Influence of Pyrolysis Temperature on the Properties and Electrochemical Performance of Cedar Wood-Derived Biochar for Supercapacitor Electrodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biochar Preparation
2.3. Physicochemical Characterization of Biochar
2.4. Structural and Morphological Characterization of Biochar
2.5. Electrical Characterization of Biochar
2.6. Preparation of Biochar-Based Electrodes and Assembly of Supercapacitor
2.7. Electrochemical Characterization of Biochar
3. Results and Discussion
3.1. Effect of Pyrolysis Temperature on the Properties of Cedar Wood-Based Biochar
3.2. Electrochemical Characterization of Biochar-Based Electrodes
3.3. Electrochemical Characterization of Biochar-Based Supercapacitors
3.4. Electrochemical Stability of Biochar-Based Supercapacitors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Temesgen, T.; Bekele, E.T.; Gonfa, B.A.; Tufa, L.T.; Sabir, F.K.; Tadesse, S.; Dessie, Y. Advancements in Biomass Derived Porous Carbon Materials and Their Surface Influence Effect on Electrode Electrochemical Performance for Sustainable Supercapacitors: A Review. J. Energy Storage 2023, 73, 109293. [Google Scholar] [CrossRef]
- Makinde, W.O.; Hassan, M.A.; Pan, Y.; Guan, G.; López-Salas, N.; Khalil, A.S.G. Sulfur and Nitrogen Co-Doping of Peanut Shell-Derived Biochar for Sustainable Supercapacitor Applications. J. Alloys Compd. 2024, 991, 174452. [Google Scholar] [CrossRef]
- Lin, Y.; Li, F.; Zhang, Q.; Liu, G.; Xue, C. Controllable Preparation of Green Biochar Based High-Performance Supercapacitors; Springer: Berlin/Heidelberg, Germany, 2022; Volume 28, ISBN 0123456789. [Google Scholar]
- Li, J.; Wu, Q. Water Bamboo-Derived Porous Carbons as Electrode Materials for Supercapacitors. New J. Chem. 2015, 39, 3859–3864. [Google Scholar] [CrossRef]
