindner, W. Simple and efficient preparation of (R)- and (S)-enantiomers of α-carbon deuterium-labeled α-amino acids. J. Label. Compd. Radiopharm. 2000, 43, 449–461. [Google Scholar] [CrossRef]
- Adam, M.J.; Ponce, Y.Z.; Berry, J.M.; Hoy, K. Synthesis and preliminary evaluation of L-6[123I]iododopa as a potential SPECT brain imaging agent. J. Label. Compd. Radiopharm. 1990, 28, 155–166. [Google Scholar] [CrossRef]
- Laumen, K.; Ghisalba, O. Easy access to enantiomerically pure nonproteinogenic amino acids. Eng. Life Sci. 2006, 6, 193–194. [Google Scholar] [CrossRef]
- Smith, K.C.; White, R.L.; Le, Y.; Vining, L.C. Isolation of N-Acetyl-3,4-dihydroxy-L-phenylalanine from Streptomyces akiyoshiensis. J. Nat. Prod. 1995, 58, 1274–1277. [Google Scholar] [CrossRef]
- Ishiyama, T.; Murata, M.; Miyaura, N. Palladium(0)-Catalyzed Cross-Coupling Reaction of Alkoxydiboron with Haloarenes: A Direct Procedure for Arylboronic Esters. J. Org. Chem. 1995, 60, 7508–7510. [Google Scholar] [CrossRef]
- Xu, J.; Sinelnikov, R.; Huang, Y. Capturing Guest Dynamics in Metal-Organic Framework CPO-27-M (M = Mg. Zn) by (2)H Solid-State NMR Spectroscopy. Langmuir 2016, 32, 5468–5479. [Google Scholar] [CrossRef] [PubMed]
