Brain-Computer Interfaces for Stroke Motor Rehabilitation
Abstract
1. Introduction
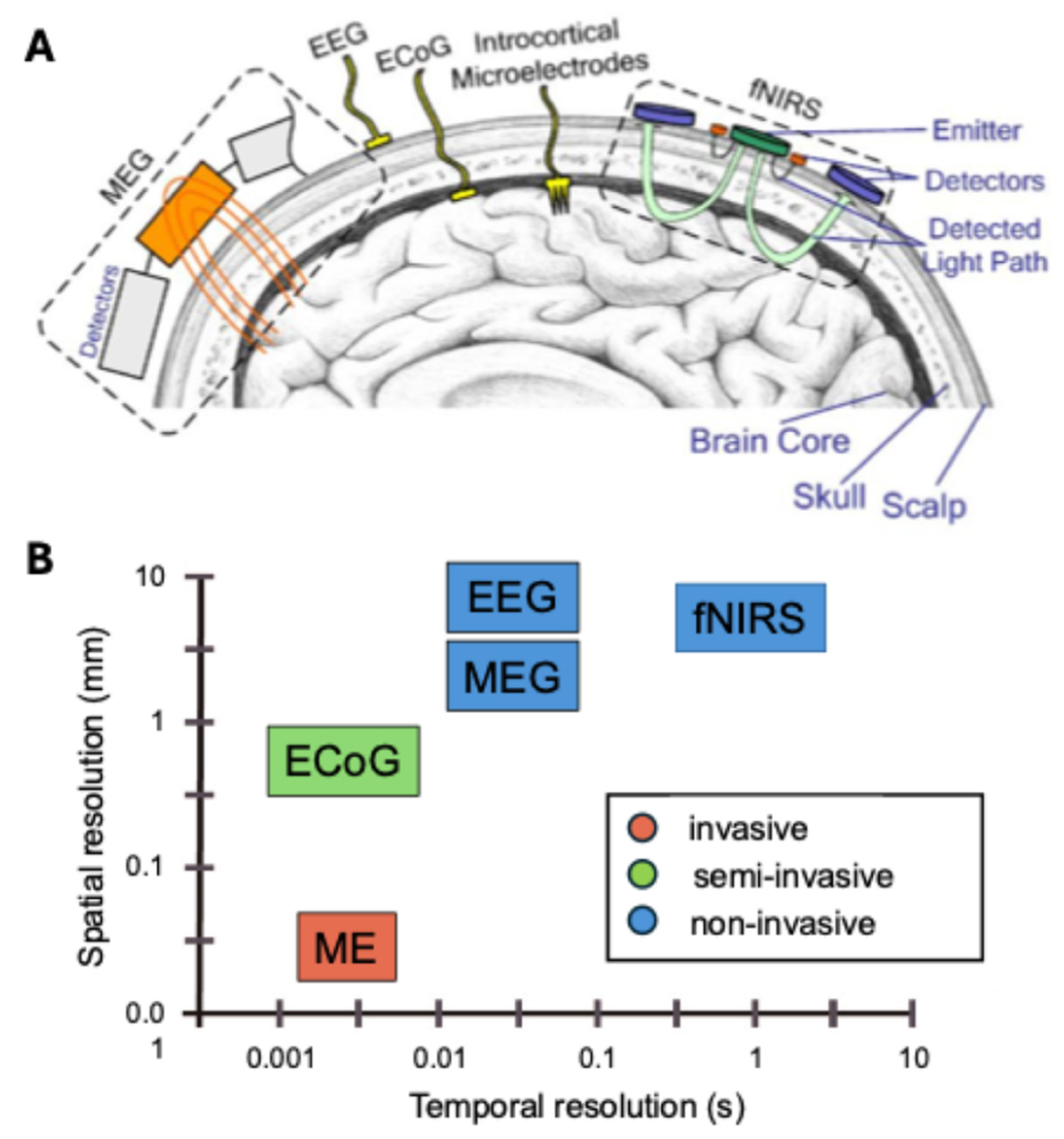
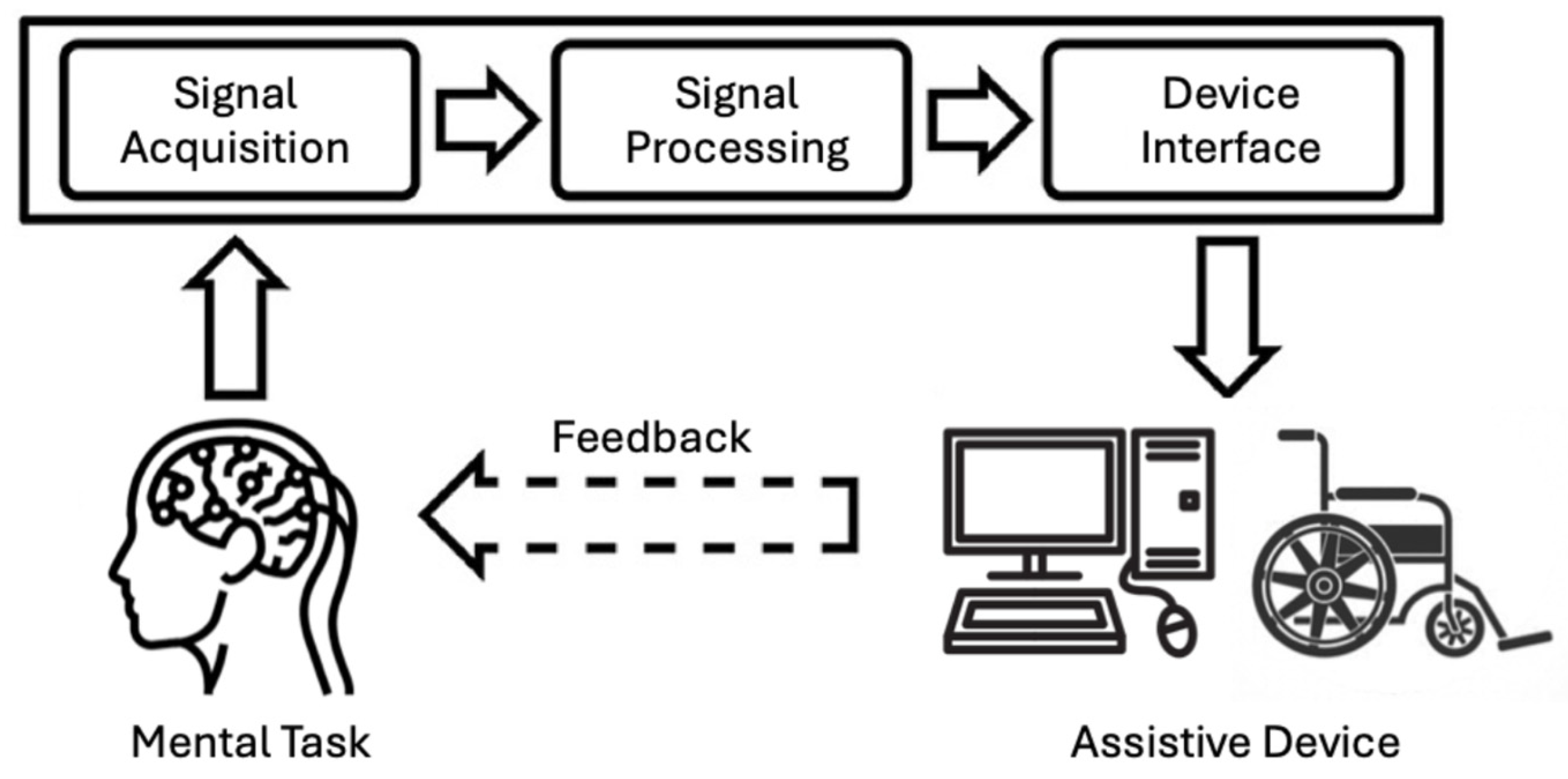
2. Brain–Computer Interface (BCI) Training Protocols
2.1. Motor Imagery-Based BCIs
2.2. Movement-Attempt-Based BCIs
2.3. Sensorimotor-Rhythm-Based BCIs
3. Combining BCIs with External Devices
3.1. Functional Electrical Stimulation (FES)
3.2. Robotic Exoskeletons
3.3. Sensory Feedback Devices
4. Clinical Applicability of BCI Training in Stroke
4.1. Evidence for Short-Term Effects
4.2. Evidence for Long-Term Effects
4.3. Safety and Viability
5. Challenges and Future Directions
5.1. Multimodal Rehabilitation Approaches
5.2. Long-Term Efficacy
5.3. Adaptability and Personalization
5.4. Technological and Logistical Barriers
5.5. Ethical and Regulatory Considerations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ARAT | Action research arm test |
| BCI | Brain–computer interface |
| DESIRED | Detailed standard for reporting of EEG data |
| ECoG | Electrocorticography |
| EEG | Electroencephalography |
| ERD | Event-related desynchronization |
| ERP | Event-related potential |
| ERS | Event-related synchronization |
| FES | Functional electrical stimulation |
| fNIRS | Functional near-infrared spectroscopy |
| FMA-UE | Fugl-Meyer assessment of upper extremity |
| GUI | Graphical user interface |
| MA | Movement attempt |
| ME | Microelectrode |
| MI | Motor imagery |
| MEG | Magnetoencephalography |
| rTMS | Repetitive transcranial magnetic stimulation |
| SMD | Standardized mean difference |
| SMR | Sensorimotor rhythm |
| tDCS | Transcranial direct current stimulation |
| TMS | Transcranial magnetic stimulation |
| VR | Virtual reality |
References
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Brewer, L.; Horgan, F.; Hickey, A.; Williams, D. Stroke Rehabilitation: Recent Advances and Future Therapies. QJM Int. J. Med. 2013, 106, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Grefkes, C.; Fink, G.R. Recovery from Stroke: Current Concepts and Future Perspectives. Neurol. Res. Pract. 2020, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Baniqued, P.D.E.; Stanyer, E.C.; Awais, M.; Alazmani, A.; Jackson, A.E.; Mon-Williams, M.A.; Mushtaq, F.; Holt, R.J. Brain-Computer Interface Robotics for Hand Rehabilitation after Stroke: A Systematic Review. J. Neuroeng. Rehabil. 2021, 18, 15. [Google Scholar] [CrossRef]
- Sebastián-Romagosa, M.; Cho, W.; Ortner, R.; Sieghartsleitner, S.; Von Oertzen, T.J.; Kamada, K.; Laureys, S.; Allison, B.Z.; Guger, C. Brain-Computer Interface Treatment for Gait Rehabilitation in Stroke Patients. Front. Neurosci. 2023, 17, 1256077. [Google Scholar] [CrossRef]
- Sebastián-Romagosa, M.; Cho, W.; Ortner, R.; Murovec, N.; Von Oertzen, T.; Kamada, K.; Allison, B.Z.; Guger, C. Brain Computer Interface Treatment for Motor Rehabilitation of Upper Extremity of Stroke Patients—A Feasibility Study. Front. Neurosci. 2020, 14, 591435. [Google Scholar] [CrossRef]
- Saha, S.; Mamun, K.A.; Ahmed, K.; Mostafa, R.; Naik, G.R.; Darvishi, S.; Khandoker, A.H.; Baumert, M. Progress in Brain Computer Interface: Challenges and Opportunities. Front. Syst. Neurosci. 2021, 15, 578875. [Google Scholar] [CrossRef]
- Alder, G.; Taylor, D.; Rashid, U.; Olsen, S.; Brooks, T.; Terry, G.; Niazi, I.K.; Signal, N. A Brain Computer Interface Neuromodulatory Device for Stroke Rehabilitation: Iterative User-Centered Design Approach. JMIR Rehabil. Assist. Technol. 2023, 10, e49702. [Google Scholar] [CrossRef]
- Kohl, S.H.; Mehler, D.M.A.; Lührs, M.; Thibault, R.T.; Konrad, K.; Sorger, B. The Potential of Functional Near-Infrared Spectroscopy-Based Neurofeedback—A Systematic Review and Recommendations for Best Practice. Front. Neurosci. 2020, 14, 594. [Google Scholar] [CrossRef]
- Lazarou, I.; Nikolopoulos, S.; Petrantonakis, P.C.; Kompatsiaris, I.; Tsolaki, M. EEG-Based Brain-Computer Interfaces for Communication and Rehabilitation of People with Motor Impairment: A Novel Approach of the 21st Century. Front. Hum. Neurosci. 2018, 12, 14. [Google Scholar] [CrossRef]
- Miller, K.J.; Hermes, D.; Staff, N.P. The Current State of Electrocorticography-Based Brain–Computer Interfaces. Neurosurg. Focus. 2020, 49, E2. [Google Scholar] [CrossRef]
