Effects of Aging on Motor Unit Properties in Isometric Elbow Flexion
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experiments
2.2.1. Force Signal Recording
2.2.2. High-Density sEMG Recording
2.2.3. Experimental Protocol
2.3. Data Analysis
2.3.1. Preprocessing
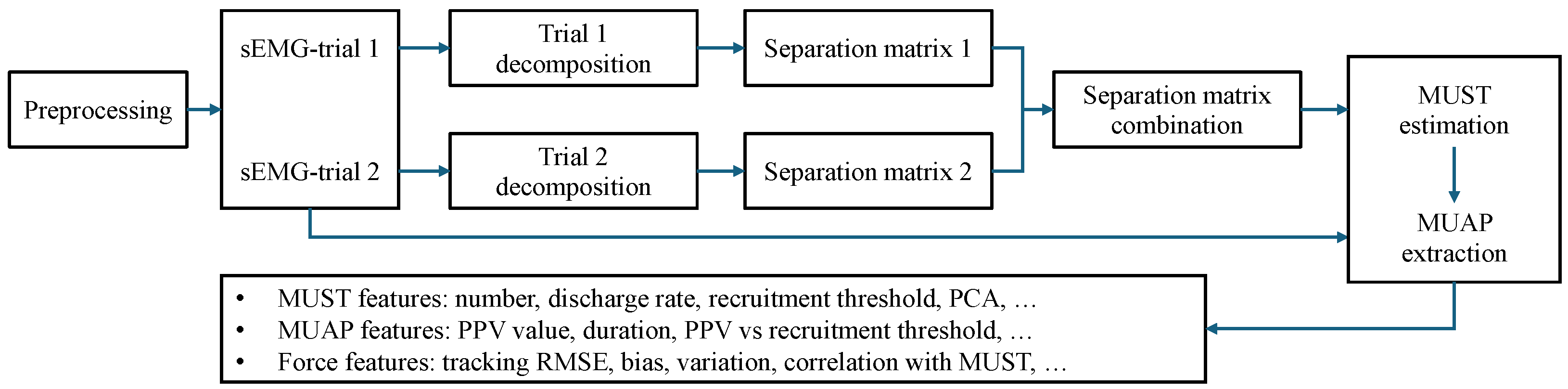
2.3.2. sEMG Decomposition
2.3.3. Feature Extraction
2.4. Statistics
3. Results
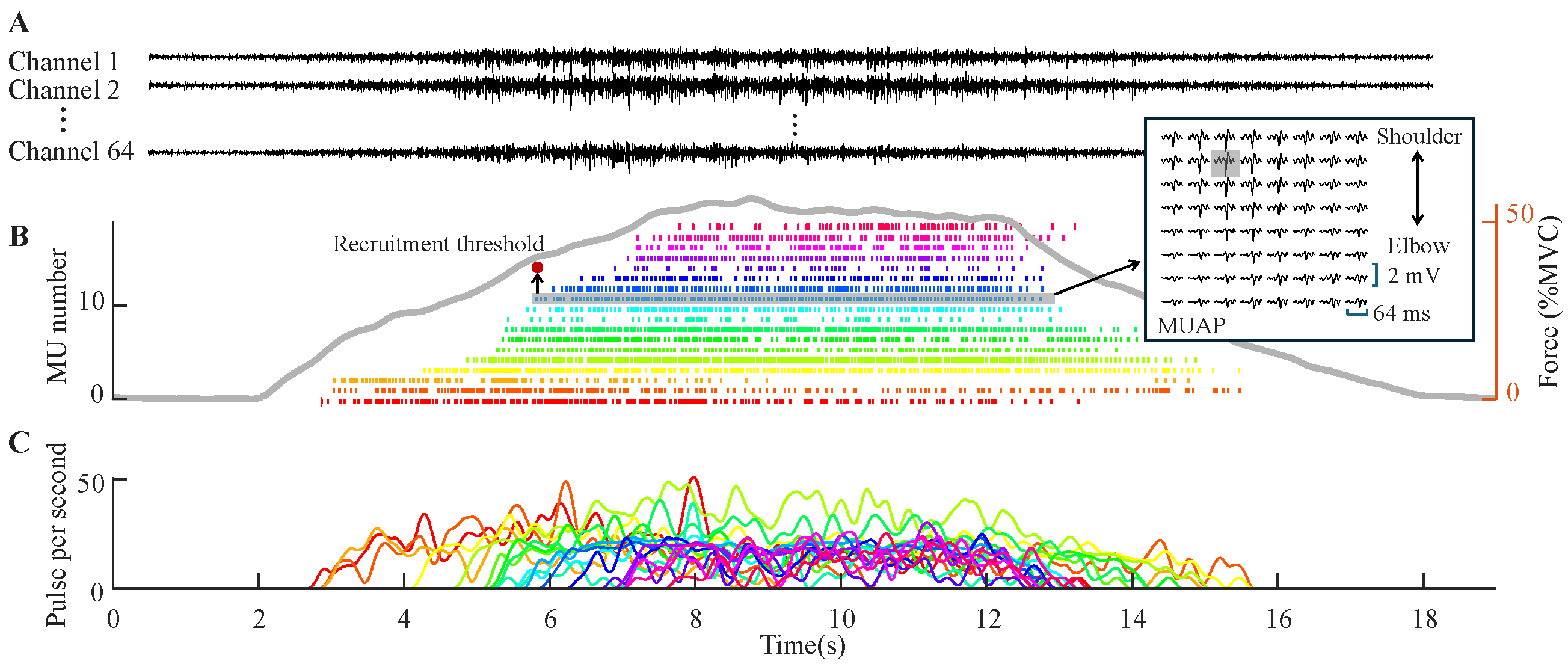
3.1. Motor Unit Decomposition Across Ages
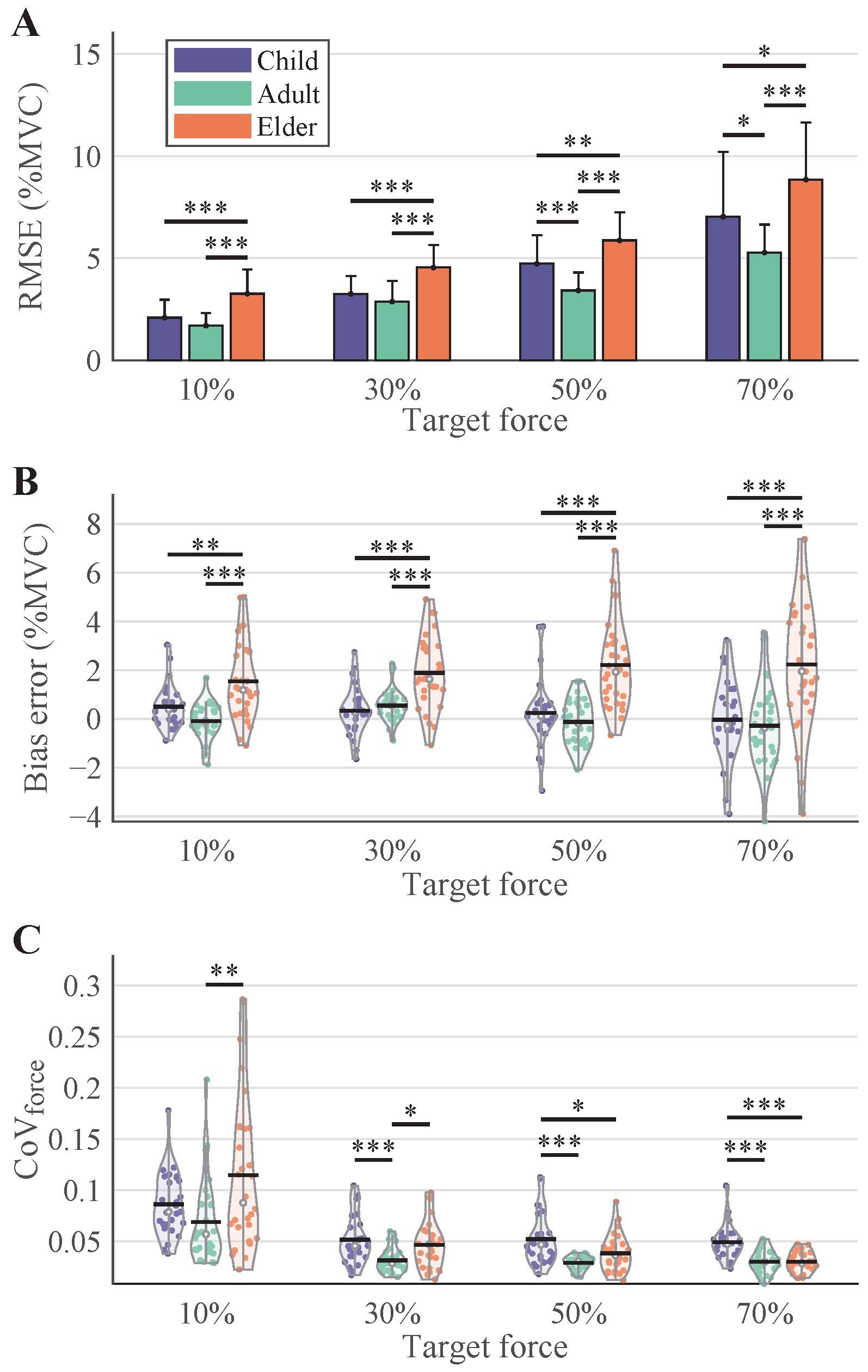
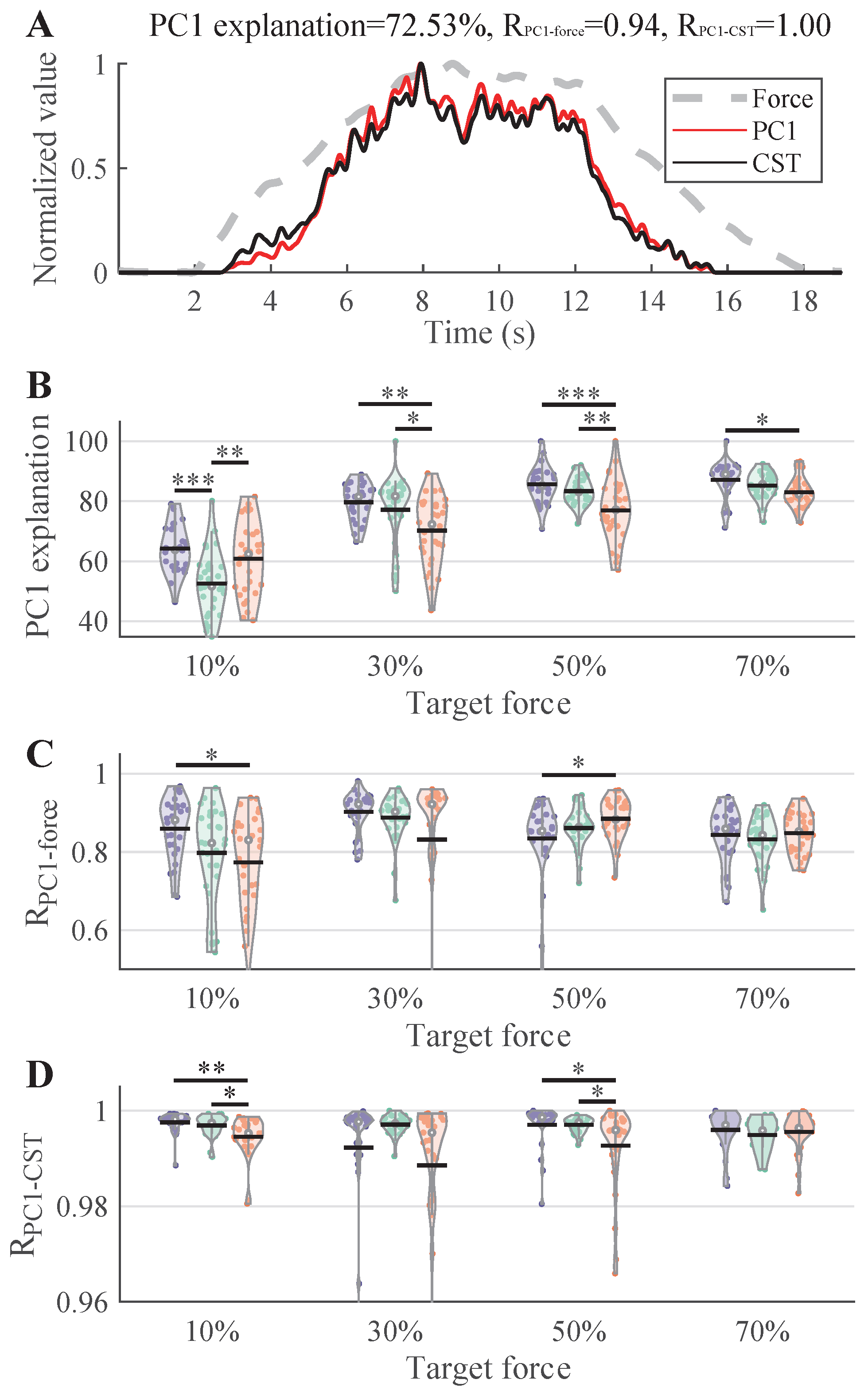
3.2. Force Tracking Performance
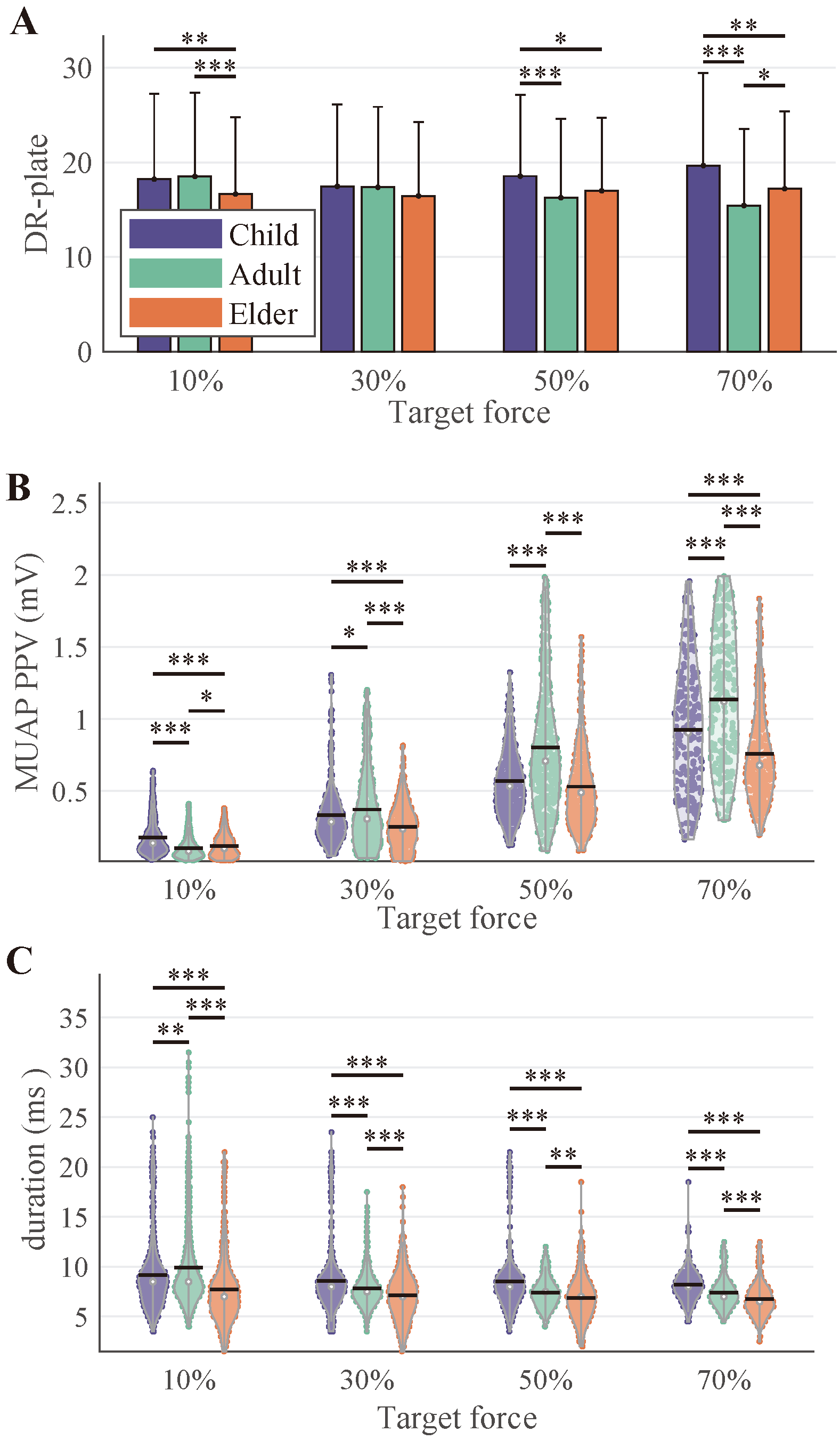
3.3. Motor Unit Discharge and MUAP Morphology Properties
3.4. Common Neural Drive from MUSTs
4. Discussion
4.1. Decomposition Performance Across Age Groups
4.2. Age-Related Modulation of Force Generation and MU Properties
4.3. Common Neural Drive and Synergy
4.4. Applications and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rose, M.; Flatt, T.; Graves, J.L., Jr.; Greer, L.F.; Martínez, D.E.; Matos, M.; Mueller, L.; Shmookler Reis, R.J.; Shahrestani, P. What is Aging? Front. Genet. 2012, 3, 134. [Google Scholar] [CrossRef]
- Hunter, S.K.; Pereira, H.M.; Keenan, K.G. The aging neuromuscular system and motor performance. J. Appl. Physiol. 2016, 121, 982–995. [Google Scholar] [CrossRef]
- Kedlian, V.R.; Wang, Y.; Liu, T.; Chen, X.; Bolt, L.; Tudor, C.; Shen, Z.; Fasouli, E.S.; Prigmore, E.; Kleshchevnikov, V.; et al. Human skeletal muscle aging atlas. Nat. Aging 2024, 4, 727–744. [Google Scholar] [CrossRef]
