Lower Limb Inter-Joint Coordination and End-Point Control During Gait in Adolescents with Early Treated Unilateral Developmental Dysplasia of the Hip
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocol
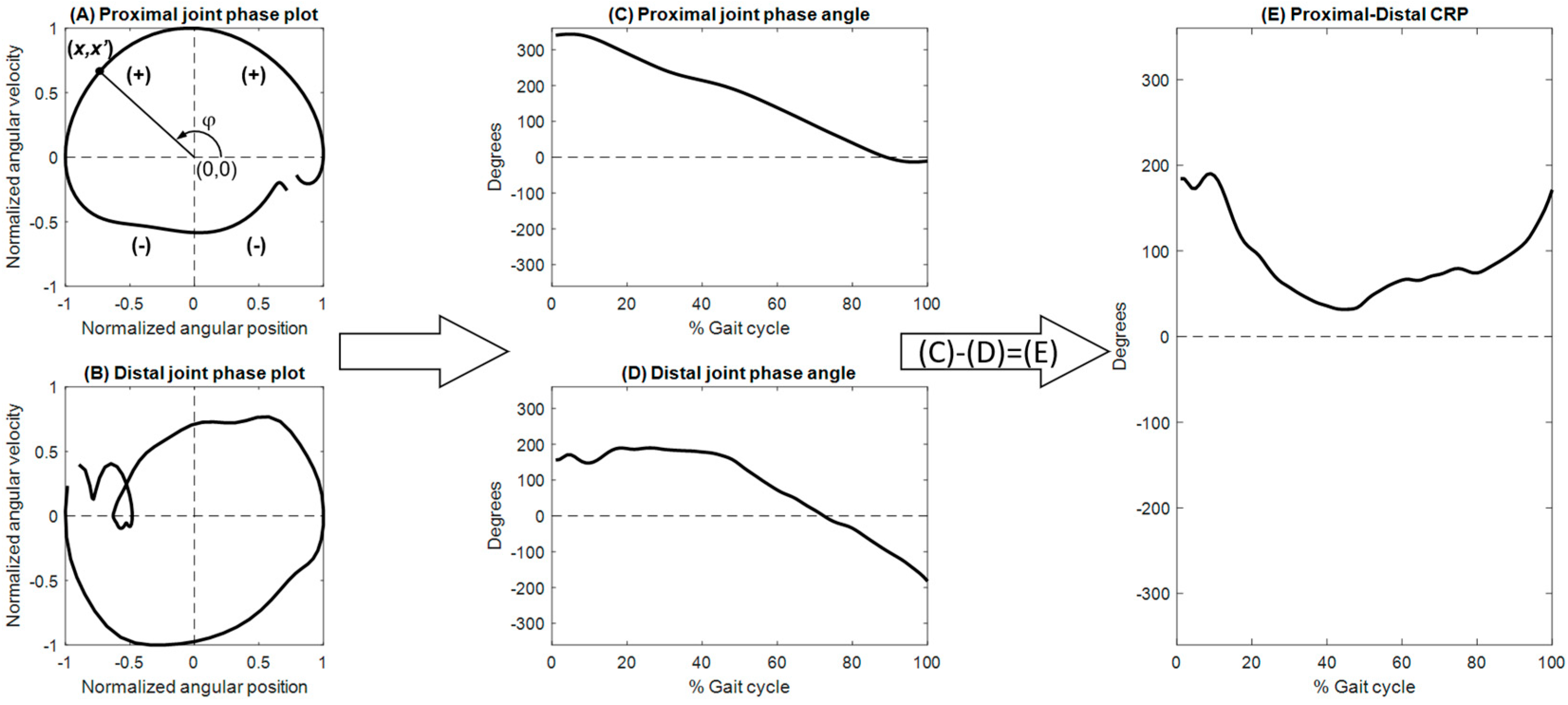
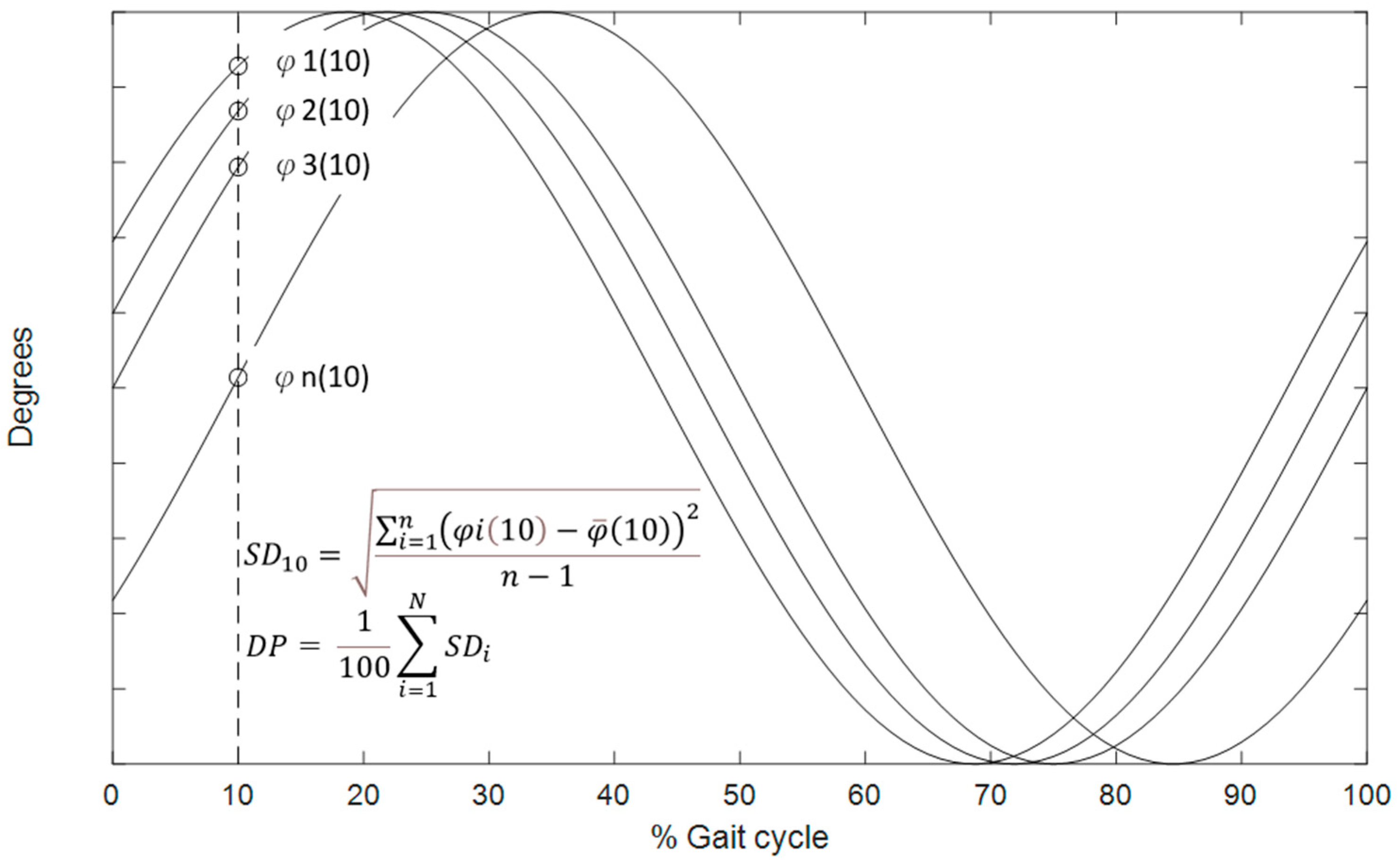
2.3. Data Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nandhagopal, T.; De Cicco, F.L. Developmental Dysplasia of the Hip; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Han, J.; Li, Y. Progress in screening strategies for neonatal developmental dysplasia of the hip. Front. Surg. 2022, 9, 995949. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, M.; Loureiro, A.; Carty, C.; Mills, P.; Barrett, R. Hip joint mechanics during walking in individuals with mild-to-moderate hip osteoarthritis. Gait Posture 2017, 53, 162–167. [Google Scholar] [CrossRef]
- Scott, E.J.; Dolan, L.A.; Weinstein, S.L. Closed Vs. Open Reduction/Salter Innominate Osteotomy for Developmental Hip Dislocation After Age 18 Months: Comparative Survival at 45-Year Follow-up. J. Bone Jt. Surg. Am. 2020, 102, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Pemberton, P.A. Pericapsular osteotomy of the ilium for the treatment of congenitally dislocated hips. Clin. Orthop. Relat. Res. 1974, 98, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Young, J.R.; Anderson, M.J.; O’Connor, C.M.; Kazley, J.M.; Mantica, A.L.; Dutt, V. Team approach: Developmental dysplasia of the hip. JBJS Rev. 2020, 8, e20. [Google Scholar] [CrossRef]
- Çiçekli, Ö.; Doğan, M. Evaluation of surgical outcome in advanced age patients with developmental hip dysplasia. Int. J. Surg. 2018, 52, 44–49. [Google Scholar] [CrossRef]
- Chang, C.F.; Wang, T.M.; Wang, J.H.; Huang, S.C.; Lu, T.W. Adolescents After Pemberton’s Osteotomy for Developmental Dysplasia of the Hip Displayed Greater Joint Loading than Healthy Controls in Affected and Unaffected Limbs during Gait. J. Orthop. Res. 2011, 29, 1034–1041. [Google Scholar] [CrossRef]
