Curcumin-Loaded Drug Delivery Systems for Acute and Chronic Wound Management: A Review
Abstract
1. Introduction
2. Physiology of Wound Healing
3. Acute Wound Management
4. Chronic Wound Management
4.1. Chronic Wound Causes and Complications
4.2. Pathophysiology of Chronic Wounds
4.3. Management Techniques
5. Biological Activity of Curcumin in Wound Healing
5.1. Bioactive Curcumin
5.2. Antioxidant Activity
5.3. Anti-Inflammatory Activity
5.4. Antibacterial Activity
5.5. Safety of Curcumin and Clinical Trials
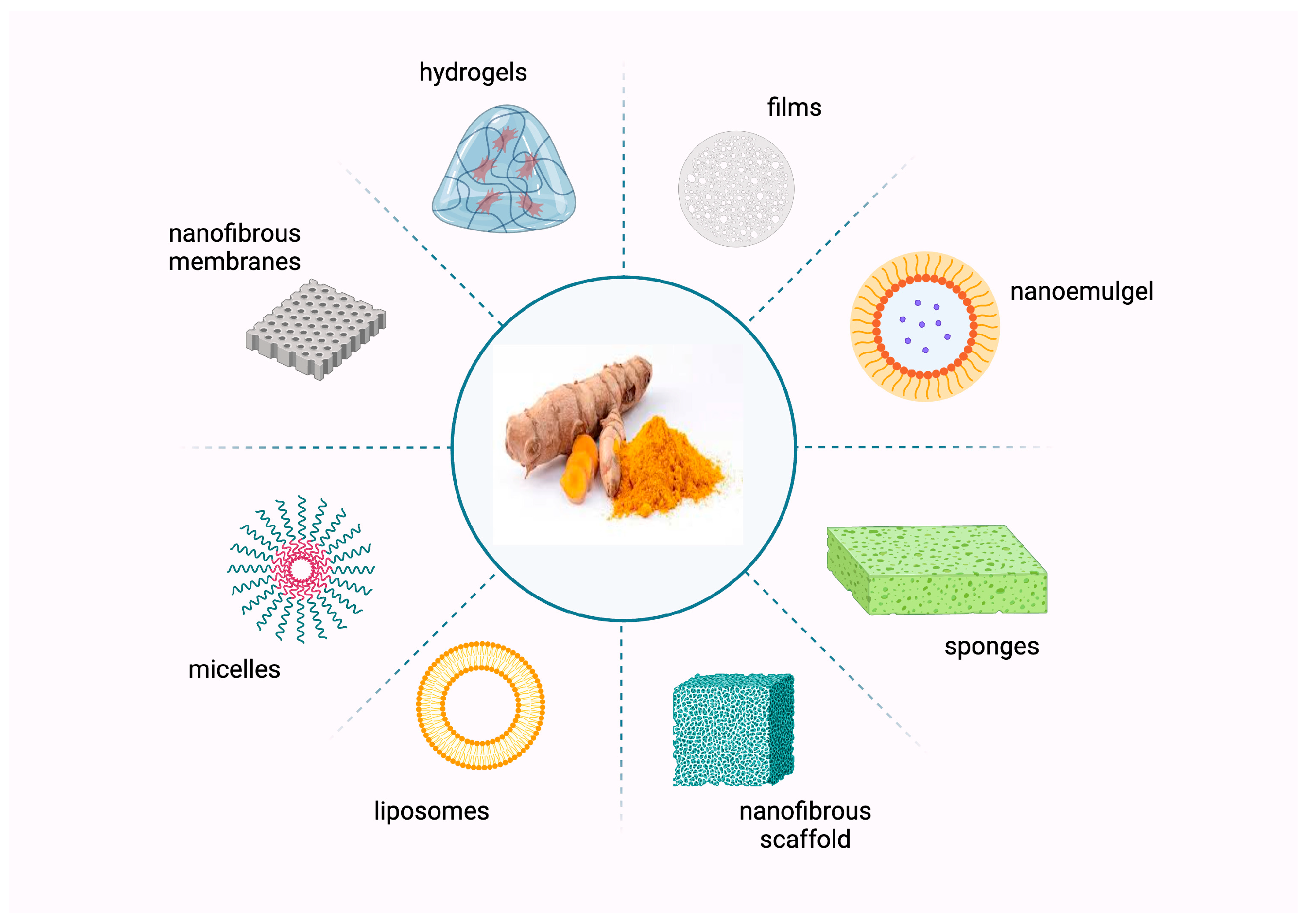
6. Curcumin-Loaded Delivery Systems for Wound Healing
6.1. Nanofibrous Scaffolds
6.2. Hydrogel
6.3. Films
6.4. Polymeric Micelles
| Dressing Type | Composition | Preparation Method | Study Method | Key Findings | References |
|---|---|---|---|---|---|
| Nanofibrous membrane | Curcumin, honey, PVA, cellulose acetate | Electrospinning | In vitro | Curcumin and honey were encapsulated in PVA and cellulose acetate individually and also together. It was found that the dressings facilitated cellular activities and provided antimicrobial activity against common infection. | [147] |
| Curcumin, gelatine, sodium bicarbonate, honey | Electrospinning | In vitro and in vivo with Wistar male albino rat model | The results of antioxidant and antibacterial activities showed better outcomes with the addition of curcumin and honey. In vivo study showed healed wounds on day 17. | [148] | |
| Curcumin, chitosan, gelatine, PCL | Electrospinning | In vitro and in vivo with rat dorsal skin defects model | In vitro studies demonstrated that the product exhibits strong antioxidant and antibacterial activity. In vivo studies showed it promotes granulation tissue formation, collagen deposition, and remodelling of epithelial tissue. Additionally, it accelerates wound healing by enhancing the expression of CD31 and TGF-β in the early stages of the healing process. | [130] | |
| Curcumin, surfactin, PCL, gelatine | Electrospinning | In vitro and in vivo with male Wistar rat model | In vitro studies demonstrated that the dressing exhibited over 99% antibacterial activity after 24 h. An increase in curcumin concentration resulted in reduced elastic modulus and increased tensile strength. In vivo studies demonstrated a significant improvement in the healing rate compared to control groups lacking curcumin. | [149] | |
| Curcumin, heparin, PLGA | Electrospinning | In vitro and in vivo with diabetic Sprague Dawley rat model | The dressings possessed high tensile strength and low cytotoxicity, along with increased hydrophilicity. In in vivo studies, the dressings were found to accelerate the re-epithelialization rate, promote higher angiogenesis, and enhance collagen deposition at the wound site. | [150] | |
| Curcumin, AgNPs, chitosan, polyethylene oxide | Electrospinning | In vitro and in vivo with male Kunming mouse model | This product demonstrated effective activity against both Gram-negative and Gram-positive bacteria in in vitro studies. In vivo studies showed improved wound closure rates compared to the commercial product AquacelAg. | [151] | |
| Nanofibrous scaffold | Curcumin, carboxymethyl guar gum, graphene oxide | Electrospinning | In vitro and in vivo with rabbit model | In vitro wound healing assays using 3T3 L1 cell lines demonstrated 100% wound closure within 48 h. In vivo studies revealed that the nanofibrous scaffold containing curcumin exhibited antibacterial, anti-inflammatory, and antioxidant effects on chronic wounds. | [152] |
| Curcumin, cellulose acetate, poly (ε-caprolactone) | Electrospinning | In vitro | Curcumin has a dual role as a drug and as a hydrophilicity-enhancing agent because of the formation of hydrogen bonds between its components. This enhances the swelling capacity by around 700% or 950%, depending on the percentage of added curcumin. The medicated scaffolds that were created increased the expression of actin in fibroblasts compared to the unmedicated ones. | [153] | |
| Sodium alginate and collagen | Physical mixing | In vitro and in vivo with female rat model | In vivo studies showed that the scaffold loaded with curcumin had a 90% wound healing rate at day 14 compared to 80% when a scaffold without curcumin was applied. | [71] | |
| Nanofibrous mat | Curcumin, PCL, PVA, silk fibroin | Electrospinning | In vitro and in vivo with streptozotocin-induced diabetic mice model | Diameters of fibres: 200–350 nm. Tensile strength: 12.41–16.80 MPa. The product demonstrated faster wound healing compared to traditional formulations and has significant potential for healing diabetic wounds. | [154] |
| Nanoemulgel | Curcumin, Labrafac PG, Tween® 80, PEG 400 | Ultrasonic emulsification method | In vitro and in vivo with Wistar rat model | Droplet size: 56.25 ± 0.69 nm. Polydispersity index: 0.05 ± 0.01. Zeta potential: −20.26 ± 0.65 mV. The selected nanoemulsion was integrated into a 0.5% Carbopol® 940 hydrogel matrix to create nanoemulgels for topical use. The developed curcumin nanoemulgel displayed thixotropic rheological behaviour and demonstrated a significant improvement in skin penetrability compared to curcumin dispersed in a traditional hydrogel system. The nanoemulgel design exhibited outstanding skin penetrability and showed promising wound healing capabilities in in vivo animal studies. | [155] |
| Curcumin, resveratrol | Emulsification | In vitro and in vivo with burn-induced male Wister rat model | Particle size: 167–180 nm. Zeta potential: −17 to −20 mV. In vivo studies have shown the enhanced burn healing potential of the combination of nutraceuticals, as well as the promising delivery characteristics of the nanoemulgel dosage form. | [156] | |
| Nanoemulsion | Curcumin, clove oil, Tween® 80, PEG400 | Ultrasonic emulsification method | In vitro and in vivo with Albino rat model | The optimised curcumin-loaded nanoemulsion was non-toxic and had a drug content of 98.11 ± 0.16%, pH of 7.4 ± 0.07, zeta potential of −11.67 ± 0.11, refractive index of 1.71 ± 0.034, and viscosity of 37 ± 7 cp. In addition, this nanoemulsion improved wound healing in rats by promoting the proliferation of epithelial cells and demonstrated significant anti-inflammatory effects in a rat model. | [157] |
