Pregnancy-Related Spinal Biomechanics: A Review of Low Back Pain and Degenerative Spine Disease
Abstract
1. Introduction
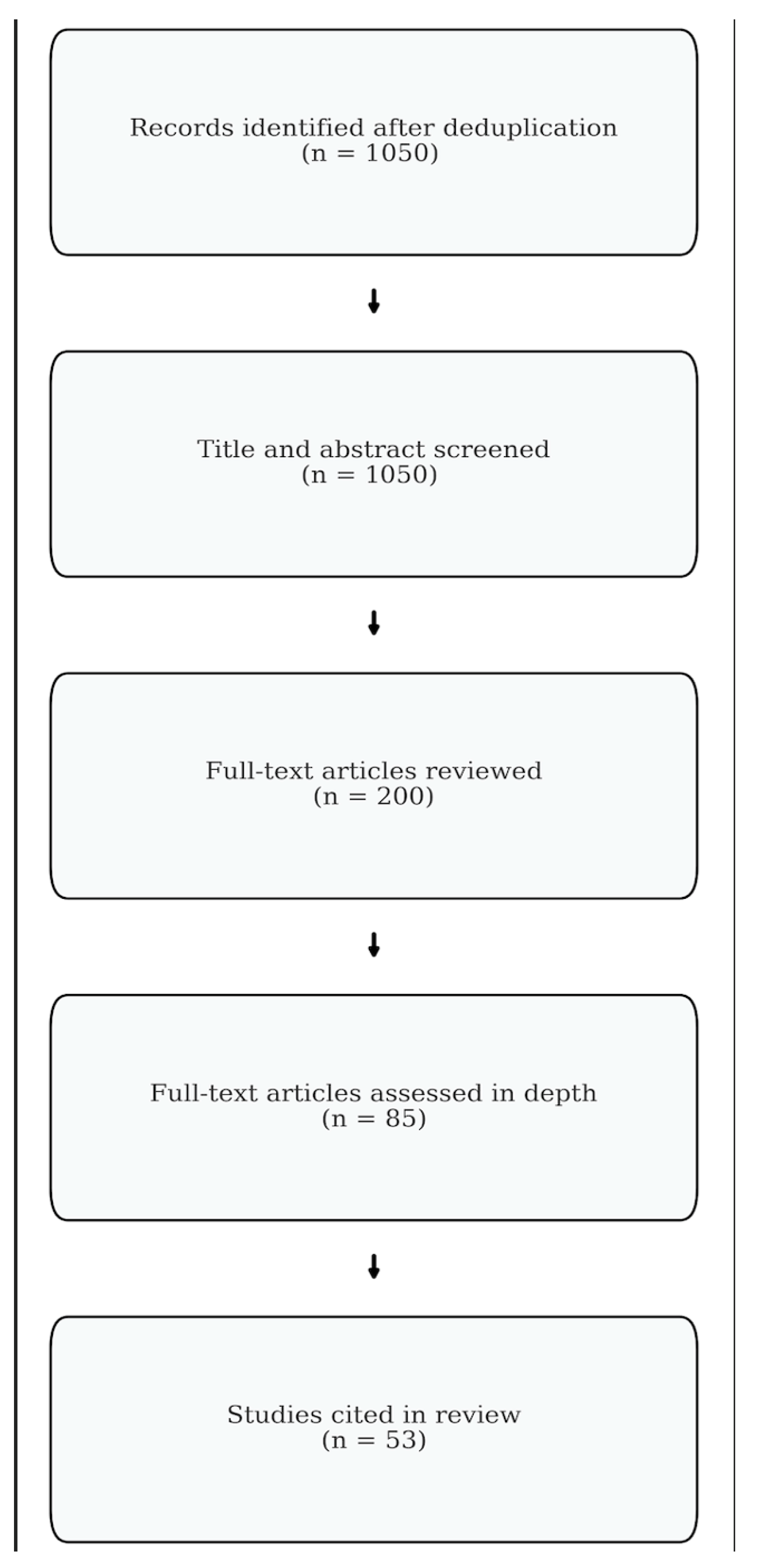
2. Methods
2.1. Study Design
2.2. Search Strategy
2.3. Study Selection
3. Results
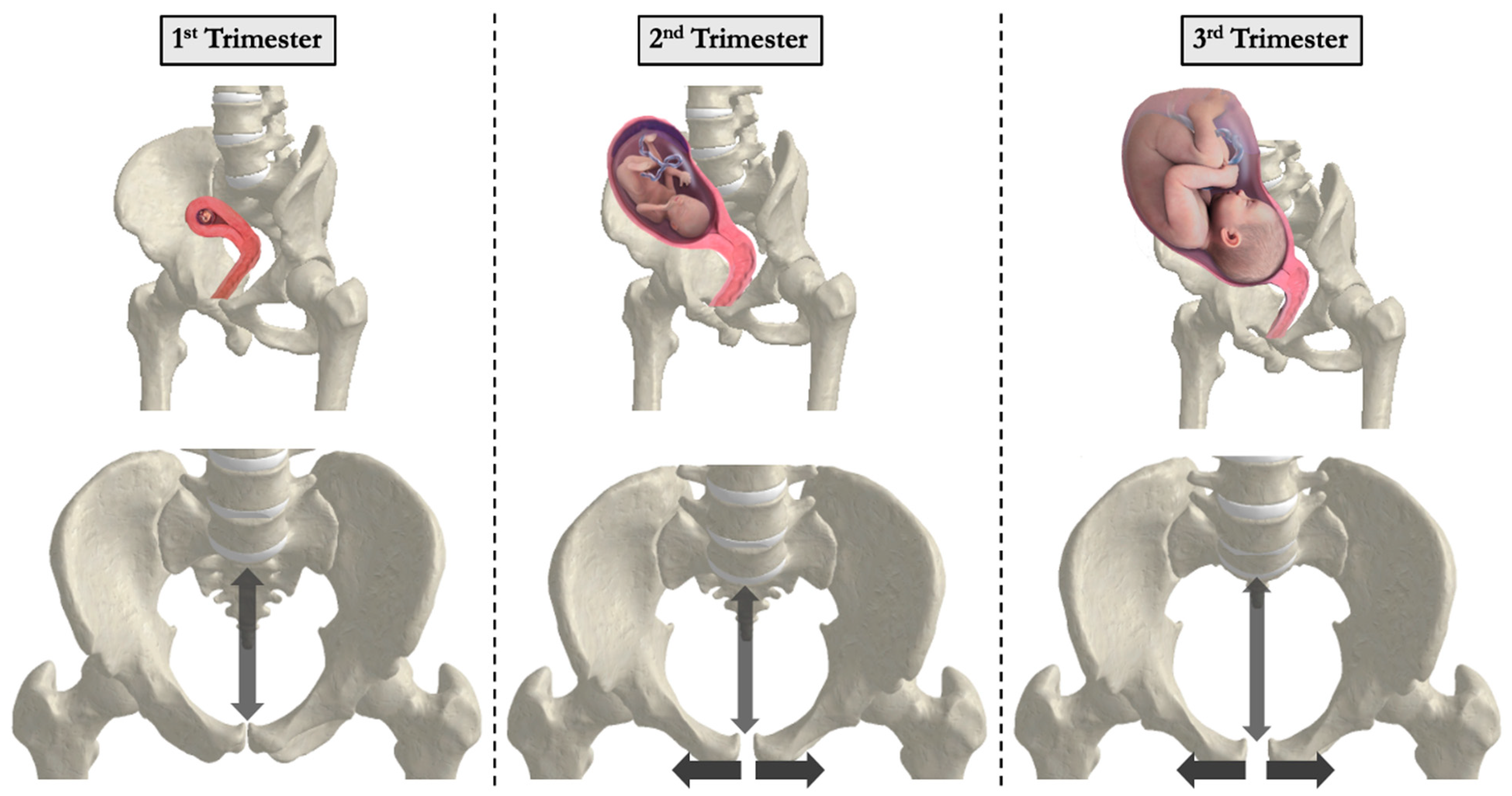
3.1. Anatomic and Physiologic Changes During Pregnancy
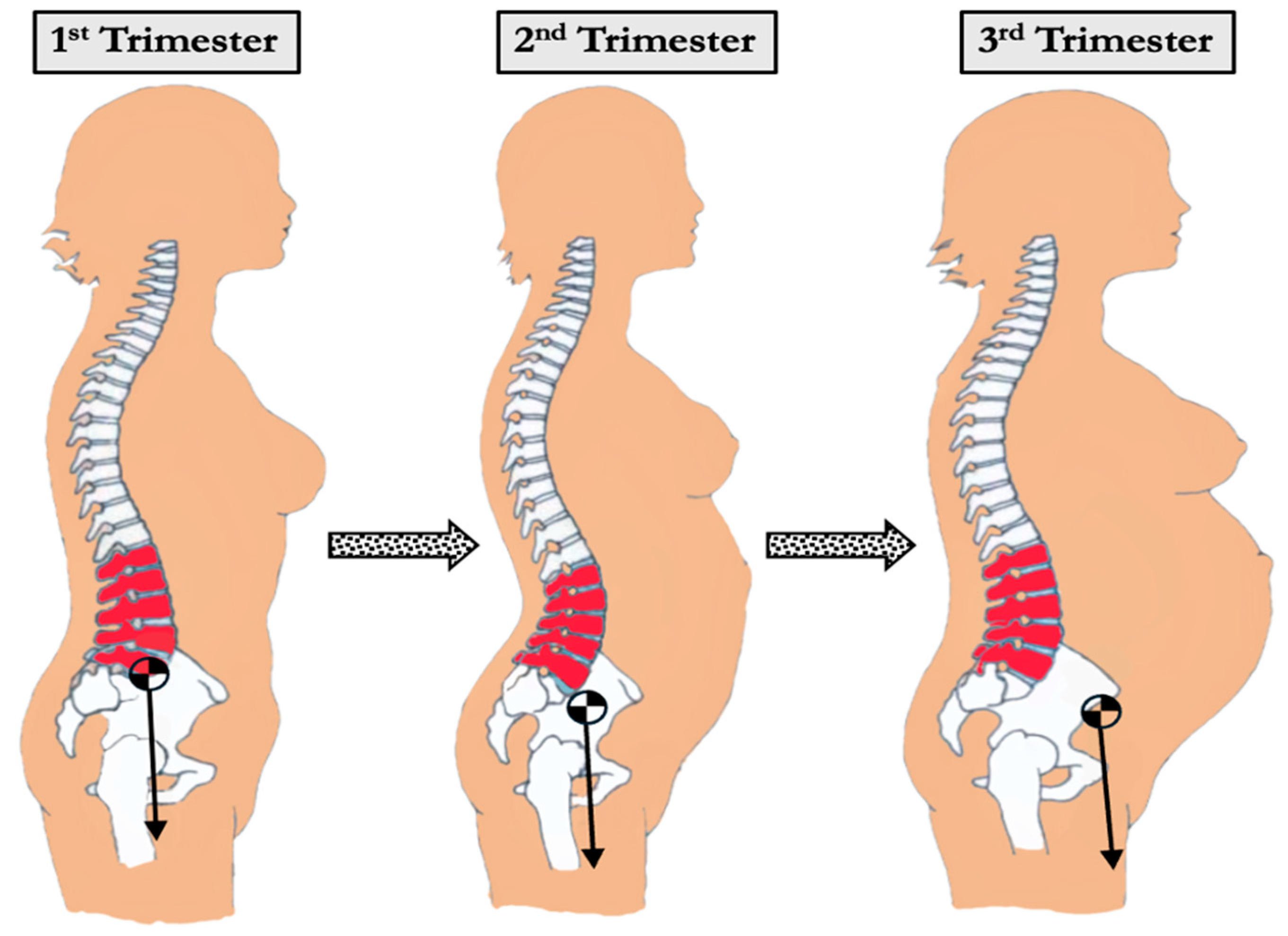
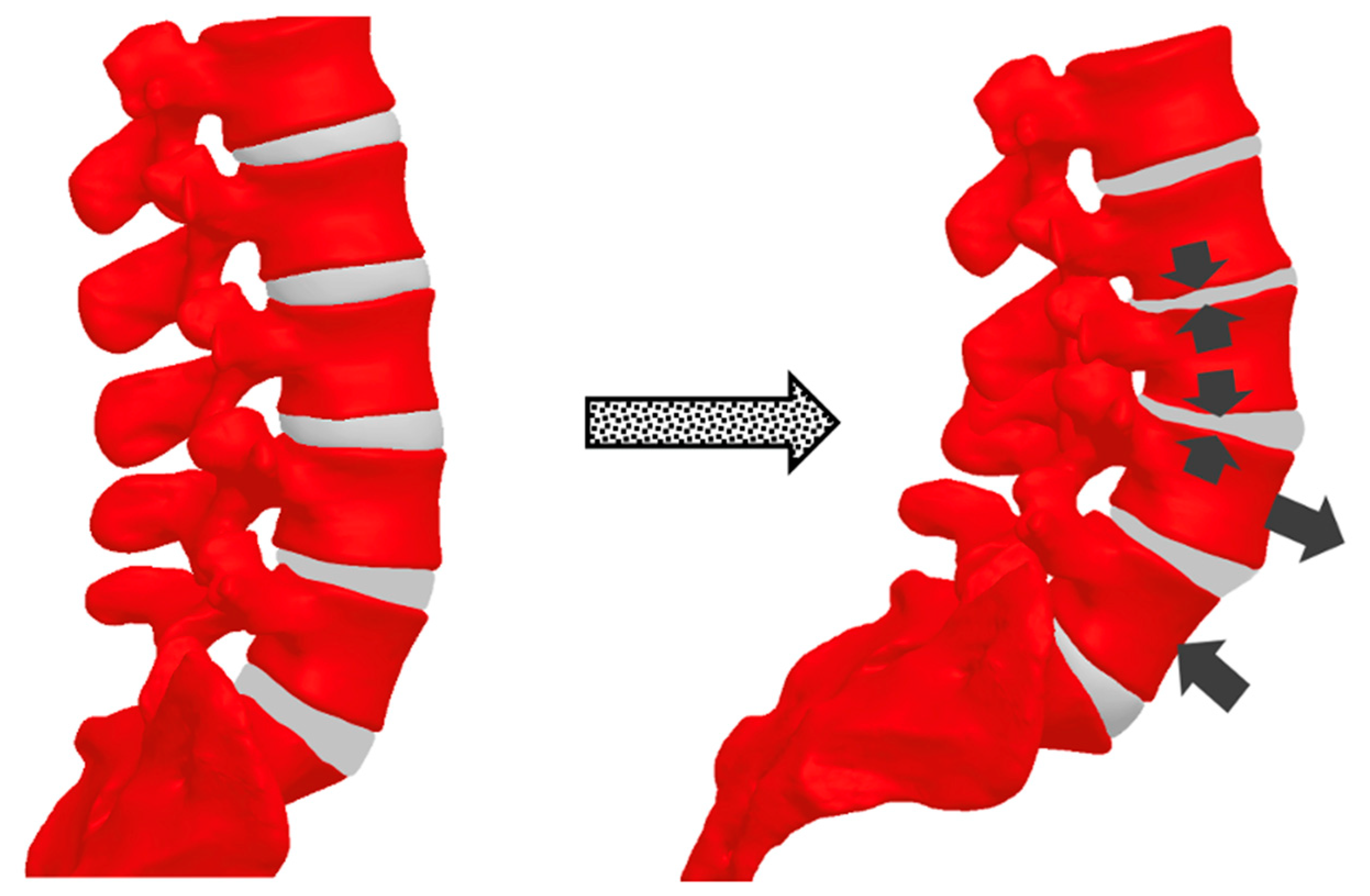
3.2. Biomechanical Changes During Pregnancy
3.3. Etiology of Pregnancy-Related Back Pain
3.4. Degenerative Spine Disease
3.5. Treatment of Pregnancy-Related Lower Back Pain
4. Future Directions
5. Limitations
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Fast, A.; Shapiro, D.; Ducommun, E.J.; Friedmann, L.W.; Bouklas, T.; Floman, Y. Low-back Pain in Pregnancy. Spine 1987, 12, 368. [Google Scholar] [CrossRef]
- Olsson, C.; Lena, N.W. Health-related quality of life and physical ability among pregnant women with and without back pain in late pregnancy. Acta Obs. Gynecol. Scand. 2004, 83, 351–357. [Google Scholar] [CrossRef]
- Yamada, T.; Yamato, Y.; Hasegawa, T.; Yoshida, G.; Yasuda, T.; Banno, T.; Arima, H.; Oe, S.; Mihara, Y.; Ushirozako, H.; et al. Association between Pelvic Parameters and Vaginal Delivery. Asian Spine J. 2021, 16, 248–253. [Google Scholar] [CrossRef]
- Oura, P.; Paananen, M.; Auvinen, J.; Niinimäki, J.; Niinimäki, M.; Karppinen, J.; Junno, J.-A. Gravidity, Parity, and Vertebral Dimensions in the Northern Finland Birth Cohort 1966. Spine 2018, 43, E1102. [Google Scholar] [CrossRef]
- Calguneri, M.B.H.A.; Bird, H.A.; Wright, V. Changes in joint laxity occurring during pregnancy. Ann. Rheum. Dis. 1982, 41, 126–128. [Google Scholar] [CrossRef]
- Hartmann, S.; Bung, P. Physical exercise during pregnancy—Physiological considerations and recommendations. J. Perinat. Med. 1999, 27, 204–215. [Google Scholar] [CrossRef]
- Artal, R.; O’Toole, M. Guidelines of the American College of Obstetricians and Gynecologists for exercise during pregnancy and the postpartum period. Br. J. Sports Med. 2003, 37, 6–12. [Google Scholar] [CrossRef]
- Dehghan, F.; Haerian, B.S.; Muniandy, S.; Yusof, A.; Dragoo, J.L.; Salleh, N. The effect of relaxin on the musculoskeletal system. Scand. J. Med. Sci. Sports 2014, 24, e220–e229. [Google Scholar] [CrossRef]