- Rawat, S.; Wang, C.T.; Lay, C.H.; Hotha, S.; Bhaskar, T. Sustainable Biochar for Advanced Electrochemical/Energy Storage Applications. J. Energy Storage 2023, 63, 107115. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Du, Y.; Ma, X.; Lin, J.; Chen, S. Apple-Pomace-Based Porous Biochar as Electrode Materials for Supercapacitors. Diam. Relat. Mater. 2022, 130, 109507. [Google Scholar] [CrossRef]
- Fawzy, S.; Osman, A.I.; Yang, H.; Doran, J.; Rooney, D.W. Industrial Biochar Systems for Atmospheric Carbon Removal: A Review; Springer International Publishing: Cham, Switzerland, 2021; Volume 1, ISBN 0123456789. [Google Scholar]
- He, X.; Li, W.; Xia, Y.; Huang, H.; Xia, X.; Gan, Y.; Zhang, J.; Zhang, W. Pivotal Factors of Wood-Derived Electrode for Supercapacitor: Component Striping, Specific Surface Area and Functional Group at Surface. Carbon N. Y. 2023, 210, 118090. [Google Scholar] [CrossRef]
- Naseem, K.; Tahir, A.; Khan, A.S.; Qin, F.; Usman, M.; Karamat, S.; Al-Salihi, M. Unlocking the Potential of Magnetic Bio-Char as a Low-Cost Cathode Material for the Supercapacitor Application. Mater. Today Commun. 2024, 39, 108764. [Google Scholar] [CrossRef]
- Yumak, T. Surface Characteristics and Electrochemical Properties of Activated Carbon Obtained from Different Parts of Pinus Pinaster. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126982. [Google Scholar] [CrossRef]
- Norouzi, O.; Pourhosseini, S.E.M.; Naderi, H.R.; Di Maria, F.; Dutta, A. Integrated Hybrid Architecture of Metal and Biochar for High Performance Asymmetric Supercapacitors. Sci. Rep. 2021, 11, 5387. [Google Scholar] [CrossRef]
- Norouzi, O.; Di Maria, F.; Dutta, A. Biochar-Based Composites as Electrode Active Materials in Hybrid Supercapacitors with Particular Focus on Surface Topography and Morphology. J. Energy Storage 2020, 29, 101291. [Google Scholar] [CrossRef]