- Zhao, Z.-P.; Nie, C.; Jiang, C.-T.; Cao, S.-H.; Tian, K.-X.; Yu, S.; Gu, J.-W. Modulating Brain Activity with Invasive Brain-Computer Interface: A Narrative Review. Brain Sci. 2023, 13, 134. [Google Scholar] [CrossRef]
- Grübler, G.; Al-Khodairy, A.; Leeb, R.; Pisotta, I.; Riccio, A.; Rohm, M.; Hildt, E. Psychosocial and Ethical Aspects in Non-Invasive EEG-Based BCI Research—A Survey Among BCI Users and BCI Professionals. Neuroethics 2014, 7, 29–41. [Google Scholar] [CrossRef]
- Maiseli, B.; Abdalla, A.T.; Massawe, L.V.; Mbise, M.; Mkocha, K.; Nassor, N.A.; Ismail, M.; Michael, J.; Kimambo, S. Brain-Computer Interface: Trend, Challenges, and Threats. Brain Inform. 2023, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Vimal Cruz, M.; Jamal, S.; Sethuraman, S.C. A Comprehensive Survey of Brain-Computer Interface Technology in Healthcare: Research Perspectives. J. Med. Signals Sens. 2025, 15, 16. [Google Scholar] [CrossRef]
- Dong, E.; Zhang, H.; Zhu, L.; Du, S.; Tong, J. A Multi-Modal Brain–Computer Interface Based on Threshold Discrimination and Its Application in Wheelchair Control. Cogn. Neurodyn. 2022, 16, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Wolpaw, J.R.; Birbaumer, N.; McFarland, D.J.; Pfurtscheller, G.; Vaughan, T.M. Brain-Computer Interfaces for Communication and Control. Clin. Neurophysiol. 2002, 113, 767–791. [Google Scholar] [CrossRef]
- Lebedev, M.A.; Nicolelis, M.A.L. Brain-Machine Interfaces: From Basic Science to Neuroprostheses and Neurorehabilitation. Physiol. Rev. 2017, 97, 767–837. [Google Scholar] [CrossRef]
- Farina, D.; Mrachacz-Kersting, N. Brain-Computer Interfaces and Plasticity of the Human Nervous System. J. Physiol. 2021, 599, 2349–2350. [Google Scholar] [CrossRef]
- Khorev, V.; Kurkin, S.; Badarin, A.; Antipov, V.; Pitsik, E.; Andreev, A.; Grubov, V.; Drapkina, O.; Kiselev, A.; Hramov, A. Review on the Use of Brain Computer Interface Rehabilitation Methods for Treating Mental and Neurological Conditions. J. Integr. Neurosci. 2024, 23, 125. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, F.; Jia, F.; Wu, Y.; Wang, B.; Gao, L.; Chu, F.; Tang, W. Efficacy of Brain-Computer Interfaces on Upper Extremity Motor Function Rehabilitation after Stroke: A Systematic Review and Meta-Analysis. NeuroRehabilitation 2024, 54, 199–212. [Google Scholar] [CrossRef]
- Peksa, J.; Mamchur, D. State-of-the-Art on Brain-Computer Interface Technology. Sensors 2023, 23, 6001. [Google Scholar] [CrossRef]
- Bonnet, M.; Decety, J.; Jeannerod, M.; Requin, J. Mental Simulation of an Action Modulates the Excitability of Spinal Reflex Pathways in Man. Brain Res. Cogn. Brain Res. 1997, 5, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.; Halsband, U. Motor Imagery. J. Physiol. Paris. 2006, 99, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Al-Qaysi, Z.T.; Ahmed, M.A.; Hammash, N.M.; Hussein, A.F.; Albahri, A.S.; Suzani, M.S.; Al-Bander, B.; Shuwandy, M.L.; Salih, M.M. Systematic Review of Training Environments with Motor Imagery Brain–Computer Interface: Coherent Taxonomy, Open Issues and Recommendation Pathway Solution. Health Technol. 2021, 11, 783–801. [Google Scholar] [CrossRef]
- Ma, Z.-Z.; Wu, J.-J.; Cao, Z.; Hua, X.-Y.; Zheng, M.-X.; Xing, X.-X.; Ma, J.; Xu, J.-G. Motor Imagery-Based Brain–Computer Interface Rehabilitation Programs Enhance Upper Extremity Performance and Cortical Activation in Stroke Patients. J. Neuroeng. Rehabil. 2024, 21, 91. [Google Scholar] [CrossRef]
- Wen, D.; Fan, Y.; Hsu, S.-H.; Xu, J.; Zhou, Y.; Tao, J.; Lan, X.; Li, F. Combining Brain–Computer Interface and Virtual Reality for Rehabilitation in Neurological Diseases: A Narrative Review. Ann. Phys. Rehabil. Med. 2021, 64, 101404. [Google Scholar] [CrossRef]
- Vidaurre, C.; Blankertz, B. Towards a Cure for BCI Illiteracy. Brain Topogr. 2010, 23, 194–198. [Google Scholar] [CrossRef]
- Lotte, F.; Larrue, F.; Mühl, C. Flaws in Current Human Training Protocols for Spontaneous Brain-Computer Interfaces: Lessons Learned from Instructional Design. Front. Hum. Neurosci. 2013, 7, 568. [Google Scholar] [CrossRef]