| Nanocomposite | Curcumin, zinc, aluminium | Chemical precipitation | In vitro and in vivo with male albino rat model | In vitro drug release: 56.78 ± 1.51% within 24 h. In vivo studies demonstrated excellent wound healing capabilities, high muscle tensile strength, and strong anti-inflammatory properties. | [72] |
| Film | Curcumin, chitosan, PEG, AgNPs | Chemical ross-linking | In vitro and in vivo with Wistar albino rat model | Particle size: 13.48 nm. The viability of Vero cells reached 96.5% with a curcumin concentration of 100 μg/mL. The dressing exhibited improved inhibition of S. aureus and E. coli at 24 h and 48 h. Additionally, a wound contraction of 98% was observed on day 12. | [141] |
| Curcumin, bacterial cellulose, alginate, gelatine | Mechanical blending and casting | In vitro | Water contact angles: 50–70°. Water vapour permeability: 300–800 g/m2/24 h. The curcumin-loaded films were non-toxic to human keratinocytes and had antibacterial activity against E. coli and S. aureus, with enhanced fluid uptake capability up to 700%. | [142] | |
| Hydrogel membrane | Curcumin, chitosan, sodium alginate | Physical cross-linking | In vitro and in vivo with male Sprague Dawley rat model | In vitro drug release: 41 ± 4.2% within 24 h. Tensile strength: 16 MPa. In vivo testing showed 75 ± 2.3% reepithelialisation within 14 days compared to gauze-covered wounds. | [158] |
| Thermosensitive hydrogels | Curcumin, poloxamer 188, poloxamer 407 | Cold swelling | In vitro and in vivo with Sprague Dawley rat model | Pore size: 5 to 10 μm. Live/dead assay showed that the curcumin-containing hydrogel extracts were non-toxic to cells. In vivo studies demonstrated the hydrogel’s ability to promote wound healing. | [159] |
| Hydrogel | Curcumin, chitosan, Lipoid S 100, polysorbate 20, stearylamine, sodium deoxycholate | Film hydration, hand-stirring | In vitro and ex vivo with full-thickness human skin model | Liposomes with a positive charge, created using stearylamine as a positive charge promoter, exhibited superior bioadhesion and improved, sustained penetration through full-thickness human skin compared to neutral and anionic liposomes. | [160] |
| Sponge | Curcumin, cellulose, β-cyclodextrin, chitosan | Synthesis, cross-linking, freeze-drying | In vitro | Binding with β-cyclodextrin increased the solubility of curcumin. Adding cyclodextrin complex and chitosan improved the sponge’s mechanical properties. The sponge was non-toxic to NCTC L929 and NHDF cells and showed increased antibacterial activities with the addition of chitosan. The authors believed that it could be used for chronic wounds. | [161] |
| Liposomes | Curcumin, Pluronic F127, liposomes | Encapsulation | In vitro | The impact of curcumin-loaded liposomes on a human keratinocyte cell line was examined, revealing no effect on cell viability. However, curcumin-loaded liposomes were found to enhance the cell migration rate and increase the expression of nuclear factor erythroid-related factor 2 and kelch-like erythroid cell-derived protein 1. This indicates a promising formulation for improved wound healing. | [162] |
| Nanoparticles | Curcumin, oleic acid, silica gel 60, Carbopol-934 | Sonication, physical mixing | In vitro and in vivo with male Wistar rat model | Mesoporous silica loaded with curcumin was created by simply mixing a curcumin solution with mesoporous silica powder at 50 °C. In vivo rat studies had two groups. One group was treated with curcumin-loaded mesoporous silica, while the other group was treated with sulfadiazine. The curcumin-treated group displayed more significant improvements in the healing process, attributed to the formulation’s anti-inflammatory effects and its capacity to enhance angiogenesis, epithelization, and collagen synthesis. | [163] |
| Carbon dots | Curcumin, carbon dots, ethylenediamine, bovine gelatine (Type B) | Cross-linking | In vitro and in vivo with male Sprague Dawley rat model | The solubility and stability of free curcumin were enhanced by formulating it as carbon dots. Carbon dots exhibited improved proliferative, proangiogenic, and antibacterial activity, making them suitable for wound healing applications. In vivo studies demonstrated accelerated wound contraction, increased angiogenesis, and complete formation of the epithelium. | [164] |
| Polymeric micelles | Curcumin, alginate | Emulsion | In vitro and in vivo with rat model | A concentration of 7.5 mg of curcumin-loaded micelles did not significantly reduce cell viability. The minimum inhibitory concentration values of curcumin were 153, 245, and 319 μg/mL against S. aureus, Pseudomonas aeruginosa, and E. coli. In vivo studies revealed that the curcumin-loaded micelles led to an increase in protein, collagen, and TGFβ1 expression. | [145] |
| Curcumin, chitosan, alginate, maltodextrin, pluronic F127, pluronic P123, Tween®80 | Thin-film hydration | In vivo with Bisphenol A-induced diabetic rat model | The blood glucose level and lipid profile of rats were observed to decrease significantly after treatment with curcumin-loaded micelles. Curcumin-loaded polymeric micelles repaired the pancreatic β cells damaged by “Bisphenol A” and prevented diabetic complications. | [146] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Falcone, M.; De Angelis, B.; Pea, F.; Scalise, A.; Stefani, S.; Tasinato, R.; Zanetti, O.; Dalla Paola, L. Challenges in the management of chronic wound infections. J. Glob. Antimicrob. Resist. 2021, 26, 140–147. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef]
- Yousefian, F.; Hesari, R.; Jensen, T.; Obagi, S.; Rgeai, A.; Damiani, G.; Bunick, C.G.; Grada, A. Antimicrobial Wound Dressings: A Concise Review for Clinicians. Antibiotics 2023, 12, 1434. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Elkhateeb, O.; Badawy, M.E.I.; Tohamy, H.G.; Abou-Ahmed, H.; El-Kammar, M.; Elkhenany, H. Curcumin-infused nanostructured lipid carriers: A promising strategy for enhancing skin regeneration and combating microbial infection. BMC Vet. Res. 2023, 19, 206. [Google Scholar] [CrossRef]