- Ivanenko, Y.P.; Cappellini, G.; Dominici, N.; Poppele, R.E.; Lacquaniti, F. Modular control of limb movements during human locomotion. J. Neurosci. 2007, 27, 11149–11161. [Google Scholar] [CrossRef]
- Hicheur, H.; Terekhov, A.V.; Berthoz, A. Intersegmental coordination during human locomotion: Does planar covariation of elevation angles reflect central constraints? J. Neurophysiol. 2006, 96, 1406–1419. [Google Scholar] [CrossRef]
- Chiu, S.L.; Lu, T.W.; Chou, L.S. Altered Inter-joint Coordination during Walking in Patients with Total Hip Arthroplasty. Gait Posture 2010, 32, 656–660. [Google Scholar] [CrossRef]
- Winter, D.A. Foot trajectory in human gait: A precise and multifactorial motor control task. Phys. Ther. 1992, 72, 45–53. [Google Scholar] [CrossRef]
- Chang, C.-F.; Wang, T.-M.; Wang, J.-H.; Huang, S.-C.; Lu, T.-W. Residual gait deviations in adolescents treated during infancy for unilateral developmental dysplasia of the hip using Pemberton’s osteotomy. Gait Posture 2012, 35, 561–566. [Google Scholar] [CrossRef]
- Jørgensen, A.E.M.; Kjær, M.; Heinemeier, K.M. The effect of aging and mechanical loading on the metabolism of articular cartilage. J. Rheumatol. 2017, 44, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Tajima, T.; Tateuchi, H.; Koyama, Y.; Ikezoe, T.; Ichihashi, N. Gait strategies to reduce the dynamic joint load in the lower limbs during a loading response in young healthy adults. Human. Mov. Sci. 2018, 58, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.; Teng, H.-L.; Powers, C.M. Lower extremity stiffness predicts ground reaction force loading rate in heel strike runners. Med. Sci. Sports Exerc. 2019, 51, 1692–1697. [Google Scholar] [CrossRef]
- Lee, W.C.; Chen, T.Y.; Hung, L.W.; Wang, T.M.; Chang, C.H.; Lu, T.W. Increased Loading Rates During Gait Correlate With Morphology of Unaffected Hip in Juveniles With Treated Developmental Hip Dysplasia. Front. Bioeng. Biotechnol. 2021, 9, 704266. [Google Scholar] [CrossRef] [PubMed]