- Ang, K.K.; Guan, C.; Phua, K.S.; Wang, C.; Zhou, L.; Tang, K.Y.; Ephraim Joseph, G.J.; Kuah, C.W.K.; Chua, K.S.G. Brain-Computer Interface-Based Robotic End Effector System for Wrist and Hand Rehabilitation: Results of a Three-Armed Randomized Controlled Trial for Chronic Stroke. Front. Neuroeng. 2014, 7, 30. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, W.; Li, W.; Zhang, S.; Lv, P.; Yin, Y. Effects of Motor Imagery Based Brain-Computer Interface on Upper Limb Function and Attention in Stroke Patients with Hemiplegia: A Randomized Controlled Trial. BMC Neurol. 2023, 23, 136. [Google Scholar] [CrossRef]
- Vourvopoulos, A.; Jorge, C.; Abreu, R.; Figueiredo, P.; Fernandes, J.-C.; Bermúdez i Badia, S. Efficacy and Brain Imaging Correlates of an Immersive Motor Imagery BCI-Driven VR System for Upper Limb Motor Rehabilitation: A Clinical Case Report. Front. Hum. Neurosci. 2019, 13, 244. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, J.H.; McDonough, S.M.; Gilmore, D.H.; Wiggam, M.I. The Adjunctive Role of Mental Practice in the Rehabilitation of the Upper Limb after Hemiplegic Stroke: A Pilot Studya. Clin. Rehabil. 2004, 18, 60–68. [Google Scholar] [CrossRef]
- Mane, R.; Chouhan, T.; Guan, C. BCI for Stroke Rehabilitation: Motor and Beyond. J. Neural Eng. 2020, 17, 041001. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, R.; Li, H.; Xu, K.; Shi, Y.; Wang, Q.; Yang, T.; Sun, X. Exploring the Use of Brain-Computer Interfaces in Stroke Neurorehabilitation. Biomed. Res. Int. 2021, 2021, 9967348. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Wu, Q.; Wan, F.; Hu, Y. State-of-the-Art Non-Invasive Brain–Computer Interface for Neural Rehabilitation: A Review. J. Neurorestoratol. 2020, 8, 12–25. [Google Scholar] [CrossRef]
- Fu, J.; Chen, S.; Shu, X.; Lin, Y.; Jiang, Z.; Wei, D.; Gao, J.; Jia, J. Functional-Oriented, Portable Brain–Computer Interface Training for Hand Motor Recovery after Stroke: A Randomized Controlled Study. Front. Neurosci. 2023, 17, 1146146. [Google Scholar] [CrossRef]
- Bai, Z.; Fong, K.N.K.; Zhang, J.J.; Chan, J.; Ting, K.H. Immediate and Long-Term Effects of BCI-Based Rehabilitation of the Upper Extremity after Stroke: A Systematic Review and Meta-Analysis. J. Neuroeng. Rehabil. 2020, 17, 57. [Google Scholar] [CrossRef]
- Marino, M.; Mantini, D. Human Brain Imaging with High-Density Electroencephalography: Techniques and Applications. J. Physiol. 2024. [Google Scholar] [CrossRef]
- Jeunet, C.; Glize, B.; McGonigal, A.; Batail, J.-M.; Micoulaud-Franchi, J.-A. Using EEG-Based Brain Computer Interface and Neurofeedback Targeting Sensorimotor Rhythms to Improve Motor Skills: Theoretical Background, Applications and Prospects. Neurophysiol. Clin. 2019, 49, 125–136. [Google Scholar] [CrossRef]
- Lim, J.; Lin, D.; Sohn, W.J.; McCrimmon, C.M.; Wang, P.T.; Nenadic, Z.; Do, A.H. BCI-Based Neuroprostheses and Physiotherapies for Stroke Motor Rehabilitation. In Neurorehabilitation Technology; Springer: Cham, Switzerland, 2022; pp. 509–524. [Google Scholar] [CrossRef]
- Cervera, M.A.; Soekadar, S.R.; Ushiba, J.; Millán, J.d.R.; Liu, M.; Birbaumer, N.; Garipelli, G. Brain-Computer Interfaces for Post-Stroke Motor Rehabilitation: A Meta-Analysis. Ann. Clin. Transl. Neurol. 2018, 5, 651–663. [Google Scholar] [CrossRef]
- Lennon, O.; Tonellato, M.; Del Felice, A.; Di Marco, R.; Fingleton, C.; Korik, A.; Guanziroli, E.; Molteni, F.; Guger, C.; Otner, R.; et al. A Systematic Review Establishing the Current State-of-the-Art, the Limitations, and the DESIRED Checklist in Studies of Direct Neural Interfacing with Robotic Gait Devices in Stroke Rehabilitation. Front. Neurosci. 2020, 14, 578. [Google Scholar] [CrossRef]
- Marquez-Chin, C.; Popovic, M.R. Functional Electrical Stimulation Therapy for Restoration of Motor Function after Spinal Cord Injury and Stroke: A Review. Biomed. Eng. OnLine 2020, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Le Franc, S.; Herrera Altamira, G.; Guillen, M.; Butet, S.; Fleck, S.; Lécuyer, A.; Bougrain, L.; Bonan, I. Toward an Adapted Neurofeedback for Post-Stroke Motor Rehabilitation: State of the Art and Perspectives. Front. Hum. Neurosci. 2022, 16, 917909. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, S.; House, S.C.; Karlsson, P.; Saab, R.; Chau, T. Brain-Computer Interfaces for Children with Complex Communication Needs and Limited Mobility: A Systematic Review. Front. Hum. Neurosci. 2021, 15, 643294. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Dosen, S.; Muller, K.-R.; Farina, D. Myoelectric Control of Artificial Limbs—Is There a Need to Change Focus? IEEE Signal Process. Mag. 2012, 29, 150–152. [Google Scholar] [CrossRef]