- Manasa, P.; Sambasivam, S.; Ran, F. Recent Progress on Biomass Waste Derived Activated Carbon Electrode Materials for Supercapacitors Applications—A Review. J. Energy Storage 2022, 54, 105290. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Xue, B.; Li, W.; Ding, Z.; Yang, X.; Qiu, J.; Wang, Z. Rice Husk-Based Hierarchical Porous Carbon for High Performance Supercapacitors: The Structure-Performance Relationship. Carbon N. Y. 2020, 161, 432–444. [Google Scholar] [CrossRef]
- Kouchachvili, L.; Maffei, N.; Entchev, E. Infested Ash Trees as a Carbon Source for Supercapacitor Electrodes. J. Porous Mater. 2015, 22, 979–988. [Google Scholar] [CrossRef]
- Bataillou, G.; Lee, C.; Monnier, V.; Gerges, T.; Sabac, A.; Vollaire, C.; Haddour, N. Cedar Wood—Based Biochar: Properties, Characterization, and Applications as Anodes in Microbial Fuel Cell. Appl. Biochem. Biotechnol. 2022, 194, 4169–4186. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zheng, J.; Feng, L.; Du, C.; Jian, S.; Yang, W.; Wu, Y.A.; Jiang, S.; He, S.; Chen, W. Wood-Derived Biochar as Thick Electrodes for High-Rate Performance Supercapacitors. Biochar 2022, 4, 50. [Google Scholar] [CrossRef]
- Khedulkar, A.P.; Pandit, B.; Dang, V.D.; Doong, R. an Agricultural Waste to Real Worth Biochar as a Sustainable Material for Supercapacitor. Sci. Total Environ. 2023, 869, 161441. [Google Scholar] [CrossRef]
- Husain, Z.; Shakeelur Raheman, A.R.; Ansari, K.B.; Pandit, A.B.; Khan, M.S.; Qyyum, M.A.; Lam, S.S. Nano-Sized Mesoporous Biochar Derived from Biomass Pyrolysis as Electrochemical Energy Storage Supercapacitor. Mater. Sci. Energy Technol. 2022, 5, 99–109. [Google Scholar] [CrossRef]