- Bars, S.L.; Bourgeois-Gironde, S.; Wyart, V.; Sari, I.; Pacherie, E.; Chambon, V. Motor Coordination and Strategic Cooperation in Joint Action. Psychol. Sci. 2022, 33, 736–751. [Google Scholar] [CrossRef]
- St-Onge, N.; Feldman, A.G. Interjoint coordination in lower limbs during different movements in humans. Exp. Brain Res. 2003, 148, 139–149. [Google Scholar] [CrossRef]
- Kuo, C.C.; Chen, S.C.; Wang, J.Y.; Ho, T.J.; Lin, J.G.; Lu, T.W. Effects of Tai-Chi Chuan Practice on Patterns and Stability of Lower Limb Inter-Joint Coordination During Obstructed Gait in the Elderly. Front. Bioeng. Biotechnol. 2021, 9, 739722. [Google Scholar] [CrossRef]
- Erdfelder, E.; Faul, F.; Buchner, A. GPOWER: A general power analysis program. Behav. Res. Methods Instrum. Comput. 1996, 28, 1–11. [Google Scholar] [CrossRef]
- Wu, G.; Van der Helm, F.C.; Veeger, H.D.; Makhsous, M.; Van Roy, P.; Anglin, C.; Nagels, J.; Karduna, A.R.; McQuade, K.; Wang, X. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion—Part II: Shoulder, elbow, wrist and hand. J. Biomech. 2005, 38, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Siegler, S.; Allard, P.; Kirtley, C.; Leardini, A.; Rosenbaum, D.; Whittle, M.; D D’Lima, D.; Cristofolini, L.; Witte, H. ISB recommendation on definitions of joint coordinate system of various joints for the reporting of human joint motion—Part I: Ankle, hip, and spine. J. Biomech. 2002, 35, 543–548. [Google Scholar] [CrossRef]
- Kim, H.K.; Dai, X.; Lu, S.-H.; Lu, T.-W.; Chou, L.-S. Discriminating features of ground reaction forces in overweight old and young adults during walking using functional principal component analysis. Gait Posture 2022, 94, 166–172. [Google Scholar] [CrossRef]
- Kim, H.K.; Lu, S.-H.; Lu, T.-W.; Chou, L.-S. Contribution of lower extremity muscles to center of mass acceleration during walking: Effect of body weight. J. Biomech. 2023, 146, 111398. [Google Scholar] [CrossRef]
- Grood, E.S.; Suntay, W.J. A joint coordinate system for the clinical description of three-dimensional motions: Application to the knee. J. Biomech. Eng. 1983, 136, 144. [Google Scholar] [CrossRef]