- Conder, R.; Zamani, R.; Akrami, M. The Biomechanics of Pregnancy: A Systematic Review. J. Funct. Morphol. Kinesiol. 2019, 4, 72. [Google Scholar] [CrossRef]
- Branco, M.; Santos-Rocha, R.; Vieira, F. Biomechanics of Gait during Pregnancy. Sci. World J. 2014, 2014, 527940. [Google Scholar] [CrossRef]
- Pauk, J.; Swinarska, D. The impact of body mass on spine alterations in pregnant women: A preliminary study. Technol. Health Care 2018, 26 (Suppl. S2), 665–669. [Google Scholar] [CrossRef]
- Romero-Vargas, S.; Zárate-Kalfópulos, B.; Otero-Cámara, E.; Rosales-Olivarez, L.; Alpízar-Aguirre, A.; Morales-Hernández, E.; Reyes-Sánchez, A. The impact of body mass index and central obesity on the spino-pelvic parameters: A correlation study. Eur. Spine J. 2013, 22, 878–882. [Google Scholar] [CrossRef]
- Whitcome, K.K.; Shapiro, L.J.; Lieberman, D.E. Fetal load and the evolution of lumbar lordosis in bipedal hominins. Nature 2007, 450, 1075–1078. [Google Scholar] [CrossRef]
- Yoo, H.; Shin, D.; Song, C. Changes in the spinal curvature, degree of pain, balance ability, and gait ability according to pregnancy period in pregnant and nonpregnant women. J. Phys. Ther. Sci. 2015, 27, 279–284. [Google Scholar] [CrossRef]
- Wang, T.; Wang, H.; Liu, H.; Ma, L.; Liu, F.Y.; Ding, W.Y. Sagittal spinopelvic parameters in 2-level lumbar degenerative spondylolisthesis: A retrospective study. Medicine 2016, 95, e5417. [Google Scholar] [CrossRef]
- Foti, T.; Davids, J.R.; Bagley, A. A Biomechanical Analysis of Gait During Pregnancy. J. Bone Jt. Surg. Am. 2000, 82, 625. [Google Scholar] [CrossRef]
- Wu, W.; Meijer, O.G.; Lamoth, C.J.; Uegaki, K.; van Dieën, J.H.; Wuisman, P.I.; de Vries, J.I.; Beek, P.J. Gait coordination in pregnancy: Transverse pelvic and thoracic rotations and their relative phase. Clin. Biomech. 2004, 19, 480–488. [Google Scholar] [CrossRef]
- Borg-Stein, J.; Dugan, S.A.; Gruber, J. Musculoskeletal aspects of pregnancy. Am. J. Phys. Med. Rehabil. 2005, 84, 180–192. [Google Scholar] [CrossRef]
- Fast, A.; Hertz, G. Nocturnal low back pain in pregnancy: Polysomnographic correlates. Am. J. Reprod. Immunol. 1992, 28, 251–253. [Google Scholar] [CrossRef]
- Mens, J.M.A.; Pool-Goudzwaard, A.; Stam, H.J. Mobility of the Pelvic Joints in Pregnancy-Related Lumbopelvic Pain: A Systematic Review. Obs. Gynecol. Surv. 2009, 64, 200. [Google Scholar] [CrossRef]