- Qin, Z.; Ye, Y.; Zhang, D.; He, J.; Zhou, J.; Cai, J. One/Two-Step Contribution to Prepare Hierarchical Porous Carbon Derived from Rice Husk for Supercapacitor Electrode Materials. ACS Omega 2023, 8, 5088–5096. [Google Scholar] [CrossRef]
- Shanmuga Priya, M.; Divya, P.; Rajalakshmi, R. A Review Status on Characterization and Electrochemical Behaviour of Biomass Derived Carbon Materials for Energy Storage Supercapacitors. Sustain. Chem. Pharm. 2020, 16, 100243. [Google Scholar] [CrossRef]
- Karakehya, N. Effects of One-Step and Two-Step KOH Activation Method on the Properties and Supercapacitor Performance of Highly Porous Activated Carbons Prepared from Lycopodium Clavatum Spores. Diam. Relat. Mater. 2023, 135, 109873. [Google Scholar] [CrossRef]
- Rawat, S.; Boobalan, T.; Sathish, M.; Hotha, S.; Thallada, B. Utilization of CO2 Activated Litchi Seed Biochar for the Fabrication of Supercapacitor Electrodes. Biomass Bioenergy 2023, 171, 106747. [Google Scholar] [CrossRef]
- Yuan, X.; Xiao, J.; Yılmaz, M.; Zhang, T.C.; Yuan, S. N, P Co-Doped Porous Biochar Derived from Cornstalk for High Performance CO2 Adsorption and Electrochemical Energy Storage. Sep. Purif. Technol. 2022, 299, 121719. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, K.H.; Lee, S.; Lee, S.K.; Jeon, O.S.; Jeon, Y.P.; Hong, D.; Yoo, Y.J.; Park, S.Y.; Yoo, H.Y. Conversion of N-Doped Biochar from Carotenoid-Extracted Tetraselmis Suecica and Its Application to Produce Supercapacitors. J. Environ. Sci. 2025, 151, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Surendran, S.; Ji, S.; Kim, D.; Chae, Y.; Kim, J.; Je, M.; Han, M.K.; Choe, W.S.; Choi, C.H.; et al. A Sulfur Self-Doped Multifunctional Biochar Catalyst for Overall Water Splitting and a Supercapacitor from Camellia Japonica Flowers. Carbon Energy 2022, 4, 491–505. [Google Scholar] [CrossRef]
