Effect of Surface Wettability and Energy on Bacterial Adhesion to Dental Aligners: A Comparative In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
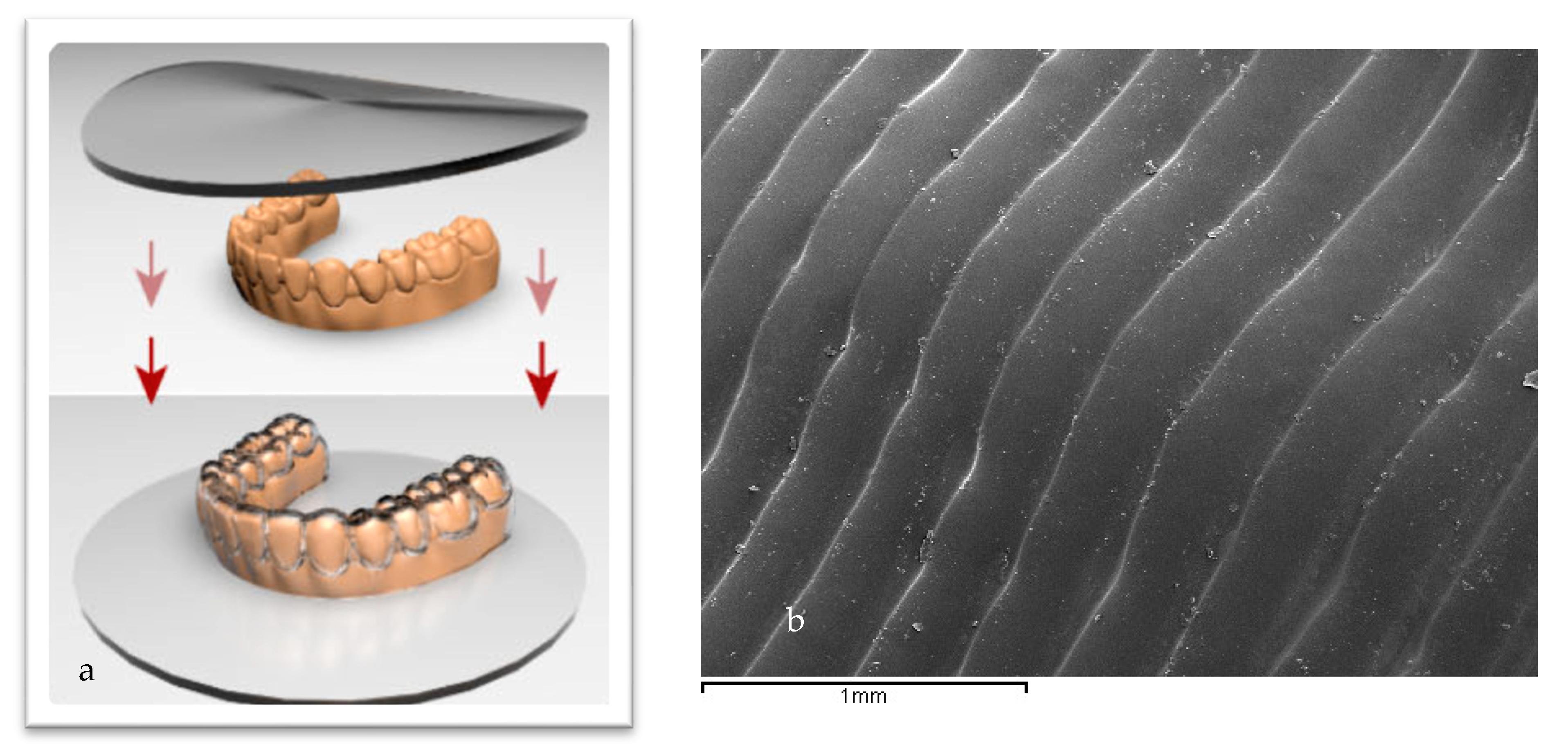
2.1. Samples Preparation
- Spark: Poliureane resin with 2-hydroxyethyl methacrylate and poly(oxy-1,2-ethanediyl), α,α′-[(1-methylethylidene)di-4,1-phenylene]bis[ω-[(2-methyl-1-oxo-2-propen-1-yl)oxy]-nickel and 2-diethylaminoethyl methacrylate—methanol and trifluoro(methanol)boron. Thickness: 0.75 mm. Commercial Manufacturer: Ormco Corporation, Brea, CA, USA
- Invisalign. Material: LD30, a multi-layer aromatic thermoplastic polyurethane from methylene diphenyl diisocyanate and 1,6-hexanediol plus additives. Thickness: 0.75 mm. Commercial Manufacturer: Align Technology Inc., San Jose, CA, USA
- Smile: Plus, C plus polypropylene/ethylene copolymer (>95%), stabilizers (<5%). Thickness: Plus: 0.035 and 0.040 C plus: 0.040 inches/1 mm. Commercial Manufacturer: Dentsply Sirona, York, PA, USA.