- Hussain, Z.; Gopalai, A.A.; Ahmad, S.A.; Salim, M.S.B.; Gouwanda, D.; Teh, P.L. Investigating age-related muscle force adaptations in males and females during sit-to-walk transition motion using EMG-informed modeling. Results Eng. 2025, 26, 104660. [Google Scholar] [CrossRef]
- Mitchell, W.K.; Atherton, P.J.; Williams, J.; Larvin, M.; Lund, J.N.; Narici, M. Sarcopenia, Dynapenia, and the Impact of Advancing Age on Human Skeletal Muscle Size and Strength; a Quantitative Review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef]
- Watanabe, K.; Holobar, A.; Kouzaki, M.; Ogawa, M.; Akima, H.; Moritani, T. Age-related changes in motor unit firing pattern of vastus lateralis muscle during low-moderate contraction. AGE 2016, 38, 48. [Google Scholar] [CrossRef]
- Holloszy, J.O. The Biology of Aging. Mayo Clin. Proc. 2000, 75, S3–S9. [Google Scholar] [CrossRef]
- Roos, M.R.; Rice, C.L.; Vandervoort, A.A. Age-related changes in motor unit function. Muscle Nerve 1997, 20, 679–690. [Google Scholar] [CrossRef]
- Holobar, A.; Farina, D. Blind source identification from the multichannel surface electromyogram. Physiol. Meas. 2014, 35, R143. [Google Scholar] [CrossRef]
- Holobar, A.; Zazula, D. Multichannel Blind Source Separation Using Convolution Kernel Compensation. IEEE Trans. Signal Process. 2007, 55, 4487–4496. [Google Scholar] [CrossRef]