- Islam, A.; Rebello, L.; Chepyala, S. Review on nanoformulations of curcumin (Curcuma longa Linn.): Special emphasis on Nanocurcumin®. Int. J. Nat. Life Sci. 2019, 3, 1–12. [Google Scholar]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative preparation of curcumin for improved oral bioavailability. Biol. Pharm. Bull. 2011, 34, 660–665. [Google Scholar] [CrossRef]
- Sohn, S.I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical Applications and Bioavailability of Curcumin-An Updated Overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Parachur, V.A.; Mohanty, N.; Swain, T.; Sahu, S. A comparative pharmacokinetic evaluation of a bioavailable curcumin formulation curene® with curcumin formulation containing turmeric volatile oil and standard curcuminoids 95% in healthy human subjects. Funct. Foods Health Dis. 2019, 9, 134–144. [Google Scholar] [CrossRef]
- Ullah, F.; Asgarov, R.; Venigalla, M.; Liang, H.; Niedermayer, G.; Munch, G.; Gyengesi, E. Effects of a solid lipid curcumin particle formulation on chronic activation of microglia and astroglia in the GFAP-IL6 mouse model. Sci. Rep. 2020, 10, 2365. [Google Scholar] [CrossRef]
- Jamwal, R. Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers. J. Integr. Med. 2018, 16, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Henriques, M.; Faustino, M.A.F.; Santos Braga, S. Curcumin innovative delivery forms: Paving the ‘Yellow Brick Road’of antitumoral phytotherapy. Appl. Sci. 2020, 10, 8990. [Google Scholar] [CrossRef]
- Hamilton, A.E.; Gilbert, R.J. Curcumin Release from Biomaterials for Enhanced Tissue Regeneration Following Injury or Disease. Bioengineering 2023, 10, 262. [Google Scholar] [CrossRef]
- Bryant, R.; Nix, D. Acute and Chronic Wounds: Current Management Concepts; Elsevier Health Sciences: Maryland Heights, MO, USA, 2015. [Google Scholar]
- Alven, S.; Nqoro, X.; Aderibigbe, B.A. Polymer-Based Materials Loaded with Curcumin for Wound Healing Applications. Polymers 2020, 12, 2286. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, J.; Xu, T.; Yao, H.; Yu, L.; Huang, D. Hemostasis Strategies and Recent Advances in Nanomaterials for Hemostasis. Molecules 2023, 28, 5264. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Barreda, D.R. Acute Inflammation in Tissue Healing. Int. J. Mol. Sci. 2022, 24, 641. [Google Scholar] [CrossRef]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Schultz, G.S.; Chin, G.A.; Moldawer, L.; Diegelmann, R.F. Principles of wound healing. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists [Internet]; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef]
- Turner, K.H.; Everett, J.; Trivedi, U.; Rumbaugh, K.P.; Whiteley, M. Requirements for Pseudomonas aeruginosa acute burn and chronic surgical wound infection. PLoS Genet. 2014, 10, e1004518. [Google Scholar] [CrossRef]
- Norman, G.; Christie, J.; Liu, Z.; Westby, M.J.; Jefferies, J.M.; Hudson, T.; Edwards, J.; Mohapatra, D.P.; Hassan, I.A.; Dumville, J.C. Antiseptics for burns. Cochrane Database Syst. Rev. 2017, 7, CD011821. [Google Scholar] [CrossRef]
- Branch-Elliman, W.; Ripollone, J.E.; O’Brien, W.J.; Itani, K.M.F.; Schweizer, M.L.; Perencevich, E.; Strymish, J.; Gupta, K. Risk of surgical site infection, acute kidney injury, and Clostridium difficile infection following antibiotic prophylaxis with vancomycin plus a beta-lactam versus either drug alone: A national propensity-score-adjusted retrospective cohort study. PLoS Med. 2017, 14, e1002340. [Google Scholar] [CrossRef]
- Costa, M.L.; Achten, J.; Knight, R.; Bruce, J.; Dutton, S.J.; Madan, J.; Dritsaki, M.; Parsons, N.; Fernandez, M.; Grant, R.; et al. Effect of Incisional Negative Pressure Wound Therapy vs Standard Wound Dressing on Deep Surgical Site Infection After Surgery for Lower Limb Fractures Associated with Major Trauma: The WHIST Randomized Clinical Trial. JAMA 2020, 323, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.M.; Holban, A.M.; Giurcaneanu, C.; Popa, L.G.; Oanea, R.M.; Lazar, V.; Chifiriuc, M.C.; Popa, M.; Popa, M.I. Microbial biofilms: Impact on the pathogenesis of periodontitis, cystic fibrosis, chronic wounds and medical device-related infections. Curr. Top. Med. Chem. 2015, 15, 1552–1576. [Google Scholar] [CrossRef] [PubMed]
- Iheozor-Ejiofor, Z.; Newton, K.; Dumville, J.C.; Costa, M.L.; Norman, G.; Bruce, J. Negative pressure wound therapy for open traumatic wounds. Cochrane Database Syst. Rev. 2018, 7, CD012522. [Google Scholar] [CrossRef] [PubMed]
- Herrod, P.J.; Boyd-Carson, H.; Doleman, B.; Blackwell, J.; Williams, J.P.; Bhalla, A.; Nelson, R.L.; Tou, S.; Lund, J.N. Prophylactic antibiotics for penetrating abdominal trauma: Duration of use and antibiotic choice. Cochrane Database Syst. Rev. 2019, 12, CD010808. [Google Scholar] [CrossRef]
- Percival, S.L. Importance of biofilm formation in surgical infection. Br. J. Surg. 2017, 104, e85–e94. [Google Scholar] [CrossRef]
- Chin, J.S.; Madden, L.; Chew, S.Y.; Becker, D.L. Drug therapies and delivery mechanisms to treat perturbed skin wound healing. Adv. Drug Deliv. Rev. 2019, 149–150, 2–18. [Google Scholar] [CrossRef]
- Liu, C.; Ponsero, A.J.; Armstrong, D.G.; Lipsky, B.A.; Hurwitz, B.L. The dynamic wound microbiome. BMC Med. 2020, 18, 358. [Google Scholar] [CrossRef]
- Verbanic, S.; Shen, Y.; Lee, J.; Deacon, J.M.; Chen, I.A. Microbial predictors of healing and short-term effect of debridement on the microbiome of chronic wounds. NPJ Biofilms Microbiomes 2020, 6, 21. [Google Scholar] [CrossRef]
- Uckay, I.; Gariani, K.; Pataky, Z.; Lipsky, B.A. Diabetic foot infections: State-of-the-art. Diabetes Obes. Metab. 2014, 16, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Agale, S.V. Chronic leg ulcers: Epidemiology, aetiopathogenesis, and management. Ulcers 2013, 2013, 413604. [Google Scholar] [CrossRef]