- Ortiz-Catalan, M.; Brånemark, R.; Håkansson, B. BioPatRec: A Modular Research Platform for the Control of Artificial Limbs Based on Pattern Recognition Algorithms. Source Code Biol. Med. 2013, 8, 11. [Google Scholar] [CrossRef]
- Daly, J.J.; Wolpaw, J.R. Brain-Computer Interfaces in Neurological Rehabilitation. Lancet Neurol. 2008, 7, 1032–1043. [Google Scholar] [CrossRef]
- Friedenberg, D.A.; Schwemmer, M.A.; Landgraf, A.J.; Annetta, N.V.; Bockbrader, M.A.; Bouton, C.E.; Zhang, M.; Rezai, A.R.; Mysiw, W.J.; Bresler, H.S.; et al. Neuroprosthetic-Enabled Control of Graded Arm Muscle Contraction in a Paralyzed Human. Sci. Rep. 2017, 7, 8386. [Google Scholar] [CrossRef]
- Mettler, J.A.; Bennett, S.M.; Doucet, B.M.; Magee, D.M. Neuromuscular Electrical Stimulation and Anabolic Signaling in Patients with Stroke. J. Stroke Cerebrovasc. Dis. 2017, 26, 2954–2963. [Google Scholar] [CrossRef]
- Yen, H.-C.; Chen, W.-S.; Jeng, J.-S.; Luh, J.-J.; Lee, Y.-Y.; Pan, G.-S. Standard Early Rehabilitation and Lower Limb Transcutaneous Nerve or Neuromuscular Electrical Stimulation in Acute Stroke Patients: A Randomized Controlled Pilot Study. Clin. Rehabil. 2019, 33, 1344–1354. [Google Scholar] [CrossRef]
- Nozoe, M.; Kanai, M.; Kubo, H.; Takeuchi, Y.; Kobayashi, M.; Yamamoto, M.; Furuichi, A.; Yamazaki, M.; Shimada, S.; Mase, K. Efficacy of Neuromuscular Electrical Stimulation for Preventing Quadriceps Muscle Wasting in Patients with Moderate or Severe Acute Stroke: A Pilot Study. NeuroRehabilitation 2017, 41, 143–149. [Google Scholar] [CrossRef]
- Biasiucci, A.; Leeb, R.; Iturrate, I.; Perdikis, S.; Al-Khodairy, A.; Corbet, T.; Schnider, A.; Schmidlin, T.; Zhang, H.; Bassolino, M.; et al. Brain-Actuated Functional Electrical Stimulation Elicits Lasting Arm Motor Recovery after Stroke. Nat. Commun. 2018, 9, 2421. [Google Scholar] [CrossRef]
- Alashram, A.R.; Padua, E.; Annino, G. Effects of Brain-Computer Interface Controlled Functional Electrical Stimulation on Motor Recovery in Stroke Survivors: A Systematic Review. Curr. Phys. Med. Rehabil. Rep. 2022, 10, 299–310. [Google Scholar] [CrossRef]
- Biswas, P.; Dodakian, L.; Wang, P.T.; Johnson, C.A.; See, J.; Chan, V.; Chou, C.; Lazouras, W.; McKenzie, A.L.; Reinkensmeyer, D.J.; et al. A Single-Center, Assessor-Blinded, Randomized Controlled Clinical Trial to Test the Safety and Efficacy of a Novel Brain-Computer Interface Controlled Functional Electrical Stimulation (BCI-FES) Intervention for Gait Rehabilitation in the Chronic Stroke Population. BMC Neurol. 2024, 24, 200. [Google Scholar] [CrossRef]
- Krueger, J.; Krauth, R.; Reichert, C.; Perdikis, S.; Vogt, S.; Huchtemann, T.; Dürschmid, S.; Sickert, A.; Lamprecht, J.; Huremovic, A.; et al. Hebbian Plasticity Induced by Temporally Coincident BCI Enhances Post-Stroke Motor Recovery. Sci. Rep. 2024, 14, 18700. [Google Scholar] [CrossRef] [PubMed]
- Semprini, M.; Lencioni, T.; Hinterlang, W.; Vassallo, C.; Scarpetta, S.; Maludrottu, S.; Iandolo, R.; Carè, M.; Laffranchi, M.; Chiappalone, M.; et al. User-Centered Design and Development of TWIN-Acta: A Novel Control Suite of the TWIN Lower Limb Exoskeleton for the Rehabilitation of Persons Post-Stroke. Front. Neurosci. 2022, 16, 915707. [Google Scholar] [CrossRef]
- Cheng, N.; Phua, K.S.; Lai, H.S.; Tam, P.K.; Tang, K.Y.; Cheng, K.K.; Yeow, R.C.-H.; Ang, K.K.; Guan, C.; Lim, J.H. Brain-Computer Interface-Based Soft Robotic Glove Rehabilitation for Stroke. IEEE Trans. Biomed. Eng. 2020, 67, 3339–3351. [Google Scholar] [CrossRef]
- Louie, D.R.; Eng, J.J. Powered Robotic Exoskeletons in Post-Stroke Rehabilitation of Gait: A Scoping Review. J. Neuroeng. Rehabil. 2016, 13, 53. [Google Scholar] [CrossRef]
- Colucci, A.; Vermehren, M.; Cavallo, A.; Angerhöfer, C.; Peekhaus, N.; Zollo, L.; Kim, W.-S.; Paik, N.-J.; Soekadar, S.R. Brain-Computer Interface-Controlled Exoskeletons in Clinical Neurorehabilitation: Ready or Not? Neurorehabil. Neural Repair 2022, 36, 747–756. [Google Scholar] [CrossRef]