- Negro, F.; Muceli, S.; Castronovo, A.M.; Holobar, A.; Farina, D. Multi-channel intramuscular and surface EMG decomposition by convolutive blind source separation. J. Neural Eng. 2016, 13, 026027. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, P. 2CFastICA: A novel method for high density surface EMG decomposition based on kernel constrained fastICA and correlation constrained fastICA. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 2177–2186. [Google Scholar] [CrossRef]
- Holobar, A.; Minetto, M.A.; Botter, A.; Negro, F.; Farina, D. Experimental analysis of accuracy in the identification of motor unit spike trains from high-density surface EMG. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 221–229. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, X.; Lu, Z.; Li, X.; Zhou, P. Two-Source Validation of Progressive FastICA Peel-Off for Automatic Surface EMG Decomposition in Human First Dorsal Interosseous Muscle. Int. J. Neural Syst. 2018, 28, 1850019. [Google Scholar] [CrossRef]
- Henry, M.; Darendeli, A.; Tvrdy, T.; Daneshgar, S.; Enoka, R.M. Influence of age and feedback modality on the proprioceptive sense of force: Insights from motor unit recordings. J. Neurophysiol. 2025, 133, 1103–1115. [Google Scholar] [CrossRef]
- Nguyen, D.A.T.; Lewis, R.H.C.; Gandevia, S.C.; Butler, J.E.; Hudson, A.L. Discharge properties of human diaphragm motor units with ageing. J. Physiol. 2019, 597, 5079–5092. [Google Scholar] [CrossRef]
- Klass, M.; Baudry, S.; Duchateau, J. Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J. Appl. Physiol. 2008, 104, 739–746. [Google Scholar] [CrossRef]
- Erim, Z.; Beg, M.F.; Burke, D.T.; de Luca, C.J. Effects of Aging on Motor-Unit Control Properties. J. Neurophysiol. 1999, 82, 2081–2091. [Google Scholar] [CrossRef]