- Ritchie, J.R. Orthopedic considerations during pregnancy. Clin. Obs. Gynecol. 2003, 46, 456–466. [Google Scholar] [CrossRef]
- Gutke, A.; Östgaard, H.C.; Öberg, B. Predicting persistent pregnancy-related low back pain. Spine 2008, 33, E386–E393. [Google Scholar] [CrossRef]
- Michoński, J.; Walesiak, K.; Pakuła, A.; Glinkowski, W.; Sitnik, R. Monitoring of spine curvatures and posture during pregnancy using surface topography—Case study and suggestion of method. Scoliosis Spinal Disord. 2016, 11 (Suppl. S2), 31. [Google Scholar] [CrossRef]
- McCrory, J.L.; Chambers, A.J.; Daftary, A.; Redfern, M.S. The pregnant “waddle”: An evaluation of torso kinematics in pregnancy. J. Biomech. 2014, 47, 2964–2968. [Google Scholar] [CrossRef]
- Bird, A.R.; Menz, H.B.; Hyde, C.C. The effect of pregnancy on footprint parameters: A prospective investigation. J. Am. Podiatr. Med. Assoc. 1999, 89, 405–409. [Google Scholar] [CrossRef]
- Krkeljas, Z. Changes in gait and posture as factors of dynamic stability during walking in pregnancy. Hum. Mov. Sci. 2018, 58, 315–320. [Google Scholar] [CrossRef]
- Matsuda, N.; Kitagaki, K.; Perrein, E.; Tsuboi, Y.; Ebina, A.; Kondo, Y.; Murata, S.; Isa, T.; Okumura, M.; Kawaharada, R.; et al. Association Between Excessive Weight Gain During Pregnancy and Persistent Low Back and Pelvic Pain After Delivery. Spine 2020, 45, 319. [Google Scholar] [CrossRef]
- Vermani, E.; Mittal, R.; Weeks, A. Pelvic Girdle Pain and Low Back Pain in Pregnancy: A Review. Pain Pract. 2010, 10, 60–71. [Google Scholar] [CrossRef]
- Casagrande, D.; Gugala, Z.; Clark, S.M.; Lindsey, R.W. Low Back Pain and Pelvic Girdle Pain in Pregnancy. J. Am. Acad. Orthop. Surg. 2015, 23, 539–549. [Google Scholar] [CrossRef]
- Mens, J.M.A.; Vleeming, A.; Snijders, C.J.; Koes, B.W.; Stam, H.J. Understanding peripartum pelvic pain: Implications of a patient survey. Spine 1996, 21, 1363–1369. [Google Scholar] [CrossRef]
- Gilleard, W.L.; Brown, J.M. Structure and function of the abdominal muscles in primigravid subjects during pregnancy and the immediate postbirth period. Phys. Ther. 1996, 76, 750–762. [Google Scholar] [CrossRef]
- Ireland, M.L.; Ott, S.M. The effects of pregnancy on the musculoskeletal system. Clin. Orthop. Relat. Res. 2000, 372, 169–179. [Google Scholar] [CrossRef]
