Review of Cardiovascular Mock Circulatory Loop Designs and Applications
Abstract
1. Introduction
- Cardiovascular Devices Testing;
- Clinical Training and Education;
- Hemodynamics and Blood Flow Studies;
- Disease Modeling.
2. Methodology
- Google Scholar;
- PubMed;
- IEEE Xplore Digital Library;
- Web of Science;
- Google Patents.
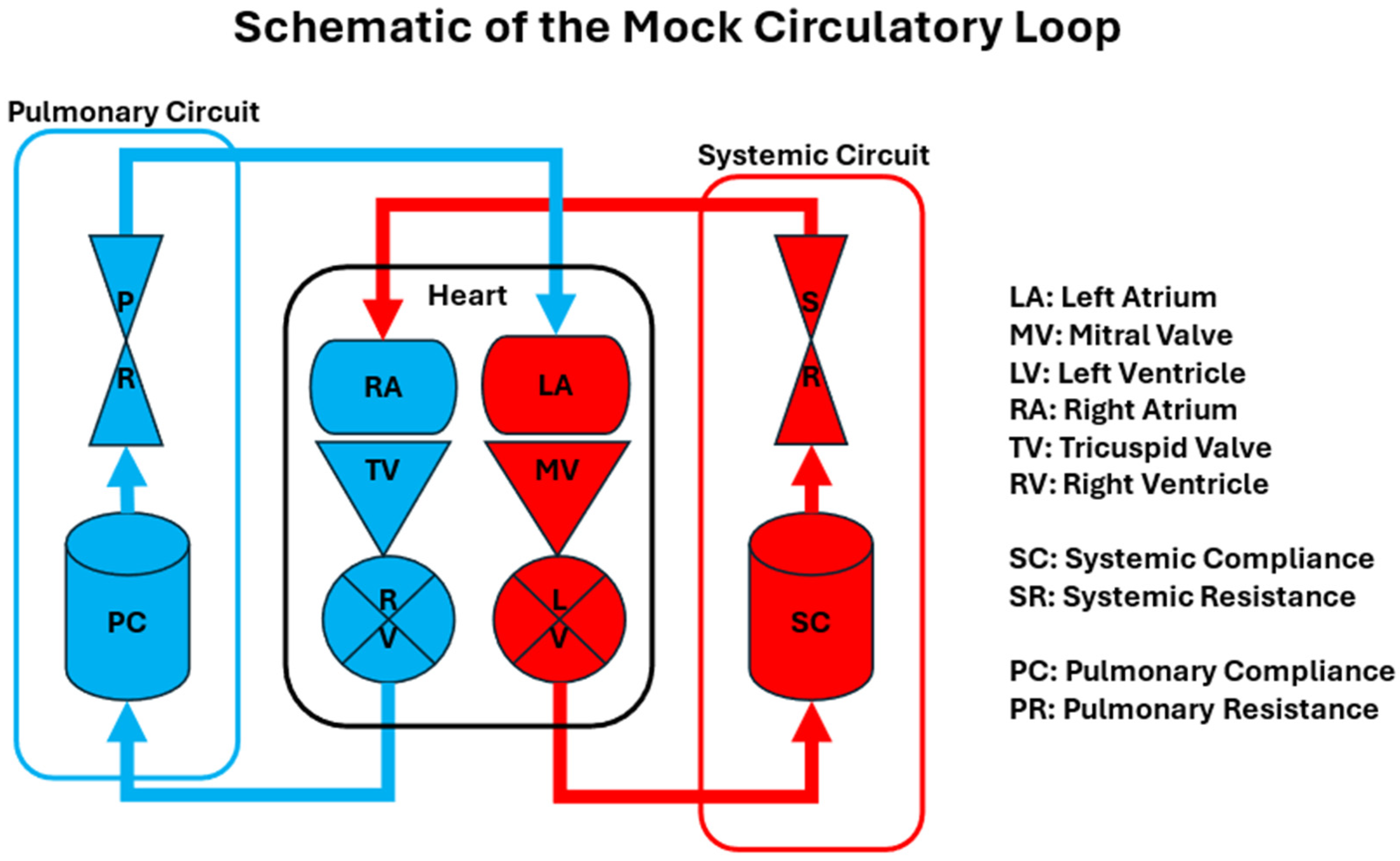
3. Design of Mock Circulatory Loops
- Pulmonary Circuit: Simulates the blood flow between the heart and lungs.
- Heart: Includes representations of the left and right atria and ventricles, often equipped with the mechanisms to mimic cardiac contractions.
- Systematic Circuit: Represents the circulation of blood to the rest of the body, including arterial compliance chambers and systemic resistances.
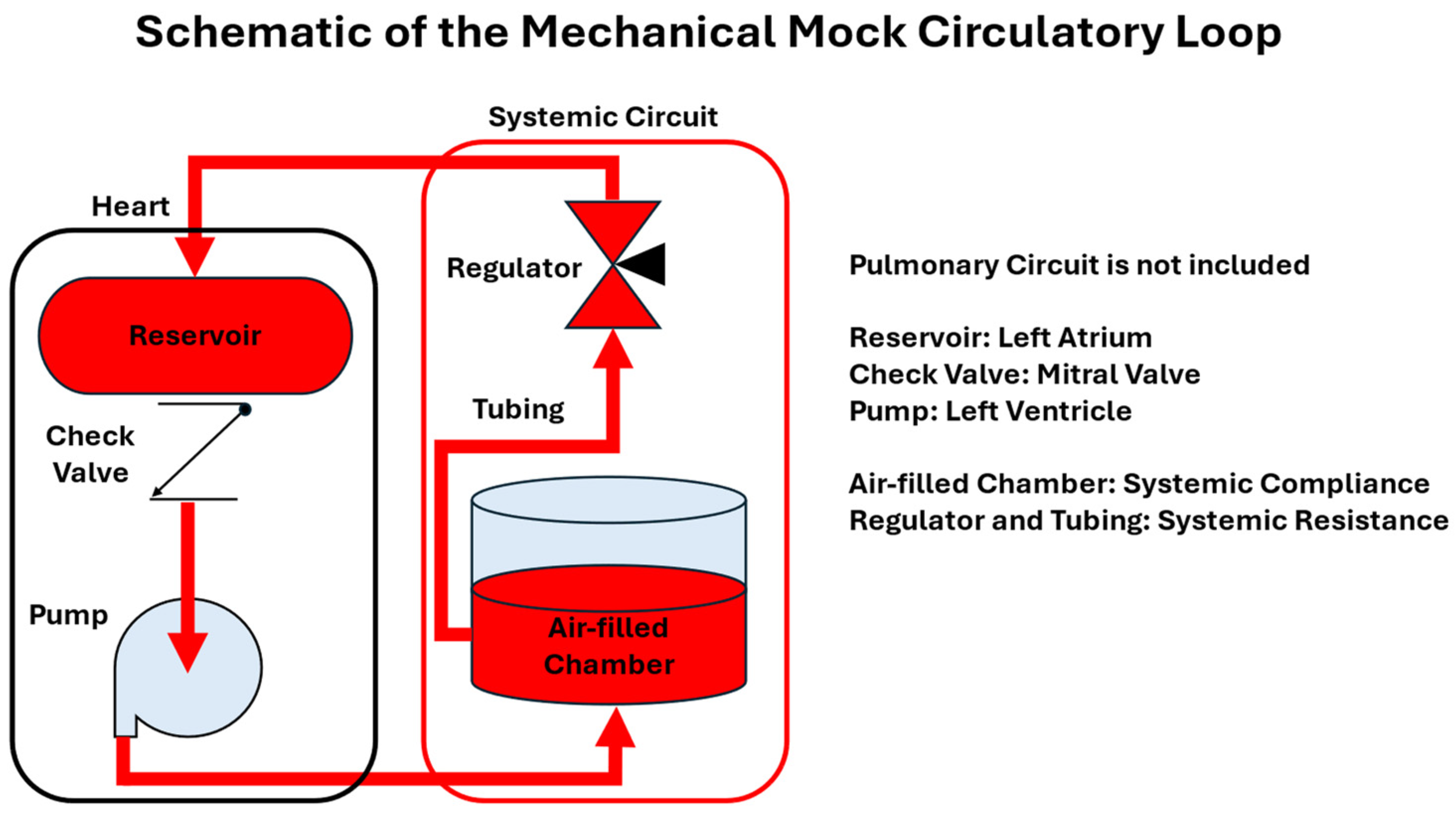
- Mechanical (Hydraulic) MCLs: These systems utilize physical components to replicate cardiovascular functions. They are known for their high repeatability but may lack flexibility in simulating autoregulatory responses.
