DSOF: A Rapid Method to Determine the Abundance of Microalgae and Methanotrophic Bacteria in Coculture Using a Combination of Differential Sedimentation, Optical Density, and Fluorescence
Abstract
1. Introduction
2. Materials and Methods
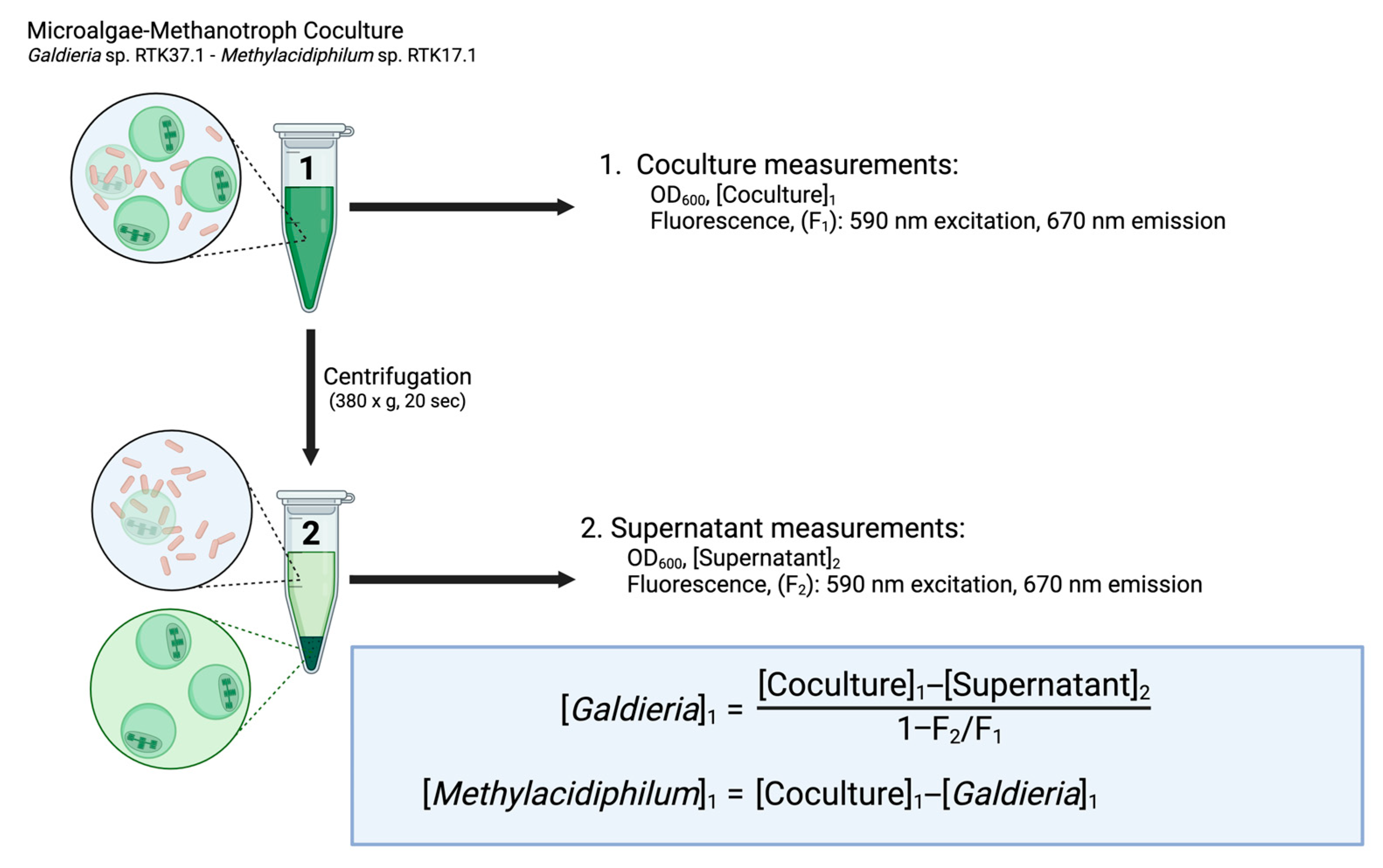
2.1. Technical Description of the DSOF Method
2.2. Growth Medium and Culture Maintenance
2.3. Biomass Determination
2.4. DSOF Evaluation in Model Suspensions
2.5. DSOF Evaluation in Live Batch Cocultures
3. Results and Discussion
3.1. Evaluation of the DSOF Method in Model Suspensions
3.2. Application of the DSOF Method to Monitor Growth Dynamics in Batch Cocultures
3.3. Outlook and Limitations of the DSOF Method
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
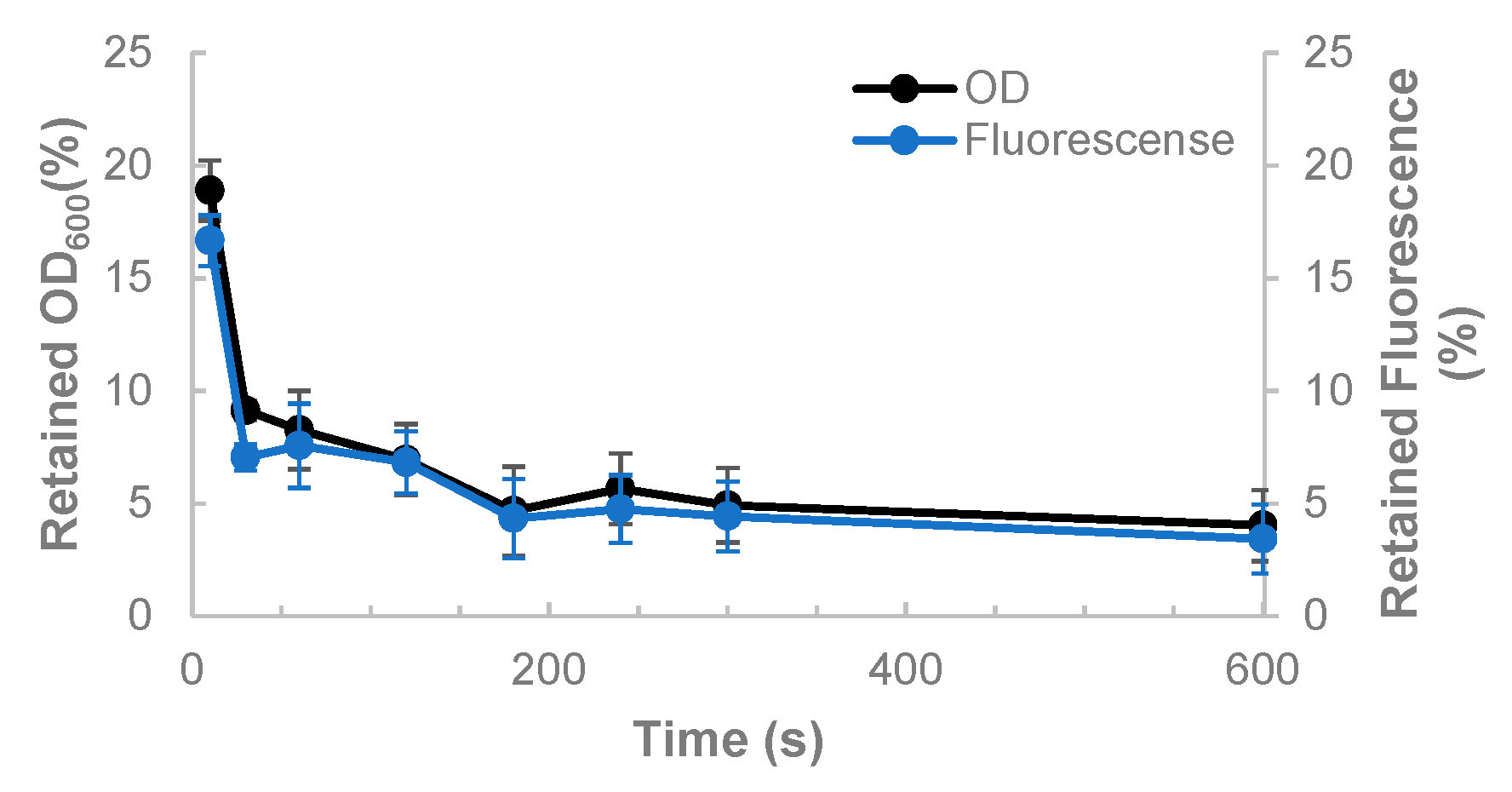
- OD600 values are additive for Methylacidiphilum sp. RTK17.1 and Galdieria sp. RTK37.1

- There is no significant centrifugation of methanotrophic cells
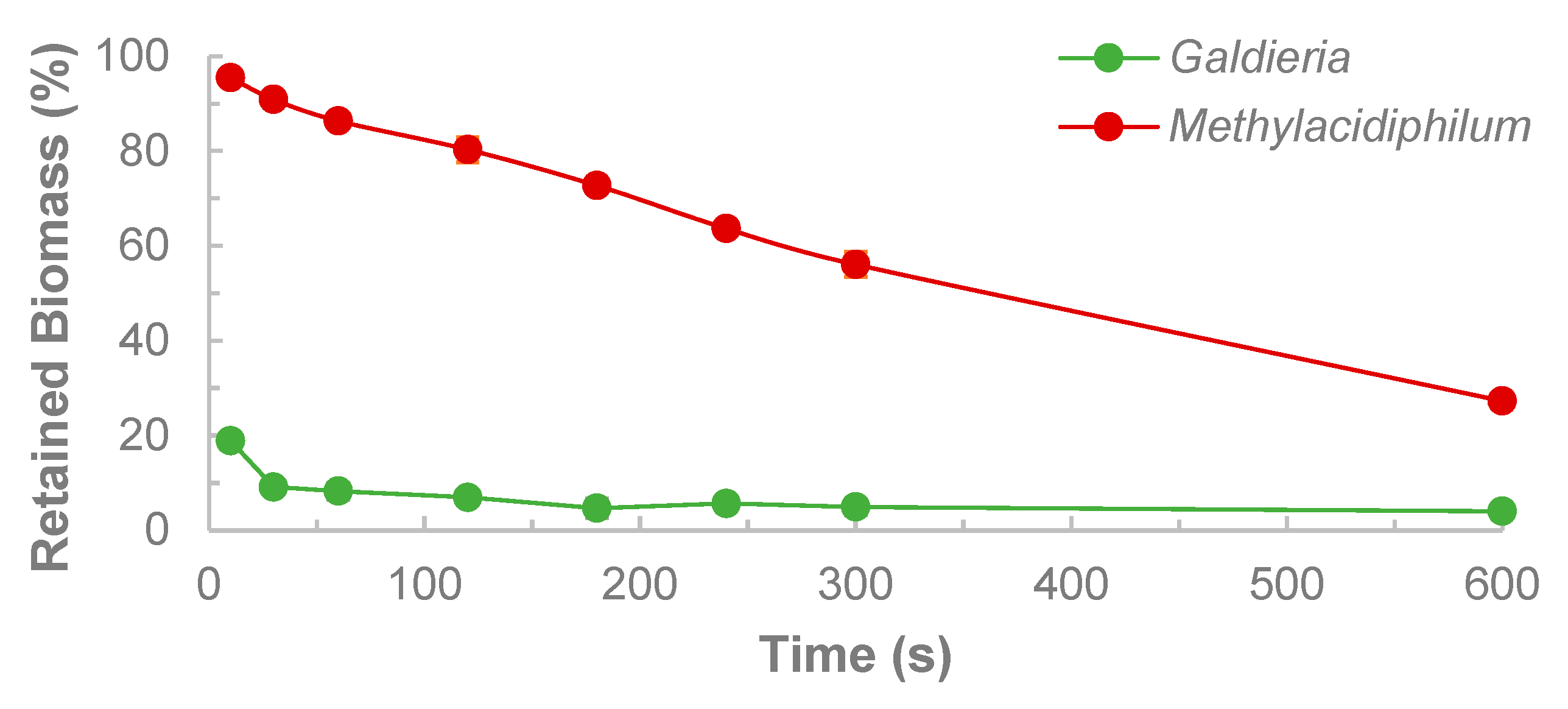
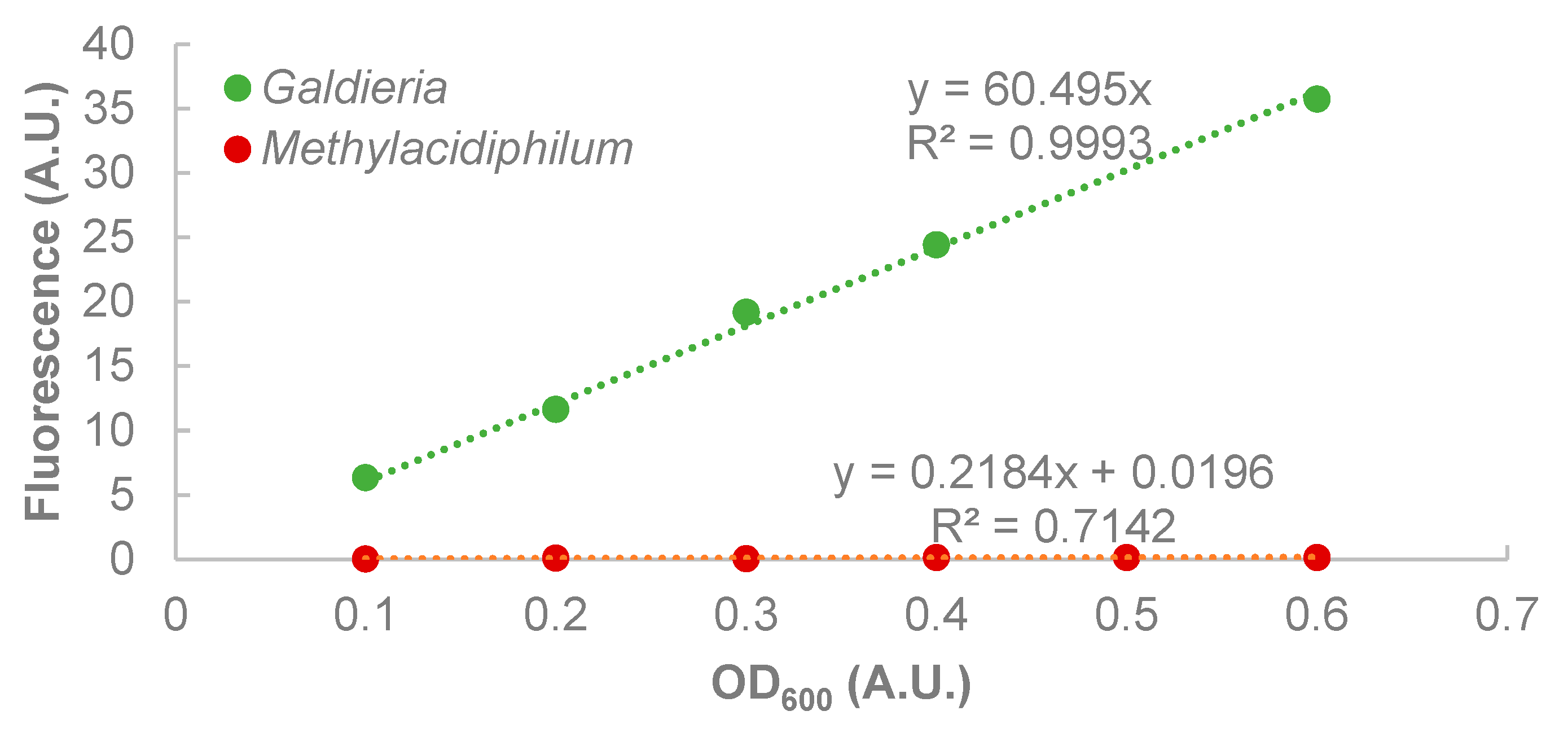
- The fraction of microalgae remaining in the supernatant can be approximated by the proportion of fluorescence of the supernatant and the original coculture

| Desired Condition | Headspace Concentrations (% v/v) | ||
|---|---|---|---|
| O2 | CH4 | CO2 | |
| 1. Methylacidiphilum growth was suppressed | 14.32 ± 0.01 | 0 | 17.29 ± 0.23 |
| 2. Galdieria growth suppressed | 14.27 ± 0.05 | 10.00 ± 0.04 | 6.55 ± 0.07 |
| 3. Galdieria and Methylacidiphilum growth allowed | 15.06 ± 0.09 | 6.94 ± 0.15 | 10.60 ± 0.04 |
| Strain | Guild | Morphology & Size (l × w) | Reference |
|---|---|---|---|
| Synechococcus spp. Methylomicrobium alcaliphilum | phototroph methanotroph | coccus: 0.6–2.1 µm rod: 1.2–3.0 µm | [66,67] |
| Chlorella sorokiniana Methylococcus capsulatus | phototroph methanotroph | coccus: 2–5 µm coccus: 0.7–1.0 µm | [68,69] |
| Galdieria spp. Methylacidiphilum spp. | phototroph methanotroph | ovoid: 3.8–5.0 µm rod: 0.8–0.65 µm | [45,70] |
References
- van der Ha, D.; Bundervoet, B.; Verstraete, W.; Boon, N. A sustainable, carbon neutral methane oxidation by a partnership of methane oxidizing communities and microalgae. Water Res. 2011, 45, 2845–2854. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.-H.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S. Algae–bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, P.; Gómez-Borraz, T.L.; Revah, S.; Morales, M. Methanotroph-microalgae co-culture for greenhouse gas mitigation: Effect of initial biomass ratio and methane concentration. Chemosphere 2020, 259, 127418. [Google Scholar] [CrossRef]