- Piasecki, M.; Ireland, A.; Stashuk, D.; Hamilton-Wright, A.; Jones, D.A.; McPhee, J.S. Age-related neuromuscular changes affecting human vastus lateralis. J. Physiol. 2016, 594, 4525–4536. [Google Scholar] [CrossRef]
- McNeil, C.J.; Doherty, T.J.; Stashuk, D.W.; Rice, C.L. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve 2005, 31, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.; McKiel, A.; Herda, T.; Klentrou, P.; Holmes, M.; Gabriel, D.; Falk, B. Developmental changes in motor unit activity patterns: Child-adult comparison using discrete motor unit analysis. Appl. Physiol. Nutr. Metab. 2024, 49, 904–919. [Google Scholar] [CrossRef]
- Miller, J.D.; Sterczala, A.J.; Trevino, M.A.; Wray, M.E.; Dimmick, H.L.; Herda, T.J. Motor unit action potential amplitudes and firing rates during repetitive muscle actions of the first dorsal interosseous in children and adults. Eur. J. Appl. Physiol. 2019, 119, 1007–1018. [Google Scholar] [CrossRef]
- Negro, F.; Farina, D. Factors Influencing the Estimates of Correlation between Motor Unit Activities in Humans. PLoS ONE 2012, 7, e44894. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.; Negro, F.; Dideriksen, J.L. The effective neural drive to muscles is the common synaptic input to motor neurons. J. Physiol. 2014, 592, 3427–3441. [Google Scholar] [CrossRef]
- Benamati, A.; Ricotta, J.M.; De, S.D.; Latash, M.L. Three levels of neural control contributing to performance-stabilizing synergies in multi-finger tasks. Neuroscience 2024, 551, 262–275. [Google Scholar] [CrossRef]