- Computational (Numerical) MCLs (cMCLs): These models employ computational methods to simulate cardiovascular dynamics, offering flexibility and programmability. However, they lack the physical properties necessary for in vitro testing of actual cardiovascular devices.
- Hybrid MCLs (hMCLs): Combining elements of the mechanical and computational models, hybrid MCLs aim to leverage the advantages of each approach to provide more comprehensive simulations.
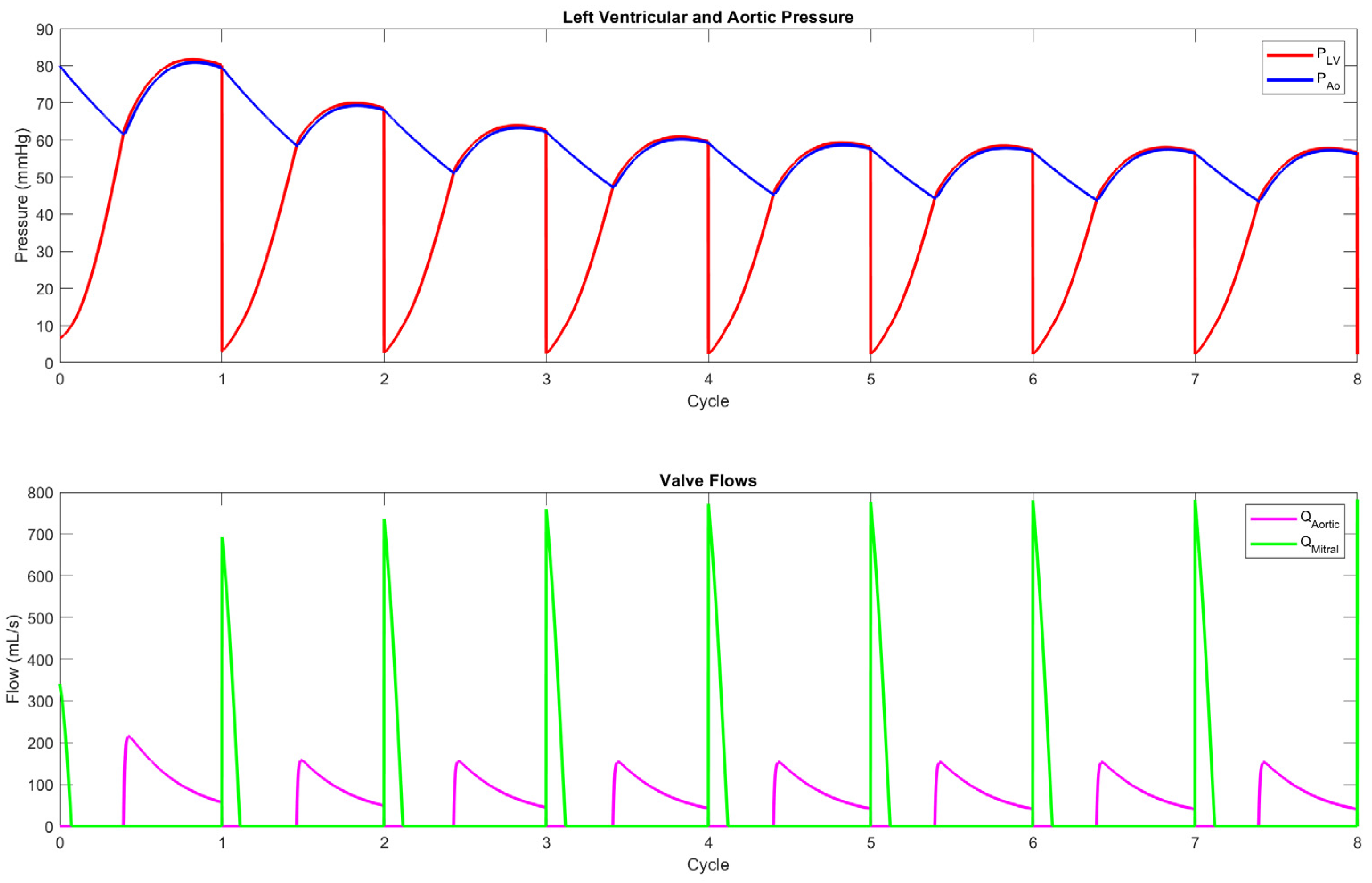
3.1. Mechanical Mock Circulatory Loops
3.2. Computational Mock Circulatory Loops
3.3. Hybrid Mock Circulatory Loops
3.4. Mock Circulatory Loops Design Summary
4. Applications of Mock Circulatory Loops
- Cardiovascular Devices Testing;
- Clinical Training and Education;
- Hemodynamics and Blood Flow Studies;
- Disease Modeling.