- Schilrreff, P.; Alexiev, U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. Int. J. Mol. Sci. 2022, 23, 4928. [Google Scholar] [CrossRef]
- Barker, J.C.; Khansa, I.; Gordillo, G.M. A Formidable Foe Is Sabotaging Your Results: What You Should Know about Biofilms and Wound Healing. Plast. Reconstr. Surg. 2017, 139, 1184e–1194e. [Google Scholar] [CrossRef]
- Spampinato, S.F.; Caruso, G.I.; De Pasquale, R.; Sortino, M.A.; Merlo, S. The Treatment of Impaired Wound Healing in Diabetes: Looking among Old Drugs. Pharmaceuticals 2020, 13, 60. [Google Scholar] [CrossRef]
- Nunan, R.; Harding, K.G.; Martin, P. Clinical challenges of chronic wounds: Searching for an optimal animal model to recapitulate their complexity. Dis. Model. Mech. 2014, 7, 1205–1213. [Google Scholar] [CrossRef]
- Krizanova, O.; Penesova, A.; Hokynkova, A.; Pokorna, A.; Samadian, A.; Babula, P. Chronic venous insufficiency and venous leg ulcers: Aetiology, on the pathophysiology-based treatment. Int. Wound J. 2024, 21, e14405. [Google Scholar] [CrossRef]
- Crawford, J.M.; Lal, B.K.; Duran, W.N.; Pappas, P.J. Pathophysiology of venous ulceration. J. Vasc. Surg. Venous Lymphat. Disord. 2017, 5, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.; Chauhan, N. Pressure ulcers: Back to the basics. Indian J. Plast. Surg. 2012, 45, 244–254. [Google Scholar] [CrossRef]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Rehak, L.; Giurato, L.; Meloni, M.; Panunzi, A.; Manti, G.M.; Uccioli, L. The Immune-Centric Revolution in the Diabetic Foot: Monocytes and Lymphocytes Role in Wound Healing and Tissue Regeneration—A Narrative Review. J. Clin. Med. 2022, 11, 889. [Google Scholar] [CrossRef]
- Liu, M.; Duan, X.P.; Li, Y.M.; Yang, D.P.; Long, Y.Z. Electrospun nanofibers for wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 1413–1423. [Google Scholar] [CrossRef]
- Fadilah, N.I.M.; Phang, S.J.; Kamaruzaman, N.; Salleh, A.; Zawani, M.; Sanyal, A.; Maarof, M.; Fauzi, M.B. Antioxidant Biomaterials in Cutaneous Wound Healing and Tissue Regeneration: A Critical Review. Antioxidants 2023, 12, 787. [Google Scholar] [CrossRef]
- Shah, A.; Amini-Nik, S. The Role of Phytochemicals in the Inflammatory Phase of Wound Healing. Int. J. Mol. Sci. 2017, 18, 1068. [Google Scholar] [CrossRef]
- Siddiqui, A.R.; Bernstein, J.M. Chronic wound infection: Facts and controversies. Clin. Dermatol. 2010, 28, 519–526. [Google Scholar] [CrossRef]
- Percival, S.; Cutting, K. Microbiology of Wounds; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun nanofibers as dressings for chronic wound care: Advances, challenges, and future prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef] [PubMed]
- Maaz Arif, M.; Khan, S.M.; Gull, N.; Tabish, T.A.; Zia, S.; Ullah Khan, R.; Awais, S.M.; Arif Butt, M. Polymer-based biomaterials for chronic wound management: Promises and challenges. Int. J. Pharm. 2021, 598, 120270. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E.; Liu, P.Y.; Schultz, G.S.; Martins-Green, M.M.; Tanaka, R.; Weir, D.; Gould, L.J.; Armstrong, D.G.; Gibbons, G.W.; Wolcott, R.; et al. Chronic wounds: Treatment consensus. Wound Repair. Regen. 2022, 30, 156–171. [Google Scholar] [CrossRef]
- Zhong, C.; Wu, Y.; Lin, H.; Liu, R. Advances in the antimicrobial treatment of osteomyelitis. Compos. Part. B Eng. 2023, 249, 110428. [Google Scholar] [CrossRef]
- Rai, R. Standard guidelines for management of venous leg ulcer. Indian Dermatol. Online J. 2014, 5, 408–411. [Google Scholar] [CrossRef]
- Danish, M.I. Short Textbook of Medical Diagnosis and Management; Scientific International Pvt. Ltd.: Delhi, India, 2018. [Google Scholar]
- Nuutila, K.; Eriksson, E. Moist Wound Healing with Commonly Available Dressings. Adv. Wound Care 2021, 10, 685–698. [Google Scholar] [CrossRef]
- Sheokand, B.; Vats, M.; Kumar, A.; Srivastava, C.M.; Bahadur, I.; Pathak, S.R. Natural polymers used in the dressing materials for wound healing: Past, present and future. J. Polym. Sci. 2023, 61, 1389–1414. [Google Scholar] [CrossRef]
- Kumari, A.; Raina, N.; Wahi, A.; Goh, K.W.; Sharma, P.; Nagpal, R.; Jain, A.; Ming, L.C.; Gupta, M. Wound-Healing Effects of Curcumin and Its Nanoformulations: A Comprehensive Review. Pharmaceutics 2022, 14, 2288. [Google Scholar] [CrossRef]
- Indira Priyadarsini, K. Chemical and structural features influencing the biological activity of curcumin. Curr. Pharm. Des. 2013, 19, 2093–2100. [Google Scholar] [PubMed]
- Cornago, P.; Claramunt, R.M.; Bouissane, L.; Alkorta, I.; Elguero, J. A study of the tautomerism of β-dicarbonyl compounds with special emphasis on curcuminoids. Tetrahedron 2008, 64, 8089–8094. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocinska, K.; Zielinska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Kulac, M.; Aktas, C.; Tulubas, F.; Uygur, R.; Kanter, M.; Erboga, M.; Ceber, M.; Topcu, B.; Ozen, O.A. The effects of topical treatment with curcumin on burn wound healing in rats. J. Mol. Histol. 2013, 44, 83–90. [Google Scholar] [CrossRef]
- Emiroglu, G.; Ozergin Coskun, Z.; Kalkan, Y.; Celebi Erdivanli, O.; Tumkaya, L.; Terzi, S.; Ozgur, A.; Demirci, M.; Dursun, E. The Effects of Curcumin on Wound Healing in a Rat Model of Nasal Mucosal Trauma. Evid. Based Complement. Altern. Med. 2017, 2017, 9452392. [Google Scholar] [CrossRef]
- Heydari, P.; Zargar Kharazi, A.; Asgary, S.; Parham, S. Comparing the wound healing effect of a controlled release wound dressing containing curcumin/ciprofloxacin and simvastatin/ciprofloxacin in a rat model: A preclinical study. J. Biomed. Mater. Res. A 2022, 110, 341–352. [Google Scholar] [CrossRef]
- Wu, J.; Deng, L.; Yin, L.; Mao, Z.; Gao, X. Curcumin promotes skin wound healing by activating Nrf2 signaling pathways and inducing apoptosis in mice. Turk. J. Med. Sci. 2023, 53, 1127–1135. [Google Scholar] [CrossRef]
- Kant, V.; Gopal, A.; Kumar, D.; Pathak, N.N.; Ram, M.; Jangir, B.L.; Tandan, S.K.; Kumar, D. Curcumin-induced angiogenesis hastens wound healing in diabetic rats. J. Surg. Res. 2015, 193, 978–988. [Google Scholar] [CrossRef]