- Holobar, A.; Zazula, D. Correlation-based decomposition of surface electromyograms at low contraction forces. Med. Biol. Eng. Comput. 2004, 42, 487–495. [Google Scholar] [CrossRef]
- Kay, S.M. Fundamentals of Statistical Signal Processing: Estimation Theory; Prentice-Hall, Inc.: Hoboken, NJ, USA, 1993. [Google Scholar]
- Holobar, A.; Zazula, D. Gradient convolution kernel compensation applied to surface electromyograms. In International Conference on Independent Component Analysis and Signal Separation; Springer: Berlin/Heidelberg, Germany, 2007; pp. 617–624. [Google Scholar]
- Holobar, A.; Minetto, M.; Farina, D. Accurate identification of motor unit discharge patterns from high-density surface EMG and validation with a novel signal-based performance metric. J. Neural Eng. 2014, 11, 016008. [Google Scholar] [CrossRef]
- Farina, D.; Negro, F.; Gazzoni, M.; Enoka, R.M. Detecting the unique representation of motor-unit action potentials in the surface electromyogram. J. Neurophysiol. 2008, 100, 1223–1233. [Google Scholar] [CrossRef]
- Lodha, N.; Naik, S.K.; Coombes, S.A.; Cauraugh, J.H. Force control and degree of motor impairments in chronic stroke. Clin. Neurophysiol. 2010, 121, 1952–1961. [Google Scholar] [CrossRef]
- Ballardini, G.; Ponassi, V.; Galofaro, E.; Carlini, G.; Marini, F.; Pellegrino, L.; Morasso, P.; Casadio, M. Interaction between position sense and force control in bimanual tasks. J. Neuroeng. Rehabil. 2019, 16, 137. [Google Scholar] [CrossRef]