- Szkwara, J.M.; Hing, W.; Pope, R.; Rathbone, E. Compression shorts reduce prenatal pelvic and low back pain: A prospective quasi-experimental controlled study. PeerJ 2019, 7, e7080. [Google Scholar] [CrossRef]
- Nascimento, S.L.; Surita, F.G.; Cecatti, J.G. Physical exercise during pregnancy: A systematic review. Curr. Opin. Obs. Gynecol. 2012, 24, 387–394. [Google Scholar] [CrossRef]
- Mørkved, S.; Bø, K. Effect of pelvic floor muscle training during pregnancy and after childbirth on prevention and treatment of urinary incontinence: A systematic review. Br. J. Sports Med. 2014, 48, 299–310. [Google Scholar] [CrossRef]
- Woodley, S.J.; Lawrenson, P.; Boyle, R.; Cody, J.D.; Mørkved, S.; Kernohan, A.; Hay-Smith, E.J.C. Pelvic floor muscle training for preventing and treating urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst. Rev. 2020, 5, CD007471. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zheng, J.J.; Yu, Z.W.; Bi, X.; Lou, S.-J.; Liu, J.; Cai, B.; Hua, Y.-H.; Wu, M.; Wei, M.-L.; et al. A meta-analysis of core stability exercise versus general exercise for chronic low back pain. PLoS ONE 2012, 7, e52082. [Google Scholar] [CrossRef]
- Pennick, V.; Liddle, S.D. Interventions for preventing and treating pelvic and back pain in pregnancy. Cochrane Database Syst. Rev. 2013, 8, CD001139. [Google Scholar] [CrossRef]
- Black, R.A.; Hill, D.A. Over-the-counter medications in pregnancy. Am. Fam. Physician 2003, 67, 2517–2524. [Google Scholar]
- Rathmell, J.P.; Viscomi, C.M.; Ashburn, M.A. Management of nonobstetric pain during pregnancy and lactation. Anesth. Analg. 1997, 85, 1074–1087. [Google Scholar] [CrossRef]
- Betsch, M.; Wehrle, R.; Dor, L.; Rapp, W.; Jungbluth, P.; Hakimi, M.; Wild, M. Spinal posture and pelvic position during pregnancy: A prospective rasterstereographic pilot study. Eur. Spine J. 2015, 24, 1282–1288. [Google Scholar] [CrossRef]
- Sanderson, P.L.; Fraser, R.D. The influence of pregnancy on the development of degenerative spondylolisthesis. J. Bone Jt. Surg. Br. 1996, 78, 951–954. [Google Scholar] [CrossRef]
- Cholewicki, J.; Lee, A.S.; Popovich, J.M., Jr.; Mysliwiec, L.W.; Winkelpleck, M.D.; Flood, J.N.; Pathak, P.K.; Kaaikala, K.H.; Reeves, N.P.; Kothe, R. Degenerative Spondylolisthesis Is Related to Multiparity and Hysterectomies in Older Women. Spine 2017, 42, 1643–1647. [Google Scholar] [CrossRef]