- Graziani, G.; Schiavo, S.; Nicolai, M.A.; Buono, S.; Fogliano, V.; Pinto, G.; Pollio, A. Microalgae as human food: Chemical and nutritional characteristics of the thermo-acidophilic microalga Galdieria sulphuraria. Food Funct. 2013, 4, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Sydney, E.B.; Schafranski, K.; Barretti, B.R.V.; Sydney, A.C.N.; Zimmerman, J.F.D.; Cerri, M.L.; Demiate, I.M. Biomolecules from extremophile microalgae: From genetics to bioprocessing of a new candidate for large-scale production. Process Biochem. 2019, 87, 37–44. [Google Scholar] [CrossRef]
- Cartin-Caballero, C.; Collet, C.; Gapes, D.; Gostomski, P.A.; Stott, M.B.; Carere, C.R. Simultaneous co-cultivation of the thermoacidophilic methanotroph, Methylacidiphilum sp. RTK17.1, and the microalga, Galdieria sp. RTK37.1, for single cell protein production. Eng. Microbiol. 2025, 5, 100229. [Google Scholar] [CrossRef]
- Wang, J.; Roberts, N.; Hilliard, M.; He, Q.P. A Microalgae-Methanotroph Coculture is a Promising Platform for Fuels and Chemical Production from Wastewater. Front. Energy Res. 2020, 8, 563352. [Google Scholar] [CrossRef]
- Montenegro-Herrera, C.A.; Portillo, F.V.-L.; Hernández-Chávez, G.T.; Martinez, A. Single-cell protein production potential with the extremophilic red microalgae Galdieria sulphuraria: Growth and biochemical characterization. J. Appl. Phycol. 2022, 34, 1341–1352. [Google Scholar] [CrossRef]
- Retta, B.; Iovinella, M.; Ciniglia, C. Significance and Applications of the Thermo-Acidophilic Microalga Galdieria sulphuraria (Cyanidiophytina, Rhodophyta). Plants 2024, 13, 1786. [Google Scholar] [CrossRef]
- Tsapekos, P.; Zhu, X.; Pallis, E.; Angelidaki, I. Proteinaceous methanotrophs for feed additive using biowaste as carbon and nutrients source. Bioresour. Technol. 2020, 313, 123646. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Cho, K.-S.; Kim, T.G. Density-dependent enhancement of methane oxidation activity and growth of Methylocystis sp. by a non-methanotrophic bacterium Sphingopyxis sp. Biotechnol. Rep. 2014, 4, 128–133. [Google Scholar] [CrossRef]
- Padmaperuma, G.; Kapoore, R.V.; Gilmour, D.J.; Vaidyanathan, S. Microbial consortia: A critical look at microalgae co-cultures for enhanced biomanufacturing. Crit. Rev. Biotechnol. 2018, 38, 690–703. [Google Scholar] [CrossRef]
- Santos, C.A.; Reis, A. Microalgal symbiosis in biotechnology. Appl. Microbiol. Biotechnol. 2014, 98, 5839–5846. [Google Scholar] [CrossRef]
- Sátiro, J.; Neto, A.D.S.; Tavares, J.; Marinho, I.; Magnus, B.; Kato, M.; Albuquerque, A.; Florencio, L. Impact of inoculum on domestic wastewater treatment in high-rate ponds in pilot-scale: Assessment of organic matter and nutrients removal, biomass growth, and content. Algal Res. 2025, 86, 103923. [Google Scholar] [CrossRef]