2.2. Physicochemical of the Surface Characterization
2.3. Bacteria Culture
2.4. Biofilm Formation
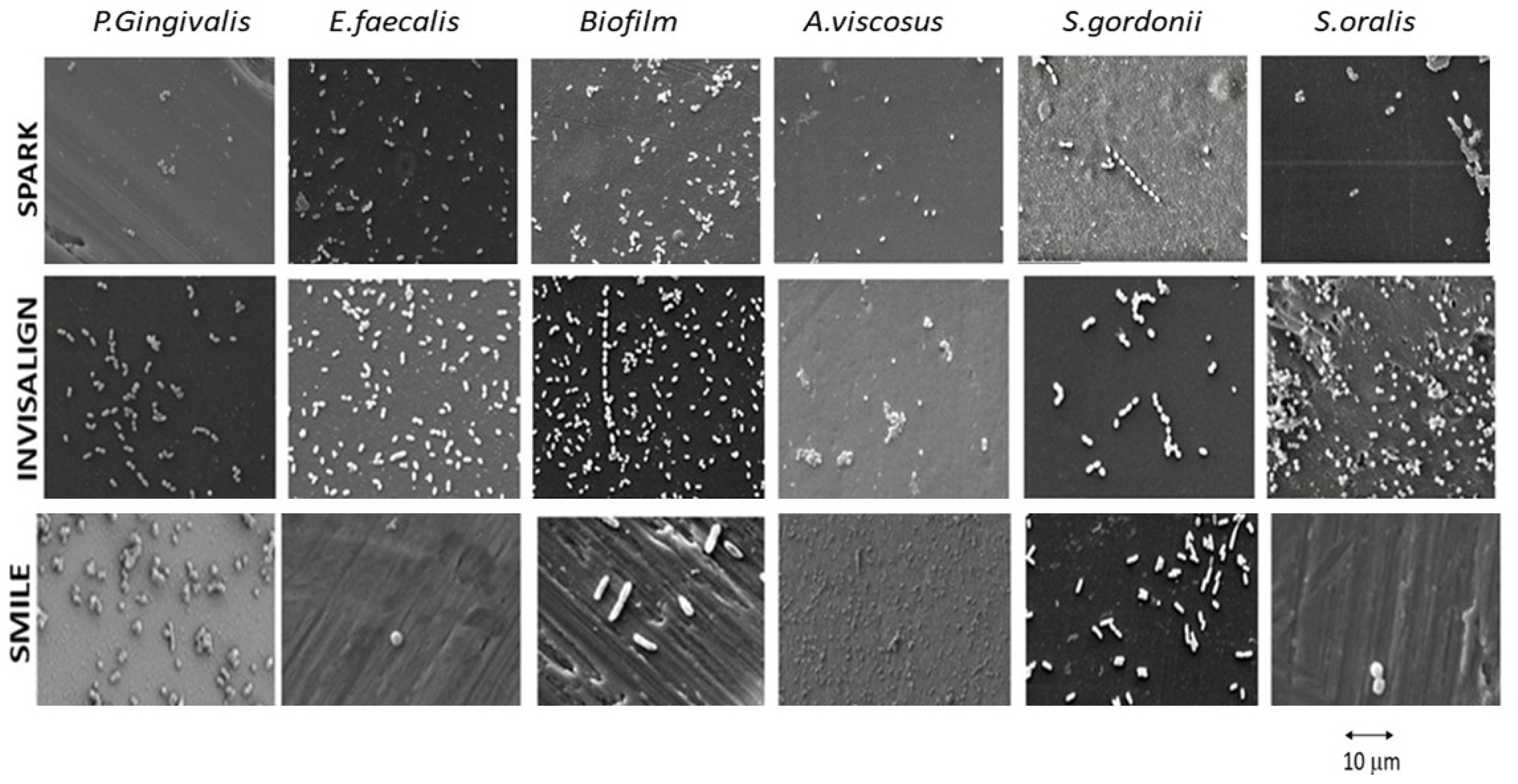
2.5. SEM Observations
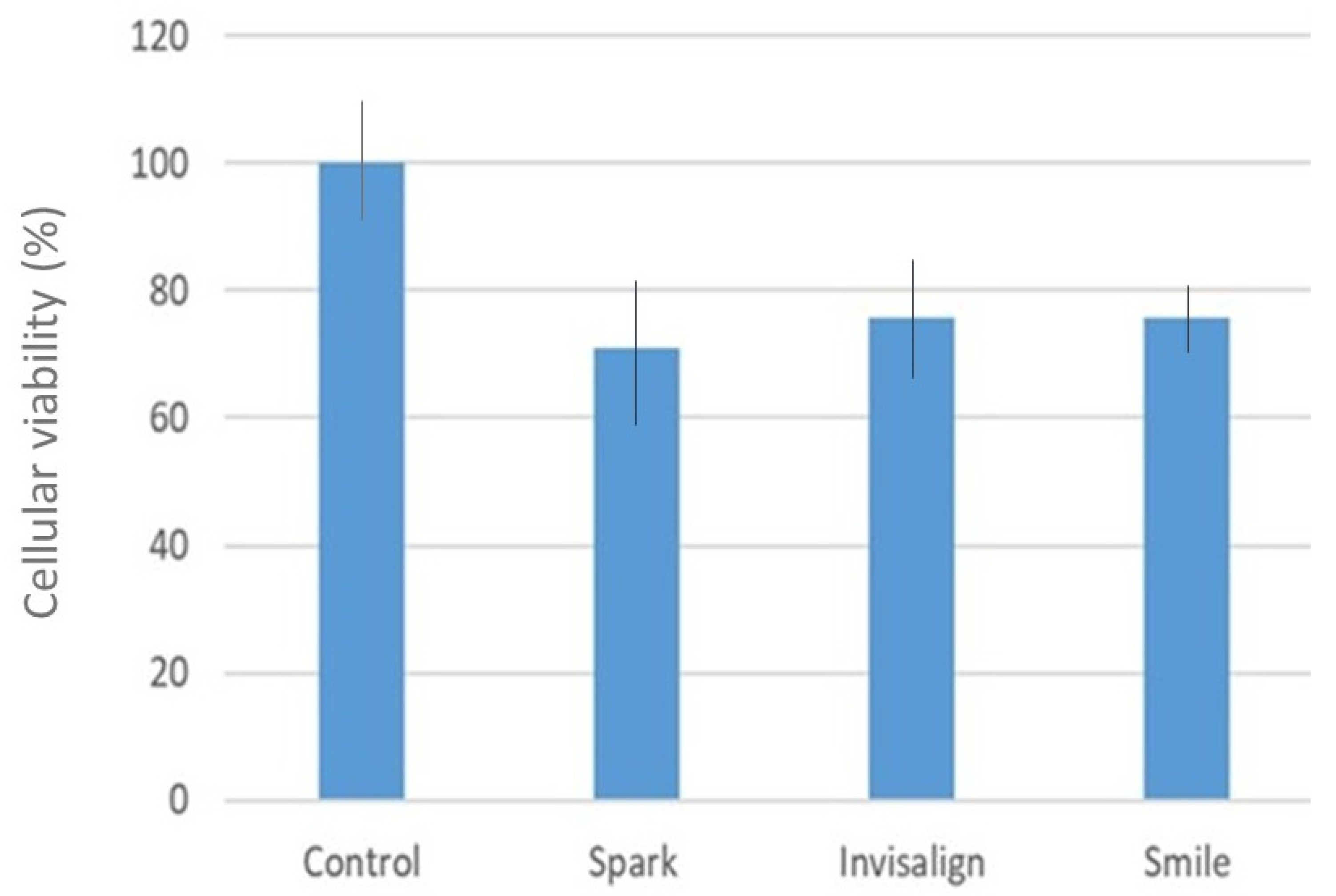
2.6. Evaluation of the Cytocompatibilty
2.7. Statistical Analysis
3. Results
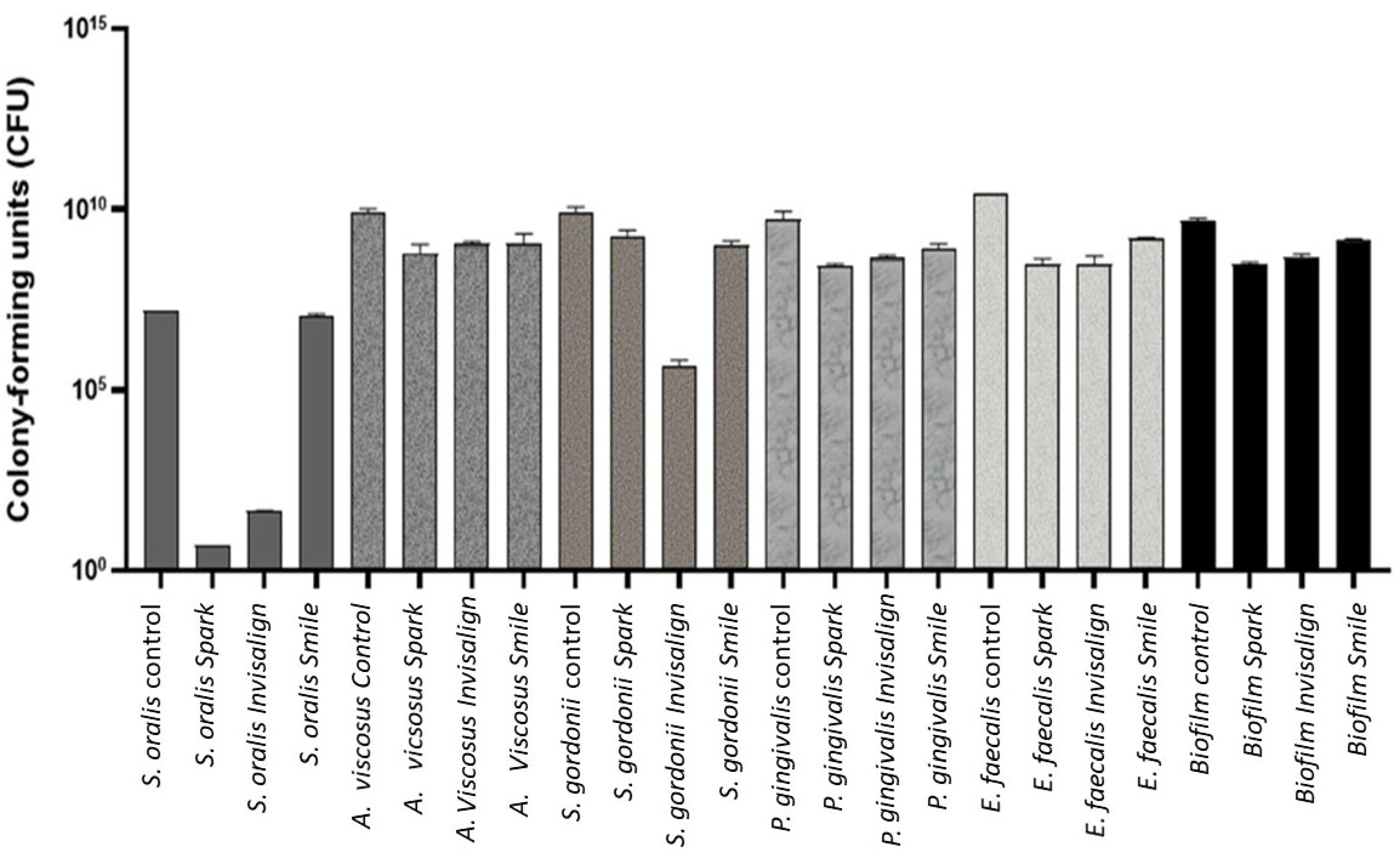
Quantification of Colony-Forming Units (CFU)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kilian, M. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef]
- Rath-Deschner, B.; Nogueira, A.V.B.; Beisel-Memmert, S.; Nokhbehsaim, M.; Eick, S.; Cirelli, J.A.; Deschner, J.; Jäger, A.; Damanaki, A. Interaction of periodontitis and orthodontic tooth movement-an in vitro and in vivo study. Clin. Oral Investig. 2022, 26, 171–181. [Google Scholar] [CrossRef]
- Carli, E.; Pasini, M.; Lardani, L.; Giuca, G.; Miceli, M. Impact of Self-Ligating Orthodontic Brackets on Dental Biofilm and Periodontal Pathogens in Adolescents. J. Biol. Regul. Homeost. Agents 2021, 35, 107–115. [Google Scholar] [CrossRef]
- Karkhanechi, M.; Chow, D.; Sipkin, J.; Sherman, D.; Boylan, R.J.; Norman, R.G.; Craig, R.G.; Cisneros, G.J. Periodontal Status of Adult Patients Treated with Fixed Buccal Appliances and Removable Aligners over One Year of Active Orthodontic Therapy. Angle Orthod. 2013, 83, 146–151. [Google Scholar] [CrossRef]
- Huang, G.; Cao, G.; Liu, J.; Liu, M. Global trends in incidence of caries in permanent teeth of children aged 5 through 14 years, 1990 through 2019. J. Am. Dent. Assoc. 2024, 155, 667–678.e21. [Google Scholar] [CrossRef]
- Batoni, G.; Pardini, M.; Giannotti, A.; Ota, F.; Giuca, M.R.; Gabriele, M.; Campa, M.; Senesi, S. Effect of removable orthodontic appliances on oral colonisation by mutans streptococci in children. Eur. J. Oral Sci. 2001, 109, 388–392. [Google Scholar] [CrossRef]
- Albhaisi, Z.; Al-Khateeb, S.N.; Abu Alhaija, E.S. Enamel demineralization during clear aligner orthodontic treatment compared with fixed appliance therapy, evaluated with quantitative light induced fluorescence: A randomized clinical trial. Am. J. Orthod. Dentofac. Orthop 2020, 157, 594–601. [Google Scholar] [CrossRef]