- Khudozhitkov, A.E.; Toktarev, A.V.; Arzumanov, S.S.; Gabrienko, A.A.; Kolokolov, D.I.; Stepanov, A.G. (2) H Solid-State NMR Spectroscopy Reveals the Dynamics of a Pyridine Probe Interacting with Coordinatively Unsaturated Metal Sites of MIL-100(Al) Metal-Organic Frameworks. Chemistry 2019, 25, 10808–10812. [Google Scholar] [CrossRef]
- Boyle, T.P.; Bremner, J.B.; Coates, J.; Deadman, J.; Keller, P.A.; Pyne, S.G.; Rhodes, D.I. New cyclic peptides via ring-closing metathesis reactions and their anti-bacterial activities. Tetrahedron 2008, 64, 11270–11290. [Google Scholar] [CrossRef]
- Tenenbaum, S.W.; Witherup, T.H.; Abbott, E.H. Selectivity in vitamin B6 model reactions. Selective α or β deuteration of amino acids under transamination or racemization conditions. Biochim. Biophys. Acta Gen. Subj. 1974, 362, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Abbott, E.H.; Martell, A.E. Nuclear magnetic resonance investigation of the metal ion and proton-catalyzed reaction of some vitamin B6 Schiff bases. J. Am. Chem. Soc. 1969, 91, 6931–6939. [Google Scholar] [CrossRef]
- Kato, T.; Tsuchiya, Y. Resolution of N-acylamino acids with optically active neutral amino acid amides. Agric. Biol. Chem. 1962, 26, 467–472. [Google Scholar] [CrossRef]