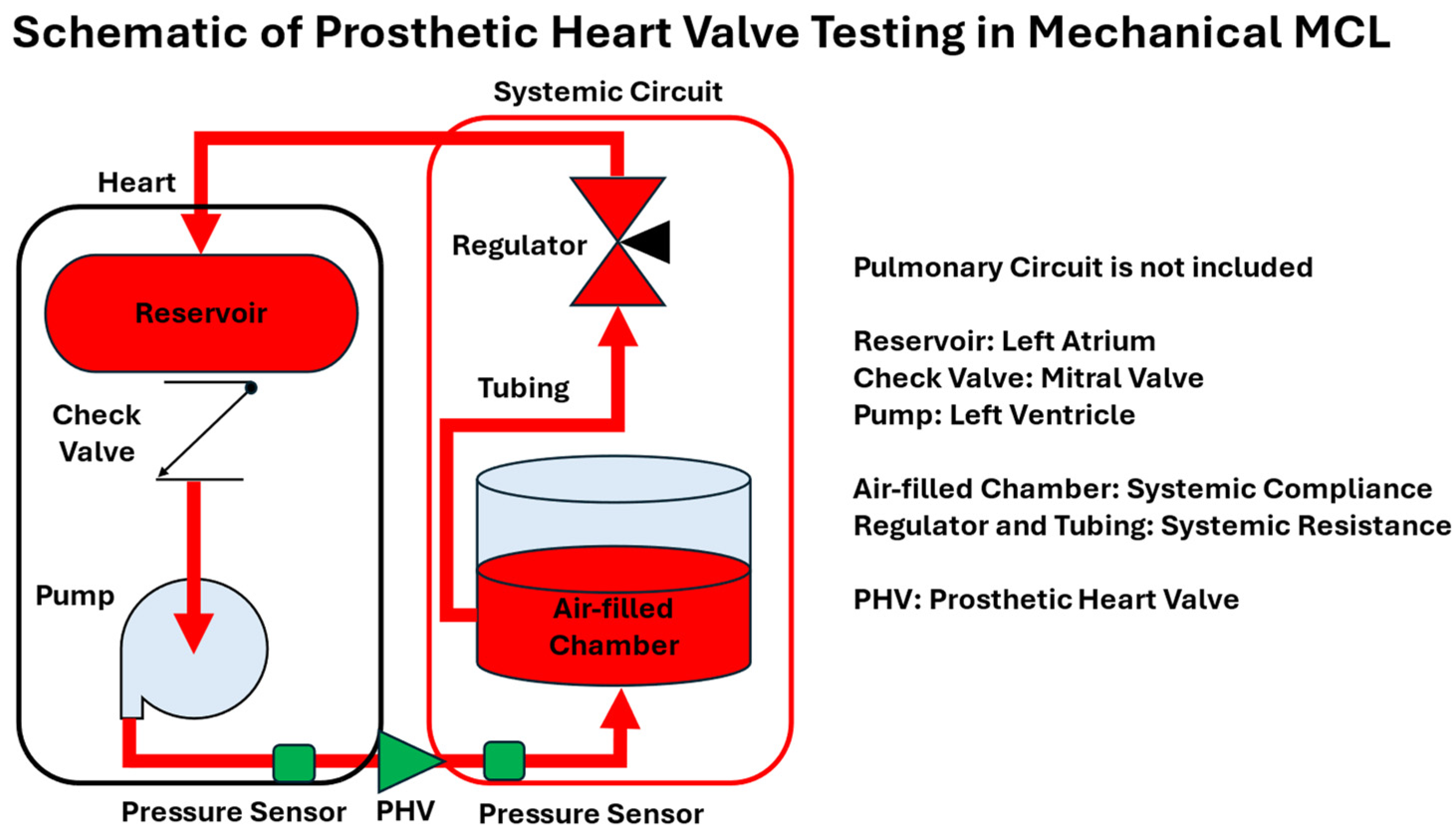
4.1. Cardiovascular Devices Testing
4.2. Clinical Training and Education
4.3. Hemodynamics and Blood Flow Studies
4.4. Disease Modeling
5. Summary and Discussion
6. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| cMCL | Computational Mock Circulatory Loop |
| ECCO2R | Extracorporeal Carbon Dioxide Removal |
| ECLS | Extracorporeal Life Support |
| ECMO | Extracorporeal Membrane Oxygenation |
| FDA | Food and Drug Administration |
| hMCL | Hybrid Mock Circulatory Loop |
| IABP | Intra-Aortic Ballon Pump |
| IAEP | Intra-Aortic Entrainment Pump |
| ISO | International Organization for Standardization |
| MAP | Mean Arterial Pressure |
| MCL | Mock Circulatory Loop |
| MCS | Mechanical Circulatory Support |
| PHV | Prosthetic Heart Valve |
| PWM | Pulse-Width Modulation |
| TAH | Total Artificial Heart |
| VAD | Ventricular Assist Device |
References
- Lu, P.J.; Chan, M.Y.; Tsui, S.; Shen, T.T.; Chang, J.C. Development of an Anatomy-Mimicking, Wave Transport-Preserving Mock Circulation Loop for Evaluating Pulsatile Hemodynamics as Supported by Cardiovascular Assist Devices. Cardiovasc. Eng. Technol. 2025; advance online publication. [Google Scholar] [CrossRef]
- Najjari, M.R.; Plesniak, M.W. PID Controller Design to Generate Pulsatile Flow Rate for In Vitro Experimental Studies of Physiological Flows. Biomed. Eng. Lett. 2017, 7, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Drost, S.; de Kruif, B.J.; Newport, D. Arduino Control of a Pulsatile Flow Rig. Med. Eng. Phys. 2018, 51, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Nestler, F.; Bradley, A.P.; Wilson, S.J.; Timms, D.L.; Frazier, O.H.; Cohn, W.E. A Hybrid Mock Circulation Loop for a Total Artificial Heart. Artif. Organs 2014, 38, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Gao, Q.; Wan, M.; Zhang, K. Mock Circulatory Loop Applications for Testing Cardiovascular Assist Devices and In Vitro Studies. Front. Physiol. 2023, 14, 902319. [Google Scholar] [CrossRef]
- Telyshev, D.; Pugovkin, A.; Selishchev, S.; Rüschen, D.; Leonhardt, S. Hybrid Mock Circulatory Loop for Training and Study Purposes. In Proceedings of the 2018 Ural Symposium on Biomedical Engineering, Radioelectronics and Information Technology (USBEREIT), Yekaterinburg, Russia, 7–8 May 2018. [Google Scholar]
- Barua, S.; Stevens, M.; Jain, P.; Vazquez, G.M.; Boss, L.; Muthiah, K.; Hayward, C. A Mock Circulatory Loop Analysis of Cardiorenal Hemodynamics with Intra-Aortic Mechanical Circulatory Support. ASAIO J. 2025, 71, 128–135. [Google Scholar] [CrossRef]
- Chen, D.; Liang, S.; Li, Z.; Mei, Y.; Dong, H.; Ma, Y.; Zhao, J.; Xu, S.; Zheng, J.; Xiong, J. A Mock Circulation Loop for In Vitro Hemodynamic Evaluation of Aorta: Application in Aortic Dissection. J. Endovasc. Ther. 2022, 29, 132–142. [Google Scholar] [CrossRef]
- Rapp, E.S.; Pawar, S.R.; Longoria, R.G. Hybrid Mock Circulatory Loop Simulation of Extreme Cardiac Events. IEEE Trans. Biomed. Eng. 2022, 69, 2883–2892. [Google Scholar] [CrossRef]
- D’Souza, G.A.; Rinaldi, J.E.; Meki, M.; Crusan, A.; Richardson, E.; Shinnar, M.; Herbertson, L.H. Using a Mock Circulatory Loop as a Regulatory Science Tool to Simulate Different Heart Failure Conditions. J. Biomech. Eng. 2024, 146, 011004. [Google Scholar] [CrossRef]
- Rocchi, M.; Ingram, M.; Claus, P.; D’hooge, J.; Meyns, B.; Fresiello, L. Use of 3D Anatomical Models in Mock Circulatory Loops for Cardiac Medical Device Testing. Artif. Organs 2023, 47, 260–272. [Google Scholar] [CrossRef]
- The U.S. FDA. Mock Circulation Loop Design for Simulating Peripheral Pressure Waveforms for Three Hemodynamic States. Available online: https://cdrh-rst.fda.gov/sites/default/files/2024-05/Instructions%20for%20Full%20Description%20of%20the%20MCL%20Design%20Development.pdf (accessed on 30 March 2025).