- Woltring, H.J. A Fortran package for generalized, cross-validatory spline smoothing and differentiation. Adv. Eng. Softw. 1986, 8, 104–113. [Google Scholar] [CrossRef]
- Stergiou, N.; Scholten, S.D.; Jensen, J.L.; Blanke, D. Intralimb coordination following obstacle clearance during running: The effect of obstacle height. Gait Posture 2001, 13, 210–220. [Google Scholar] [CrossRef]
- Kurz, M.J.; Stergiou, N. Effect of Normalization and Phase Angle Calculations on Continuous Relative Phase. J. Biomech. 2002, 35, 369–374. [Google Scholar] [CrossRef]
- Burgess-Limerick, R.; Abernethy, B.; Neal, R.J. Relative Phase Quantifies Interjoint Coordination. J. Biomech. 1993, 26, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Cutti, A.G.; Garofalo, P.; Raggi, M.; Heijboer, M.; Cappello, A.; Davalli, A. First in Vivo Assessment of “Outwalk”: A Novel Protocol for Clinical Gait Analysis Based on Inertial and Magnetic Sensors. Med. Biol. Eng. Comput. 2010, 48, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, A.; Pohl, M.B.; Woods, K.; Noehren, B. Variables during swing associated with decreased impact peak and loading rate in running. J. Biomech. 2014, 47, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Silverman, A.K.; Fey, N.P.; Portillo, A.; Walden, J.G.; Bosker, G.; Neptune, R.R. Compensatory mechanisms in below-knee amputee gait in response to increasing steady-state walking speeds. Gait Posture 2008, 28, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Woodacre, T.; Ball, T.; Cox, P. Epidemiology of developmental dysplasia of the hip within the UK: Refining the risk factors. J. Child. Orthop. 2016, 10, 633–642. [Google Scholar] [CrossRef] [PubMed]



| Control Group | DDH Group | Pg | Ps | |||
|---|---|---|---|---|---|---|
| Affected | Unaffected | Affected | Unaffected | |||
| Gait speed (m/s) | 1.22 (0.24) | 1.13 (0.13) | 1.10 (0.23) | 0.306 | 0.314 | 0.819 |
| Stride length (mm) | 112.31 (9.93) | 110.91 (8.52) | 111.02 (7.00) | 0.730 | 0.741 | 0.904 |
| Cadence (1/s) | 118.72 (12.82) | 111.10 (10.94) | 113.63 (12.40) | 0.162 | 0.365 | 0.353 |