- Xiaorui, L.; Haiping, Y. A State-of-the-Art Review of N Self-Doped Biochar Development in Supercapacitor Applications. Front. Energy Res. 2023, 11, 1135093. [Google Scholar] [CrossRef]
- Carrasco-Marín, F.; López-Ramón, M.V.; Moreno-Castilla, C. Applicability of the Dubinin-Radushkevich Equation to CO2 Adsorption on Activated Carbons. Langmuir 1993, 9, 2758–2760. [Google Scholar] [CrossRef]
- Sigmund, G.; Hüffer, T.; Hofmann, T.; Kah, M. Biochar Total Surface Area and Total Pore Volume Determined by N2 and CO2 Physisorption Are Strongly Influenced by Degassing Temperature. Sci. Total Environ. 2017, 580, 770–775. [Google Scholar] [CrossRef]
- Miccoli, I.; Edler, F.; Pfnür, H.; Tegenkamp, C. The 100th Anniversary of the Four-Point Probe Technique: The Role of Probe Geometries in Isotropic and Anisotropic Systems. J. Phys. Condens. Matter 2015, 27, 223201. [Google Scholar] [CrossRef]
- Choudhury, N.A.; Northrop, P.W.C. Chitosan Hydrogel-Based Electrode Binder and Electrolyte Membrane for EDLCs: Experimental Studies and Model Validation. J. Appl. Electrochem. 2012, 42, 935–943. [Google Scholar] [CrossRef]
- Yang, C.; Liu, J.; Lu, S. Geoderma Pyrolysis Temperature Affects Pore Characteristics of Rice Straw and Canola Stalk Biochars and Biochar-Amended Soils. Geoderma 2021, 397, 115097. [Google Scholar] [CrossRef]
- Zha, Z.; Zhang, Z.; Xiang, P.; Zhu, H.; Zhou, B.; Sun, Z.; Zhou, S. One-Step Preparation of Eggplant-Derived Hierarchical Porous Graphitic Biochar as Efficient Oxygen Reduction Catalyst in Microbial Fuel Cells. RSC Adv. 2020, 11, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Timms, W.; Mahmud, M.A.P. Science of the Total Environment Optimising Water Holding Capacity and Hydrophobicity of Biochar for Soil Amendment—A Review. Sci. Total Environ. 2022, 851, 158043. [Google Scholar] [CrossRef] [PubMed]