- Del Vecchio, A.; Casolo, A.; Negro, F.; Scorcelletti, M.; Bazzucchi, I.; Enoka, R.; Felici, F.; Farina, D. The increase in muscle force after 4 weeks of strength training is mediated by adaptations in motor unit recruitment and rate coding. J. Physiol. 2019, 597, 1873–1887. [Google Scholar] [CrossRef]
- Qiu, F.; Liu, X.; Xu, Y.; Shi, L.; Sheng, X.; Chen, C. Neural inputs from spinal motor neurons to lateralis vastus muscle: Comparison between sprinters and nonathletes. Front. Physiol. 2022, 13, 994857. [Google Scholar] [CrossRef]
- Holobar, A.; Glaser, V. Cumulative Spike Train Outperforms the Root-Mean-Square Metric in Muscle Excitation Estimation from Dynamic High-Density EMG. In Converging Clinical and Engineering Research on Neurorehabilitation III; Biosystems & Biorobotics; Springer: Cham, Switzerland, 2019; Volume 228, pp. 1143–1147. [Google Scholar]
- Glaser, V.; Holobar, A.; Zazula, D. Real-Time Motor Unit Identification From High-Density Surface EMG. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 949–958. [Google Scholar] [CrossRef]
- Okudaira, M.; Hirono, T.; Takeda, R.; Nishikawa, T.; Ueda, S.; Mita, Y.; Holobar, A.; Yoshimura, A.; Watanabe, K. Longitudinal development of muscle strength and relationship with motor unit activity and muscle morphological characteristics in youth athletes. Exp. Brain Res. 2023, 241, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Narici, M.V.; Maganaris, C.N.; Reeves, N.D.; Capodaglio, P. Effect of aging on human muscle architecture. J. Appl. Physiol. 