- Miethke, R.R.; Vogt, S. A comparison of the periodontal health of patients during treatment with the Invisalign system and with fixed orthodontic appliances. J. Orofac. Orthop. 2005, 66, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Cintora-López, P.; Arrieta-Blanco, P.; Martin-Vacas, A.; Paz-Cortés, M.M.; Gil, J.; Aragoneses, J.M. In vitro analysis of the influence of the thermocycling and the applied force on orthodontic clear aligners. Front. Bioeng. Biotechnol. 2023, 20, 1321495. [Google Scholar] [CrossRef] [PubMed]
- Tamburrino, F.; D’Anto, V.; Bucci, R.; Alessandri-Bonetti, G.; Barone, S.; Razionale, A.V. Mechanical properties of thermoplastic polymers for aligner manufacturing: In vitro study. Dent. J. 2020, 8, 47. [Google Scholar] [CrossRef]
- Condo, R.; Mampieri, G.; Giancotti, A.; Cerroni, L.; Pasquantonio, G.; Divizia, A. SEM Characterization and ageing analysis on two generations of Invisible aligners. BMC Oral Health 2021, 21, 316. [Google Scholar] [CrossRef]
- Zhang, N.; Bai, Y.; Ding, X.; Zhang, Y. Preparation and characterization of thermoplastic materials for invisible orthodontics, Dent. Mater. J. 2011, 30, 954–959. [Google Scholar]
- Eslami, S.; Kopp, S.; Goteni, M.; Dahmer, J.; Sayahpour, B. Alterations in the surface roughness and porosity parameters of directly printed and Invisalign aligners after 1 week of intraoral usage: An in vivo prospective investigation. Am. J. Orthod. Dentofac. Orthop. 2024, 165, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, A.; Mousoulea, S.; Gkantidis, N.; Kloukos, D. Clinical effectiveness of Invisalign® orthodontic treatment: A systematic review. Prog. Orthod. 2018, 19, 37. [Google Scholar] [CrossRef]
- Ferreira, L.; Otero, E.; Vargas, A.; Moreira da Silva, E.; Nunes da Silva, J.; Elzubair, A.; Siqueira de Morais, L.; Souza, L.; Serra, G.; Gomes de Souza, M.M. Effect of oral exposure on chemical, physical, mechanical, and morphologic properties of clear orthodontic aligners. Am. J. Orthod. Dentofac. Orthop. 2023, 164, e51–e63. [Google Scholar] [CrossRef] [PubMed]
- Staderini, E.; Chiusolo, G.; Guglielmi, F.; Papi, M.; Perini, G.; Tepedino, M.; Gallenzi, P. Effects of Thermoforming on the Mechanical, Optical, Chemical, and Morphological Properties of PET-G: In Vitro Study. Polymers 2024, 16, 203. [Google Scholar] [CrossRef]