- Gezahegn, S.; Sain, M.; Thomas, S.C. Variation in Feedstock Wood Chemistry Strongly Influences Biochar Liming Potential. Soil Syst. 2019, 3, 26. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Lyczakowski, J.J.; Bourdon, M.; Terrett, O.M.; Helariutta, Y.; Wightman, R.; Dupree, P. Structural Imaging of Native Cryo-Preserved Secondary Cell Walls Reveals the Presence of Macrofibrils and Their Formation Requires Normal Cellulose, Lignin and Xylan Biosynthesis. Front. Plant Sci. 2019, 10, 1398. [Google Scholar] [CrossRef]
- Fuertes, A.B.; Arbestain, M.C.; Sevilla, M.; MacIá-Agulló, J.A.; Fiol, S.; López, R.; Smernik, R.J.; Aitkenhead, W.P.; Arce, F.; MacIas, F. Chemical and Structural Properties of Carbonaceous Products Obtained by Pyrolysis and Hydrothermal Carbonisation of Corn Stover. Aust. J. Soil Res. 2010, 48, 618–626. [Google Scholar] [CrossRef]
- Prabakar, P.; Mustafa Mert, K.; Muruganandam, L.; Sivagami, K. A Comprehensive Review on Biochar for Electrochemical Energy Storage Applications: An Emerging Sustainable Technology. Front. Energy Res. 2024, 12, 1448520. [Google Scholar] [CrossRef]
- Mehdi, R.; Khoja, A.H.; Naqvi, S.R.; Gao, N.; Amin, N.A.S. A Review on Production and Surface Modifications of Biochar Materials via Biomass Pyrolysis Process for Supercapacitor Applications. Catalysts 2022, 12, 798. [Google Scholar] [CrossRef]
- Liu, S.H.; You, S.S.; Lin, C.W.; Cheng, Y.S. Optimizing Biochar and Conductive Carbon Black Composites as Cathode Catalysts for Microbial Fuel Cells to Improve Isopropanol Removal and Power Generation. Renew. Energy 2022, 199, 1318–1328. [Google Scholar] [CrossRef]
- Salleh, N.A.; Kheawhom, S.; Mohamad, A.A. Chitosan as Biopolymer Binder for Graphene in Supercapacitor Electrode. Results Phys. 2021, 25, 104244. [Google Scholar] [CrossRef]