2003, 95, 2229–2234. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.i.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Dobrzynska, Z.; Celichowski, J. Changes in contractile properties and action potentials of motor units in the rat medial gastrocnemius muscle during maturation. J. Physiol. Pharmacol. 2016, 67, 139–150. [Google Scholar]
- Henneman, E. Relation between Size of Neurons and Their Susceptibility to Discharge. Science 1957, 126, 1345–1347. [Google Scholar] [CrossRef]
- Bell, M.; Al Masruri, G.; Fernandez, J.; Williams, S.A.; Agur, A.M.; Stott, N.S.; Hajarizadeh, B.; Mirjalili, A. Typical m. triceps surae morphology and architecture measurement from 0 to 18 years: A narrative review. J. Anat. 2022, 240, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T.B.; Thompson, B.J. Influence of age on passive stiffness and size, quality, and strength characteristics. Muscle Nerve 2017, 55, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Lulic-Kuryllo, T.; Greig Inglis, J. Sex differences in motor unit behaviour: A review. J. Electromyogr. Kinesiol. 2022, 66, 102689. [Google Scholar] [CrossRef]
- Yacyshyn, A.F.; Mohammadalinejad, G.; Afsharipour, B.; Duchcherer, J.; Bashuk, J.; Bennett, D.J.; Negro, F.; Quinlan, K.A.; Gorassini, M.A. Sex-related differences in motoneuron firing behaviour during typical development. J. Neurophysiol. 2025, 133, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, A.; Negro, F.; Holobar, A.; Casolo, A.; Folland, J.P.; Felici, F.; Farina, D. You are as fast as your motor neurons: Speed of recruitment and maximal discharge of motor neurons determine the maximal rate of force development in humans. J. Physiol. 2019, 597, 2445–2456. [Google Scholar] [CrossRef]