- Ryokawa, H.; Miyazaki, Y.; Fujishima, A.; Miyazaki, T.; Maki, K. The mechanical properties of dental thermoplastic materials in a simulated intraoral environment. Orthod. Waves 2006, 65, 64–72. [Google Scholar] [CrossRef]
- Yu, X.; Li, G.; Zheng, Y.; Gao, J.; Fu, Y.; Wang, Q.; Huang, L.; Pan, X.; Ding, J. ‘Invisible’ orthodontics by polymeric ‘clear’ aligners molded on 3D-printed personalized dental models. Regen. Biomater. 2022, 9, rbac007. [Google Scholar] [CrossRef]
- Vázquez, B.; Ginebra, M.P.; Gil, F.J.; Planell, J.A.; López Bravo, A.; San Román, J. Radiopaque acrylic cements prepared with a new acrylic derivative of iodo-quinoline. Biomaterials 1999, 20, 2047–2053. [Google Scholar] [CrossRef]
- Fernández, J.C.; Pastor, F.; Mora, J.M.B.; Demiquels, E.; Espinar, E.; Gil, J. Conformation Effect on the Mechanical and Microbiological Behavior of Invisible Orthodontic Aligners. Materials 2024, 17, 1360. [Google Scholar] [CrossRef] [PubMed]
- Buxadera-Palomero, J.; Calvo, C.; Torrent-Camarero, S.; Gil, F.J.; Mas-Moruno, C.; Canal, C.; Rodríguez, D. Biofunctional polyethylene glycol coatings on titanium: An in vitro-based comparison of functionalization methods. Colloids Surf. B Biointerfaces 2017, 152, 367–375. [Google Scholar] [CrossRef]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth. Adv. Colloid Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef]
- Spengler, C.; Nolle, F.; Mischo, J.; Faidt, T.; Grandthyll, S.; Thewes, N. Strength of bacterial adhesion on nanostructured surfaces quantified by substrate morphometry. Nanoscale 2019, 11, 19713–19722. [Google Scholar] [CrossRef]
- Chang, Y.R.; Weeks, E.R.; Ducker, W.A. Surface topography hinders bacterial surface motility. ACS Appl. Mater. Interfaces 2018, 10, 9225–9234. [Google Scholar] [CrossRef] [PubMed]
- Stepanovic, S.; Vukovic, D.; Jezek, P.; Pavlovic, M.; Svabic-Vlahovic, M. Influence of dynamic conditions on biofilm formation by Staphylococci. Eur. J. Clin. Microbiol. Infect. Dis. 2001, 20, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Bilgili, D.; Dundar, A.; Barutcugil, C.; Tayfun, D.; Ozyurt, O.K. Surface properties and bacterial adhesion of bulk-fill composite resins. J. Dent. 2020, 95, 103317. [Google Scholar] [CrossRef]