- Calf, G.; Garnett, J. Catalytic deuterium exchange reactions with organics. XXIII. Deformylation during the deuteration of aromatic α-hydroxy acids and related compounds. Aust. J. Chem. 1966, 19, 211–220. [Google Scholar] [CrossRef]
- Woodworth, R.C.; Dobson, C.M. Selective substitution of deuterium and tritium into aromatic amino acids catalyzed by Raney nickel. FEBS Lett. 1979, 101, 329–332. [Google Scholar] [CrossRef]
- Taglang, C.; Martinez-Prieto, L.M.; del Rosal, I.; Maron, L.; Poteau, R.; Philippot, K.; Chaudret, B.; Perato, S.; Sam Lone, A.; Puente, C.; et al. Enantiospecific C-H activation using ruthenium nanocatalysts. Angew. Chem. Int. Ed. 2015, 54, 10474–10477. [Google Scholar] [CrossRef]
- Shao, F.; Wang, K.; Jiang, W.; Xu, Z.; Li, X.; Zhong, X.; Wang, J. Phytic Acid-Modulated Ru Catalyzes Regioselective Deuteration of 1,6-Hexamethylenediamine. ACS Catal. 2023, 13, 15746–15757. [Google Scholar] [CrossRef]
- Moozeh, K.; So, S.M.; Chin, J. Catalytic Stereoinversion of L-Alanine to Deuterated D-Alanine. Angew. Chem. 2015, 127, 9513–9517. [Google Scholar] [CrossRef]
- Michelotti, A.; Rodrigues, F.; Roche, M. Development and Scale-Up of Stereoretentive α-Deuteration of Amines. Org. Process Res. Dev. 2017, 21, 1741–1744. [Google Scholar] [CrossRef]