- Cardoso-Daodu, I.M.; Ilomuanya, M.O.; Azubuike, C.P. Development of curcumin-loaded liposomes in lysine-collagen hydrogel for surgical wound healing. Beni-Suef Univ. J. Basic. Appl. Sci. 2022, 11, 100. [Google Scholar] [CrossRef]
- Mokhtari, M.; Razzaghi, R.; Momen-Heravi, M. The effects of curcumin intake on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2021, 35, 2099–2107. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Ghosh, C.; Hwang, S.G.; Tran, L.D.; Park, J.S. Characteristics of curcumin-loaded poly (lactic acid) nanofibers for wound healing. J. Mater. Sci. 2013, 48, 7125–7133. [Google Scholar] [CrossRef]
- Zahiri, M.; Khanmohammadi, M.; Goodarzi, A.; Ababzadeh, S.; Sagharjoghi Farahani, M.; Mohandesnezhad, S.; Bahrami, N.; Nabipour, I.; Ai, J. Encapsulation of curcumin loaded chitosan nanoparticle within poly (epsilon-caprolactone) and gelatin fiber mat for wound healing and layered dermal reconstitution. Int. J. Biol. Macromol. 2020, 153, 1241–1250. [Google Scholar] [CrossRef]
- Mobaraki, M.; Bizari, D.; Soltani, M.; Khshmohabat, H.; Raahemifar, K.; Akbarzade Amirdehi, M. The Effects of Curcumin Nanoparticles Incorporated into Collagen-Alginate Scaffold on Wound Healing of Skin Tissue in Trauma Patients. Polymers 2021, 13, 4291. [Google Scholar] [CrossRef]
- Mahmoud, R.; Safwat, N.; Fathy, M.; Mohamed, N.A.; El-Dek, S.; El-Banna, H.A.; Farghali, A.; El-Ela, F.I.A. Novel anti-inflammatory and wound healing controlled released LDH-Curcumin nanocomposite via intramuscular implantation, in-vivo study. Arab. J. Chem. 2022, 15, 103646. [Google Scholar] [CrossRef]
- Qian, S.; Wang, J.; Liu, Z.; Mao, J.; Zhao, B.; Mao, X.; Zhang, L.; Cheng, L.; Zhang, Y.; Sun, X.; et al. Secretory Fluid-Aggregated Janus Electrospun Short Fiber Scaffold for Wound Healing. Small 2022, 18, e2200799. [Google Scholar] [CrossRef]
- Aslam, Z.; Roome, T.; Razzak, A.; Aslam, S.M.; Zaidi, M.B.; Kanwal, T.; Sikandar, B.; Bertino, M.F.; Rehman, K.; Shah, M.R. Investigation of wound healing potential of photo-active curcumin-ZnO-nanoconjugates in excisional wound model. Photodiagnosis Photodyn. Ther. 2022, 39, 102956. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Pourakbari, B.; Bahador, A. Contribution of antimicrobial photo-sonodynamic therapy in wound healing: An in vivo effect of curcumin-nisin-based poly (L-lactic acid) nanoparticle on Acinetobacter baumannii biofilms. BMC Microbiol. 2022, 22, 28. [Google Scholar] [CrossRef]
- Ravanfar, K.; Amniattalab, A.; Mohammadi, R. Curcumin-Polyethylene Glycol Loaded on Chitosan-Gelatin Nanoparticles Enhances Burn Wound Healing in Rat. J. Burn. Care Res. 2022, 43, 1399–1409. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Cueto, R.; Effi, C.; Zhang, Y.; Tan, H.; Qin, X.; Ji, Y.; Yang, X.; Wang, H. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 2019, 26, 101284. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yang, F.; Zhou, W.; Xiao, N.; Luo, M.; Tang, Z. The initiation of oxidative stress and therapeutic strategies in wound healing. Biomed. Pharmacother. 2023, 157, 114004. [Google Scholar] [CrossRef] [PubMed]
- Borra, S.K.; Mahendra, J.; Gurumurthy, P.; Jayamathi; Iqbal, S.S.; Mahendra, L. Effect of curcumin against oxidation of biomolecules by hydroxyl radicals. J. Clin. Diagn. Res. 2014, 8, CC01–CC05. [Google Scholar] [CrossRef] [PubMed]
- Huyut, Z.; Beydemir, S.; Gulcin, I. Antioxidant and Antiradical Properties of Selected Flavonoids and Phenolic Compounds. Biochem. Res. Int. 2017, 2017, 7616791. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Tzvetkov, N.T.; El-Tawil, O.S.; Bungau, S.G.; Abdel-Daim, M.M.; Atanasov, A.G. Antioxidants: Scientific Literature Landscape Analysis. Oxid. Med. Cell Longev. 2019, 2019, 8278454. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gulcin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Barclay, L.R.; Vinqvist, M.R.; Mukai, K.; Goto, H.; Hashimoto, Y.; Tokunaga, A.; Uno, H. On the antioxidant mechanism of curcumin: Classical methods are needed to determine antioxidant mechanism and activity. Org. Lett. 2000, 2, 2841–2843. [Google Scholar] [CrossRef]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Subudhi, U.; Chainy, G.B. Expression of hepatic antioxidant genes in l-thyroxine-induced hyperthyroid rats: Regulation by vitamin E and curcumin. Chem. Biol. Interact. 2010, 183, 304–316. [Google Scholar] [CrossRef]
- Panchatcharam, M.; Miriyala, S.; Gayathri, V.S.; Suguna, L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol. Cell Biochem. 2006, 290, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Motohashi, H.; Yamamoto, M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 2011, 16, 123–140. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Curcumin Activates the Nrf2 Pathway and Induces Cellular Protection Against Oxidative Injury. Curr. Mol. Med. 2020, 20, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Z.; Wang, J.; Li, R.; Li, T.; Chang, M.; Yan, F.; Wang, Y. Encapsulation of Curcumin Nanoparticles with MMP9-Responsive and Thermos-Sensitive Hydrogel Improves Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 16315–16326. [Google Scholar] [CrossRef]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Devel Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Merrell, J.G.; McLaughlin, S.W.; Tie, L.; Laurencin, C.T.; Chen, A.F.; Nair, L.S. Curcumin loaded poly (ε-caprolactone) nanofibers: Diabetic wound dressing with antioxidant and anti-inflammatory properties. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1149. [Google Scholar] [CrossRef]
- Antoine, F.; Girard, D. Curcumin increases gelatinase activity in human neutrophils by a p38 mitogen-activated protein kinase (MAPK)-independent mechanism. J. Immunotoxicol. 2015, 12, 188–193. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Zhu, R.; Liu, Q.; Fei, J.; Wang, S. Anti-inflammatory activity of curcumin-loaded solid lipid nanoparticles in IL-1beta transgenic mice subjected to the lipopolysaccharide-induced sepsis. Biomaterials 2015, 53, 475–483. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Wu, J.; Bai, B.; Chen, H.; Xiao, Z.; Chen, L.; Zhao, Y.; Lum, H.; Wang, Y.; et al. New MD2 inhibitors derived from curcumin with improved anti-inflammatory activity. Eur. J. Med. Chem. 2018, 148, 291–305. [Google Scholar] [CrossRef]