- Durajski, A.P.; Gruszka, K.; Giza, K.; Niegodajew, P. The Energy Storage Properties of Supercapacitors with Carbon-Based Electrodes. Acta Phys. Pol. A 2020, 138, 148–151. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, L.; Wang, X.; Holm, N.; Rajagopalan, K.; Chen, F.; Ma, S. Highly Ordered Macroporous Woody Biochar with Ultra-High Carbon Content as Supercapacitor Electrodes. Electrochim. Acta 2013, 113, 481–489. [Google Scholar] [CrossRef]
- Cuña, A.; Tancredi, N.; Bussi, J.; Barranco, V.; Centeno, T.A.; Quevedo, A.; Rojo, J.M. Biocarbon Monoliths as Supercapacitor Electrodes: Influence of Wood Anisotropy on Their Electrical and Electrochemical Properties. J. Electrochem. Soc. 2014, 161, A1806–A1811. [Google Scholar] [CrossRef]
- Caguiat, J.N.; Arpino, G.; Krigstin, S.G.; Kirk, D.W.; Jia, C.Q. Dependence of Supercapacitor Performance on Macro-Structure of Monolithic Biochar Electrodes. Biomass Bioenergy 2018, 118, 126–132. [Google Scholar] [CrossRef]
- Salleh, N.A.; Kheawhom, S.; Hamid, N.A.A.; Rahiman, W.; Mohamad, A.A. Electrode Polymer Binders for Supercapacitor Applications: A Review. J. Mater. Res. Technol. 2023, 23, 3470–3491. [Google Scholar] [CrossRef]
- Zhang, L.; Chai, L.; Qu, Q.; Zhang, L.; Shen, M.; Zheng, H. Chitosan, a New and Environmental Benign Electrode Binder for Use with Graphite Anode in Lithium-Ion Batteries. Electrochim. Acta 2013, 105, 378–383. [Google Scholar] [CrossRef]
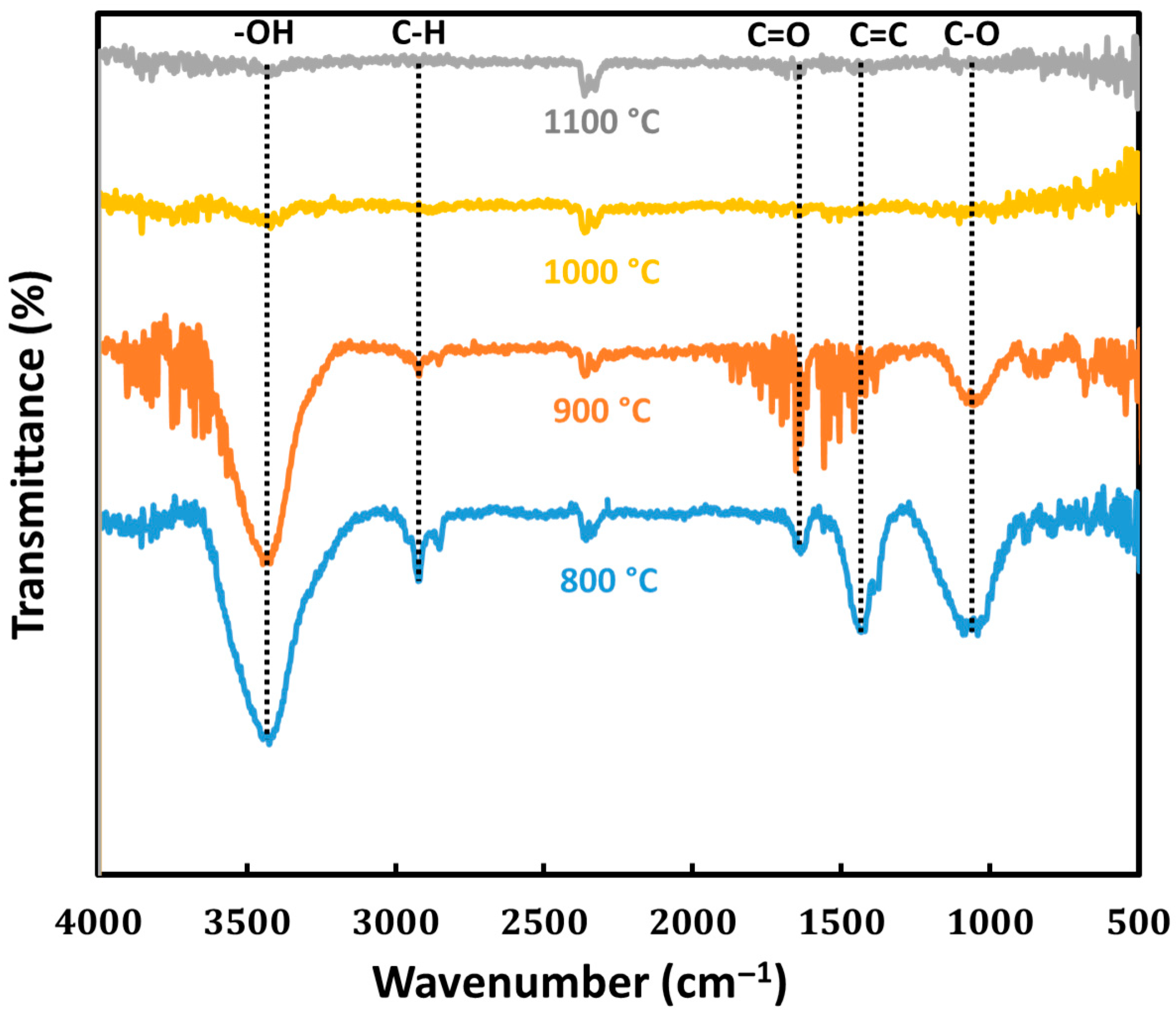

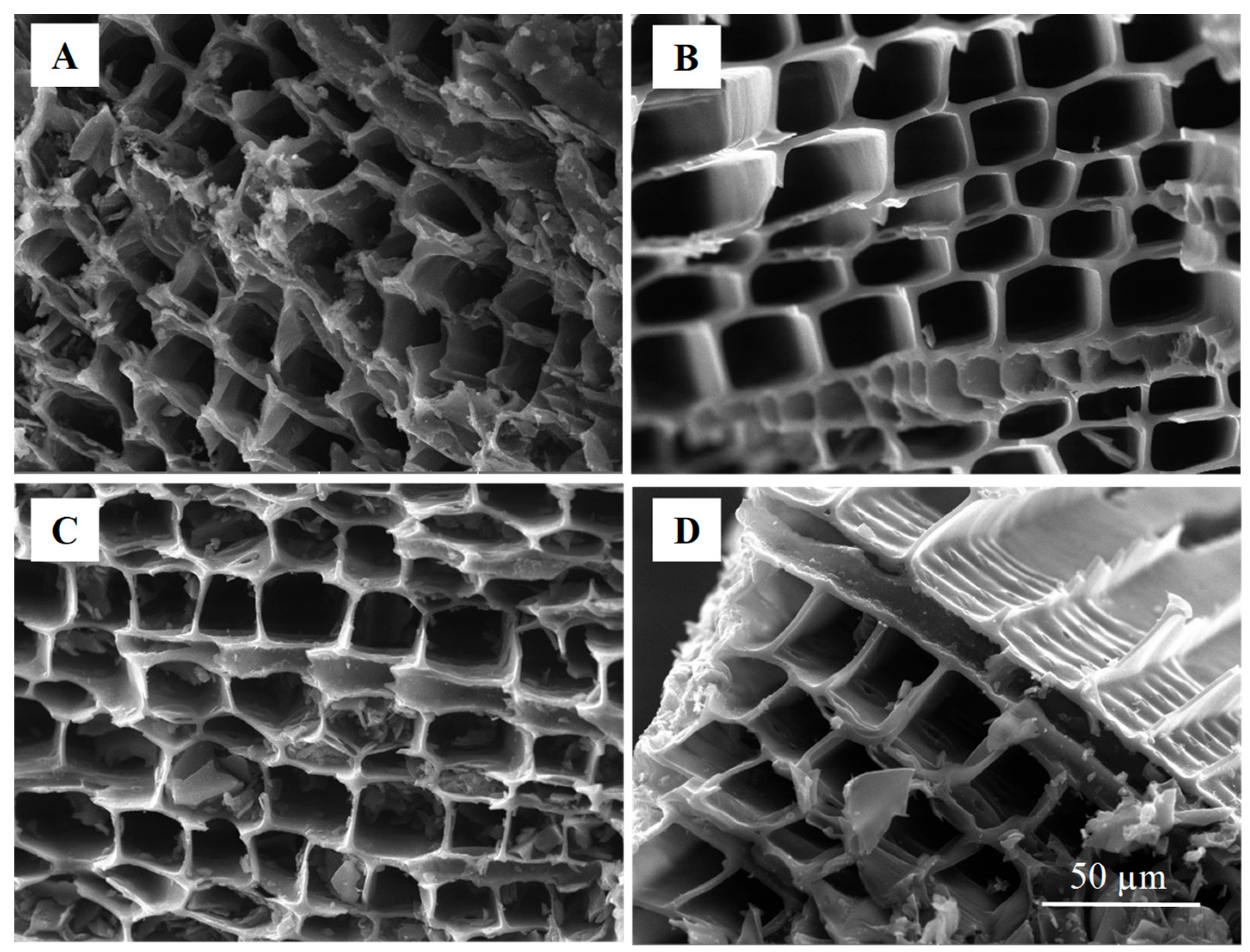

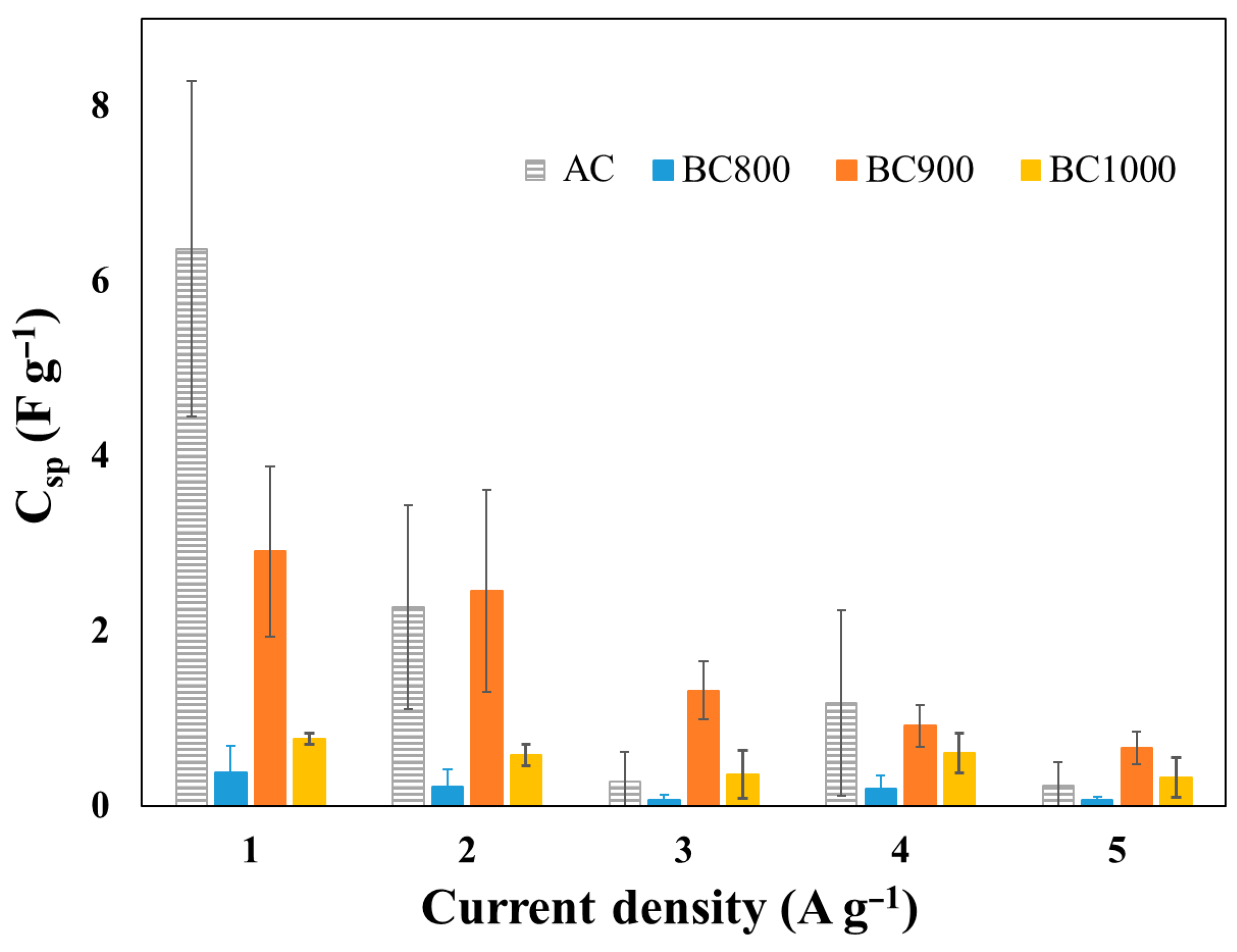
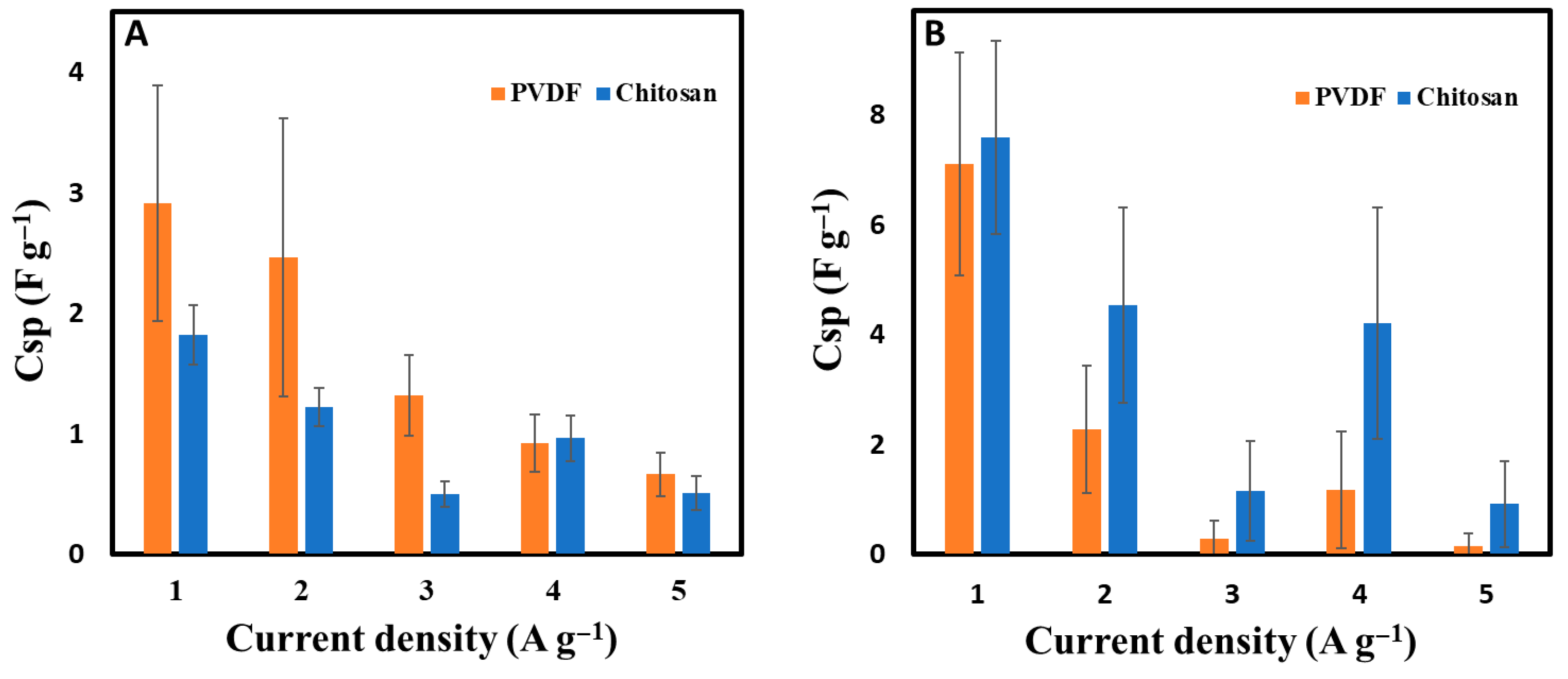
| Material | Specific Surface Area (N2, m2 g−1) | Specific Surface Area (CO2, m2 g−1) | Resistivity 10−3 (Ω m) | Contact Angle (°) | pH |
|---|---|---|---|---|---|
| BC800 | 370 | 454 ± 8 | 18 ± 1 | 26 ± 3 | 7.8 ± 0.1 |