- Darwish, T.A.; Yepuri, N.R.; Holden, P.J.; James, M. Quantitative analysis of deuterium using the isotopic effect on quaternary 13C NMR chemical shifts. Anal. Chim. Acta 2016, 927, 89–98. [Google Scholar] [CrossRef]
- Lockwood, T.E.; Angeloski, A. DGet! An open source deuteration calculator for mass spectrometry data. J. Cheminform. 2024, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Winnicka, E.; Kanska, M. Synthesis of L-tryptophan labeled with hydrogen isotopes in the indole ring. J. Radioanal. Nucl. Chem. 2009, 279, 675–678. [Google Scholar] [CrossRef]
- Kanska, M.; Dragulska, S.; Pajak, M.; Palka, K.; Winnicka, E. Isotope effects in the hydroxylation of L-tyrosine catalyzed by tyrosinase. J. Radioanal. Nucl. Chem. 2015, 305, 371–378. [Google Scholar] [CrossRef]
- Maegawa, T.; Akashi, A.; Esaki, H.; Aoki, F.; Sajiki, H.; Hirota, K. Efficient and selective deuteration of phenylalanine derivatives catalyzed by Pd/C. Synlett 2005, 2005, 845–847. [Google Scholar] [CrossRef]
- Sheng, F.-F.; Gu, J.-G.; Liu, K.-H.; Zhang, H.-H. Synthesis of β-Deuterated Amino Acids via Palladium-Catalyzed H/D Exchange. J. Org. Chem. 2022, 87, 16084–16089. [Google Scholar] [CrossRef]
- Li, A.; Song, X.; Ren, Q.; Bao, P.; Long, X.; Huang, F.; Yuan, L.; Zhou, J.S.; Qin, X. Cobalt-Catalyzed Asymmetric Deuteration of α-Amidoacrylates for Stereoselective Synthesis of α,β-Dideuterated α-Amino Acids. Angew. Chem. Int. Ed. 2023, 62, e202301091. [Google Scholar] [CrossRef]