- Çevik, S.; Yılmaz, H.; Kaplan, A.; Yetkinel, S.; Evran, Ş.; Çalış, F.; Akkaya, E.; Katar, S.; Baygül, A.; Hanımoğlu, H. Association between parity and lumbar spine degenerative disorders in young women. Br. J. Neurosurg. 2020, 34, 172–175. [Google Scholar] [CrossRef]
- Güngör, E.; Karakuzu Güngör, Z. Obstetric-related lower back pain: The effect of number of pregnancy on development of chronic lower back pain, worsening of lumbar disc degeneration and alteration of lumbar sagittal balance. J. Orthop. Surg. Res. 2024, 19, 174. [Google Scholar] [CrossRef]
- Schuller, S.; Charles, Y.P.; Steib, J.P. Sagittal spinopelvic alignment and body mass index in patients with degenerative spondylolisthesis. Eur. Spine J. 2011, 20, 713–719. [Google Scholar] [CrossRef]
- Barrey, C.; Roussouly, P.; Le Huec, J.C.; D’Acunzi, G.; Perrin, G. Compensatory mechanisms contributing to keep the sagittal balance of the spine. Eur. Spine J. 2013, 22 (Suppl. S6), 834–841. [Google Scholar] [CrossRef]
- Bailey, J.F.; Sparrey, C.J.; Williams, F.M.K.; Curran, P.F.; Lotz, J.C.; Kramer, P.A. The Effect of Parity on Age-Related Degenerative Changes in Sagittal Balance. Spine 2020, 45, E210. [Google Scholar] [CrossRef]
- Weis, C.A.; Pohlman, K.; Draper, C.; daSilva-Oolup, S.; Stuber, K.; Hawk, C. Chiropractic Care for Adults with Pregnancy-Related Low Back, Pelvic Girdle Pain, or Combination Pain: A Systematic Review. J. Manip. Physiol. Ther. 2020, 43, 714–731. [Google Scholar] [CrossRef]
- Shiri, R.; Coggon, D.; Falah-Hassani, K. Exercise for the prevention of low back and pelvic girdle pain in pregnancy: A meta-analysis of randomized controlled trials. Eur. J. Pain 2018, 22, 19–27. [Google Scholar] [CrossRef]
- Sapsford, R. Rehabilitation of pelvic floor muscles utilizing trunk stabilization. Man. Ther. 2004, 9, 3–12. [Google Scholar] [CrossRef]
- Fitzgerald, C.M.; Bennis, S.; Marcotte, M.L.; Shannon, M.B.; Iqbal, S.; Adams, W.H. The impact of a sacroiliac joint belt on function and pain using the active straight leg raise in pregnancy-related pelvic girdle pain. PM&R 2022, 14, 19–29. [Google Scholar] [CrossRef]
- Field, T. Prenatal exercise research. Infant. Behav. Dev. 2012, 35, 397–407. [Google Scholar] [CrossRef]