- Frolov, A.A.; Mokienko, O.; Lyukmanov, R.; Biryukova, E.; Kotov, S.; Turbina, L.; Nadareyshvily, G.; Bushkova, Y. Post-Stroke Rehabilitation Training with a Motor-Imagery-Based Brain-Computer Interface (BCI)-Controlled Hand Exoskeleton: A Randomized Controlled Multicenter Trial. Front. Neurosci. 2017, 11, 400. [Google Scholar] [CrossRef]
- Gaxiola-Tirado, J.A.; Ianez, E.; Ortiz, M.; Gutierrez, D.; Azorin, J.M. Effects of an Exoskeleton-Assisted Gait Motor Imagery Training in Functional Brain Connectivity. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2019, 2019, 429–432. [Google Scholar] [CrossRef]
- Moucheboeuf, G.; Griffier, R.; Gasq, D.; Glize, B.; Bouyer, L.; Dehail, P.; Cassoudesalle, H. Effects of Robotic Gait Training after Stroke: A Meta-Analysis. Ann. Phys. Rehabil. Med. 2020, 63, 518–534. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.V.M.; Furlan, L.; Fregni, F.; Battistella, L.R.; Simis, M. Robotic-Assisted Gait Training (RAGT) in Stroke Rehabilitation: A Pilot Study. Arch. Rehabil. Res. Clin. Transl. 2023, 5, 100255. [Google Scholar] [CrossRef]
- Oh, K.; Park, J.; Jo, S.H.; Hong, S.-J.; Kim, W.-S.; Paik, N.-J.; Park, H.-S. Improved Cortical Activity and Reduced Gait Asymmetry during Poststroke Self-Paced Walking Rehabilitation. J. Neuroeng. Rehabil. 2021, 18, 60. [Google Scholar] [CrossRef] [PubMed]
- Kitai, K.; Odagiri, M.; Yamauchi, R.; Kodama, T. Evaluation of Intervention Effectiveness of Sensory Compensatory Training with Tactile Discrimination Feedback on Sensorimotor Dysfunction of the Hand after Stroke. Brain Sci. 2021, 11, 1314. [Google Scholar] [CrossRef] [PubMed]
- Serrada, I.; Hordacre, B.; Hillier, S.L. Does Sensory Retraining Improve Sensation and Sensorimotor Function Following Stroke: A Systematic Review and Meta-Analysis. Front. Neurosci. 2019, 13, 402. [Google Scholar] [CrossRef]
- Belkacem, A.N.; Jamil, N.; Palmer, J.A.; Ouhbi, S.; Chen, C. Brain Computer Interfaces for Improving the Quality of Life of Older Adults and Elderly Patients. Front. Neurosci. 2020, 14, 692. [Google Scholar] [CrossRef]
- Hughes, C.; Herrera, A.; Gaunt, R.; Collinger, J. Bidirectional Brain-Computer Interfaces. Handb. Clin. Neurol. 2020, 168, 163–181. [Google Scholar] [CrossRef]
- Awuah, W.A.; Ahluwalia, A.; Darko, K.; Sanker, V.; Tan, J.K.; Tenkorang, P.O.; Ben-Jaafar, A.; Ranganathan, S.; Aderinto, N.; Mehta, A.; et al. Bridging Minds and Machines: The Recent Advances of Brain-Computer Interfaces in Neurological and Neurosurgical Applications. World Neurosurg. 2024, 189, 138–153. [Google Scholar] [CrossRef]
- Shu, X.; Chen, S.; Meng, J.; Yao, L.; Sheng, X.; Jia, J.; Farina, D.; Zhu, X. Tactile Stimulation Improves Sensorimotor Rhythm-Based BCI Performance in Stroke Patients. IEEE Trans. Biomed. Eng. 2018, 66, 1987–1995. [Google Scholar] [CrossRef]
- Ziadeh, H.; Gulyas, D.; Nielsen, L.D.; Lehmann, S.; Nielsen, T.B.; Kjeldsen, T.K.K.; Hougaard, B.I.; Jochumsen, M.; Knoche, H. “Mine Works Better”: Examining the Influence of Embodiment in Virtual Reality on the Sense of Agency During a Binary Motor Imagery Task with a Brain-Computer Interface. Front. Psychol. 2021, 12, 806424. [Google Scholar] [CrossRef]
- Ang, K.K.; Chua, K.S.G.; Phua, K.S.; Wang, C.; Chin, Z.Y.; Kuah, C.W.K.; Low, W.; Guan, C. A Randomized Controlled Trial of EEG-Based Motor Imagery Brain-Computer Interface Robotic Rehabilitation for Stroke. Clin. EEG Neurosci. 2015, 46, 310–320. [Google Scholar] [CrossRef]
- Frolov, A.A.; Mokienko, O.A.; Lyukmanov, R.K.h.; Chernikova, L.A.; Kotov, S.V.; Turbina, L.G.; Bobrov, P.D.; Biryukova, E.V.; Kondur, A.A.; Ivanova, G.E.; et al. Preliminary Results of a Controlled Study of BCI–Exoskeleton Technology Efficacy in Patients with Poststroke Arm Paresis. Bull. Russ. State Med. Univ. 2016, 16–23. [Google Scholar] [CrossRef][Green Version]
- Kim, T.; Kim, S.; Lee, B. Effects of Action Observational Training Plus Brain-Computer Interface-Based Functional Electrical Stimulation on Paretic Arm Motor Recovery in Patient with Stroke: A Randomized Controlled Trial. Occup. Ther. Int. 2016, 23, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Y.; Wu, Y.; Liu, S.; Jia, J.; Zhang, L. Neurophysiological Substrates of Stroke Patients with Motor Imagery-Based Brain-Computer Interface Training. Int. J. Neurosci. 2014, 124, 403–415. [Google Scholar] [CrossRef]