- Chatterjee, B.; Krishnakumar, V.; Gunanathan, C. Selective α-Deuteration of Amines and Amino Acids Using D2O. Org. Lett. 2016, 18, 5892–5895. [Google Scholar] [CrossRef]
- Blomquist, A.T.; Cedergren, R.J. Deuterated amino acids. IV. Synthesis of L-phenyl-d5-alanine-2,3,3-d3. Can. J. Chem. 1968, 46, 1053–1056. [Google Scholar] [CrossRef]
- Blomquist, A.T.; Cedergren, R.J.; Hiscock, B.F.; Tripp, S.L.; Harpp, D.N. Synthesis of highly deuterated amino acids. Proc. Natl. Acad. Sci. USA 1966, 55, 453–456. [Google Scholar] [CrossRef]
- O’Reilly, E.; Balducci, D.; Paradisi, F. A stereoselective synthesis of α-deuterium-labeled (S)-α-amino acids. Amino Acids 2010, 39, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Barraclough, P.; Dieterich, P.; Spray, C.A.; Young, D.W. Two separate and distinct syntheses of stereospecifically deuterated samples of (2S)-proline. Org. Biomol. Chem. 2006, 4, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.E.; Leeson, P.D.; Gani, D. Stereospecific synthesis of α-deuteriated α-amino acids: Regiospecific deuteration of chiral 3-isopropyl-2,5-dimethoxy-3,6-dihydropyrazines. J. Chem. Soc. Perkin Trans. 1995, 1, 157–165. [Google Scholar] [CrossRef]