- Alibolandi, M.; Mohammadi, M.; Taghdisi, S.M.; Abnous, K.; Ramezani, M. Synthesis and preparation of biodegradable hybrid dextran hydrogel incorporated with biodegradable curcumin nanomicelles for full thickness wound healing. Int. J. Pharm. 2017, 532, 466–477. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.G.; Chen, W.M.; Yu, A.X. Efficacy of thermosensitive chitosan/beta-glycerophosphate hydrogel loaded with beta-cyclodextrin-curcumin for the treatment of cutaneous wound infection in rats. Exp. Ther. Med. 2018, 15, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Sharif Zak, M.; Majdi, H.; Mostafavi, E.; Barati, M.; Lotfimehr, H.; Ghaseminasab, K.; Pazoki-Toroudi, H.; Webster, T.J.; Akbarzadeh, A. The effect of chrysin-curcumin-loaded nanofibres on the wound-healing process in male rats. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Huang, C.; Huang, H.; Zhao, Y.; Khan, M.R.U.; Zhao, H.; Huang, L. Antibacterial Mechanism of Curcumin: A Review. Chem. Biodivers. 2020, 17, e2000171. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.H.; Kim, S.B.; Kong, R.; Choi, J.G.; Kim, Y.C.; Shin, D.W.; Kang, O.H.; Kwon, D.Y. Curcumin reverse methicillin resistance in Staphylococcus aureus. Molecules 2014, 19, 18283–18295. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.T.; Moayer, F. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly(epsilon-caprolactone) electrospun nanofibers. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 1183–1191. [Google Scholar] [CrossRef]
- Sharma, D.; Dhingra, S.; Banerjee, A.; Saha, S.; Bhattacharyya, J.; Satapathy, B.K. Designing suture-proof cell-attachable copolymer-mediated and curcumin- beta-cyclodextrin inclusion complex loaded aliphatic polyester-based electrospun antibacterial constructs. Int. J. Biol. Macromol. 2022, 216, 397–413. [Google Scholar] [CrossRef]
- Dos Santos, D.M.; de Annunzio, S.R.; Carmello, J.C.; Pavarina, A.C.; Fontana, C.R.; Correa, D.S. Combining Coaxial Electrospinning and 3D Printing: Design of Biodegradable Bilayered Membranes with Dual Drug Delivery Capability for Periodontitis Treatment. ACS Appl. Bio Mater. 2022, 5, 146–159. [Google Scholar] [CrossRef]
- Chagas, P.A.M.; Schneider, R.; dos Santos, D.M.; Otuka, A.J.G.; Mendonça, C.R.; Correa, D.S. Bilayered electrospun membranes composed of poly(lactic-acid)/natural rubber: A strategy against curcumin photodegradation for wound dressing application. React. Funct. Polym. 2021, 163, 104889. [Google Scholar] [CrossRef]
- Jose, J.; Pai, A.R.; Gopakumar, D.A.; Dalvi, Y.; Ruby, V.; Bhat, S.G.; Pasquini, D.; Kalarikkal, N.; Thomas, S. Novel 3D porous aerogels engineered at nano scale from cellulose nano fibers and curcumin: An effective treatment for chronic wounds. Carbohydr. Polym. 2022, 287, 119338. [Google Scholar] [CrossRef]
- Zhang, Q.; Zou, Y.H.; Tang, L.Q.; Liu, X.L.; Hu, M.Q.; Han, H.; Li, Y.; Wang, F.J.; Wang, L.; Mao, J.F. Stage-controlled antibacterial surgical sutures based on curcumin@ZIF-8 functional coating for improved wound healing. Prog. Org. Coat. 2023, 184, 107829. [Google Scholar] [CrossRef]
- Cai, J.; Zhong, H.; Tang, W.; Wen, F.; Lv, Y.; Huang, X.; Luo, J.; Li, P. Multiple response behaviors of curcumin-loaded ammonium alginate/polyvinyl alcohol hydrogel and its application. Biomass Convers. Biorefin. 2023, 14, 16121–16139. [Google Scholar] [CrossRef]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, C.; Zhang, X.; Lin, Y.; Yang, H.; Zhang, Y.S.; Ding, J. An oxidative stress-responsive electrospun polyester membrane capable of releasing anti-bacterial and anti-inflammatory agents for postoperative anti-adhesion. J. Control Release 2021, 335, 359–368. [Google Scholar] [CrossRef]
- Bakhshi, M.; Mahboubi, A.; Jaafari, M.R.; Ebrahimi, F.; Tofangchiha, M.; Alizadeh, A. Comparative Efficacy of 1% Curcumin Nanomicelle Gel and 2% Curcumin Gel for Treatment of Recurrent Aphthous Stomatitis: A Double-Blind Randomized Clinical Trial. J. Evid. Based Dent. Pr. 2022, 22, 101708. [Google Scholar] [CrossRef]
- Milano, F.; Mari, L.; van de Luijtgaarden, W.; Parikh, K.; Calpe, S.; Krishnadath, K.K. Nano-curcumin inhibits proliferation of esophageal adenocarcinoma cells and enhances the T cell mediated immune response. Front. Oncol. 2013, 3, 137. [Google Scholar] [CrossRef]
- Epelbaum, R.; Schaffer, M.; Vizel, B.; Badmaev, V.; Bar-Sela, G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr. Cancer 2010, 62, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Agah, E.; Nafissi, S.; Jaafari, M.R.; Harirchian, M.H.; Sarraf, P.; Faghihi-Kashani, S.; Hosseini, S.J.; Ghoreishi, A.; Aghamollaii, V.; et al. Safety and Efficacy of Nanocurcumin as Add-On Therapy to Riluzole in Patients With Amyotrophic Lateral Sclerosis: A Pilot Randomized Clinical Trial. Neurotherapeutics 2018, 15, 430–438. [Google Scholar] [CrossRef]
- Bateni, Z.; Rahimi, H.R.; Hedayati, M.; Afsharian, S.; Goudarzi, R.; Sohrab, G. The effects of nano-curcumin supplementation on glycemic control, blood pressure, lipid profile, and insulin resistance in patients with the metabolic syndrome: A randomized, double-blind clinical trial. Phytother. Res. 2021, 35, 3945–3953. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef]
- Lombardi, N.; Crescioli, G.; Maggini, V.; Ippoliti, I.; Menniti-Ippolito, F.; Gallo, E.; Brilli, V.; Lanzi, C.; Mannaioni, G.; Firenzuoli, F.; et al. Acute liver injury following turmeric use in Tuscany: An analysis of the Italian Phytovigilance database and systematic review of case reports. Br. J. Clin. Pharmacol. 2021, 87, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Parsons, H.A.; Baracos, V.E.; Hong, D.S.; Abbruzzese, J.; Bruera, E.; Kurzrock, R. The effects of curcumin (diferuloylmethane) on body composition of patients with advanced pancreatic cancer. Oncotarget 2016, 7, 20293–20304. [Google Scholar] [CrossRef]