- Mihara, M.; Hattori, N.; Hatakenaka, M.; Yagura, H.; Kawano, T.; Hino, T.; Miyai, I. Near-Infrared Spectroscopy-Mediated Neurofeedback Enhances Efficacy of Motor Imagery-Based Training in Poststroke Victims: A Pilot Study. Stroke 2013, 44, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Pichiorri, F.; Morone, G.; Petti, M.; Toppi, J.; Pisotta, I.; Molinari, M.; Paolucci, S.; Inghilleri, M.; Astolfi, L.; Cincotti, F.; et al. Brain-Computer Interface Boosts Motor Imagery Practice during Stroke Recovery. Ann. Neurol. 2015, 77, 851–865. [Google Scholar] [CrossRef]
- Ramos-Murguialday, A.; Broetz, D.; Rea, M.; Läer, L.; Yilmaz, O.; Brasil, F.L.; Liberati, G.; Curado, M.R.; Garcia-Cossio, E.; Vyziotis, A.; et al. Brain-Machine Interface in Chronic Stroke Rehabilitation: A Controlled Study. Ann. Neurol. 2013, 74, 100–108. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The Post-Stroke Hemiplegic Patient. 1. a Method for Evaluation of Physical Performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Volland, G.; Kashman, N.; Weber, K. Adult Norms for the Box and Block Test of Manual Dexterity. Am. J. Occup. Ther. 1985, 39, 386–391. [Google Scholar] [CrossRef]
- Ma, Z.-Z.; Wu, J.-J.; Hua, X.-Y.; Zheng, M.-X.; Xing, X.-X.; Ma, J.; Shan, C.-L.; Xu, J.-G. Evidence of Neuroplasticity with Brain–Computer Interface in a Randomized Trial for Post-Stroke Rehabilitation: A Graph-Theoretic Study of Subnetwork Analysis. Front. Neurol. 2023, 14, 1135446. [Google Scholar] [CrossRef] [PubMed]
- Abbott, L.F.; Nelson, S.B. Synaptic Plasticity: Taming the Beast. Nat. Neurosci. 2000, 3, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, W.; Moxon, K.; Ehrman, S.; Yarborough, M.; Panontin, T.L.; Nathan-Roberts, D. Feedback Modalities in Brain–Computer Interfaces: A Systematic Review. Proc. Human Factors Ergon. Soc. Annu. Meet. 2020, 64, 1186–1190. [Google Scholar] [CrossRef]
- Grevet, E.; Forge, K.; Tadiello, S.; Izac, M.; Amadieu, F.; Brunel, L.; Pillette, L.; Py, J.; Gasq, D.; Jeunet-Kelway, C. Modeling the Acceptability of BCIs for Motor Rehabilitation after Stroke: A Large Scale Study on the General Public. Front. Neuroergonomics 2023, 3, 1082901. [Google Scholar] [CrossRef]
- Ramos-Murguialday, A.; Curado, M.R.; Broetz, D.; Yilmaz, Ö.; Brasil, F.L.; Liberati, G.; Garcia-Cossio, E.; Cho, W.; Caria, A.; Cohen, L.G.; et al. Brain-Machine Interface in Chronic Stroke: Randomized Trial Long-Term Follow-Up. Neurorehabil. Neural Repair 2019, 33, 188–198. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Zhao, D.; Zhong, L.; Wang, Y.; Hao, M.; Ma, J. Efficacy and Safety of Brain–Computer Interface for Stroke Rehabilitation: An Overview of Systematic Review. Front. Hum. Neurosci. 2025, 19, 1525293. [Google Scholar] [CrossRef]
- Carvalho, R.; Dias, N.; Cerqueira, J.J. Brain-machine Interface of Upper Limb Recovery in Stroke Patients Rehabilitation: A Systematic Review. Physiother. Res. Int. 2019, 24, e1764. [Google Scholar] [CrossRef]
- Ramadan, R.A.; Altamimi, A.B. Unraveling the Potential of Brain-Computer Interface Technology in Medical Diagnostics and Rehabilitation: A Comprehensive Literature Review. Health Technol. 2024, 14, 263–276. [Google Scholar] [CrossRef]
- Brukamp, K. Brain-Computer Interfaces for Communication in Severe Acquired Brain Damage: Challenges and Strategies in Clinical Research and Development. In Universal Access in Human-Computer Interaction. Design Approaches and Supporting Technologies; Lecture Notes in Computer Science; Springer: Cham, Switzerland, 2020; pp. 113–123. [Google Scholar] [CrossRef]
- Petric-Gray, N.; Whittet, C.; Liu, T.; Vuckovic, A. The Design of a Customised Portable BCI Headset for Home Based Neurorehabilitation. In Proceedings of the 2019 UK/China Emerging Technologies (UCET), Glasgow, UK, 21–22 August 2019; pp. 1–2. [Google Scholar] [CrossRef]
- Klein, E. Ethics and the Emergence of Brain-Computer Interface Medicine. Handb. Clin. Neurol. 2020, 168, 329–339. [Google Scholar] [CrossRef]