- Rose, J.E.; Leeson, P.D.; Gani, D. Regiospecific deuteration of chiral 3-isopropyl-2,5-dimethoxy-3,6-dihydropyrazines in the stereospecific synthesis of α-deuteriated α-amino acids. J. Chem. Society. Perkin Trans. 1992, 1, 1563–1565. [Google Scholar] [CrossRef]
- Maeda, Y.; Tago, K.; Eguchi, T.; Kakinuma, K. Versatile synthon for chirally β-deuterated L-amino acids and synthesis of (3R)-and (3S)-[3-2H1]-l-serine. Biosci. Biotechnol. Biochem. 1996, 60, 1248–1254. [Google Scholar] [CrossRef]
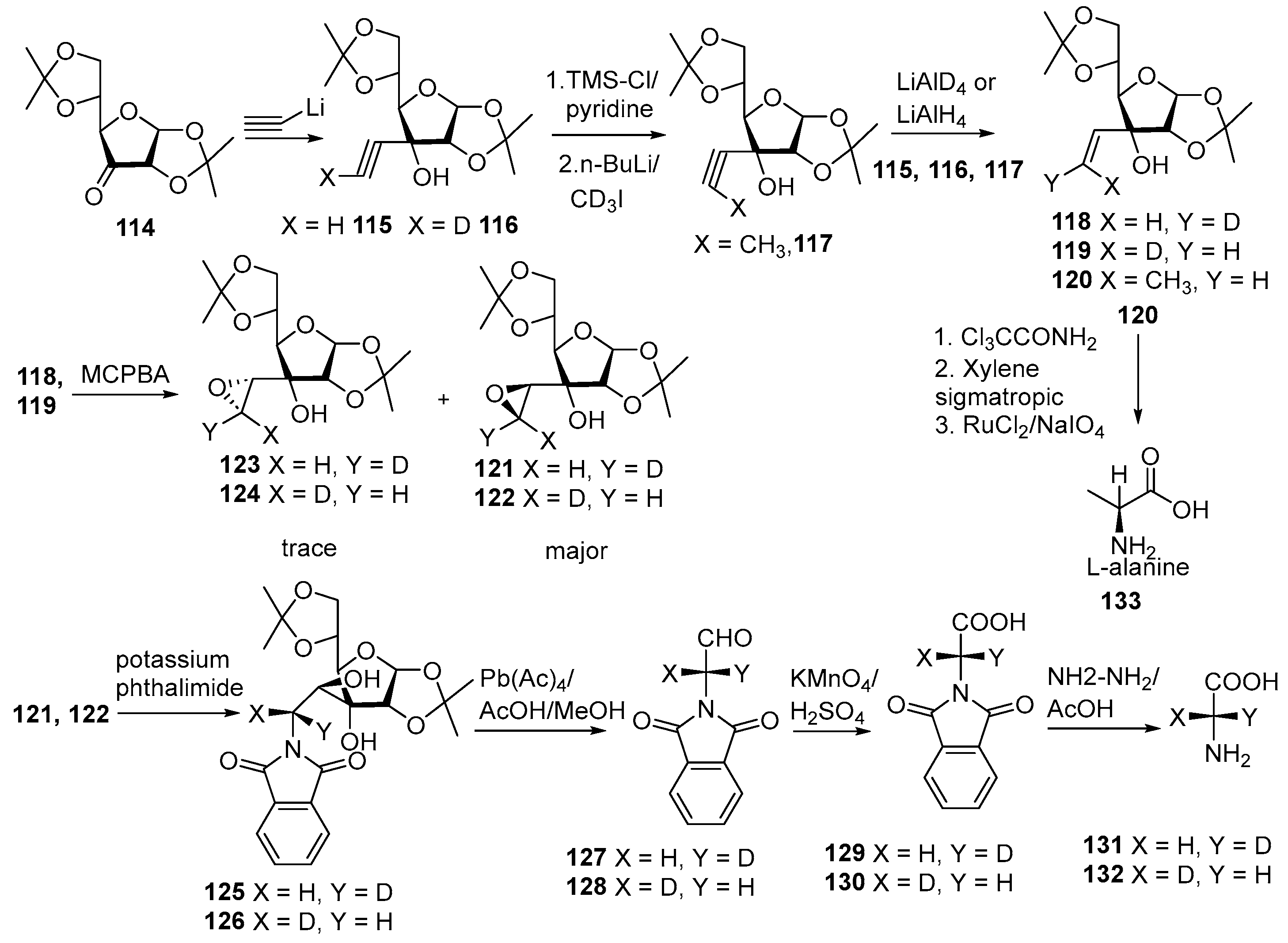
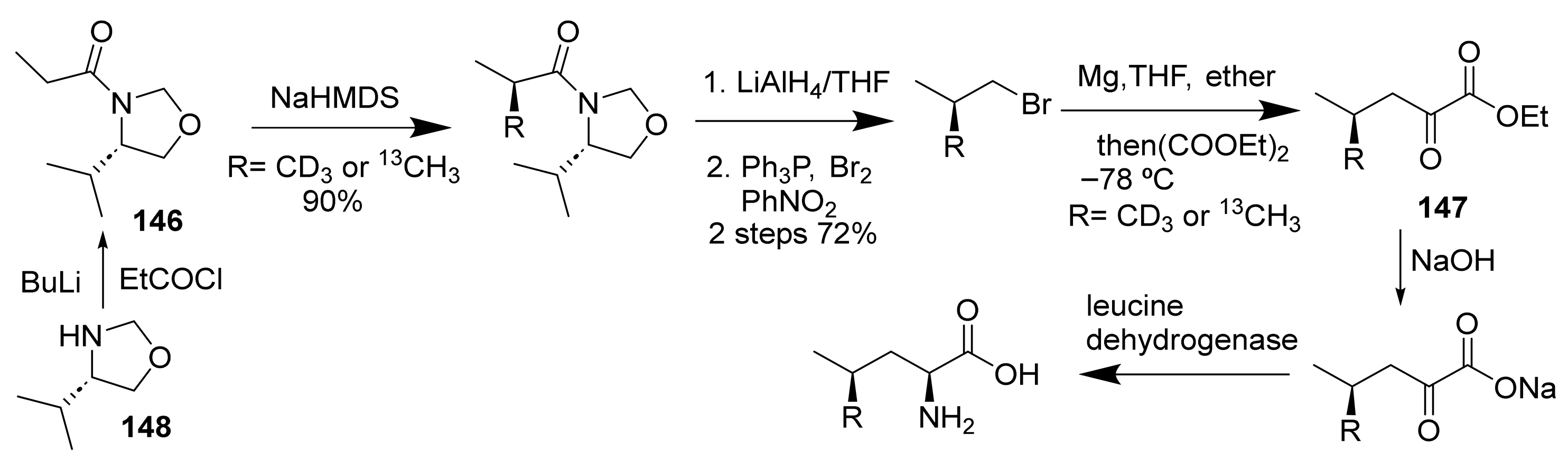
- Kakinuma, K.; Koudate, T.; Li, H.-Y.; Eguchi, T. Enantiocontrol by intrinsic antiparallel double repulsion on diacetone-D-glucose template. Enantioselective synthesis of alanine and chirally deuterated glycine. Tetrahedron Lett. 1991, 32, 5801–5804. [Google Scholar] [CrossRef]
- Kelly, N.M.; Reid, R.G.; Willis, C.L.; Winton, P.L. Methods for the Synthesis of L-Leucine Selectively Labelled with Carbon-13 or Deuterium in either Diastereotopic Methyl Group. Tetrahedron Lett. 1995, 36, 8315–8318. [Google Scholar] [CrossRef]
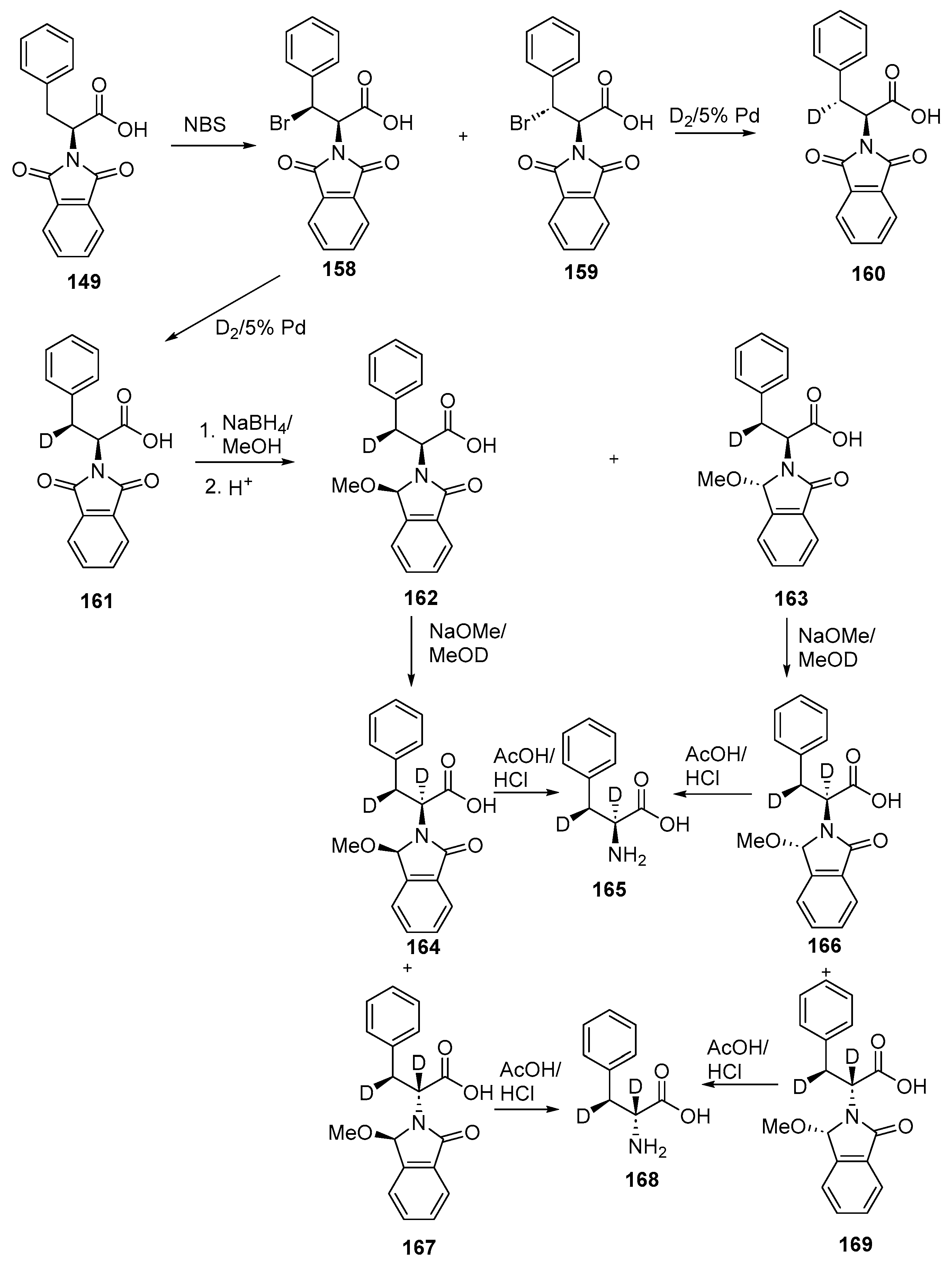
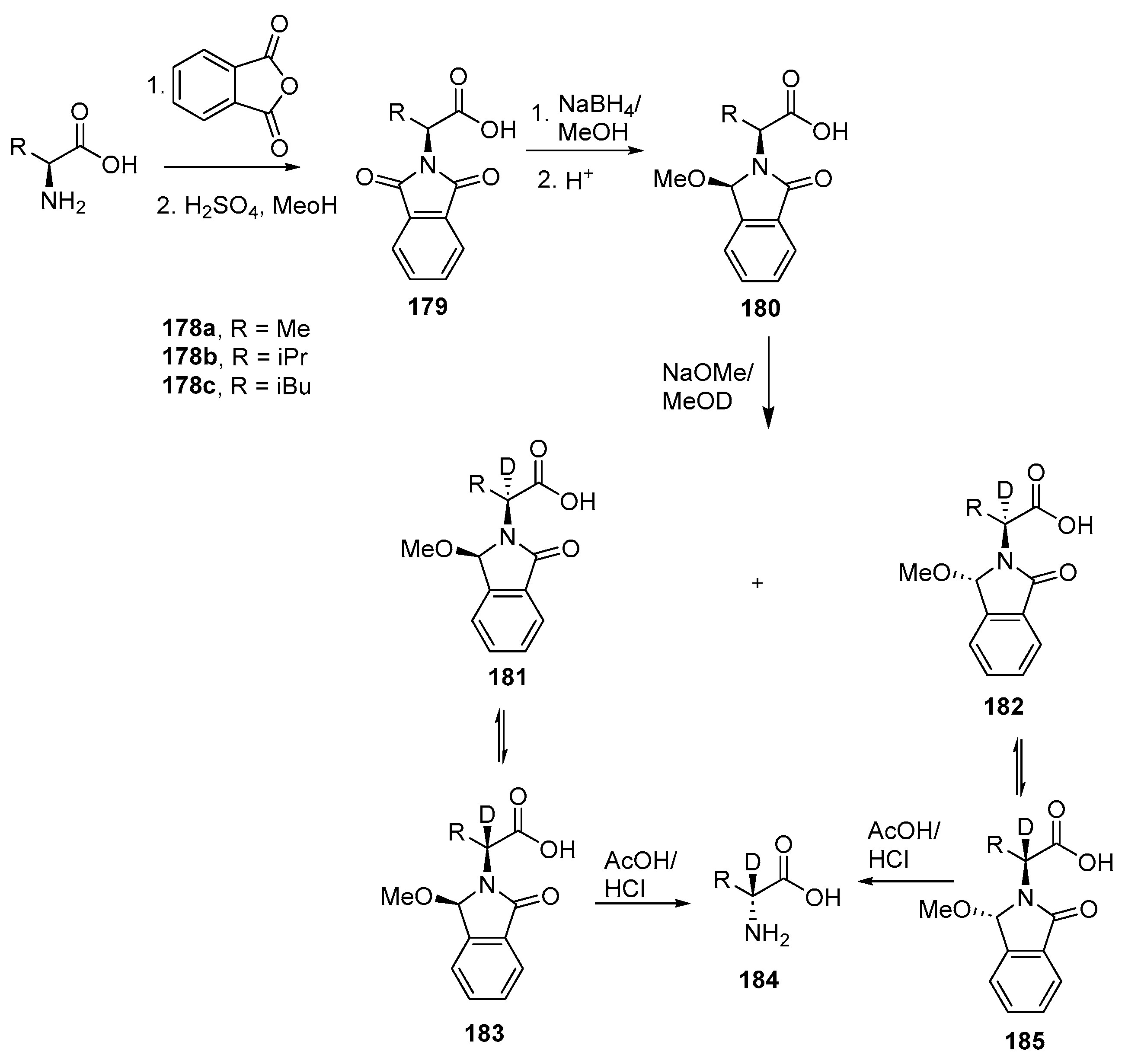