- McMillan, I.K.R.; Dalley, R.; Matthews, M.B. The Movement of Aortic and Pulmonary Valves Studied Post Mortem by Colour Cinematography. Br. Heart J. 1952, 14, 42–46. [Google Scholar] [CrossRef]
- Liotta, D.; Wieting, D.W.; Hall, C.W.; Chidoni, J.J.; DeBakey, M.E. In Vitro and In Vivo Flow Studies in Blood Pumps. Am. Soc. Artif. Intern. Organs 1967, 4, 94–97. [Google Scholar]
- Kolff, W.J.; Jacobsen, S.C. Mock Circulation. U.S. Patent 3,631,607A, 4 January 1972. [Google Scholar]
- Low, J.; Huben, M.V. Mock Circulatory Apparatus. U.S. Patent 20070054256A1, 8 March 2007. [Google Scholar]
- Kolff, W.J.; Bishop, N.D.; Topaz, S.R.; Chiang, B.Y. Mock Circulation System. U.S. Patent 5,632,623A, 27 May 1997. [Google Scholar]
- Gurdin, J.M.; Verkaik, J.E. Circulatory Heart Model. U.S. Patent 8,678,830B2, 25 March 2014. [Google Scholar]
- Leonhardt, H.J. Circulatory Assist Pump. U.S. Patent 20230201563A1, 29 June 2023. [Google Scholar]
- Vozenilek, J.; Cusack, T.; Admani, S.; Bethke, E.; Regan, M. Device, System, and Method for Simulating Blood Flow. U.S. Patent 8926333B2, 6 January 2015. [Google Scholar]
- Pickard, M.L. Apparatus and Method for Testing Prosthetic Heart Valves. U.S. Patent 4,682,491A, 28 July 1987. [Google Scholar]
- Gregory, S.; Timms, D.; Stevens, M.; Pearcy, M. An Advanced Mock Circulation Loop for In Vitro Cardiovascular Device Evaluation. Artif. Organs 2020, 44, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Khienwad, T.; Praemai, W.; Phornphop, N. Novel Design of a Frank-Starling Physiological Mock Circulatory Loop for In-Vitro Testing of Rotary Blood Pump. Int. J. Appl. Biomed. Eng. 2019, 12, 56–63. [Google Scholar]
- Baloa, L.A.; Boston, J.R.; Antaki, J.F. Elastance-Based Control of a Mock Circulatory System. Ann. Biomed. Eng. 2001, 29, 244–251. [Google Scholar] [CrossRef]
- Kasavaraj, A.; Said, C.; Boss, L.A.; Vazquez, G.M.; Stevens, M.; Jiang, J.; Adji, A.; Hayward, C.; Jain, P. Increased VA-ECMO Pump Speed Reduces Left Atrial Pressure: Insights from a Novel Biventricular Heart Model. Bioengineering 2025, 12, 237. [Google Scholar] [CrossRef]
- Gregory, S.D.; Stevens, M.; Timms, D.; Pearcy, M. Replication of the Frank-Starling Response in a Mock Circulation Loop. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 6825–6828. [Google Scholar] [CrossRef]
- Schampaert, S.; Pennings, K.A.M.A.; van de Molengraft, M.J.G.; Pijls, N.H.J.; van de Vosse, F.N.; Rutten, M.C.M. A Mock Circulation Model for Cardiovascular Device Evaluation. Physiol. Meas. 2014, 35, 687–694. [Google Scholar] [CrossRef]
- Mushi, S.; Yu, Y. Control of a Mock Circulatory System to Simulate the Short-Term Baroreflex. In Proceedings of the 2008 American Control Conference, Seattle, WA, USA, 11–13 June 2008; pp. 3701–3706. [Google Scholar]
- De Lazzari, B.; Badagliacca, R.; Filomena, D.; Papa, S.; Vizza, C.D.; Capoccia, M.; De Lazzari, C. CARDIOSIM©: The First Italian Software Platform for Simulation of the Cardiovascular System and Mechanical Circulatory and Ventilatory Support. Bioengineering 2022, 9, 383. [Google Scholar] [CrossRef]
- Quarteroni, A.; Formaggia, L. Mathematical Modelling and Numerical Simulation of the Cardiovascular System. In Handbook of Numerical Analysis; Elsevier: Amsterdam, The Netherlands, 2004; Volume 12, pp. 3–127. [Google Scholar]
- Conlon, M.J.; Russell, D.L.; Mussivand, T. Development of a Mathematical Model of the Human Circulatory System. Ann. Biomed. Eng. 2006, 34, 1400–1413. [Google Scholar] [CrossRef]
- Gregory, S.D. Simulation and Development of a Mock Circulation Loop with Variable Compliance. Master’s Thesis, Queensland University of Technology, Brisbane, Australia, 2009. [Google Scholar]
- Taylor, C.E.; Miller, G.E. Mock Circulatory Loop Compliance Chamber Employing a Novel Real-Time Control Process. ASME J. Med. Devices 2012, 6, 450031–450038. [Google Scholar] [CrossRef]
- Liu, X.; Mo, C.; Li, J.; Yu, H.; Hu, S.; Zhu, D.; Zhang, P.; Li, Y. Development of a Lumped Parameter Model of Human Whole Body Circulatory Loop. IEEE Access 2024, 12, 188505–188518. [Google Scholar] [CrossRef]
- Shi, Y.; Lawford, P.; Hose, D.R. Construction of Lumped-Parameter Cardiovascular Models Using the CellML Language. J. Med. Eng. Technol. 2018, 42, 525–531. [Google Scholar] [CrossRef]
- Korakianitis, T.; Shi, Y. Numerical simulation of cardiovascular dynamics with healthy and diseased heart valves. J. Biomech. 2006, 39, 1964–1982. [Google Scholar] [CrossRef]
- Bardi, F.; Gasparotti, E.; Vignali, E.; Avril, S.; Celi, S. A Hybrid Mock Circulatory Loop for Fluid Dynamic Characterization of 3D Anatomical Phantoms. IEEE Trans. Biomed. Eng. 2023, 70, 1651–1661. [Google Scholar] [CrossRef]
- Cuenca-Navalon, E.; Finocchiaro, T.; Laumen, M.; Fritschi, A.; Schmitz-Rode, T.; Steinseifer, U. Design and Evaluation of a Hybrid Mock Circulatory Loop for Total Artificial Heart Testing. Int. J. Artif. Organs 2014, 37, 71–80. [Google Scholar] [CrossRef]
- Li, T.; Li, H.; Cui, W.; Xie, N.; Li, X.; Wang, Y. Design and Intelligent Control of Mock Circulation System to Reproduce Patient-Specific Physiological Indexes. Biomed. Signal Process. Control 2022, 78, 103842. [Google Scholar] [CrossRef]
- Timms, D.L.; Gregory, S.D.; Stevens, M.; Pearcy, M. A Compact Mock Circulation Loop for the In Vitro Testing of Cardiovascular Devices. Artif. Organs 2011, 35, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Iscan, M.; Yesildirek, A. A New Cardiovascular Mock Loop Driven by Novel Active Capacitance in Normal and Abnormal Conditions. Appl. Bionics Biomech. 2023, 2023, 2866637. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.E. Implementing a Fully Automated Mock Circulatory Loop to Simulate Cardiovascular Conditions. MathWorks Tech. Artic. 2013. [Google Scholar]
- ISO 14708-5:2020; Implants for Surgery—Active Implantable Medical Devices; Part 5: Circulatory Support Devices. ISO: Geneva, Switzerland, 2020.