- Garg, A.X.; Devereaux, P.J.; Hill, A.; Sood, M.; Aggarwal, B.; Dubois, L.; Hiremath, S.; Guzman, R.; Iyer, V.; James, M.; et al. Oral curcumin in elective abdominal aortic aneurysm repair: A multicentre randomized controlled trial. CMAJ 2018, 190, E1273–E1280. [Google Scholar] [CrossRef] [PubMed]
- Skiba, M.B.; Luis, P.B.; Alfafara, C.; Billheimer, D.; Schneider, C.; Funk, J.L. Curcuminoid Content and Safety-Related Markers of Quality of Turmeric Dietary Supplements Sold in an Urban Retail Marketplace in the United States. Mol. Nutr. Food Res. 2018, 62, e1800143. [Google Scholar] [CrossRef]
- Nikpour, M.; Delavar, M.A.; Khafri, S.; Ghanbarpour, A.; Moghadamnia, A.A.; Esmaeilzadeh, S.; Behmanesh, F. The Use of Honey and Curcumin for Episiotomy Pain Relief and Wound Healing: A Three-Group Double-Blind Randomized Clinical Trial. Nurs. Midwifery Stud. 2019, 8, 64–69. [Google Scholar] [CrossRef]
- Adamu, B.F.; Gao, J.; Jhatial, A.K.; Kumelachew, D.M. A review of medicinal plant-based bioactive electrospun nano fibrous wound dressings. Mater. Des. 2021, 209, 109942. [Google Scholar] [CrossRef]
- Gaspar-Pintiliescu, A.; Stanciuc, A.M.; Craciunescu, O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int. J. Biol. Macromol. 2019, 138, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Bt Hj Idrus, R.; Fauzi, M.B. Plant- vs. Bacterial-Derived Cellulose for Wound Healing: A Review. Int. J. Environ. Res. Public Health 2020, 17, 6803. [Google Scholar] [CrossRef]
- Pandey, V.K.; Ajmal, G.; Upadhyay, S.N.; Mishra, P.K. Nano-fibrous scaffold with curcumin for anti-scar wound healing. Int. J. Pharm. 2020, 589, 119858. [Google Scholar] [CrossRef]
- Tejada, S.; Manayi, A.; Daglia, M.; Nabavi, S.F.; Sureda, A.; Hajheydari, Z.; Gortzi, O.; Pazoki-Toroudi, H.; Nabavi, S.M. Wound Healing Effects of Curcumin: A Short Review. Curr. Pharm. Biotechnol. 2016, 17, 1002–1007. [Google Scholar] [CrossRef]
- Dong, Y.P.; Zheng, Y.Q.; Zhang, K.Y.; Yao, Y.M.; Wang, L.H.; Li, X.R.; Yu, J.Y.; Ding, B. Electrospun Nanofibrous Materials for Wound Healing. Adv. Fiber Mater. 2020, 2, 212–227. [Google Scholar] [CrossRef]
- Gupta, K.C.; Haider, A.; Choi, Y.R.; Kang, I.K. Nanofibrous scaffolds in biomedical applications. Biomater. Res. 2014, 18, 5. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Feng, Z.; Han, B.; Yu, D.G.; Wang, K. Advances in the Preparation of Nanofiber Dressings by Electrospinning for Promoting Diabetic Wound Healing. Biomolecules 2022, 12, 1727. [Google Scholar] [CrossRef] [PubMed]
- Yusuf Aliyu, A.; Adeleke, O.A. Nanofibrous Scaffolds for Diabetic Wound Healing. Pharmaceutics 2023, 15, 986. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Pan, H.; Ji, D.; Li, Y.; Duan, H.; Pan, W. Curcumin-loaded sandwich-like nanofibrous membrane prepared by electrospinning technology as wound dressing for accelerate wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 127, 112245. [Google Scholar] [CrossRef]
- Pillai, M.M.; Dandia, H.; Checker, R.; Rokade, S.; Sharma, D.; Tayalia, P. Novel combination of bioactive agents in bilayered dermal patches provides superior wound healing. Nanomedicine 2022, 40, 102495. [Google Scholar] [CrossRef]
- Gorain, B.; Pandey, M.; Leng, N.H.; Yan, C.W.; Nie, K.W.; Kaur, S.J.; Marshall, V.; Sisinthy, S.P.; Panneerselvam, J.; Molugulu, N.; et al. Advanced drug delivery systems containing herbal components for wound healing. Int. J. Pharm. 2022, 617, 121617. [Google Scholar] [CrossRef] [PubMed]
- Anumolu, S.S.; DeSantis, A.S.; Menjoge, A.R.; Hahn, R.A.; Beloni, J.A.; Gordon, M.K.; Sinko, P.J. Doxycycline loaded poly(ethylene glycol) hydrogels for healing vesicant-induced ocular wounds. Biomaterials 2010, 31, 964–974. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Vimala, K.; Ravindra, S.; Narayana Reddy, N.; Venkata Subba Reddy, G.; Mohana Raju, K. Fabrication of silver nanocomposite films impregnated with curcumin for superior antibacterial applications. J. Mater. Sci. Mater. Med. 2011, 22, 1863–1872. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Zhang, B.; Li, M.; Diao, K.; Zhang, Z.; Li, J.; Xu, Y.; Wang, X.; Chen, H. In situ injectable nano-composite hydrogel composed of curcumin, N,O-carboxymethyl chitosan and oxidized alginate for wound healing application. Int. J. Pharm. 2012, 437, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Nan, K.H.; Li, L.L.; Zhang, Z.L.; Chen, H. evaluation of curcumin nanoformulation loaded methoxy poly(ethylene glycol)-graft-chitosan composite film for wound healing application. Carbohydr. Polym. 2012, 88, 84–90. [Google Scholar] [CrossRef]
- Gong, C.; Wu, Q.; Wang, Y.; Zhang, D.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials 2013, 34, 6377–6387. [Google Scholar] [CrossRef]
- Pham, L.; Dang, L.H.; Truong, M.D.; Nguyen, T.H.; Le, L.; Le, V.T.; Nam, N.D.; Bach, L.G.; Nguyen, V.T.; Tran, N.Q. A dual synergistic of curcumin and gelatin on thermal-responsive hydrogel based on Chitosan-P123 in wound healing application. Biomed. Pharmacother. 2019, 117, 109183. [Google Scholar] [CrossRef]
- Chen, L.; Pan, Z.; Zhu, J.; Mao, Y.; Sun, J. Novel fabrication of dual nanoparticle loaded-co-polymeric dressing for effective healing efficiency in wound care after fracture surgery. J. Biomater. Sci. Polym. Ed. 2021, 32, 2009–2027. [Google Scholar] [CrossRef]
- Chiaoprakobkij, N.; Suwanmajo, T.; Sanchavanakit, N.; Phisalaphong, M. Curcumin-Loaded Bacterial Cellulose/Alginate/Gelatin as A Multifunctional Biopolymer Composite Film. Molecules 2020, 25, 3800. [Google Scholar] [CrossRef]
- Jhaveri, A.M.; Torchilin, V.P. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front. Pharmacol. 2014, 5, 77. [Google Scholar] [CrossRef]
- Wu, J.; Li, H.; Zhang, N.; Zheng, Q. Micelle-Containing Hydrogels and Their Applications in Biomedical Research. Gels 2024, 10, 471. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X. Curcumin Loading on Alginate Nano-Micelle for Anti-Infection and Colonic Wound Healing. J. Biomed. Nanotechnol. 2021, 17, 1160–1169. [Google Scholar] [CrossRef]