| Target Force | MVC Force (kg) | MU Number | PNR (dB) | |
|---|---|---|---|---|
| Child | 10% | 7.8 ± 2.2 | 15 ± 6 | 29.8 ± 3.0 |
| 30% | 10 ± 5 | 29.8 ± 3.4 | ||
| 50% | 11 ± 6 | 29.7 ± 3.3 | ||
| 70% | 8 ± 6 | 29.8 ± 3.4 | ||
| Adult | 10% | 21.8 ± 7.8 | 19 ± 5 | 29.3 ± 2.8 |
| 30% | 11 ± 6 | 29.5 ± 2.7 | ||
| 50% | 12 ± 5 | 30.2 ± 3.1 | ||
| 70% | 10 ± 4 | 29.9 ± 2.8 | ||
| Elder | 10% | 14.6 ± 5.1 | 13 ± 6 | 29.1 ± 2.8 |
| 30% | 11 ± 5 | 29.4 ± 2.8 | ||
| 50% | 10 ± 5 | 29.9 ± 2.8 | ||
| 70% | 11 ± 5 | 30.0 ± 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, F.; Liu, X.; Chen, C. Effects of Aging on Motor Unit Properties in Isometric Elbow Flexion. Bioengineering 2025, 12, 869. https://doi.org/10.3390/bioengineering12080869
Qiu F, Liu X, Chen C. Effects of Aging on Motor Unit Properties in Isometric Elbow Flexion. Bioengineering. 2025; 12(8):869. https://doi.org/10.3390/bioengineering12080869
Chicago/Turabian StyleQiu, Fang, Xiaodong Liu, and Chen Chen. 2025. "Effects of Aging on Motor Unit Properties in Isometric Elbow Flexion" Bioengineering 12, no. 8: 869. https://doi.org/10.3390/bioengineering12080869
APA StyleQiu, F., Liu, X., & Chen, C. (2025). Effects of Aging on Motor Unit Properties in Isometric Elbow Flexion. Bioengineering, 12(8), 869. https://doi.org/10.3390/bioengineering12080869