- Imachi, K.; Tofy, M. Outline of the International Organization for Standardization Standard for Circulatory Support Devices (ISO 14708-5). Artif. Organs 2010, 34, 695–698. [Google Scholar] [CrossRef]
- Transonic Website. Mock Circulatory Loops Guide. Available online: https://info.transonic.com/hubfs/13%20Bio%20Guides/1%20Mock%20Circulatory%20Loops.pdf (accessed on 30 March 2025).
- Pantalos, G.M.; Koenig, S.C.; Gillars, K.J.; Giridharan, G.A.; Ewert, D.L. Characterization of an Adult Mock Circulation for Testing Cardiac Support Devices. ASAIO J. 2004, 50, 37–46. [Google Scholar] [CrossRef]
- Liu, Y.; Allaire, P.; Wood, H.; Olsen, D. Design and Initial Testing of a Mock Human Circulatory Loop for Left Ventricular Assist Device Performance Testing. Artif. Organs 2005, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Agrafiotis, E.; Zimpfer, D.; Mächler, H.; Holzapfel, G.A. Review of Systemic Mock Circulation Loops for Evaluation of Implantable Cardiovascular Devices and Biological Tissues. J. Endovasc. Ther. 2024, 15266028241235876. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Bianccucci, B.A.; Deutsch, S.; Fontaine, A.A.; Tarbell, J.M. Observation and Quantification of Gas Bubble Formation on a Mechanical Heart Valve. J. Biomech. Eng. 2000, 122, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Yang, X.; Liu, Y.; Fan, Y. An in Vitro Feasibility Study of the Influence of Configurations and Leaflet Thickness on the Hydrodynamics of Deformed Transcatheter Aortic Valve: Hydrodynamics of Deformed TAV. Artif. Organs 2017, 41, 735–743. [Google Scholar] [CrossRef]
- Walker, A.R. In Vitro Evaluation of Mechanical Heart Valve Performance Using a Novel Test Chamber in an Automated Mock Circulatory Loop. Ph.D. Thesis, Virginia Commonwealth University, Richmond, Virginia, 2010. [Google Scholar]
- Vismara, R.; Fiore, G.B.; Mangini, A.; Contino, M.; Lemma, M.; Redaelli, A.; Antona, C. A Novel Approach to the In Vitro Hydrodynamic Study of the Aortic Valve: Mock Loop Development and Test. ASAIO J. 2010, 56, 279–284. [Google Scholar] [CrossRef]
- Johannes, G.; Julian, Z.; Markus, B.; Stefan, S.; Martin, F.; Gabriele, K.; Andreas, B.; Philippe, G. Development and Validation of a Life-Sized Mock Circulatory Loop of the Human Circulation for Fluid-Mechanical Studies. ASAIO J. 2019, 65, 788–797. [Google Scholar] [CrossRef]
- Ferrari, G.; Balasubramanian, P.; Tubaldi, E.; Giovanniello, F.; Amabili, M. Experiments on Dynamic Behaviour of a Dacron Aortic Graft in a Mock Circulatory Loop. J. Biomech. 2019, 86, 132–140. [Google Scholar] [CrossRef]
- Moyer, J.C.; Chivukula, V.K.; Taheri-Tehrani, P.; Sandhu, S.; Blaha, C.; Fissell, W.H.; Roy, S. An Arteriovenous Mock Circulatory Loop and Accompanying Bond Graph Model for In Vitro Study of Peripheral Intravascular Bioartificial Organs. Artif. Organs 2024, 48, 336–346. [Google Scholar] [CrossRef]
- Wang, J.; Hao, Z.; Yin, B.; Tan, W.; Zhu, C. Effect of Guidewire on the Accuracy of Trans-Stenotic Pressure Measurement—A Computational Study. Phys. Fluids 2025, 36, 011905. [Google Scholar] [CrossRef]
- Pantalos, G.M.; Ionan, C.; Koenig, S.C.; Gillars, K.J.; Horrell, T.; Sahetya, S.; Colyer, J.; Gray, L.A., Jr. Expanded Pediatric Cardiovascular Simulator for Research and Training. ASAIO J. 2010, 56, 67–72. [Google Scholar] [CrossRef]
- Carson, D.J.; Lieber, B.B.; Sadasivan, C.; Florella, D.J.; Woo, H.H.; Romeo, M. Cardiac Simulation Device. U.S. Patent US9183763B2, 10 November 2015. [Google Scholar]
- Liang, S.; Li, L.; Yang, B.; Yuan, P.; Li, S.; Shi, Y.; Gao, F.; Deng, Y.; Jiao, L.; Chen, D. Qualitative and Quantitative Assessment of Invasive Surgery Skill Based on a Custom Training Simulator. J. Endovasc. Ther. 2023, 32, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Cupp, G.; Armour, N.; Warren, K.; Stone, C.; Lee, D.; Gilbert, N.; Hammond, C.; Moore, J.; Kang, Y. An Inexpensive Cardiovascular Flow Simulator for Cardiac Catheterization Procedure Using a Pulmonary Artery Catheter. Front. Med. Technol. 2021, 3, 764007. [Google Scholar] [CrossRef] [PubMed]
- Perloff, J.K. Physical Examination of the Heart and Circulation; PMPH-USA: Shelton, CT, USA, 2009. [Google Scholar]
- Jeong, J.H.; Kim, Y.M.; Lee, B.; Hong, J.; Kim, J.; Woo, S.Y.; Yang, T.H.; Park, Y.H. Design and Evaluation of Enhanced Mock Circulatory Platform Simulating Cardiovascular Physiology for Medical Palpation Training. Appl. Sci. 2020, 10, 5433. [Google Scholar] [CrossRef]