- Easton, C.J.; Fryer, N.L.; Kelly, J.B.; Kociuba, K. Stereocontrolled synthesis of deuterated phenylalanine derivatives through manipulation of an N-phthaloyl protecting group for the recall of stereochemistry. Application in the study of phenylalanine ammonia lyase. ARKIVOC 2001, 2001, 63–76. [Google Scholar] [CrossRef]
- Oba, M.; Terauchi, T.; Hashimoto, J.; Tanaka, T.; Nishiyama, K. Stereoselective synthesis of (2S,3S,4R,5S)-proline-3,4,5-d3. Tetrahedron Lett. 1997, 38, 5515–5518. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.H.; Kim, D.; Kim, Y.; Kim, S.; Kim, S. Simple and Efficient Enantioselective α-Deuteration Method of α-Amino Acids without External Chiral Sources. JACS Au 2024, 4, 2246–2251. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Shinohara, Y.; Tagoku, K.; Hashimoto, T. Synthesis of L-[3,3,4,4,S-methyl-2H7]methionine for use as a substrate for the methionine loading test. J. Label. Compd. Radiopharm. 2001, 44, 21–30. [Google Scholar] [CrossRef]
- Dippe, M.; Brandt, W.; Rost, H.; Porzel, A.; Schmidt, J.; Wessjohann, L.A. Rationally engineered variants of S-adenosylmethionine (SAM) synthase: Reduced product inhibition and synthesis of artificial cofactor homologues. Chem. Commun. 2015, 51, 3637–3640. [Google Scholar] [CrossRef] [PubMed]