- Tavares-Camardella, L.; Vasconcellos-Vilella, O.; Breuning, H. Accuracy of printed dental models made with 2 prototype technologies and different designs of model bases. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 1178–1187. [Google Scholar] [CrossRef]
- Papadopoulou, A.K.; Cantele, A.; Polychronis, G.; Zinelis, S.; Eliades, T. Changes in Roughness and Mechanical Properties of Invisalign® Appliances after One- and Two-Weeks Use. Materials 2019, 12, 2406. [Google Scholar] [CrossRef] [PubMed]
- Koletsi, D.; Panayi, N.; Laspos, C.; Athanasiou, A.E.; Zinelis, S.; Eliades, T. In vivo aging-induced surface roughness alterations of Invisalign and 3D-printed aligners. J. Orthod. 2022, 50, 352–360. [Google Scholar] [CrossRef]
- Alhendi, A.; Khounganianb, R.; Almudhic, A. Cytotoxicity assessment of different clear aligner systems: An in vitro study. Angle Orthod. 2022, 92, 655–660. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 123–132. [Google Scholar] [CrossRef]
- Perkowski, K.; Baltaza, W.; Conn, D.B.; Marczyńska-Stolarek, M.; Chomicz, L. Examination of oral biofilm microbiota in patients using fixed orthodontic appliances in order to prevent risk factors for health complications. Ann. Agric. Environ. Med. 2019, 26, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Heimisdottir, K.; Gebauer, U.; Persson, G.R. Clinical and microbiological findings at sites treated with orthodontic fixed appliances in adolescents. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Vico, R.-M.; Iglesias-Linares, A.; Ballesta-Mudarra, S.; Ortiz-Ariza, E.; Solano-Reina, E.; Perea, E.-J. Short-term effect of removal of fixed orthodontic appliances on gingival health and subgingival microbiota: A prospective cohort study. Acta Odontol. Scand. 2015, 73, 496–502. [Google Scholar] [CrossRef]
- Papageorgiou, S.N.; Xavier, G.M.; Cobourne, M.T.; Eliades, T. Effect of orthodontic treatment on the subgingival microbiota: A systematic review and meta-analysis. Orthod. Craniofacial Res. 2018, 21, 175–185. [Google Scholar] [CrossRef]
- ASTM D5465-16; Standard Practices for Determining Microbial Colony Counts from Waters Analyzed by Plating Methods. ASTM: West Conshohocken, PA, USA, 2020.