- Malfertheiner, M.V.; Broman, L.M.; Vercaemst, L.; Belliato, M.; Aliberti, A.; Di Nardo, M.; Swol, J.; Barrett, N.; Pappalardo, F.; Bělohlávek, J.; et al. Ex Vivo Models for Research in Extracorporeal Membrane Oxygenation: A Systematic Review of the Literature. Perfusion 2020, 35 (Suppl. S1), 38–49. [Google Scholar] [CrossRef]
- Geier, A.; Kunert, A.; Albrecht, G.; Liebold, A.; Hoenicka, M. Influence of Cannulation Site on Carotid Perfusion During Extracorporeal Membrane Oxygenation in a Compliant Human Aortic Model. Ann. Biomed. Eng. 2017, 45, 2281–2297. [Google Scholar] [CrossRef]
- Mahmoud, A.; Alsalemi, A.; Bensaali, F.; Hssain, A.A.; Hassan, I. A Review of Human Circulatory System Simulation: Bridging the Gap between Engineering and Medicine. Membranes 2021, 11, 744. [Google Scholar] [CrossRef]
- Packy, A.; D’Souza, G.A.; Farahmand, M.; Herbertson, L.; Scully, C.G. Simulating Radial Pressure Waveforms with a Mock Circulatory Flow Loop to Characterize Hemodynamic Monitoring Systems. Cardiovasc. Eng. Technol. 2022, 13, 279–290. [Google Scholar] [CrossRef]
- Franchini, G.; Giovanniello, F.; Amabili, M. Viscoelasticity of Human Descending Thoracic Aorta in a Mock Circulatory Loop. J. Mech. Behav. Biomed. Mater. 2022, 130, 105205. [Google Scholar] [CrossRef]
- Schwärzel, L.S.; Jungmann, A.M.; Schmoll, N.; Seiler, F.; Muellenbach, R.M.; Schenk, J.; Dinh, Q.T.; Bals, R.; Lepper, P.M.; Omlor, A.J. A Mock Circulation Loop to Test Extracorporeal CO2 Elimination Setups. Intensiv. Care Med. Exp. 2020, 8, 52. [Google Scholar] [CrossRef]
- Stephens, A.F.; Wanigasekara, D.; Pellegrino, V.A.; Burrell, A.J.C.; Marasco, S.F.; Kaye, D.M.; Steinseifer, U.; Gregory, S.D. Comparison of Circulatory Unloading Techniques for Venoarterial Extracorporeal Membrane Oxygenation. ASAIO J. 2021, 67, 623–631. [Google Scholar] [CrossRef]
- Rozencwajg, S.; Wu, E.L.; Heinsar, S.; Stevens, M.; Chinchilla, J.; Fraser, J.F.; Pauls, J.P. A Mock Circulation Loop to Evaluate Differential Hypoxemia During Peripheral Venoarterial Extracorporeal Membrane Oxygenation. Perfusion 2024, 39, 66–75. [Google Scholar] [CrossRef]
- Mi, J.; Zhao, Z.; Wang, H.; Tang, H. Study of the Relationship Between Pulmonary Artery Pressure and Heart Valve Vibration Sound Based on Mock Loop. Bioengineering 2023, 10, 985. [Google Scholar] [CrossRef]
- Papaioannou, T.G.; Mathioulakis, D.S.; Tsangaris, S.G. Simulation of Systolic and Diastolic Left Ventricular Dysfunction in a Mock Circulation: The Effect of Arterial Compliance. J. Med. Eng. Technol. 2003, 27, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Yu, H.; Chen, J.; Talamantes, J.; Rollins, D.M.; Fang, X.; Long, J.; Xu, C.; Sawchuk, A.P. A Mock Circulation Loop to Characterize In Vitro Hemodynamics in Human Systemic Arteries with Stenosis. Fluids 2023, 8, 198. [Google Scholar] [CrossRef]
- Koenig, S.C.; Pantalos, G.M.; Gillars, K.J.; Ewert, D.L.; Litwak, K.N.; Etoch, S.W. Hemodynamic and Pressure–Volume Responses to Continuous and Pulsatile Ventricular Assist in an Adult Mock Circulation. ASAIO J. 2004, 50, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Z.; Chen, D. A mock circulation loop for in vitro haemodynamic evaluation of aorta. J. Phys. Conf. Ser. 2020, 1600, 012066. [Google Scholar] [CrossRef]
- Contarino, C.; Chifari, F.; D’Souza, G.A.; Herbertson, L.H. Validation of a Multiscale Computational Model Using a Mock Circulatory Loop to Simulate Cardiogenic Shock. ASAIO J. 2023, 69, e502–e512. [Google Scholar] [CrossRef]
- Taylor, C.E.; Gerald, E.M. Implementation of an Automated Peripheral Resistance Device in a Mock Circulatory Loop with Characterization of Performance Values Using Simulink Simscape and Parameter Estimation. J. Med. Devices 2012, 6, 045001. [Google Scholar] [CrossRef]
- Schroeder, M.J.; Perreault, B.; Ewert, D.L.; Koenig, S.C. HEART: An Automated Beat-to-Beat Cardiovascular Analysis Package Using Matlab®. Comput. Biol. Med. 2004, 34, 371–388. [Google Scholar] [CrossRef]
- Willemet, M.; Chowienczyk, P.; Alastruey, J. A Database of Virtual Healthy Subjects to Assess the Accuracy of Foot-to-Foot Pulse Wave Velocities for Estimation of Aortic Stiffness. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H663–H675. [Google Scholar] [CrossRef]
- Banik, S. Relationship of Mean Arterial Pressure with Other Cardiac and Biological Factors. Clin. Pract. 2023, 20, 5–11. [Google Scholar]
- Fei, Y. Understanding the Association Between Mean Arterial Pressure and Mortality in Young Adults. Postgrad. Med. J. 2020, 96, 453–454. [Google Scholar] [CrossRef] [PubMed]
- Hemodynamic Monitoring. Thoracic Key. Available online: https://thoracickey.com/hemodynamic-monitoring/ (accessed on 30 March 2025).