| Biomechanical Change | Mechanism | Clinical Significance | References |
|---|---|---|---|
| Increased Lumbar Lordosis | Forward shift in the center of mass and compensatory hyperextension of the lower spine | May elevate facet joint loads; can predispose to low back pain and spondylolisthesis | Whitcome et al., 2007 [13]; Yoo et al., 2015 [14] |
| Altered Pelvic Parameters (↑ Sacral Slope, ↑ Pelvic Tilt) | Hormonal relaxation of ligaments and change in weight distribution lead to greater pelvic incidence and sacral slope | Excessive pelvic tilt alters lumbopelvic biomechanics; may contribute to postpartum spinal issues and degenerative changes over time | Yamada et al., 2021 [3]; Wang et al., 2016 [15] |
| Changes in Gait and Balance | Abdomen enlargement shifts center of gravity anteriorly, altering stride length, cadence, and stance width | Altered balance can increase mechanical load on lower spine and pelvis; may cause postural instability and higher fall risk | Foti et al., 2000 [16]; Wu et al., 2004 [17] |
| Reduced Lumbopelvic Stabilization | Abdominal expansion reduces stability of transverse abdominis, multifidus, and pelvic floor | Reduced spinal support increases stress on lumbar structures, potentially aggravating low back pain | Fast et al. 1987 [1]; Borg-Stein et al., 2005 [18]; Fast et al. 1992 [19] |
| Pelvic Girdle and SI Joint Laxity | Elevated relaxin and progesterone levels lead to ligamentous laxity in the pubic symphysis and SI joints | Instability in the pelvic ring can co-occur with low back pain and is unique to pregnancy (not observed with non-pregnant weight gain) | Wu et al., 2004 [17]; Mens et al., 2009 [20] |
| Anterior Loading from the Growing Uterus | Progressive uterine and fetal enlargement places an anterior pull on the lower spine | Increases shear forces across the lumbar region; can amplify lordotic posture and contribute to degenerative disc or facet changes | Ritchie JR et al., 2003 [21] |
| Intervention | Mechanism | Evidence | References |
|---|---|---|---|