- Surdu, A.E.; Popa, C.O.; Luchian, I.O. Identification of bacteria involved in periodontal disease using molecular biology techniques. Rev. Chim. 2017, 68, 2407–2412. [Google Scholar]
- Kuang, P.; Constant, K. Increased Wettability and Surface Free Energy of Polyurethane by Ultraviolet Ozone Treatment. In Wetting and Wettability; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef]
- Bauer, M.G.; Reithmeir, R.; Lutz, T.M.; Lieleg, O. Wetting behavior and stability of surface-modified polyurethane materials. Plasma Process. Polym. 2021, 18, e2100126. [Google Scholar] [CrossRef]
- Singh, R.P.; Tome, N.S.; Bhadraiah, S.V. Photo-oxidation studies on polyurethane coating: Effect of additives on yellowing of polyurethane. Polym. Degrad. Stab. 2001, 73, 443–446. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Garrido, B.; Rodriguez, D.; Ruperez, E.; Gil, F.J. Biofunctionalization strategies on tantalum-based materials for osseointegrative applications. J. Mater. Sci. Mater. Med. 2015, 26, 109. [Google Scholar] [CrossRef]
- Pascual, B.; Gurruchaga, M.; Ginebra, M.P.; Gil, F.J.; Planell, J.A.; Goñi, I. Influence of the modification of P/L ratio on a new formulation of acrylic bone cement. Biomaterials 1999, 20, 465–474. [Google Scholar] [CrossRef]
- Brizuela-Velasco, A.; Álvarez-Arenal, Á.; Gil-Mur, F.J.; Herrero-Climent, M.; Chávarri-Prado, D.; Chento-Valiente, Y.; Dieguez-Pereira, M. Relationship Between Insertion Torque and Resonance Frequency Measurements, Performed by Resonance Frequency Analysis, in Micromobility of Dental Implants: An In Vitro Study. Implant. Dent. 2015, 24, 607–611. [Google Scholar] [CrossRef]
- Aparicio, C.; Manero, J.M.; Conde, F.; Pegueroles, M.; Planell, J.A.; Vallet-Regí, M.; Gil, F.J. Acceleration of apatite nucleation on microrough bioactive titanium for bone-replacing implants. J. Biomed. Mater. Res. A 2007, 82, 521–529. [Google Scholar] [CrossRef]
- Koo, G.H.; Jang, J. Surface modification of poly (lactic acid) by UV/Ozone irradiation. Fibers Polym. 2008, 9, 674–678. [Google Scholar] [CrossRef]
- Yuan, Y.; Hays, M.P.; Hardwidgeb, P.R.; Kim, J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017, 7, 14254. [Google Scholar] [CrossRef]
- Lorenzetti, M.; Dogša, I.; Stošicki, T.; Stopar, D.; Kalin, M.; Kobe, S.; Novak, S. The Influence of Surface Modification on Bacterial Adhesion to Titanium-based Substrates. ACS Appl. Mater. Interfaces 2015, 7, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Fernandez, J.C.; Pastor, F.; Barrera Mora, J.M.; Brizuela, A.; Puigdollers, A.; Espinar, E.; Gil, F.J. Bacteriostatic Poly. Ethylene Glycol Plasma Coatings for Orthodontic Titanium Mini-Implants. Materials 2022, 15, 7487. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Guillem-Marti, J.; Sevilla, P.; Manero, J.M.; Gil, F.J.; Rodriguez, D. Anhydride-Functional Silane Immobilized onto Titanium Surfaces Induces Osteoblast Cell Differentiation and Reduces Bacterial Adhesion and Biofilm Formation. Mater. Sci. Eng. C 2016, 59, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Tuson, H.H.; Weibel, D.B. Bacteria-surface Interactions. Soft Matter 2013, 9, 4368–4380. [Google Scholar] [CrossRef]
- Yan, D.; Liu, Y.; Che, X.; Mi, S.; Jiao, Y.; Guo, L.; Song, L. Changes in the microbiome of the inner surface of clear aligners after different usage periods. Curr. Microbiol. 2021, 78, 566–575. [Google Scholar] [CrossRef]
- Zhao, R.; Huang, R.; Long, H.; Li, Y.; Gao, M.; Lai, W. The dynamics of the oral microbiome and oral health among patients receiving clear aligner orthodontic treatment. Oral Dis. 2020, 26, 473–483. [Google Scholar] [CrossRef]
- Low, B.; Lee, W.; Seneviratne, C.J.; Samaranayake, L.P.; Hagg, U. Ultrastructure and morphology of biofilms on thermoplastic orthodontic appliances in ’fast’ and ’slow’ plaque formers. Eur. J. Orthod. 2011, 33, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, L.; Martini, M.; Cervinara, F.; Spedicato, G.A.; Oliverio, T.; Siciliani, G. Comparative SEM analysis of nine F22 aligner cleaning strategies. Prog. Orthod. 2017, 18, 26. [Google Scholar] [CrossRef]
- Caccianiga, P.; Nota, A.; Tecco, S.; Ceraulo, S.; Caccianiga, G. Efficacy of Home Oral-Hygiene Protocols during Orthodontic Treatment with Multibrackets and Clear Aligners: Microbiological Analysis with Phase-Contrast Microscope. Healthcare 2022, 10, 2255. [Google Scholar] [CrossRef] [PubMed]