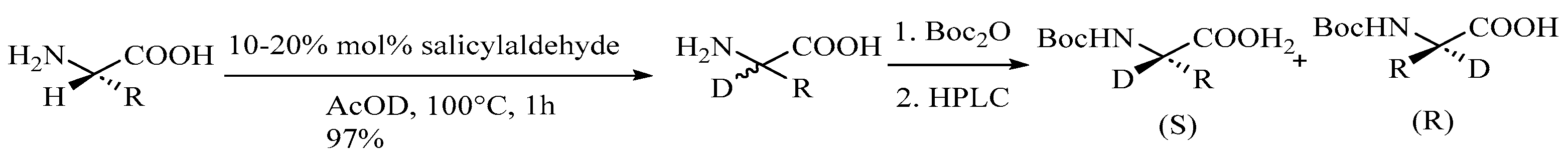







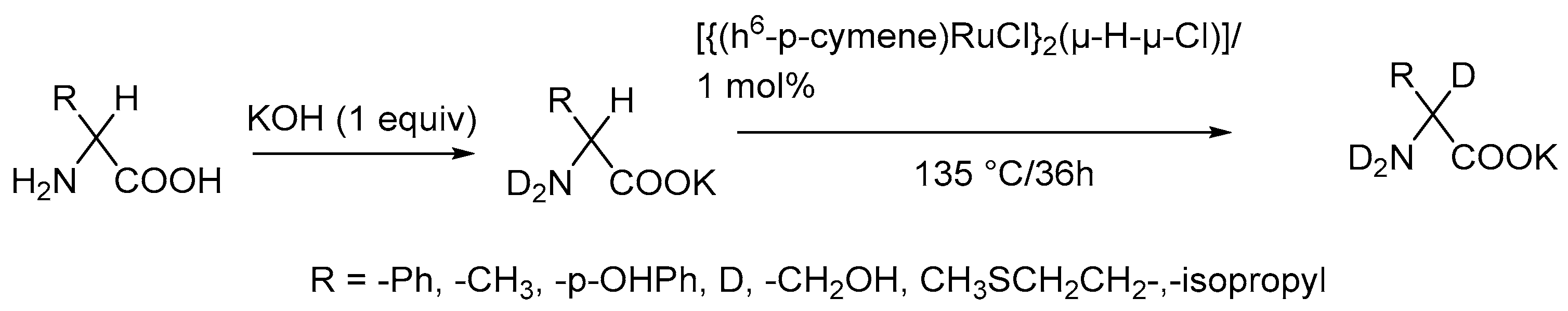
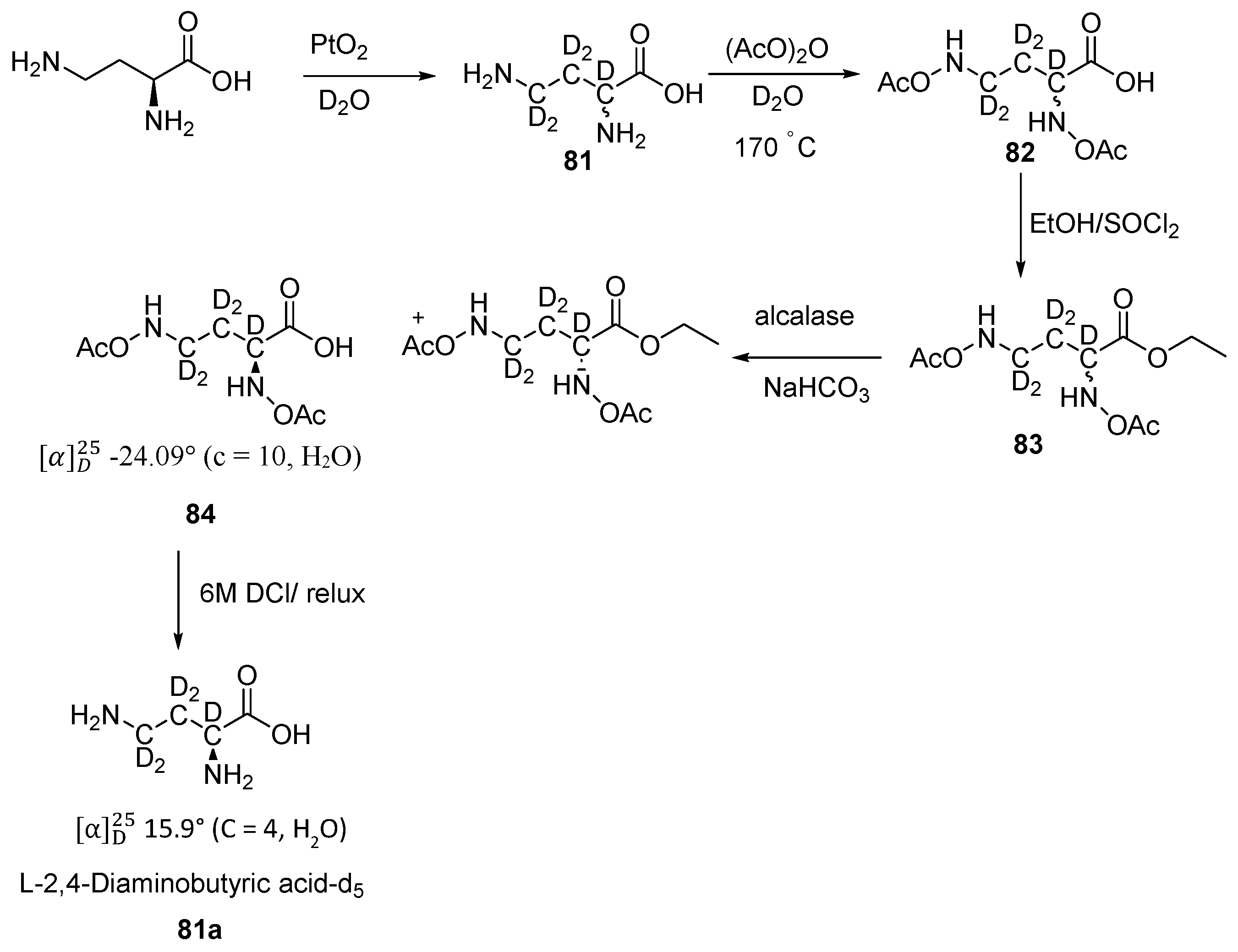
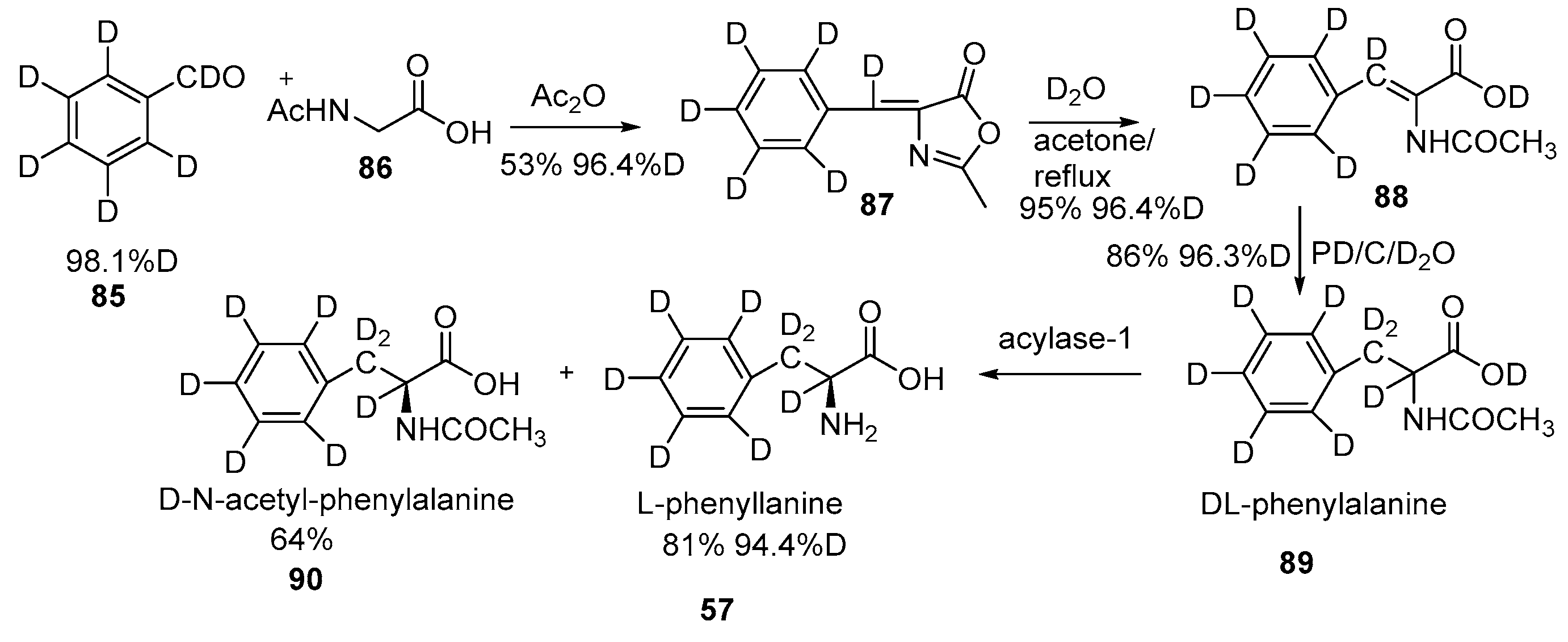
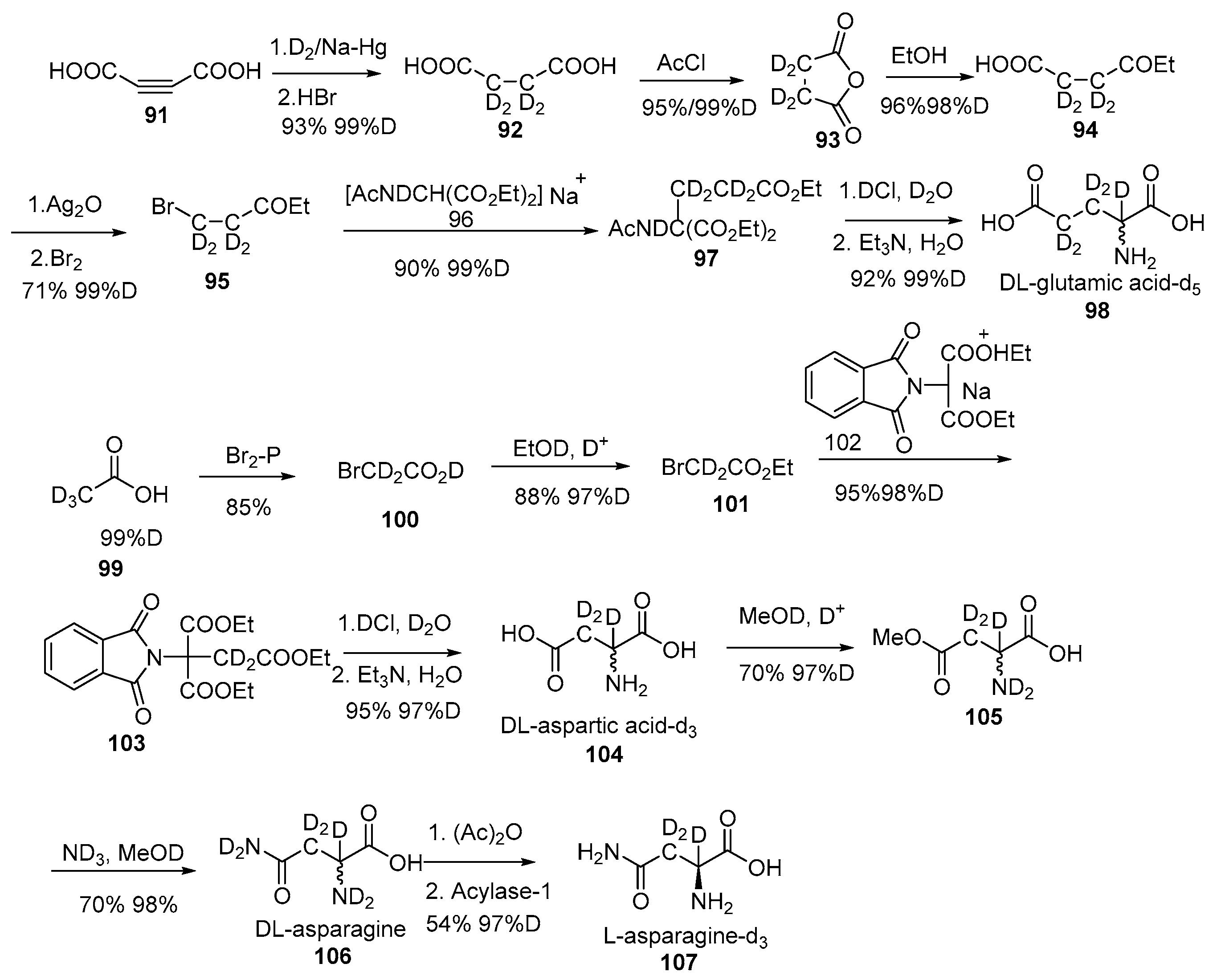
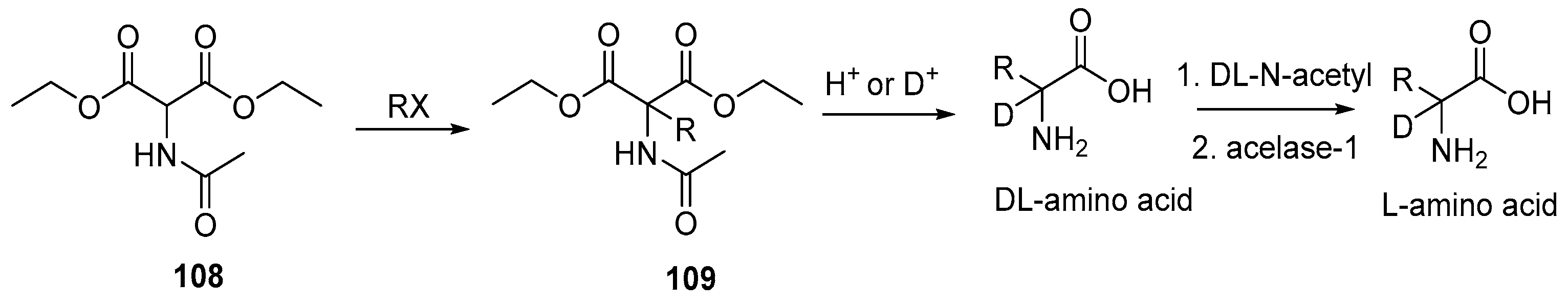





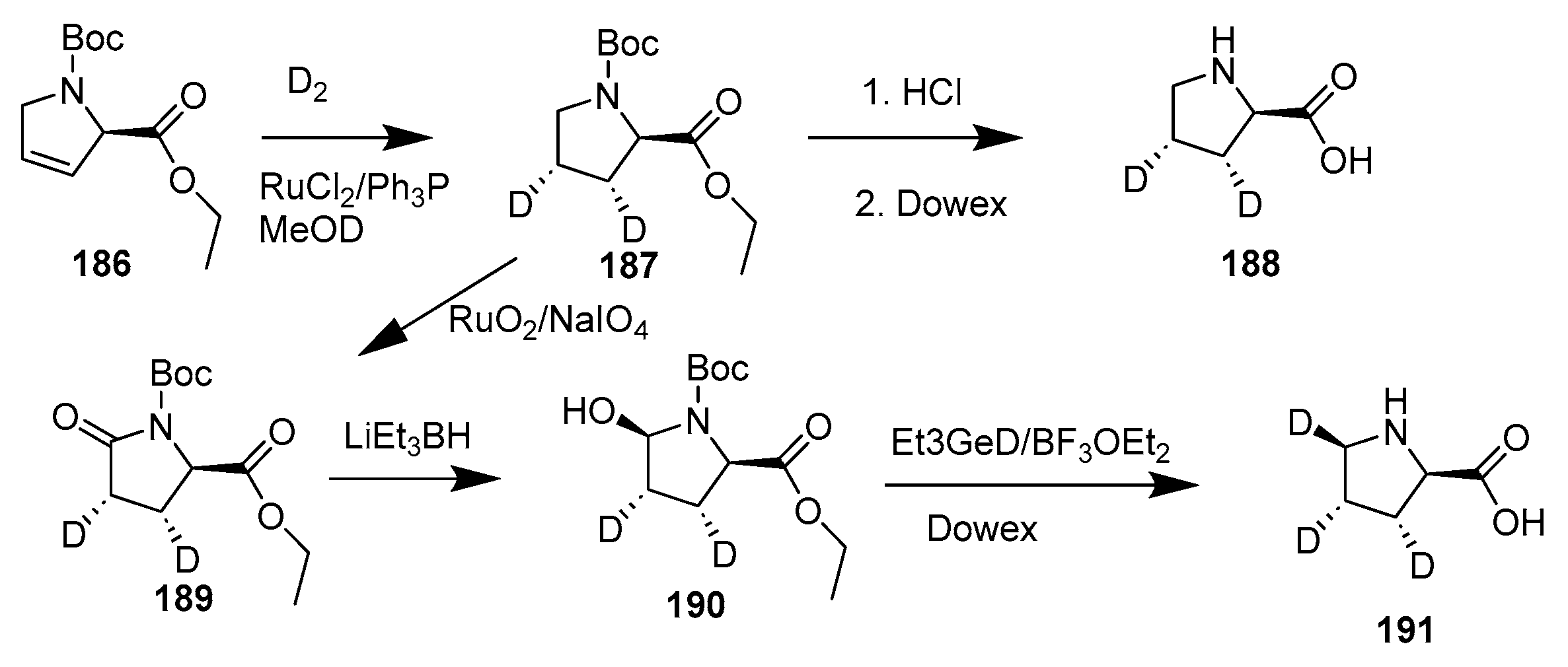
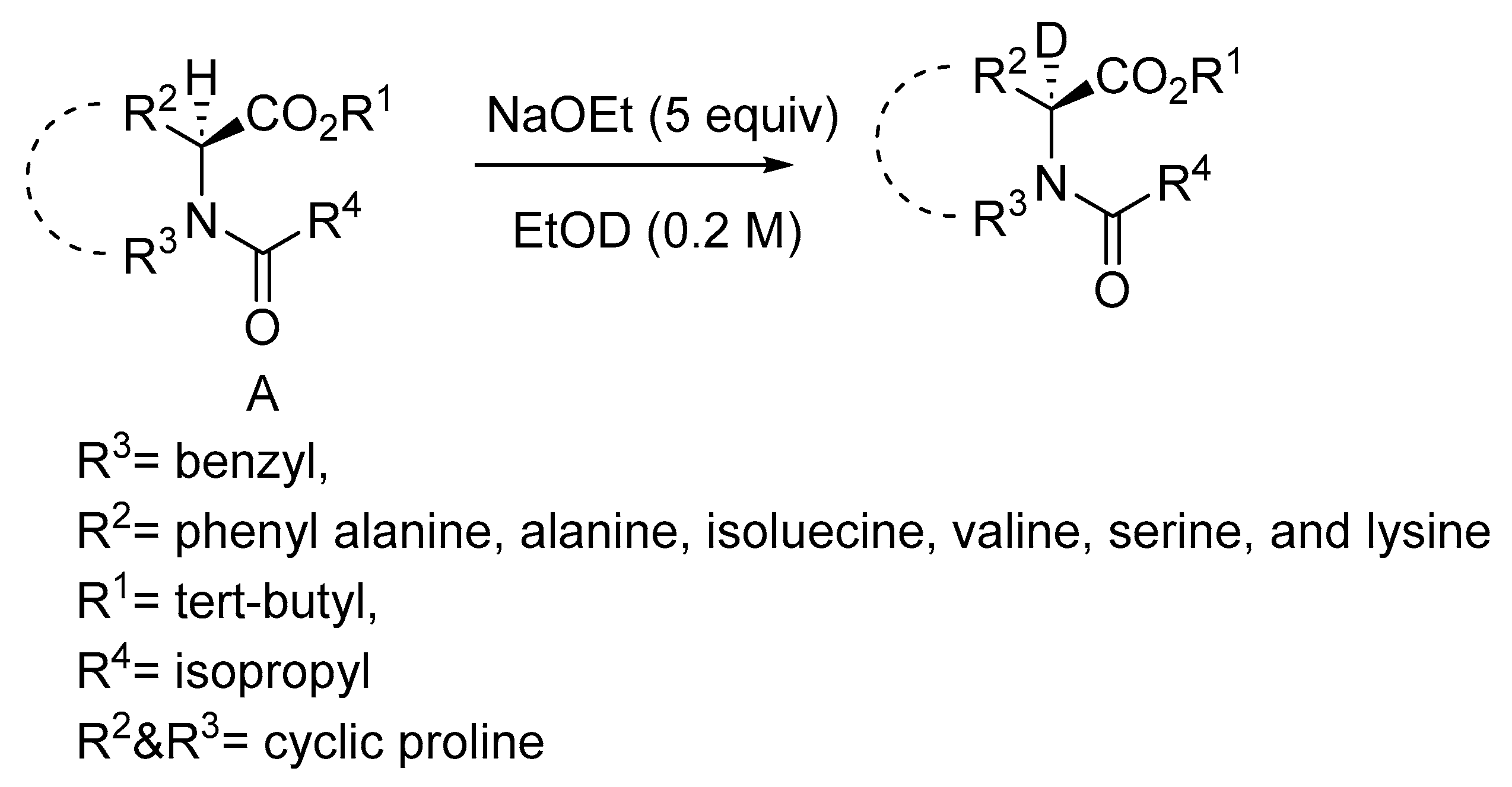
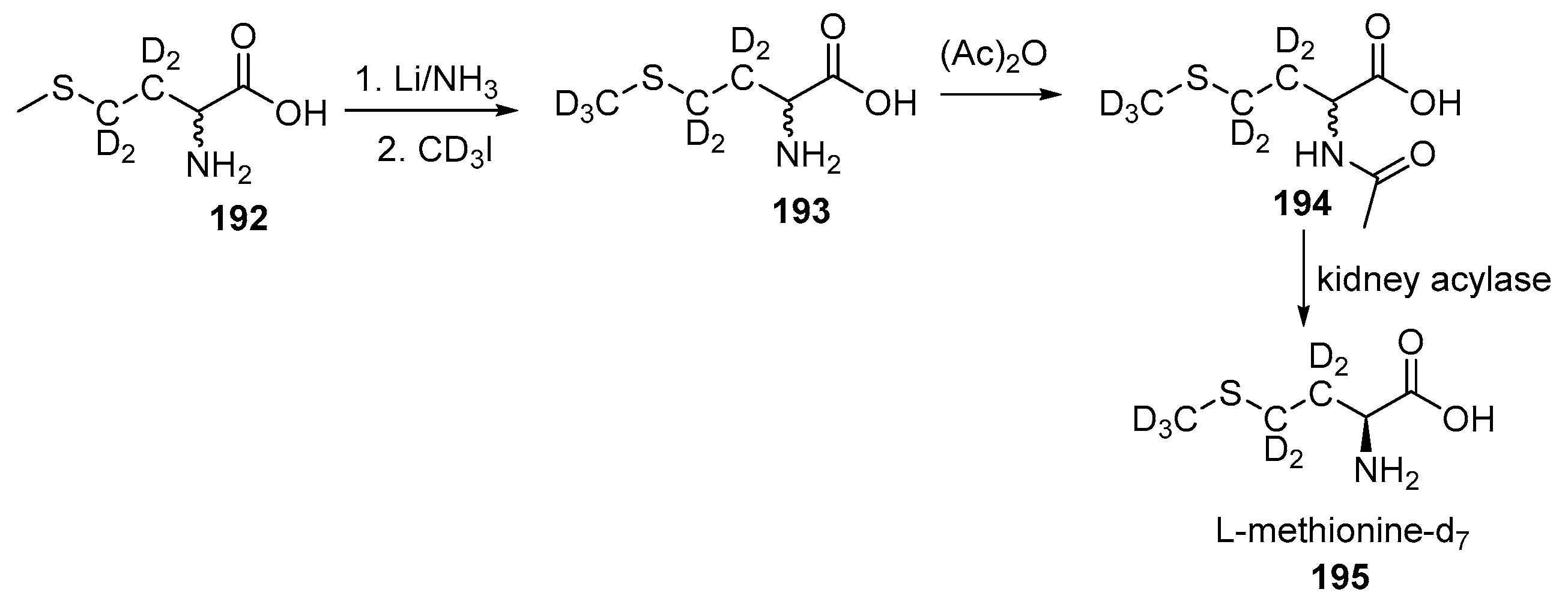

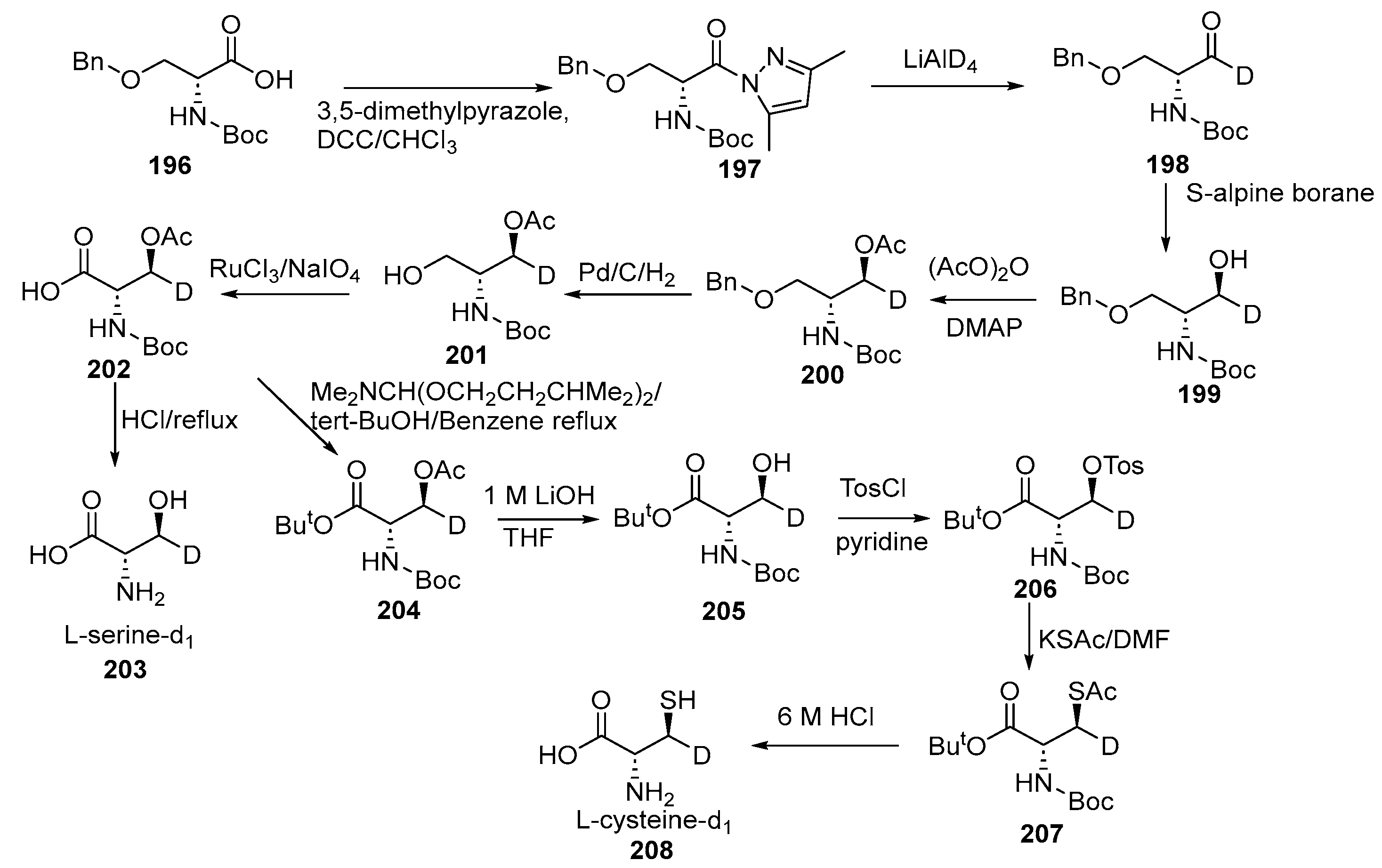
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yepuri, N.R. Chemical Deuteration of α-Amino Acids and Optical Resolution: Overview of Research Developments. Bioengineering 2025, 12, 916. https://doi.org/10.3390/bioengineering12090916
Yepuri NR. Chemical Deuteration of α-Amino Acids and Optical Resolution: Overview of Research Developments. Bioengineering. 2025; 12(9):916. https://doi.org/10.3390/bioengineering12090916
Chicago/Turabian StyleYepuri, Nageshwar R. 2025. "Chemical Deuteration of α-Amino Acids and Optical Resolution: Overview of Research Developments" Bioengineering 12, no. 9: 916. https://doi.org/10.3390/bioengineering12090916
APA StyleYepuri, N. R. (2025). Chemical Deuteration of α-Amino Acids and Optical Resolution: Overview of Research Developments. Bioengineering, 12(9), 916. https://doi.org/10.3390/bioengineering12090916