- Hill, E.A.; Chrisler, W.B.; Beliaev, A.S.; Bernstein, H.C. A flexible microbial co-culture platform for simultaneous utilization of methane and carbon dioxide from gas feedstocks. Bioresour. Technol. 2017, 228, 250–256. [Google Scholar] [CrossRef]
- Kerckhof, F.-M.; Sakarika, M.; Van Giel, M.; Muys, M.; Vermeir, P.; De Vrieze, J.; Vlaeminck, S.E.; Rabaey, K.; Boon, N. From Biogas and Hydrogen to Microbial Protein Through Co-Cultivation of Methane and Hydrogen Oxidizing Bacteria. Front. Bioeng. Biotechnol. 2021, 9, 733753. [Google Scholar] [CrossRef]
- Hammes, F.; Egli, T. Cytometric methods for measuring bacteria in water: Advantages, pitfalls and applications. Anal. Bioanal. Chem. 2010, 397, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Helmi, K.; Watt, A.; Jacob, P.; Ben-Hadj-Salah, I.; Henry, A.; Méheut, G.; Charni-Ben-Tabassi, N. Monitoring of three drinking water treatment plants using flow cytometry. Water Supply 2014, 14, 850–856. [Google Scholar] [CrossRef]
- Van Nevel, S.; Koetzsch, S.; Proctor, C.R.; Besmer, M.D.; Prest, E.I.; Vrouwenvelder, J.S.; Knezev, A.; Boon, N.; Hammes, F. Flow cytometric bacterial cell counts challenge conventional heterotrophic plate counts for routine microbiological drinking water monitoring. Water Res. 2017, 113, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Günther, S.; Hübschmann, T.; Rudolf, M.; Eschenhagen, M.; Röske, I.; Harms, H.; Müller, S. Fixation procedures for flow cytometric analysis of environmental bacteria. J. Microbiol. Methods 2008, 75, 127–134. [Google Scholar] [CrossRef]
- Badr, K.; Whelan, W.; He, Q.P.; Wang, J. Fast and easy quantitative characterization of methanotroph–photoautotroph cocultures. Biotechnol. Bioeng. 2020, 118, 703–714. [Google Scholar] [CrossRef]
- Buckeridge, E.; Caballero, C.C.; Smith, D.H.; Stott, M.B.; Carere, C.R. Substrate and nutrient manipulation during continuous cultivation of extremophilic algae, Galdieria sp. RTK37.1, substantially impacts biomass productivity and composition. Biotechnol. Bioeng. 2024, 121, 3428–3439. [Google Scholar] [CrossRef]
- Carere, C.R.; McDonald, B.; Peach, H.A.; Greening, C.; Gapes, D.J.; Collet, C.; Stott, M.B. Hydrogen oxidation influences glycogen accumulation in a Verrucomicrobial methanotroph. Front. Microbiol. 2019, 10, 1873. [Google Scholar] [CrossRef]
- Khadem, A.; Teeseling, M.C.; Niftrik, L.; Jetten, M.S.; den Camp, H.J.O.; Pol, A. Genomic and physiological analysis of carbon storage in the verrucomicrobial methanotroph “Ca. Methylacidiphilum fumariolicum” SolV. Front. Microbiol. 2012, 3, 345. [Google Scholar] [CrossRef]
- Rostkowski, K.H.; Pfluger, A.R.; Criddle, C.S. Stoichiometry and kinetics of the PHB-producing Type II methanotrophs Methylosinus trichosporium OB3b and Methylocystis parvus OBBP. Bioresour. Technol. 2013, 132, 71–77. [Google Scholar] [CrossRef]