- Moradinezhad, M.; Montazeri, E.A.; Ashtiani, A.H.; Pourlotfi, R.Z.; Rakhshan, V. Biofilm formation of Streptococcus mutans, Streptococcus sanguinis, Staphylococcus epidermidis, Staphylococcus aureus, Lactobacillus casei, and Candida Albicans on 5 thermoform and 3D printed orthodontic clear aligner and retainer materials at 3 time points: An in vitro study. BMC Oral Health 2024, 24, 1107. [Google Scholar] [CrossRef] [PubMed]
- Sfondrini, M.F.; Butera, A.; Di Michele, P.; Luccisano, C.; Ottini, B.; Sangalli, E.; Gallo, S.; Pascadopoli, M.; Gandini, P.; Scribante, A. Microbiological Changes during Orthodontic Aligner Therapy: A Prospective Clinical Trial. Appl. Sci. 2021, 11, 6758. [Google Scholar] [CrossRef]
- Mummolo, S.; Tieri, M.; Nota, A.; Caruso, S.; Darvizeh, A.; Albani, F.; Gatto, R.; Marzo, G.; Marchetti, E.; Quinzi, V.; et al. Salivary concentrations of Streptococcus mutans and Lactobacilli during an orthodontic treatment. An observational study comparing fixed and removable orthodontic appliances. Clin. Exp. Dent. Res. 2020, 6, 181–187. [Google Scholar] [CrossRef]
- Pasaoglu, B.; Demirci, M.; Erdogan, P.; Kayalar, E. Comparison of microbial adhesion and biofilm formation on different orthodontic aligners. Am. J. Orthod. Dentofac. Orthop. 2024, 167, 47–62. [Google Scholar] [CrossRef]
- Sifakakis, I.; Papaioannou, W.; Papadimitriou, A.; Kloukos, D.; Papageorgiou, S.N.; Eliades, T. Salivary levels of cariogenic bacterial species during orthodontic treatment with thermoplastic aligners or fixed appliances: A prospective cohort study. Prog. Orthod. 2018, 19, 25. [Google Scholar] [CrossRef]
- Eliades, T.; Pratsinis, H.; Athanasiou, A.E.; Eliades, G.; Kletsas, D. Cytotoxicity and estrogenicity of Invisalign appliances. Am. J. Orthod. Dentofac. Orthop. 2009, 136, 100–103. [Google Scholar] [CrossRef]
- Premaraj, T.; Simet, S.; Beatty, M.; Premaraj, S. Oral epithelial cell reaction after exposure to Invisalign plastic material. Am. J. Orthod. Dentofac. Orthop. 2014, 145, 64–71. [Google Scholar] [CrossRef]
- Hamada, S. Dermal uptake study with 4,40-diphenylmethane diisocyanate led to active sensitization. Contact Dermat. 2012, 66, 101–105. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, M.; Zhang, W.; Liu, X.; Zheng, W.; Jiang, X. Gold Nanoclusters-Coated Orthodontic Devices Can Inhibit the Formation of Streptococcus mutans Biofilm. ACS Biomater. Sci. Eng. 2020, 6, 1239–1246. [Google Scholar] [CrossRef]
- Martina, S.; Rongo, R.; Bucci, R.; Razionale, A.V.; Valletta, R.; D’Anto, V. In Vitro cytotoxicity of different thermoplastic materials for clear aligners. Angle Orthod. 2019, 89, 942–945. [Google Scholar] [CrossRef]
- Pratsinis, H.; Papageorgiou, S.N.; Panayi, N.; Iliadi, N.; Eliades, T.; Kletsas, D. Cytotoxicity and estrogenicity of a novel 3-dimensional printed orthodontic aligner. Am. J. Orthod. Dentofac. Orthop. 2022, 162, e116–e122. [Google Scholar] [CrossRef]
- Kim, J.E.; Mangal, U.; Yu, J.H. Evaluation of the effects of temperature and centrifugation time on elimination of uncured resin from 3D-printed dental aligners. Sci. Rep. 2014, 14, 15206. [Google Scholar] [CrossRef]
- Vazquez, B.; Elvira, C.; Levenfeld, B.; Pascual, B.; Goñi, I.; Gurruchaga, M.; Ginebra, M.P.; Gil, F.X.; Planell, J.A.; Liso, P.A.; et al. Application of tertiary amines with reduced toxicity to the curing process of acrylic bone cements. J. Biomed. Mater. Res. 1997, 34, 129–136. [Google Scholar] [CrossRef]
- Kotyk, M.W.; Wiltshire, W.A. An investigation into bisphenol-A leaching from orthodontic materials. Angle Orthod. 2014, 84, 516–520. [Google Scholar] [CrossRef]

| CA (°) | SFE (mJ/m2) | DISP (mJ/m2) | POL (mJ/m2) | |
|---|---|---|---|---|
| Spark | 70.5 ± 7.2 | 60.8 ± 9.8 | 28.9 ± 3.3 | 31.9 ± 3.4 |
| Invisalign | 80.6 ± 5.1 * | 66.7 ± 1.5 | 47.4 ± 0.2 * | 19,3 ± 1.5 * |
| Smile | 91.2 ± 6.6 ** | 74.2 ± 2.1 * | 50.2 ± 1.1 ** | 20.0 ± 2.2 * |
| Bacteria | Group | Mean | SD | Spark | Invisalign | SureSmile |
|---|---|---|---|---|---|---|
| S. oralis | Control | 100 | 2.70473745 | <0.0001 | <0.0001 | <0.0001 |
| S. oralis | Spark | 23.1467288 | 0.11968374 | ns | <0.0001 | |
| S. oralis | Invisalign | 28.6743516 | 0.34232693 | <0.05 | ||
| S. oralis | SureSmile | 38.5737936 | 0.15635539 | |||
| A. viscosus | Control | 100 | 10.4870816 | <0.0001 | <0.0001 | <0.0001 |
| A. viscosus | Spark | 43.1797115 | 0.53193343 | ns | <0.05 | |