- Akbar, M.U.; Zia, K.M.; Akash, M.S.H.; Nazir, A.; Zuber, M.; Ibrahim, M. In-vivo anti-diabetic and wound healing potential of chitosan/alginate/maltodextrin/pluronic-based mixed polymeric micelles: Curcumin therapeutic potential. Int. J. Biol. Macromol. 2018, 120, 2418–2430. [Google Scholar] [CrossRef]
- Gaydhane, M.K.; Kanuganti, J.S.; Sharma, C.S. Honey and curcumin loaded multilayered polyvinylalcohol/cellulose acetate electrospun nanofibrous mat for wound healing. J. Mater. Res. 2020, 35, 600–609. [Google Scholar] [CrossRef]
- Samraj, S.M.D.; Kirupha, S.D.; Elango, S.; Vadodaria, K. Fabrication of nanofibrous membrane using stingless bee honey and curcumin for wound healing applications. J. Drug Deliv. Sci. Technol. 2021, 63, 102271. [Google Scholar] [CrossRef]
- Hadizadeh, M.; Naeimi, M.; Rafienia, M.; Karkhaneh, A. A bifunctional electrospun nanocomposite wound dressing containing surfactin and curcumin: In vitro and in vivo studies. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 129, 112362. [Google Scholar] [CrossRef]
- Liao, H.T.; Lai, Y.T.; Kuo, C.Y.; Chen, J.P. A bioactive multi-functional heparin-grafted aligned poly(lactide-co-glycolide)/curcumin nanofiber membrane to accelerate diabetic wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111689. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, Y.; Lun, X.; Sheng, H.; Yan, A. Effects of wound dressing based on the combination of silver@curcumin nanoparticles and electrospun chitosan nanofibers on wound healing. Bioengineered 2022, 13, 4328–4339. [Google Scholar] [CrossRef] [PubMed]
- Orsu, P.; Haider, H.Y.; Koyyada, A. Bioengineering for curcumin loaded carboxymethyl guargum/reduced graphene oxide nanocomposites for chronic wound healing applications. Int. J. Pharm. 2021, 606, 120928. [Google Scholar] [CrossRef] [PubMed]
- Suteris, N.N.; Yasin, A.; Misnon, I.I.; Roslan, R.; Zulkifli, F.H.; Rahim, M.H.A.; Venugopal, J.R.; Jose, R. Curcumin loaded waste biomass resourced cellulosic nanofiber cloth as a potential scaffold for regenerative medicine: An in-vitro assessment. Int. J. Biol. Macromol. 2022, 198, 147–156. [Google Scholar] [CrossRef]
- Agarwal, Y.; Rajinikanth, P.S.; Ranjan, S.; Tiwari, U.; Balasubramnaiam, J.; Pandey, P.; Arya, D.K.; Anand, S.; Deepak, P. Curcumin loaded polycaprolactone-/polyvinyl alcohol-silk fibroin based electrospun nanofibrous mat for rapid healing of diabetic wound: An in-vitro and in-vivo studies. Int. J. Biol. Macromol. 2021, 176, 376–386. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Nourein, I.H.; Albarqi, H.A.; Alyami, H.S.; Alyami, M.H.; Alqahtani, A.A.; Alasiri, A.; Algahtani, T.S.; Mohammed, A.A.; et al. Preparation and Characterization of Curcumin Nanoemulgel Utilizing Ultrasonication Technique for Wound Healing: In Vitro, Ex Vivo, and In Vivo Evaluation. Gels 2021, 7, 213. [Google Scholar] [CrossRef]
- Alyoussef, A.; El-Gogary, R.I.; Ahmed, R.F.; Farid, O.A.A.; Bakeer, R.M.; Nasr, M. The beneficial activity of curcumin and resveratrol loaded in nanoemulgel for healing of burn-induced wounds. J. Drug Deliv. Sci. Technol. 2021, 62, 102360. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Al-Qudaihi, A.; Alaseel, S.E.; Fita, I.Z.; Khalid, M.S.; Pottoo, F.H. Preparation of a novel curcumin nanoemulsion by ultrasonication and its comparative effects in wound healing and the treatment of inflammation. RSC Adv. 2019, 9, 20192–20206. [Google Scholar] [CrossRef]
- Albarqi, H.A.; Alqahtani, A.A.; Ullah, I.; Khan, N.R.; Basit, H.M.; Iftikhar, T.; Wahab, A.; Ali, M.; Badar, M. Microwave-Assisted Physically Cross-Linked Chitosan-Sodium Alginate Hydrogel Membrane Doped with Curcumin as a Novel Wound Healing Platform. AAPS PharmSciTech 2022, 23, 72. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, H.; Shen, W.; Deng, J.; Bai, C.; Xiao, Y.; Lyu, L. Localized delivery of curcumin by thermosensitive hydrogels for promoting wound healing. J. Cosmet. Dermatol. 2022, 21, 5081–5091. [Google Scholar] [CrossRef] [PubMed]
- Ternullo, S.; Schulte Werning, L.V.; Holsaeter, A.M.; Skalko-Basnet, N. Curcumin-In-Deformable Liposomes-In-Chitosan-Hydrogel as a Novel Wound Dressing. Pharmaceutics 2019, 12, 8. [Google Scholar] [CrossRef]
- Kiti, K.; Suwantong, O. The potential use of curcumin-beta-cyclodextrin inclusion complex/chitosan-loaded cellulose sponges for the treatment of chronic wound. Int. J. Biol. Macromol. 2020, 164, 3250–3258. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Cai, X.; Huang, Y.; Zhou, Y. Pluronic F127-liposome-encapsulated curcumin activates Nrf2/Keap1 signaling pathway to promote cell migration of HaCaT cells. Mol. Cell Biochem. 2023, 478, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Hamam, F.; Nasr, A. Curcumin-Loaded Mesoporous Silica Particles as Wound-Healing Agent: An In vivo Study. Saudi J. Med. Med. Sci. 2020, 8, 17–24. [Google Scholar] [CrossRef]
- Sharma, A.; Panwar, V.; Salaria, N.; Ghosh, D. Protease-responsive hydrogel, cross-linked with bioactive curcumin-derived carbon dots, encourage faster wound closure. Biomater. Adv. 2022, 139, 212978. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Ratnayake, J.; Ali, A. Curcumin-Loaded Drug Delivery Systems for Acute and Chronic Wound Management: A Review. Bioengineering 2025, 12, 860. https://doi.org/10.3390/bioengineering12080860
Deng X, Ratnayake J, Ali A. Curcumin-Loaded Drug Delivery Systems for Acute and Chronic Wound Management: A Review. Bioengineering. 2025; 12(8):860. https://doi.org/10.3390/bioengineering12080860
Chicago/Turabian StyleDeng, Xiaoxuan, Jithendra Ratnayake, and Azam Ali. 2025. "Curcumin-Loaded Drug Delivery Systems for Acute and Chronic Wound Management: A Review" Bioengineering 12, no. 8: 860. https://doi.org/10.3390/bioengineering12080860
APA StyleDeng, X., Ratnayake, J., & Ali, A. (2025). Curcumin-Loaded Drug Delivery Systems for Acute and Chronic Wound Management: A Review. Bioengineering, 12(8), 860. https://doi.org/10.3390/bioengineering12080860