| Step length (mm) | 579.93 (49.23) | 579.5 (39.75) | 560.6 (31.01) | 0.983 | 0.300 | 0.054 |
| Step width (mm) | 832.52 (268.51) | 1070.30 (389.52) | 0.001 * | |||
| End-points (during the swing phase): Heel | ||||||
| Anterior-posterior component (mm) | 22.69 (9.12) | 35.83 (24.37) | 36.31 (17.71) | 0.110 | 0.035 * | 0.959 |
| Vertical component (mm) | 11.99 (5.88) | 19.98 (15.19) | 20.65 (10.78) | 0.119 | 0.030 * | 0.906 |
| End-points (during the swing phase): Toe | ||||||
| Anterior-posterior component (mm) | 28.07 (10.65) | 40.65 (31.97) | 39.38 (15.68) | 0.230 | 0.042 * | 0.907 |
| Vertical component (mm) | 5.35 (0.85) | 8.47 (5.05) | 9.80 (2.74) | 0.069 | 0.001 * | 0.451 |
| Phase | CRP | Control Group | DDH Group | Pg | Ps | CMC | RMSD | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Affected | Unaffected | Affected | Unaffected | Affected | Unaffected | Affected | Unaffected | ||||
| Initial DLS | Hip–Knee | 177.99 (13.08) | 180.75 (16.62) | 187.01 (11.07) | 0.670 | 0.096 | 0.311 | 0.94 | 0.83 | 6.05 | 11.57 |
| Knee–Ankle | 62.21 (77.97) | 43.62 (42.29) | 97.66 (57.41) | 0.495 | 0.023 * | 0.210 | 0.85 | 0.31 | 15.32 | 40.93 | |
| SLS | Hip–Knee | 63.45 (9.87) | 59.67 (9.04) | 63.48 (8.88) | 0.360 | 0.994 | 0.330 | 0.97 | 0.99 | 4.97 | 2.52 |
| Knee–Ankle | 172.30 (9.77) | 174.12 (7.44) | 179.69 (8.27) | 0.629 | 0.700 | 0.112 | 0.75 | 0.02 | 5.46 | 9.46 | |
| Terminal DLS | Hip–Knee | 56.75 (11.72) | 55.18 (10.54) | 52.68 (8.69) | 0.743 | 0.366 | 0.552 | 0.91 | 0.79 | 2.74 | 4.26 |
| Knee–Ankle | 178.24 (18.43) | 184.52 (21.92) | 196.39 (16.97) | 0.475 | 0.026 * | 0.171 | 0.76 | 0.09 | 10.01 | 20.94 | |
| Swing | Hip–Knee | 89.18 (9.03) | 91.93 (6.54) | 96.38 (13.86) | 0.424 | 0.164 | 0.346 | 0.94 | 0.86 | 8.99 | 13.53 |
| Knee–Ankle | 277.99 (35.92) | 268.57 (20.11) | 257.21 (22.49) | 0.456 | 0.119 | 0.226 | 0.75 | 0.71 | 14.19 | 15.69 | |
| Phase | CRP | Control Group | DDH Group | Pg | Ps | ||
|---|---|---|---|---|---|---|---|
| Affected | Unaffected | Affected | Unaffected | ||||