- Kruse, A.; Suica, Z.; Taeymans, J.; Schuster-Amft, C. Effect of Brain-Computer Interface Training Based on Non-Invasive Electroencephalography Using Motor Imagery on Functional Recovery after Stroke—A Systematic Review and Meta-Analysis. BMC Neurol. 2020, 20, 385. [Google Scholar] [CrossRef]
- Semprini, M.; Laffranchi, M.; Sanguineti, V.; Avanzino, L.; De Icco, R.; De Michieli, L.; Chiappalone, M. Technological Approaches for Neurorehabilitation: From Robotic Devices to Brain Stimulation and Beyond. Front. Neurol. 2018, 9, 212. [Google Scholar] [CrossRef]
- Sample, M.; Boehlen, W.; Sattler, S.; Blain-Moraes, S.; Racine, E. Brain-Computer Interfaces, Inclusive Innovation, and the Promise of Restoration: A Mixed-Methods Study with Rehabilitation Professionals. Engag. Sci. Technol. Soc. 2022, 8, 80–104. [Google Scholar] [CrossRef]
- Bolognini, N.; Pascual-Leone, A.; Fregni, F. Using Non-Invasive Brain Stimulation to Augment Motor Training-Induced Plasticity. J. Neuroeng. Rehabil. 2009, 6, 8. [Google Scholar] [CrossRef]
- Zrenner, C.; Ziemann, U. Closed-Loop Brain Stimulation. Biol. Psychiatry 2024, 95, 545–552. [Google Scholar] [CrossRef]
- Lew, B.; Alavi, N.; Randhawa, B.K.; Menon, C. An Exploratory Investigation on the Use of Closed-Loop Electrical Stimulation to Assist Individuals with Stroke to Perform Fine Movements with Their Hemiparetic Arm. Front. Bioeng. Biotechnol. 2016, 4, 20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shima, A.; Miyake, T.; Tanaka, K.; Ogawa, A.; Omae, E.; Nagamori, Y.; Miyata, Y.; Ohata, K.; Maki, T.; Ono, Y.; et al. Case Report: A Novel Approach of Closed-Loop Brain Stimulation Combined with Robot Gait Training in Post-Stroke Gait Disturbance. Front. Hum. Neurosci. 2023, 17, 1082556. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Chen, S.; Chai, G.; Sheng, X.; Jia, J.; Zhu, X. Neural Modulation By Repetitive Transcranial Magnetic Stimulation (rTMS) for BCI Enhancement in Stroke Patients. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2018, 2018, 2272–2275. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.; Teo, W.-P.; Tang, N.; Ang, K.K.; Ng, Y.S.; Zhou, J.H.; Teh, I.; Phua, K.S.; Zhao, L.; Guan, C. Using Transcranial Direct Current Stimulation to Augment the Effect of Motor Imagery-Assisted Brain-Computer Interface Training in Chronic Stroke Patients-Cortical Reorganization Considerations. Front. Neurol. 2020, 11, 948. [Google Scholar] [CrossRef]
- Jiping, Z. Brain Computer Interface System, Performance, Challenges and Applications. J. Comput. Nat. Sci. 2023, 3, 46–57. [Google Scholar] [CrossRef]
- Mang, J.; Xu, Z.; Qi, Y.; Zhang, T. Favoring the Cognitive-Motor Process in the Closed-Loop of BCI Mediated Post Stroke Motor Function Recovery: Challenges and Approaches. Front. Neurorobot 2023, 17, 1271967. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Gao, X.; Gao, S. A Benchmark Dataset for SSVEP-Based Brain-Computer Interfaces. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Roy, Y.; Banville, H.; Albuquerque, I.; Gramfort, A.; Falk, T.H.; Faubert, J. Deep Learning-Based Electroencephalography Analysis: A Systematic Review. J. Neural Eng. 2019, 16, 051001. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, M.; Ramsey, L.; Callejas, A.; Baldassarre, A.; Hacker, C.D.; Siegel, J.S.; Astafiev, S.V.; Rengachary, J.; Zinn, K.; Lang, C.E.; et al. Common Behavioral Clusters and Subcortical Anatomy in Stroke. Neuron 2015, 85, 927–941. [Google Scholar] [CrossRef]
- Santos, E.M.d.; Fernandes, C.A.; Castellano, G. Performance of Stroke Patients Using a Brain-Computer Interface during Motor Imagery: A Systematic Review. Res. Biomed. Eng. 2023, 39, 451–465. [Google Scholar] [CrossRef]
- Fernandes, J.B.; Fernandes, S.; Domingos, J.; Castro, C.; Romão, A.; Graúdo, S.; Rosa, G.; Franco, T.; Ferreira, A.P.; Chambino, C.; et al. Motivational Strategies Used by Health Care Professionals in Stroke Survivors in Rehabilitation: A Scoping Review of Experimental Studies. Front. Med. 2024, 11, 1384414. [Google Scholar] [CrossRef]
- Verrienti, G.; Raccagni, C.; Lombardozzi, G.; De Bartolo, D.; Iosa, M. Motivation as a Measurable Outcome in Stroke Rehabilitation: A Systematic Review of the Literature. Int. J. Environ. Res. Public Health 2023, 20, 4187. [Google Scholar] [CrossRef]