- Dambruin, N.A.; Pronk, J.T.; Klijn, M.E. Application of process analytical technology for real-time monitoring of synthetic co-culture bioprocesses. Anal. Bioanal. Chem. 2025. [Google Scholar] [CrossRef] [PubMed]
- García, B.G.; Fernández-Manteca, M.G.; Gómez-Galdós, C.; Álvarez, S.D.; Monteoliva, A.P.; López-Higuera, J.M.; Algorri, J.F.; Ocampo-Sosa, A.A.; Rodríguez-Cobo, L.; Cobo, A. Integration of Fluorescence Spectroscopy into a Photobioreactor for the Monitoring of Cyanobacteria. Biosensors 2025, 15, 128. [Google Scholar] [CrossRef] [PubMed]
- Mora-Sánchez, J.F.; Ribes, J.; González-Camejo, J.; Seco, A.; Ruano, M.V. Towards Optimisation of Microalgae Cultivation Through Monitoring and Control in Membrane Photobioreactor Systems. Water 2024, 16, 155. [Google Scholar] [CrossRef]
- Reyes, L.P.; Havlik, I.; Beutel, S. Software sensors in the monitoring of microalgae cultivations. Rev. Environ. Sci. Biotechnol. 2024, 23, 67–92. [Google Scholar] [CrossRef]
- Satiro, J.; Neto, A.G.D.S.; Marinho, T.; Sales, M.; Marinho, I.; Kato, M.T.; Simões, R.; Albuquerque, A.; Florencio, L. The Role of the Microalgae–Bacteria Consortium in Biomass Formation and Its Application in Wastewater Treatment Systems: A Com-prehensive Review. Appl. Sci. 2024, 14, 6083. [Google Scholar] [CrossRef]
- Stone, K.A.; Shah, D.; Kim, M.H.; Roberts, N.R.M.; He, Q.P.; Wang, J. A novel soft sensor approach for estimating individual biomass in mixed cultures. Biotechnol. Prog. 2017, 33, 347–354. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Li, N.; Wang, Y.; Yu, R.; Zhu, G.; Zeng, R.J. Mixotrophic cultivation of microalgae using biogas as the substrate. Environ. Sci. Technol. 2022, 56, 3669–3677. [Google Scholar] [CrossRef]
- Carere, C.R.; Hards, K.; Houghton, K.M.; Power, J.F.; McDonald, B.; Collet, C.; Gapes, D.J.; Sparling, R.; Boyd, E.S.; Cook, G.M.; et al. Mixotrophy drives niche expansion of verrucomicrobial methanotrophs. ISME J. 2017, 11, 2599–2610. [Google Scholar] [CrossRef]
- Carere, C.R.; Hards, K.; Wigley, K.; Carman, L.; Houghton, K.M.; Cook, G.M.; Stott, M.B. Growth on formic acid is dependent on intracellular pH homeostasis for the thermoacidophilic methanotroph Methylacidiphilum sp. RTK17.1. Front. Microbiol. 2021, 12, 651744. [Google Scholar] [CrossRef] [PubMed]
- Graverholt, O.S.; Eriksen, N.T. Heterotrophic high-cell-density fed-batch and continuous-flow cultures of Galdieria sulphuraria and production of phycocyanin. Appl. Microbiol. Biotechnol. 2007, 77, 69–75. [Google Scholar] [CrossRef]