| A. viscosus | Invisalign | 52.1486041 | 1.4706573 | ns | ||
| A. viscosus | SureSmile | 55.6004598 | 1.13615019 | |||
| S. gordonii | Control | 100 | 4.11583083 | <0.0001 | <0.0001 | <0.0001 |
| S. gordonii | Spark | 57.3586457 | 2.45147211 | <0.0001 | <0.0001 | |
| S. gordonii | Invisalign | 43.5841447 | 2.42727023 | <0.0001 | ||
| S. gordonii | SureSmile | 23.6468261 | 0.05131396 | |||
| P. gingivalis | Control | 100 | 3.69664728 | <0.0001 | ns | <0.01 |
| P. gingivalis | Spark | 17.7484069 | 0.22514496 | <0.0001 | <0.0001 | |
| P. gingivalis | Invisalign | 78.1921171 | 15.4480032 | ns | ||
| P. gingivalis | SureSmile | 71.245378 | 1.1596853 | |||
| E. faecalis | Control | 100 | 1.21932032 | <0.0001 | <0.0001 | <0.0001 |
| E. faecalis | Spark | 76.8894807 | 3.63631184 | ns | <0.0001 | |
| E. faecalis | Invisalign | 76.0612517 | 3.70975383 | ns | <0.0001 | |
| E. faecalis | SureSmile | 51.5739015 | 0.27633597 | |||
| Biofilm | Control | 100 | 0.79243285 | <0.0001 | <0.0001 | <0.0001 |
| Biofilm | Spark | 2.43285861 | 0.06415541 | <0.0001 | <0.0001 | |
| Biofilm | Invisalign | 27.5373564 | 0.1068084 | ns | ||
| Biofilm | SureSmile | 30.137912 | 0.20499682 |
| Bacteria | Group | Mean | SD | Spark | Invisalign | SureSmile |
| S. oralis | Control | 15,000,000 | 0 | ns | ns | ns |
| S. oralis | Spark | 5 | 0 | |||
| S. oralis | Invisalign | 44 | 1.414213562 | |||
| S. oralis | SureSmile | 10,650,000 | 1,909,188.309 | |||
| A. viscosus | Control | 7,808,000 | 2,375,878.785 | <0.001 | <0.01 | <0.01 |
| A. viscosus | Spark | 615,000,000 | 445,477,272.1 | ns | ns | ns |
| A. viscosus | Invisalign | 180,000,000 | 127,279,220.6 | ns | ns | ns |
| A. viscosus | SureSmile | 1,140,000,000 | 933,380,951.2 | ns | ns | ns |
| S. gordonii | Control | 8,040,000 | 3,563,818.177 | <0.01 | <0.0001 | <0.001 |
| S. gordonii | Spark | 1,845,000 | 784,888,527.1 | |||
| S. gordonii | Invisalign | 450,000 | 212,132.0344 | |||
| S. gordonii | SureSmile | 1,530,000 | 275,771,644.7 | |||
| P. gingivalis | Control | 5,280,000 | 3,394,112,550 | <0.05 | <0.05 | ns |
| P. gingivalis | Spark | 285,000,000 | 21,213,203.44 | |||
| P. gingivalis | Invisalign | 119,000,000 | 84,852,813.74 | |||
| P. gingivalis | SureSmile | 840,000,000 | 254,558,441.2 | |||
| E. faecalis | Control | 27,000,000 | 0 | <0.0001 | <0.0001 | <0.0001 |
| E. faecalis | Spark | 300,000,000 | 127,279,220.6 | |||
| E. faecalis | Invisalign | 315,000,000 | 190,918,830.9 | |||
| E. faecalis | SureSmile | 150,000,000 | 21,213,203.44 | |||
| Biofilm | Control | 4,740,000 | 933,380,951.2 | ns | ns | ns |
| Biofilm | Spark | 300,000,000 | 42,246,406.87 | ns | ns | ns |
| Biofilm | Invisalign | 480,000,000 | 84,852,813.74 | ns | ns | ns |
| Biofilm | SureSmile | 1,470,000,000 | 42,246,406.87 | ns | ns | ns |
| Group | Mean | SD | Spark | Invisalign | SureSmile |
|---|---|---|---|---|---|
| Control | 100 | 2.028959866 | 0.0083 * | 0.1002 | 0.0040 * |
| Spark | 70.88959285 | 9.749625257 | — | 0.3322 | 0.9348 |
| Invisalign | 75.71665974 | 12.87686935 | — | — | 0.1544 |
| SureSmile | 75.64914832 | 7.425628458 | — | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez Gil-Ortega, A.; Paz-Cortés, M.M.; Viñas, M.J.; Cintora-López, P.; Martín-Vacas, A.; Gil, J.; Aragoneses, J.M. Effect of Surface Wettability and Energy on Bacterial Adhesion to Dental Aligners: A Comparative In Vitro Study. Bioengineering 2025, 12, 898. https://doi.org/10.3390/bioengineering12090898
Martínez Gil-Ortega A, Paz-Cortés MM, Viñas MJ, Cintora-López P, Martín-Vacas A, Gil J, Aragoneses JM. Effect of Surface Wettability and Energy on Bacterial Adhesion to Dental Aligners: A Comparative In Vitro Study. Bioengineering. 2025; 12(9):898. https://doi.org/10.3390/bioengineering12090898
Chicago/Turabian StyleMartínez Gil-Ortega, A., M. M. Paz-Cortés, M. J. Viñas, P. Cintora-López, A. Martín-Vacas, J. Gil, and J. M. Aragoneses. 2025. "Effect of Surface Wettability and Energy on Bacterial Adhesion to Dental Aligners: A Comparative In Vitro Study" Bioengineering 12, no. 9: 898. https://doi.org/10.3390/bioengineering12090898
APA StyleMartínez Gil-Ortega, A., Paz-Cortés, M. M., Viñas, M. J., Cintora-López, P., Martín-Vacas, A., Gil, J., & Aragoneses, J. M. (2025). Effect of Surface Wettability and Energy on Bacterial Adhesion to Dental Aligners: A Comparative In Vitro Study. Bioengineering, 12(9), 898. https://doi.org/10.3390/bioengineering12090898