| Initial DLS | Hip–Knee | 16.80 (8.33) | 26.00 (9.92) | 22.22 (8.42) | 0.360 | 0.163 | 0.369 |
| Knee–Ankle | 8.81 (2.92) | 11.72 (5.51) | 10.31 (3.78) | 0.040 * | 0.156 | 0.514 | |
| SLS | Hip–Knee | 8.49 (4.02) | 6.59 (3.19) | 6.63 (3.04) | 0.254 | 0.256 | 0.976 |
| Knee–Ankle | 7.86 (2.87) | 11.69 (4.41) | 8.97 (4.30) | 0.032 * | 0.507 | 0.176 | |
| Terminal DLS | Hip–Knee | 66.44 (34.64) | 62.77 (27.48) | 55.21 (27.93) | 0.796 | 0.434 | 0.549 |
| Knee–Ankle | 12.49 (4.06) | 15.49 (10.08) | 14.44 (5.97) | 0.039 * | 0.404 | 0.777 | |
| Swing | Hip–Knee | 13.77 (6.04) | 16.02 (7.54) | 18.05 (6.37) | 0.469 | 0.138 | 0.522 |
| Knee–Ankle | 22.46 (9.74) | 29.02 (14.79) | 26.31 (15.43) | 0.255 | 0.513 | 0.692 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-F.; Lu, T.-W.; Hu, C.-H.; Wu, K.-W.; Kuo, C.-C.; Wang, T.-M. Lower Limb Inter-Joint Coordination and End-Point Control During Gait in Adolescents with Early Treated Unilateral Developmental Dysplasia of the Hip. Bioengineering 2025, 12, 836. https://doi.org/10.3390/bioengineering12080836
Chang C-F, Lu T-W, Hu C-H, Wu K-W, Kuo C-C, Wang T-M. Lower Limb Inter-Joint Coordination and End-Point Control During Gait in Adolescents with Early Treated Unilateral Developmental Dysplasia of the Hip. Bioengineering. 2025; 12(8):836. https://doi.org/10.3390/bioengineering12080836
Chicago/Turabian StyleChang, Chu-Fen, Tung-Wu Lu, Chia-Han Hu, Kuan-Wen Wu, Chien-Chung Kuo, and Ting-Ming Wang. 2025. "Lower Limb Inter-Joint Coordination and End-Point Control During Gait in Adolescents with Early Treated Unilateral Developmental Dysplasia of the Hip" Bioengineering 12, no. 8: 836. https://doi.org/10.3390/bioengineering12080836
APA StyleChang, C.-F., Lu, T.-W., Hu, C.-H., Wu, K.-W., Kuo, C.-C., & Wang, T.-M. (2025). Lower Limb Inter-Joint Coordination and End-Point Control During Gait in Adolescents with Early Treated Unilateral Developmental Dysplasia of the Hip. Bioengineering, 12(8), 836. https://doi.org/10.3390/bioengineering12080836