- Henkanatte-Gedera, S.M.; Selvaratnam, T.; Karbakhshravari, M.; Myint, M.; Nirmalakhandan, N.; Van Voorhies, W.; Lam-mers, P.J. Removal of dissolved organic carbon and nutrients from urban wastewaters by Galdieria sulphuraria: Laboratory to field scale demonstration. Algal Res. 2017, 24, 450–456. [Google Scholar] [CrossRef]
- Awala, S.I.; Gwak, J.-H.; Kim, Y.-M.; Kim, S.-J.; Strazzulli, A.; Dunfield, P.F.; Yoon, H.; Kim, G.-J.; Rhee, S.-K. Verrucomicrobial methanotrophs grow on diverse C3 compounds and use a homolog of particulate methane monooxygenase to oxidize acetone. ISME J. 2021, 15, 3636–3647. [Google Scholar] [CrossRef] [PubMed]
- Baer, S.; Heining, M.; Schwerna, P.; Buchholz, R.; Hübner, H. Optimization of spectral light quality for growth and product formation in different microalgae using a continuous photobioreactor. Algal Res. 2016, 14, 109–115. [Google Scholar] [CrossRef]
- Gregor, J.; Maršálek, B. Freshwater phytoplankton quantification by chlorophyll a: A comparative study of in vitro, in vivo and in situ methods. Water Res. 2004, 38, 517–522. [Google Scholar] [CrossRef]
- Gregor, J.; Maršálek, B. A simple in vivo fluorescence method for the selective detection and quantification of freshwater cyanobacteria and eukaryotic algae. Acta Hydrochim. Hydrobiol. 2005, 33, 142–148. [Google Scholar] [CrossRef]
- Stewart, D.E.; Farmer, F.H. Extraction, identification, and quantitation of phycobiliprotein pigments from phototrophic plankton. Limnol. Oceanogr. 1984, 29, 392–397. [Google Scholar] [CrossRef]
- Schagerl, M.; Siedler, R.; Konopáčová, E.; Ali, S.S. Estimating Biomass and Vitality of Microalgae for Monitoring Cultures: A Roadmap for Reliable Measurements. Cells 2022, 11, 2455. [Google Scholar] [CrossRef]
- Fekete, G.; Sebők, A.; Klátyik, S.; Varga, Z.I.; Grósz, J.; Czinkota, I.; Székács, A.; Aleksza, L. Comparative Analysis of Laboratory-Based and Spectroscopic Methods Used to Estimate the Algal Density of Chlorella vulgaris. Microorganisms 2024, 12, 1050. [Google Scholar] [CrossRef]
- Vítová, M.; Goecke, F.; Sigler, K.; Řezanka, T. Lipidomic analysis of the extremophilic red alga Galdieria sulphuraria in response to changes in pH. Algal Res. 2016, 13, 218–226. [Google Scholar] [CrossRef]
- Islam, T.; Jensen, S.; Reigstad, L.J.; Larsen, O.; Birkeland, N.-K. Methane oxidation at 55 degrees C and pH 2 by a thermoaci-dophilic bacterium belonging to the Verrucomicrobia phylum. Proc. Natl. Acad. Sci. USA 2008, 105, 300–304. [Google Scholar] [CrossRef] [PubMed]