- Fan, Z.; Zhang, G.; Liao, S. Pulse Wave Analysis. In Advanced Biomedical Engineering; Intech: London, UK, 2011. [Google Scholar]
- Jozwiak, M.; Monnet, X.; Teboul, J.L. Pressure Waveform Analysis. Anesth. Analg. 2018, 126, 1930–1933. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Geng, X.; Zhang, Y.; Zhang, H.; Ye, T. Pulse Wave Analysis Method of Cardiovascular Parameters Extraction for Health Monitoring. Int. J. Environ. Res. Public Health 2023, 20, 2597. [Google Scholar] [CrossRef]
- Wang, L.; Xu, L.; Feng, S.; Meng, M.; Wang, K. Multi-Gaussian Fitting for Pulse Waveform Using Weighted Least Squares and Multi-Criteria Decision-Making Method. Comput. Biol. Med. 2013, 43, 1661–1672. [Google Scholar] [CrossRef]
- Baturalp, T.B. Design and Development of a Systemic Mock Circulation Loop with a Novel Beating Left Ventricular Simulator. Ph.D. Thesis, Texas Tech University, Lubbock, Texas, 2016. [Google Scholar]
- Liang, F.; Takagi, S.; Himeno, R.; Liu, H. Multi-Scale Modeling of the Human Cardiovascular System with Applications to Aortic Valvular and Arterial Stenoses. Med. Biol. Eng. Comput. 2009, 47, 743–755. [Google Scholar] [CrossRef]
- Barrera Loredo, J. Development of an In Vitro System for Cardiovascular Flow Measurements and Computational Study of Cerebral Aneurysm Hemodynamics. Master’s Thesis, University of British Columbia, Victoria, BC, Canada, 2019. [Google Scholar]
- Cappon, F.; Wu, T.; Papaioannou, T.; Du, X.; Hsu, P.L.; Khir, A.W. Mock Circulatory Loops Used for Testing Cardiac Assist Devices: A Review of Computational and Experimental Models. Int. J. Artif. Organs 2021, 44, 793–806. [Google Scholar] [CrossRef]
- Timms, D.L.; Gregory, S.D.; Stevens, M.C.; Fraser, J.F. Haemodynamic Modeling of the Cardiovascular System Using Mock Circulation Loops to Test Cardiovascular Devices. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011. [Google Scholar]
- Hong, W.; Tewari, V.; Chen, J.; Sawchuk, A.P.; Yu, H. A Comprehensive Review of Mock Circulation Loop Systems for Experimental Hemodynamics of Cardiovascular Diseases. Fluids 2025, 10, 166. [Google Scholar] [CrossRef]
- Ochsner, G.; Amacher, R.; Amstutz, A.; Plass, A.; Daners, M.S.; Tevaearai, H.; Vandenberghe, S.; Wilhelm, M.J.; Guzzella, L. A Novel Interface for Hybrid Mock Circulations to Evaluate Ventricular Assist Devices. IEEE Trans. Biomed. Eng. 2013, 60, 507–516. [Google Scholar] [CrossRef]
- D’Souza, G.; Hirschhorn, M.; Richardson, E.; Crusan, A.; Farahmand, M.; Rinaldi, J.; Herbertson, L. Use of Mock Circulatory Loops for Standardized Performance Testing of Mechanical Circulatory Support Devices: An Interlaboratory Study. Food and Drug Administration. Available online: https://www.fda.gov/media/168734/download (accessed on 30 March 2025).
- Franzetti, G.; Diaz-Zuccarini, V.; Balabani, S. Design of an In Vitro Mock Circulatory Loop to Reproduce Patient-Specific Vascular Conditions: Toward Precision Medicine. J. Eng. Sci. Med. Diagn. Ther. 2019, 2, 041004. [Google Scholar] [CrossRef]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.C.; Reid, R.R. 3-D Bioprinting Technologies in Tissue Engineering and Regenerative Medicine: Current and Future Trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Saugel, B.; Kouz, K.; Scheeren, T.W.L.; Greiwe, G.; Hoppe, P.; Romagnoli, S.; de Backer, D. Cardiac Output Estimation Using Pulse Wave Analysis—Physiology, Algorithms, and Technologies: A Narrative Review. Br. J. Anaesth. 