- Hossain, K.M.; Islam, M.A.; Hossain, S.; Nijholt, A.; Ahad, M.A.R. Status of Deep Learning for EEG-Based Brain-Computer Interface Applications. Front. Comput. Neurosci. 2022, 16, 1006763. [Google Scholar] [CrossRef]
- Zhang, J. Clinical Applications of Machine Learning in Stroke Care. In New Frontiers in Medicine and Medical Research; BP International: London, UK, 2021; Volume 8. [Google Scholar] [CrossRef]
- Uyanik, C.; Khan, M.A.; Brunner, I.; Hansen, J.P.; Puthusserypady, S. Machine Learning for Motor Imagery Wrist Dorsiflexion Prediction in Brain-Computer Interface Assisted Stroke Rehabilitation. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, Scotland, UK, 1–15 July 2022; pp. 715–719. [Google Scholar] [CrossRef]
- Mastakouri, A.-A.; Weichwald, S.; Özdenizci, O.; Meyer, T.; Scholkopf, B.; Grosse-Wentrup, M. Personalized Brain-Computer Interface Models for Motor Rehabilitation. In Proceedings of the 2017 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Banff, AB, Canada, 5–8 October 2017; pp. 3024–3029. [Google Scholar] [CrossRef]
- Karikari, E.; Koshechkin, K.A. Review on Brain-Computer Interface Technologies in Healthcare. Biophys. Rev. 2023, 15, 1351–1358. [Google Scholar] [CrossRef]
- Pichiorri, F.; Mattia, D. Brain-Computer Interfaces in Neurologic Rehabilitation Practice. Handb. Clin. Neurol. 2020, 168, 101–116. [Google Scholar] [CrossRef]
- Burwell, S.; Sample, M.; Racine, E. Ethical Aspects of Brain Computer Interfaces: A Scoping Review. BMC Med. Ethics 2017, 18, 60. [Google Scholar] [CrossRef]
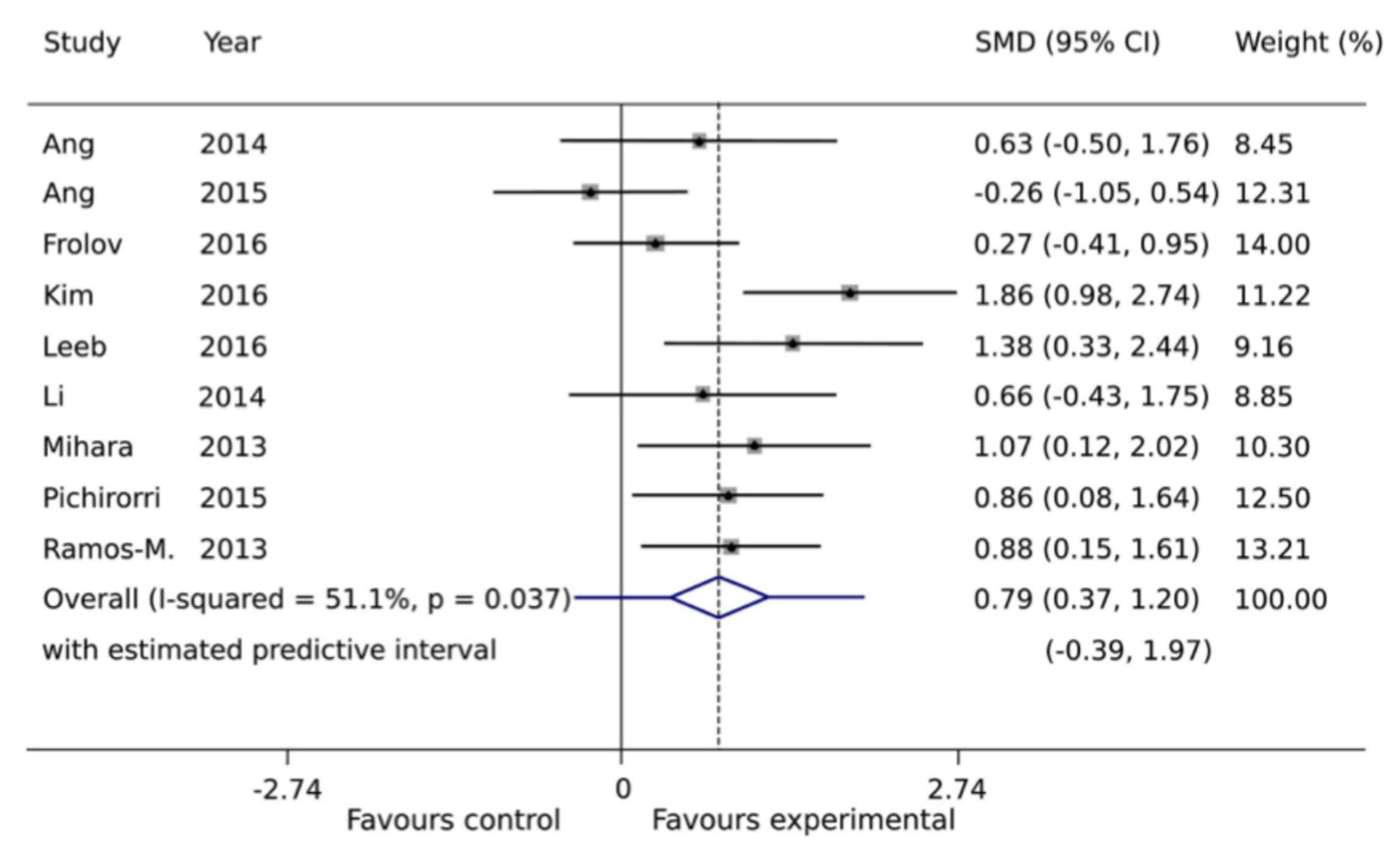
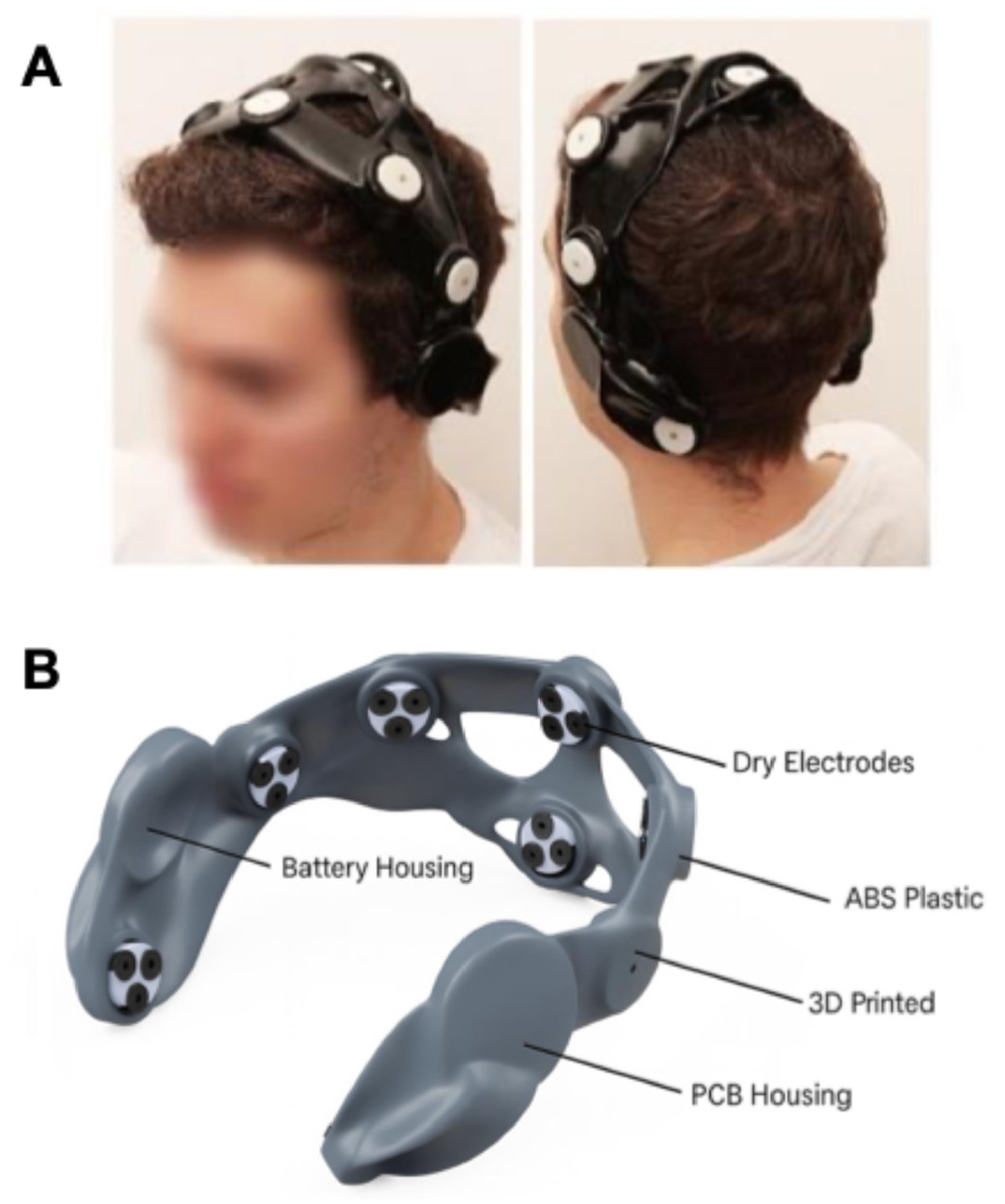
| Study (Author, Year) | Study Design | Intervention Type | Sample Size/Population | Duration/Follow-Up | Outcome Measures | Main Findings |
|---|---|---|---|---|---|---|
| Ramos-Murguialday et al., 2019 [87] | Controlled study | Motor imagery BCI with feedback | Chronic stroke patients (n ≈ 16) | 12-month follow-up | Fugl-Meyer assessment (FMA), grip force | Some patients retained motor gains at 12 months, while others showed a partial decline, highlighting the heterogeneity of long-term effects. |
| Biasiucci et al., 2018 [54] | Controlled trial | BCI-triggered functional electrical stimulation (BCI-FES) | Subacute stroke patients (n ≈ 27) | 6-month follow-up | FMA-UE | Significant motor gains sustained at 6 months, demonstrating durable neuroplastic changes. |
| Ang et al., 2015 [74] | Three-arm RCT | BCI with robotic assistance | Chronic stroke patients (n ≈ 27) | 3-month follow-up | FMA-UE, ARAT | Significant sustained improvements at 3 months post-training; combining BCI and robotics enhances recovery. |
| Zhang et al., 2024 [21] | Meta-analysis of 25 RCTs | BCI-based training | Post-stroke patients | Variable (up to 6 months) | FMA, other motor scales | BCI shows slight overall efficacy; gains may plateau without maintenance; short, intensive regimens are more effective. |
| Liu et al., 2025 [88] | Systematic review of reviews | BCI interventions | Multiple studies reviewed | Variable | Motor function scales (FMA, ARAT) | Confirms the fact that BCI improves motor recovery; calls for more multicenter, long-term trials for stronger evidence. |
| Carvalho et al., 2019 [89] | Systematic review | BCI-based training | Nine high-quality RCTs | Variable (some with follow-up) | FMA-UE | Supports BCI efficacy with neurophysiological evidence of plasticity; variability suggests a need for standardization. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonin, A.; Semprini, M.; Kiper, P.; Mantini, D. Brain-Computer Interfaces for Stroke Motor Rehabilitation. Bioengineering 2025, 12, 820. https://doi.org/10.3390/bioengineering12080820
Tonin A, Semprini M, Kiper P, Mantini D. Brain-Computer Interfaces for Stroke Motor Rehabilitation. Bioengineering. 2025; 12(8):820. https://doi.org/10.3390/bioengineering12080820
Chicago/Turabian StyleTonin, Alessandro, Marianna Semprini, Pawel Kiper, and Dante Mantini. 2025. "Brain-Computer Interfaces for Stroke Motor Rehabilitation" Bioengineering 12, no. 8: 820. https://doi.org/10.3390/bioengineering12080820
APA StyleTonin, A., Semprini, M., Kiper, P., & Mantini, D. (2025). Brain-Computer Interfaces for Stroke Motor Rehabilitation. Bioengineering, 12(8), 820. https://doi.org/10.3390/bioengineering12080820