- den Camp, H.J.O.; Islam, T.; Stott, M.B.; Harhangi, H.R.; Hynes, A.; Schouten, S.; Jetten, M.S.; Birkeland, N.K.; Pol, A.; Dunfield, P.F. Environmental, genomic and taxonomic perspectives on methanotrophic Verrucomicrobia. Environ. Microbiol. Rep. 2009, 1, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.; Gilissen, L.; Rinzema, A.; Vermuë, M.H.; Wijffels, R.H. Modeling microalgal flocculation and sedimentation. Bioresour. Technol. 2013, 144, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Dunfield, P.F.; Yuryev, A.; Senin, P.; Smirnova, A.V.; Stott, M.B.; Hou, S.; Ly, B.; Saw, J.H.; Zhou, Z.; Ren, Y.; et al. Methane oxidation by an ex-tremely acidophilic bacterium of the phylum Verrucomicrobia. Nature 2007, 450, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Quispe, J.; Concha, F.; Toledo, P.G. Discrete sedimentation model for ideal suspensions. Chem. Eng. J. 2000, 80, 135–140. [Google Scholar] [CrossRef]
- Powell, R.J.; Hill, R.T. Rapid Aggregation of Biofuel-Producing Algae by the Bacterium Bacillus sp. Strain RP1137. Appl. Environ. Microbiol. 2013, 79, 6093–6101. [Google Scholar] [CrossRef]
- Babiak, W.; Krzemińska, I. Extracellular polymeric substances (EPS) as microalgal bioproducts: A review of factors affecting EPS synthesis and application in flocculation processes. Energies 2021, 14, 4007. [Google Scholar] [CrossRef]
- Zhu, B.; Wei, D.; Pohnert, G. The thermoacidophilic red alga Galdieria sulphuraria is a highly efficient cell factory for ammonium recovery from ultrahigh-NH4+ industrial effluent with co-production of high-protein biomass by photo-fermentation. Chem. Eng. J. 2022, 438, 135598. [Google Scholar] [CrossRef]
- Cantera, S.; Sanchez-Andrea, I.; Lebrero, R.; Garcia-Encina, P.A.; Stams, A.J.M.; Munoz, R. Multi-production of high added market value metabolites from diluted methane emissions via methanotrophic extremophiles. Biores. Technol. 2018, 267, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Khadem, A.; Pol, A.; Wieczorek, A.; Mohammadi, S.S.; Francoijs, K.J.; Stunnenberg, H.G.; Jetten, M.S.; den Camp, H.J.O. Autotrophic methanotrophy in verrucomicrobia: Methylacidiphilum fumariolicum SolV uses the Calvin-Benson-Bassham cycle for carbon dioxide fixation. J. Bacteriol. 2011, 193, 4438–4446. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids. 2018, 50, 1685–1695. [Google Scholar] [CrossRef]
- Pol, A.; Heijmans, K.; Harhangi, H.R.; Tedesco, D.; Jetten, M.S.M.; den Camp, H.J.M.O. Methanotrophy below pH 1 by a new Verrucomicrobia species. Nature 2007, 450, 874. [Google Scholar] [CrossRef]
- Oesterhelt, C.; Schmälzlin, E.; Schmitt, J.M.; Lokstein, H. Regulation of photosynthesis in the unicellular acidophilic red alga Galdieria sulphuraria. Plant J. 2007, 51, 500–511. [Google Scholar] [CrossRef]
- Sinetova, M.P.; Markelova, A.G.; Los, D.A. The effect of nitrogen starvation on the ultrastructure and pigment composition of chloroplasts in the acidothermophilic microalga Galdieria sulphuraria. Russ. J. Plant Physiol. 2006, 53, 153–162. [Google Scholar] [CrossRef]