| Prenatal Exercise (PFMT, aquatics, stability ball, etc.) | Strengthens the deep core (transverse abdominis, multifidus) and pelvic floor (levator ani complex) while improving posture and offsetting excessive lumbar lordosis. Specific routines like aquatics and PFMT (“Kegels”) focus on lumbopelvic stability | Numerous RCTs and reviews link pelvic floor and core strengthening to reduced low back and pelvic pain, improved function, and better postural stability. Prenatal yoga/Pilates programs (with appropriate modifications) may also alleviate discomfort and reduce stress | Borg-Stein et al., 2005 [18]; Nascimento et al., 2012 [34]; Mørkved & Bø, 2014 [35]; Woodley et al., 2020 [36]; Wang XQ et al., 2012 [37] |
| Maternity Support Belts and Sacroiliac (SI) belts | Provides external support to the lower abdomen and lumbopelvic region. SI belts, a subtype of maternity belts, are specifically designed to stabilize the sacroiliac joints and control pelvic ring laxity. These supports reduce spinal load and improve functional mobility in patients with SI joint pain | Some trials indicate short-term pain relief and reduced postpartum pelvic pain, especially in cases of sacroiliac joint dysfunction. Evidence is variable, but SI belts are often included in conservative management | Casagrande et al., 2015 [29]; Mens et al., 2009 [20] |
| Acupuncture and Related Modalities | May modulate pain pathways and reduce pelvic girdle instability; believed to stimulate endorphin release and increase local blood flow | Cochrane reviews suggest beneficial effects for pelvic girdle and low back pain in pregnancy | Pennick & Liddle, 2013 [38] |
| Physical Therapy and Spinal Manipulation | Manual therapy techniques can address restricted spinal segments and muscular imbalances; guided exercises improve muscle activation | Mild to moderate relief in some pregnant populations; safety often considered good if performed by experienced clinicians | Ritchie, 2003 [21]; Borg-Stein et al., 2005 [18], Mens et al., 2009 [20] |
| Postural Re-education and Ergonomics | Teaches pregnant individuals to distribute weight more evenly, maintain neutral spine alignment, and use proper lifting mechanics | Anecdotally effective and often recommended, though high- quality RCT data may be sparse; widely included in comprehensive prenatal programs | Conder et al., 2019 [9]; Branco et al., 2014 [10] |