2021, 126, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Sun, Z.; Li, J.; Zhang, R.; Zhang, X.; Liu, S.; Li, J.; Xu, C.; Hu, D.; Sun, Y. Pulse Pressure and Mean Arterial Pressure in Relation to Ischemic Stroke Among Patients with Uncontrolled Hypertension in Rural Areas of China. Stroke 2008, 39, 1932–1937. [Google Scholar] [CrossRef] [PubMed]


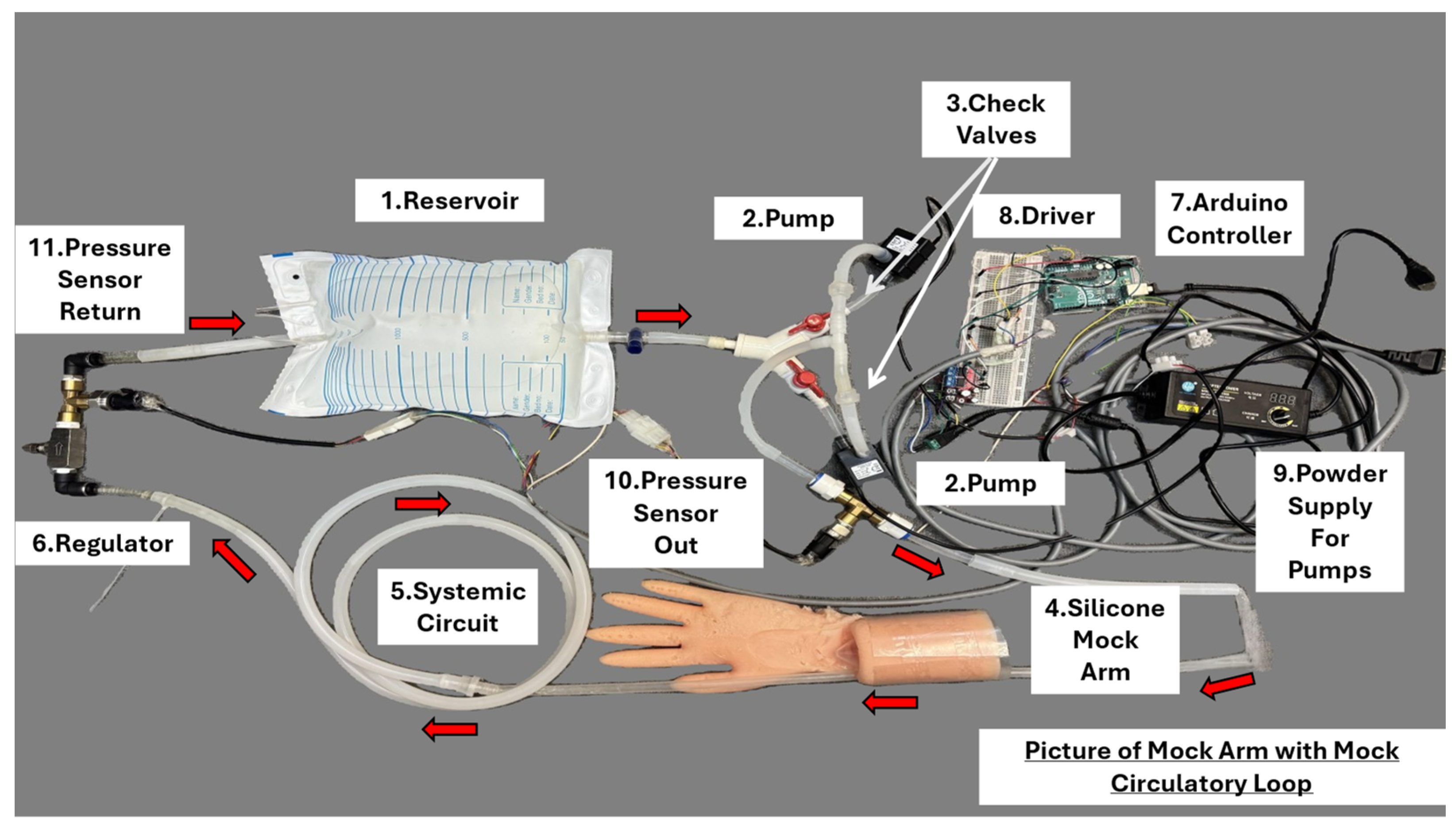

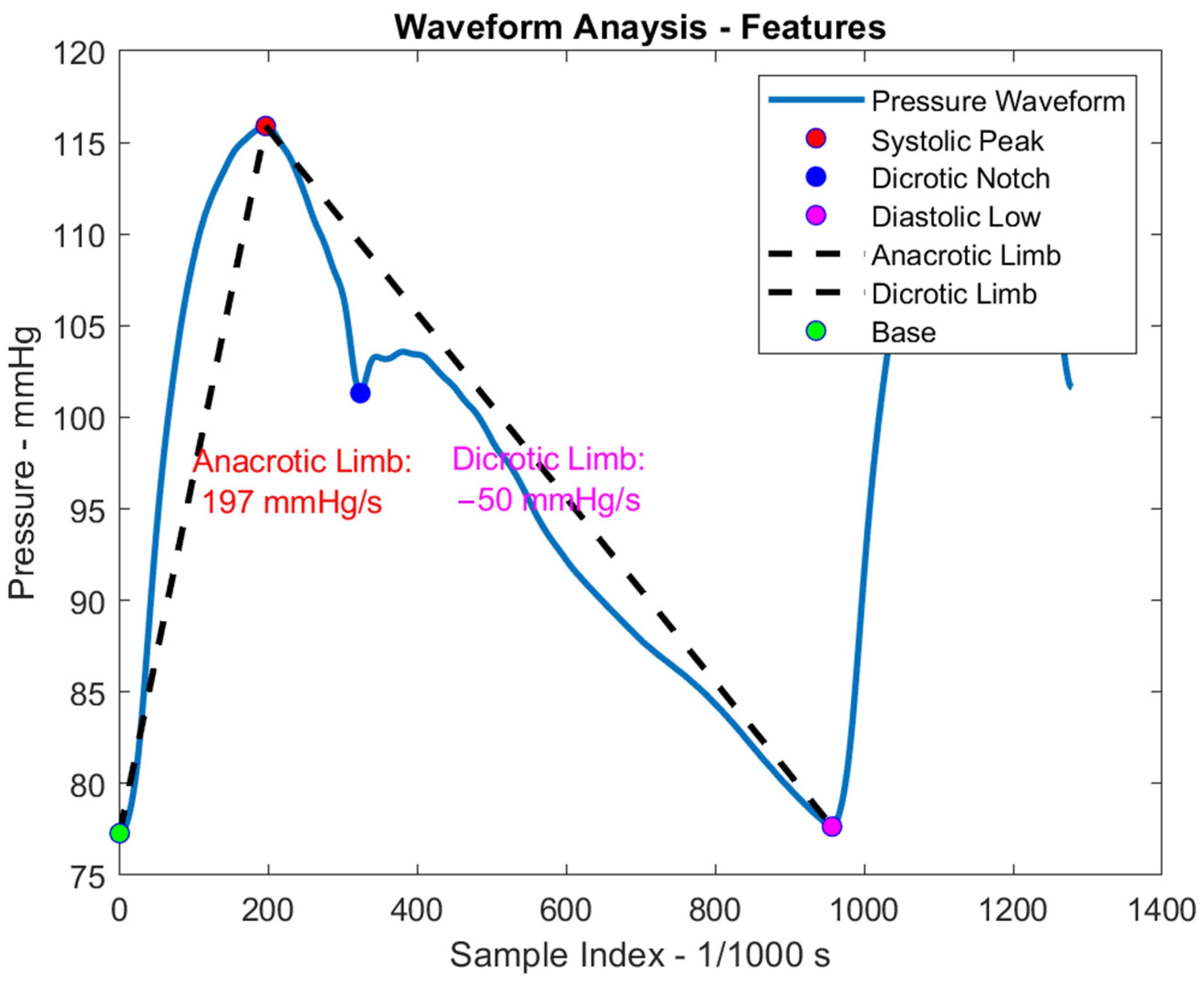
| Mechanical Model | Computational Model | Hybrid Model | |
|---|---|---|---|
| Pros | Realistic fluid behavior | High flexibility | Best of both worlds |
| Device testing compatibility | Cost-effective | Patient-specific modeling | |
| Tangible interface | Rapid simulation | Devic integration | |
| Standard compliance | Parameter analysis | Closed-loop feedback | |
| Cons | Limited flexibility | No physical interaction | Higher complexity |
| Bulky and complex | Requires validation | Cost and setup | |
| Less adaptive | Software limitations | Requires technical expertise |
| Cardiovascular Devices Testing | Clinical Training and Education | Hemodynamics and Blood Flow Studies | Disease Modeling |
|---|---|---|---|
| Ventricular Assist Device | Cardiac Surgery | Hemodynamics of Cardiovascular Conditions | Heart Failure and Cardiogenic Shock |
| Prosthetic Heart Valve | Catheterization and Intervention Surgical Simulation | Flow of Intra-Aortic Balloon Pump | Hypertension and Peripheral Resistance |
| Stent, Graft, and Vascular Implant | Medical and Palpation Education | Venoarterial Extracorporeal Membrane Oxygenation | Waveform Analysis |
| Extracorporeal Circuit, Blood Pump, and Artificial Organ | Perfusion and Extracorporeal Life Support Training | Pulmonary and Systemic Pressure–Flow | Aortic Stenosis and Valvular Performance |
| Catheter and Guidewire | Pressure, Flow Rate, Vascular Resistance, Compliance | Aneurysm and Stroke |
| Mechanical Model | Computational Model | Hybrid Model |
|---|---|---|
| Cardiovascular device testing | Disease modeling | Cardiovascular device testing |
| Clinical training and education | Device design and optimization | Patient-specific simulations |
| Waveform analysis | Hemodynamics and flow simulations | Advanced control algorithm development |
| In vitro clinical trials | In silico clinical trials | Preclinical testing under varied conditions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsui, V.K.; Ewert, D. Review of Cardiovascular Mock Circulatory Loop Designs and Applications. Bioengineering 2025, 12, 851. https://doi.org/10.3390/bioengineering12080851
Tsui VK, Ewert D. Review of Cardiovascular Mock Circulatory Loop Designs and Applications. Bioengineering. 2025; 12(8):851. https://doi.org/10.3390/bioengineering12080851
Chicago/Turabian StyleTsui, Victor K., and Daniel Ewert. 2025. "Review of Cardiovascular Mock Circulatory Loop Designs and Applications" Bioengineering 12, no. 8: 851. https://doi.org/10.3390/bioengineering12080851
APA StyleTsui, V. K., & Ewert, D. (2025). Review of Cardiovascular Mock Circulatory Loop Designs and Applications. Bioengineering, 12(8), 851. https://doi.org/10.3390/bioengineering12080851