- Salbitani, G.; Cipolletta, S.; Vona, V.; Di Martino, C.; Carfagna, S. Heterotrophic cultures of Galdieria phlegrea shift to autotrophy in the presence or absence of glycerol. J. Plant Growth Regul. 2020, 40, 371–378. [Google Scholar] [CrossRef]
- Carvalho, Â.R.; Bazana, L.C.G.; Ferrão, M.F.; Fuentefria, A.M. Curve fitting and linearization of UV–Vis spectrophotometric measurements to estimate yeast in inoculum preparation. Anal. Biochem. 2021, 625, 114216. [Google Scholar] [CrossRef]
- Myers, J.A.; Curtis, B.S.; Curtis, W.R. Improving accuracy of cell and chromophore concentration measurements using optical density. BMC Biophys. 2013, 6, 4. [Google Scholar] [CrossRef]
- Thatipamala, R.; Hill, G.A. Spectrophotometric method for high biomass concentration measurements. Biotechnol. Bioeng. 1991, 38, 1007–1011. [Google Scholar] [CrossRef]
- Hakiki, R.; Purnama, I.; Zevi, Y.; Muntalif, B.S. Limiting Factors of Simultaneous Measurement Method for Turbidity and Total Suspended Solids Based on Image Processing Approaches. J. Phys. Conf. Ser. 2024, 2705, 012021. [Google Scholar] [CrossRef]
- Kennedy, M.J.; Thakur, M.S.; Wang, D.I.C.; Stephanopoulos, G.N. Estimating cell concentration in the presence of suspended solids: A light scatter technique. Biotechnol. Bioeng. 1992, 40, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, S.; Macaloney, G.; Vaughan, J.; McNeil, B.; Harvey, L.M. Monitoring of Submerged Bioprocesses. Crit. Rev. Biotechnol. 1999, 19, 277–316. [Google Scholar] [CrossRef]
- Bertilsson, S.; Berglund, O.; Karl, D.M.; Chisholm, S.W. Elemental composition of marine Prochlorococcus and Synechococcus: Implications for the ecological stoichiometry of the sea. Limnol. Oceanogr. 2003, 48, 1721–1731. [Google Scholar] [CrossRef]
- Kalyuzhnaya, M.G.; Khmelenina, V.; Eshinimaev, B.; Sorokin, D.; Fuse, H.; Lidstrom, M.; Trotsenko, Y. Classification of ha-lo(alkali)philic and halo(alkali)tolerant methanotrophs provisionally assigned to the genera Methylomicrobium and Methylobacter and emended description of the genus Methylomicrobium. Int. J. Syst. Evol. Microbiol. 2008, 58, 591–596. [Google Scholar] [CrossRef]
- Awala, S.I.; Bellosillo, L.A.; Gwak, J.-H.; Nguyen, N.-L.; Kim, S.-J.; Lee, B.-H.; Rhee, S.-K. Methylococcus geothermalis sp. nov., a methanotroph isolated from a geothermal field in the Republic of Korea. Int. J. Syst. Evol. Microbiol. 2020, 70, 5520–5530. [Google Scholar] [CrossRef]
- Azaman, S.N.A.; Nagao, N.; Yusoff, F.; Tan, S.W.; Yeap, S.K. A comparison of the morphological and biochemical characteristics of Chlorella sorokiniana and Chlorella zofingiensis cultured under photoautotrophic and mixotrophic conditions. PeerJ 2017, 5, e3473. [Google Scholar] [CrossRef] [PubMed]