| Compression Stockings and Regular Ambulation | Reduces venous stasis in the inferior vena cava region (especially in 3rd trimester with prolonged standing) and improves blood flow to pelvic/lumbar tissues | Shown to alleviate nocturnal back pain associated with decreased basal oxygen saturation and supine positioning; may help reduce edema-related pain | Fast et al., 1992 [19]; Szkwara et al., 2019 [33] |
| Pharmacological Management | Limited safe options in pregnancy (e.g., acetaminophen, possibly muscle relaxants like cyclobenzaprine); opioids and NSAIDs often restricted by trimester-specific risks | Generally recommended only if pain is debilitating and after nonpharmacologic strategies; usage minimized for fetal safety | Black & Hill, 2003 [39]; Rathmell et al., 1997 [40] |
| Surface Topography and AI-Based Monitoring | Non-invasive imaging and predictive algorithms to track spinal curvature changes, posture, and potential LBP onset | Emerging data support early intervention if major postural alterations are detected; may reduce postpartum persistence of pain by guiding exercise | Michoński et al., 2016 [23], Betsch et al., 2015 [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoseph, E.T.; Taiwo, R.; Kiapour, A.; Touponse, G.; Massaad, E.; Theologitis, M.; Wu, J.Y.; Williamson, T.; Zygourakis, C.C. Pregnancy-Related Spinal Biomechanics: A Review of Low Back Pain and Degenerative Spine Disease. Bioengineering 2025, 12, 858. https://doi.org/10.3390/bioengineering12080858
Yoseph ET, Taiwo R, Kiapour A, Touponse G, Massaad E, Theologitis M, Wu JY, Williamson T, Zygourakis CC. Pregnancy-Related Spinal Biomechanics: A Review of Low Back Pain and Degenerative Spine Disease. Bioengineering. 2025; 12(8):858. https://doi.org/10.3390/bioengineering12080858
Chicago/Turabian StyleYoseph, Ezra T., Rukayat Taiwo, Ali Kiapour, Gavin Touponse, Elie Massaad, Marinos Theologitis, Janet Y. Wu, Theresa Williamson, and Corinna C. Zygourakis. 2025. "Pregnancy-Related Spinal Biomechanics: A Review of Low Back Pain and Degenerative Spine Disease" Bioengineering 12, no. 8: 858. https://doi.org/10.3390/bioengineering12080858
APA StyleYoseph, E. T., Taiwo, R., Kiapour, A., Touponse, G., Massaad, E., Theologitis, M., Wu, J. Y., Williamson, T., & Zygourakis, C. C. (2025). Pregnancy-Related Spinal Biomechanics: A Review of Low Back Pain and Degenerative Spine Disease. Bioengineering, 12(8), 858. https://doi.org/10.3390/bioengineering12080858