| BC900 | 1.7 | 385 ± 30 | 5.3 ± 0.1 | 51 ± 5 | 7.7 ± 0.1 |
| BC1000 | 6.7 | 431 ± 40 | 3.70 ± 0.05 | 53 ± 4 | 7.9 ± 0.1 |
| BC1100 | 7.3 | 425 ± 20 | 3.60 ± 0.06 | 53 ± 15 | 7.9 ± 0.2 |
| Electrode | Resistivity 10−3 (Ω m) | Contact Angle (°) |
|---|---|---|
| BC800/CB/PVDF | 6.43 ± 0.05 | 127 ± 3 |
| BC900/CB/PVDF | 3.35 ± 0.01 | 116 ± 8 |
| BC1000/CB/PVDF | 2.75 ± 0.03 | 17 ± 6 |
| AC/CB/PVDF | 0.62 ± 0.10 | 44 ± 6 |
| BC900/CB/chitosan | 0.61 ± 0.21 | 63 ± 4 |
| AC/CB/chitosan | 0.68 ± 0.21 | 39 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallah, L.; Gondran, C.; Monnier, V.; Vollaire, C.; Haddour, N. Influence of Pyrolysis Temperature on the Properties and Electrochemical Performance of Cedar Wood-Derived Biochar for Supercapacitor Electrodes. Bioengineering 2025, 12, 841. https://doi.org/10.3390/bioengineering12080841
Abdallah L, Gondran C, Monnier V, Vollaire C, Haddour N. Influence of Pyrolysis Temperature on the Properties and Electrochemical Performance of Cedar Wood-Derived Biochar for Supercapacitor Electrodes. Bioengineering. 2025; 12(8):841. https://doi.org/10.3390/bioengineering12080841
Chicago/Turabian StyleAbdallah, Layal, Chantal Gondran, Virginie Monnier, Christian Vollaire, and Naoufel Haddour. 2025. "Influence of Pyrolysis Temperature on the Properties and Electrochemical Performance of Cedar Wood-Derived Biochar for Supercapacitor Electrodes" Bioengineering 12, no. 8: 841. https://doi.org/10.3390/bioengineering12080841
APA StyleAbdallah, L., Gondran, C., Monnier, V., Vollaire, C., & Haddour, N. (2025). Influence of Pyrolysis Temperature on the Properties and Electrochemical Performance of Cedar Wood-Derived Biochar for Supercapacitor Electrodes. Bioengineering, 12(8), 841. https://doi.org/10.3390/bioengineering12080841