- Stadnichuk, I.N.; Rakhimberdieva, M.G.; Bolychevtseva, Y.V.; Yurina, N.P.; Karapetyan, N.V.; Selyakh, I.O. Inhibition by glucose of chlorophyll a and phycocyanobilin biosynthesis in the unicellular red alga Galdieria partita at the stage of coproporphyrinogen III formation. Plant Sci. 1998, 136, 11–23. [Google Scholar] [CrossRef]
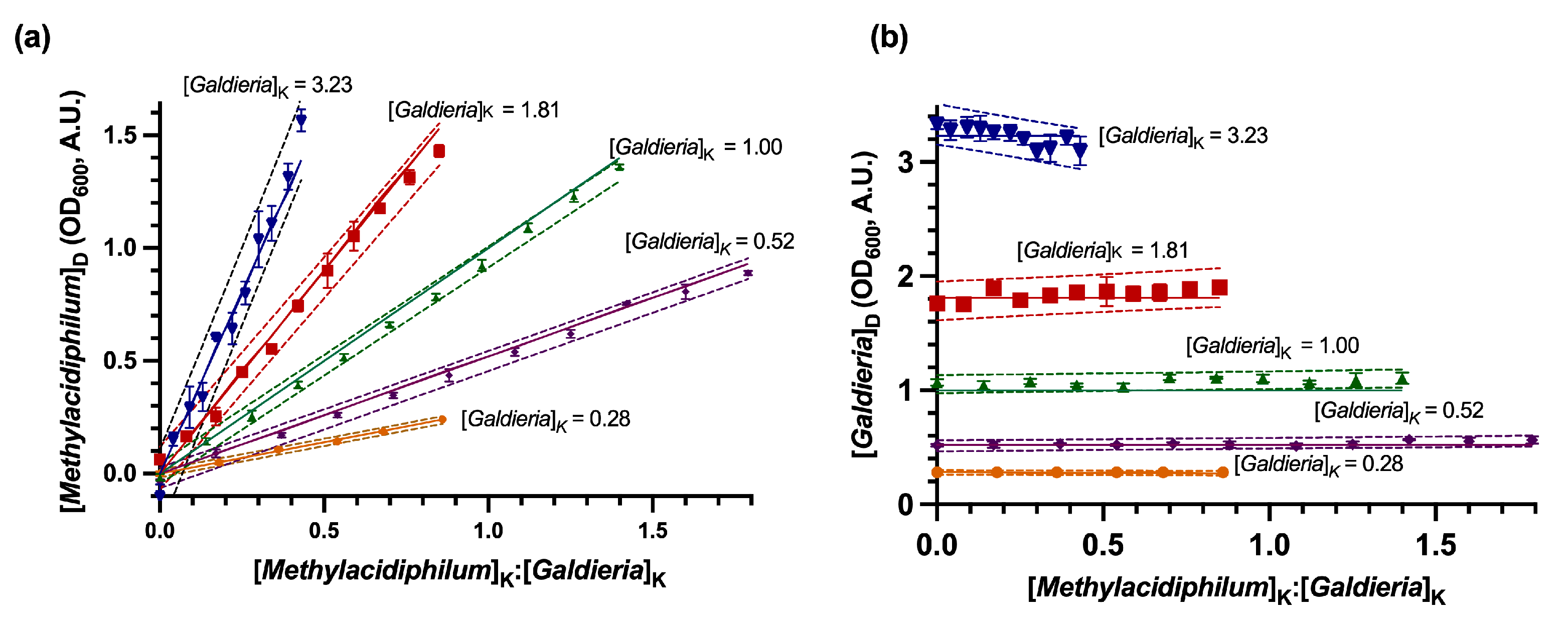
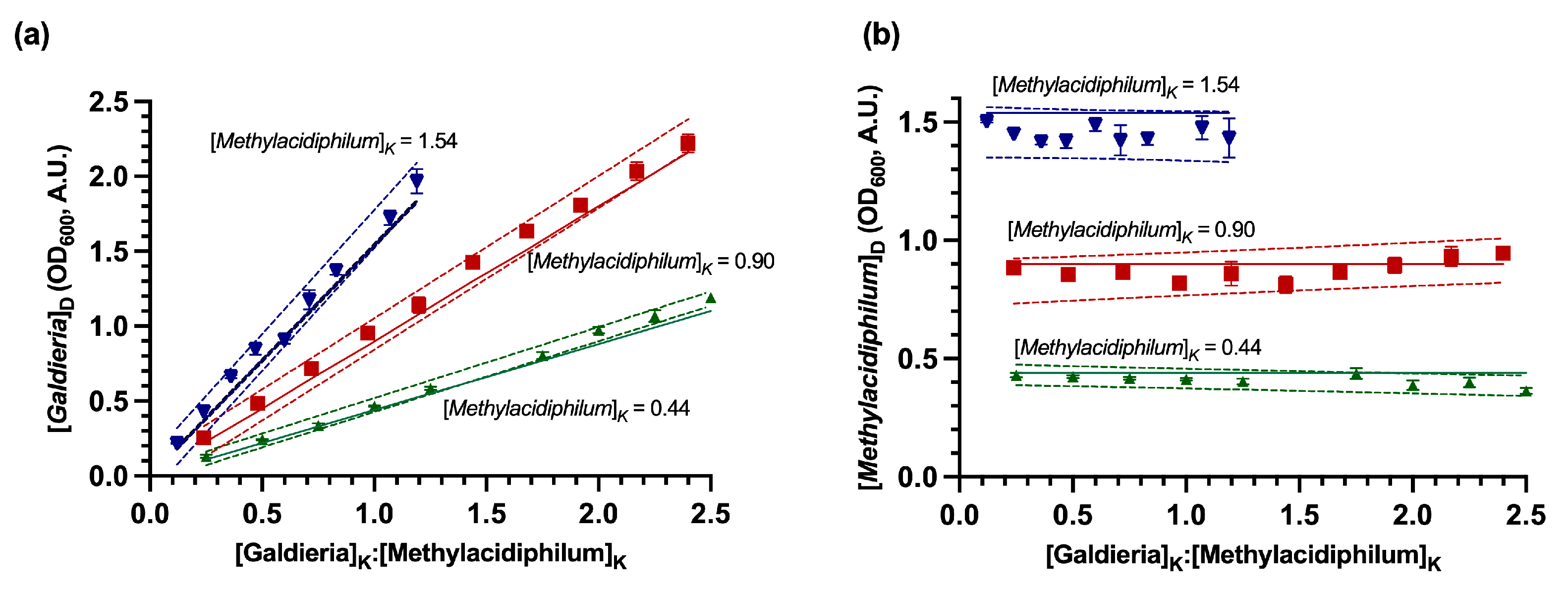


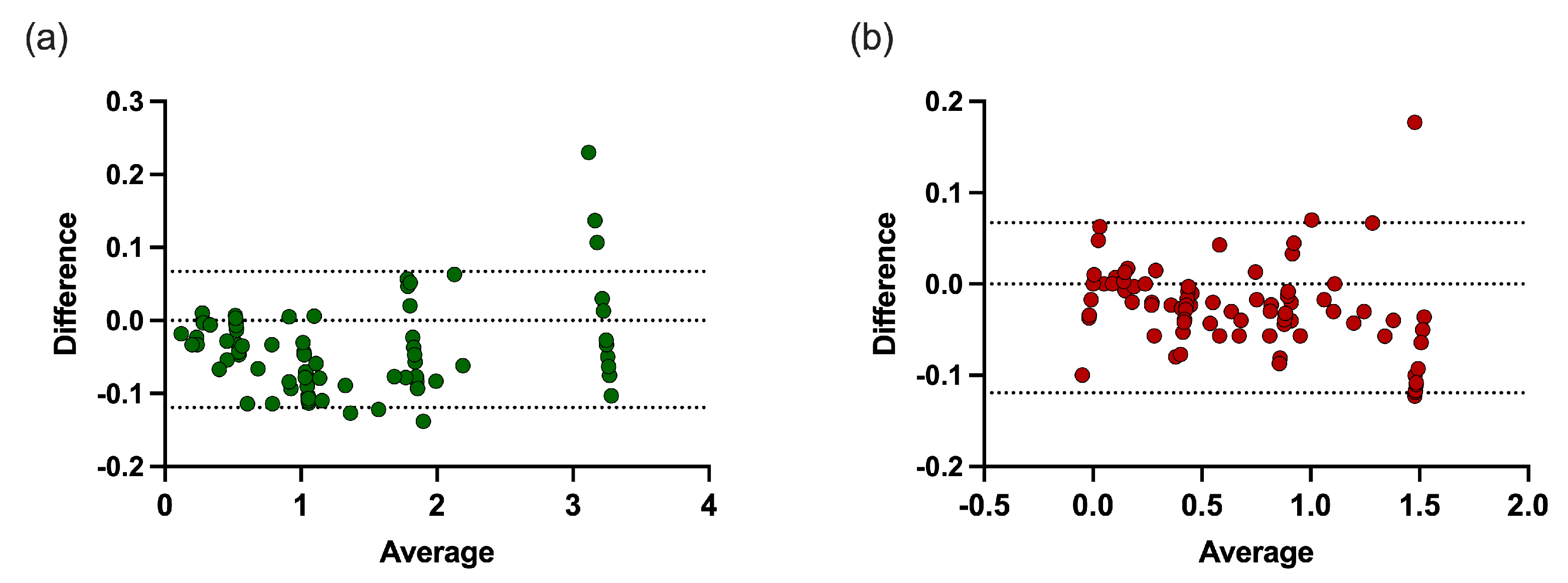
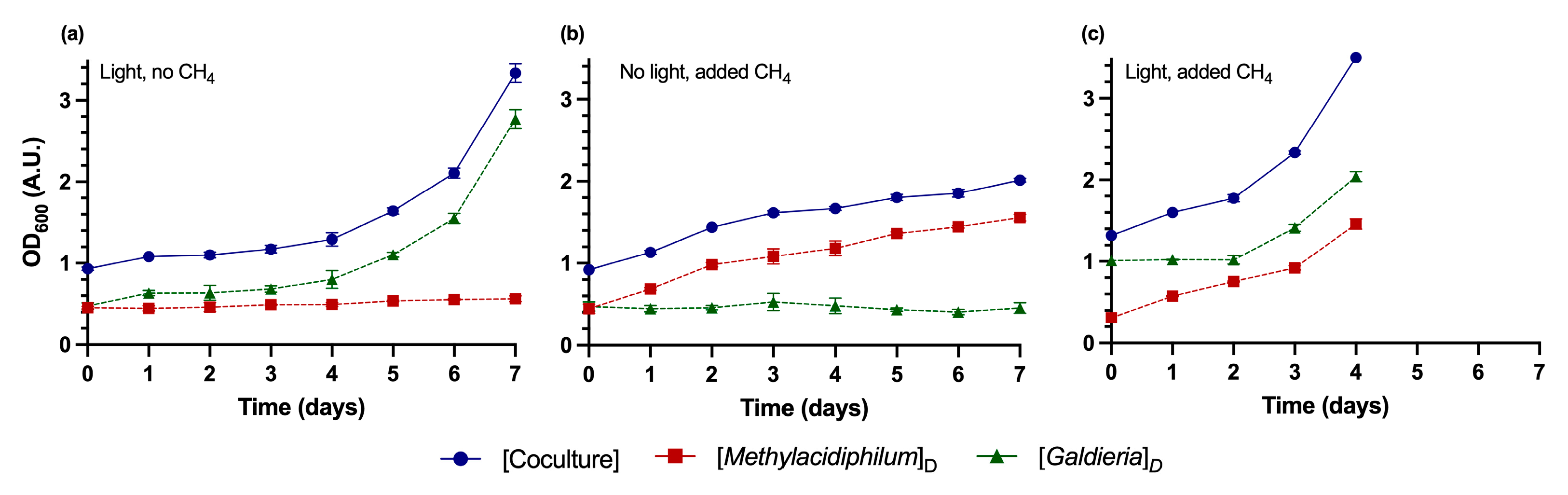
| Known Values (A.U.) | Methylacidiphilum sp. RTK17.1 Error | Galdieria sp. RTK37.1 Error | |||
|---|---|---|---|---|---|
| RTK37.1 (A.U.) | RTK17.1 (A.U.) | Absolute (A.U.) | Relative (%) | Absolute (A.U.) | Relative (%) |
| <0.5 | <0.5 | ±0.10 | <10 | ±0.05 | <20 |
| <0.5 | 0.5–1.5 | ±0.10 | <10 | ±0.10 | <10 |
| 0.5–1.0 | <1.0 | ±0.10 | <10 | ±0.10 | <10 |
| 0.5–1.0 | 1.0–1.5 | ±0.10 | <10 | ±0.10 | 10–15 |
| 1.0–2.0 | <0.5 | ±0.10 | <10 | ±0.10 | <10 |
| 1.0–2.0 | 0.5–1.0 | ±0.10 | <10 | ±0.10 | <10 |
| 1.0–2.0 | 1.0–1.5 | ±0.15 | <10 | ±0.15 | <10 |
| 2.0–3.2 | <1.0 | ±0.10 | <20 | ±0.10 | <5 |
| 2.0–3.2 | 1.0–1.5 | ±0.20 | <10 | ±0.15 | <10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cartin-Caballero, C.; Collet, C.; Gapes, D.; Gostomski, P.A.; Stott, M.B.; Carere, C.R. DSOF: A Rapid Method to Determine the Abundance of Microalgae and Methanotrophic Bacteria in Coculture Using a Combination of Differential Sedimentation, Optical Density, and Fluorescence. Bioengineering 2025, 12, 1000. https://doi.org/10.3390/bioengineering12091000
Cartin-Caballero C, Collet C, Gapes D, Gostomski PA, Stott MB, Carere CR. DSOF: A Rapid Method to Determine the Abundance of Microalgae and Methanotrophic Bacteria in Coculture Using a Combination of Differential Sedimentation, Optical Density, and Fluorescence. Bioengineering. 2025; 12(9):1000. https://doi.org/10.3390/bioengineering12091000
Chicago/Turabian StyleCartin-Caballero, Carlos, Christophe Collet, Daniel Gapes, Peter A. Gostomski, Matthew B. Stott, and Carlo R. Carere. 2025. "DSOF: A Rapid Method to Determine the Abundance of Microalgae and Methanotrophic Bacteria in Coculture Using a Combination of Differential Sedimentation, Optical Density, and Fluorescence" Bioengineering 12, no. 9: 1000. https://doi.org/10.3390/bioengineering12091000
APA StyleCartin-Caballero, C., Collet, C., Gapes, D., Gostomski, P. A., Stott, M. B., & Carere, C. R. (2025). DSOF: A Rapid Method to Determine the Abundance of Microalgae and Methanotrophic Bacteria in Coculture Using a Combination of Differential Sedimentation, Optical Density, and Fluorescence. Bioengineering, 12(9), 1000. https://doi.org/10.3390/bioengineering12091000









