Guiding Microbial Crossroads: Syngas-Driven Valorisation of Anaerobic-Digestion Intermediates into Bio-Hydrogen and Volatile Fatty Acids
Abstract
1. Introduction
2. Intermediate Products of Anaerobic Digestion: Volatile Fatty Acids and Hydrogen
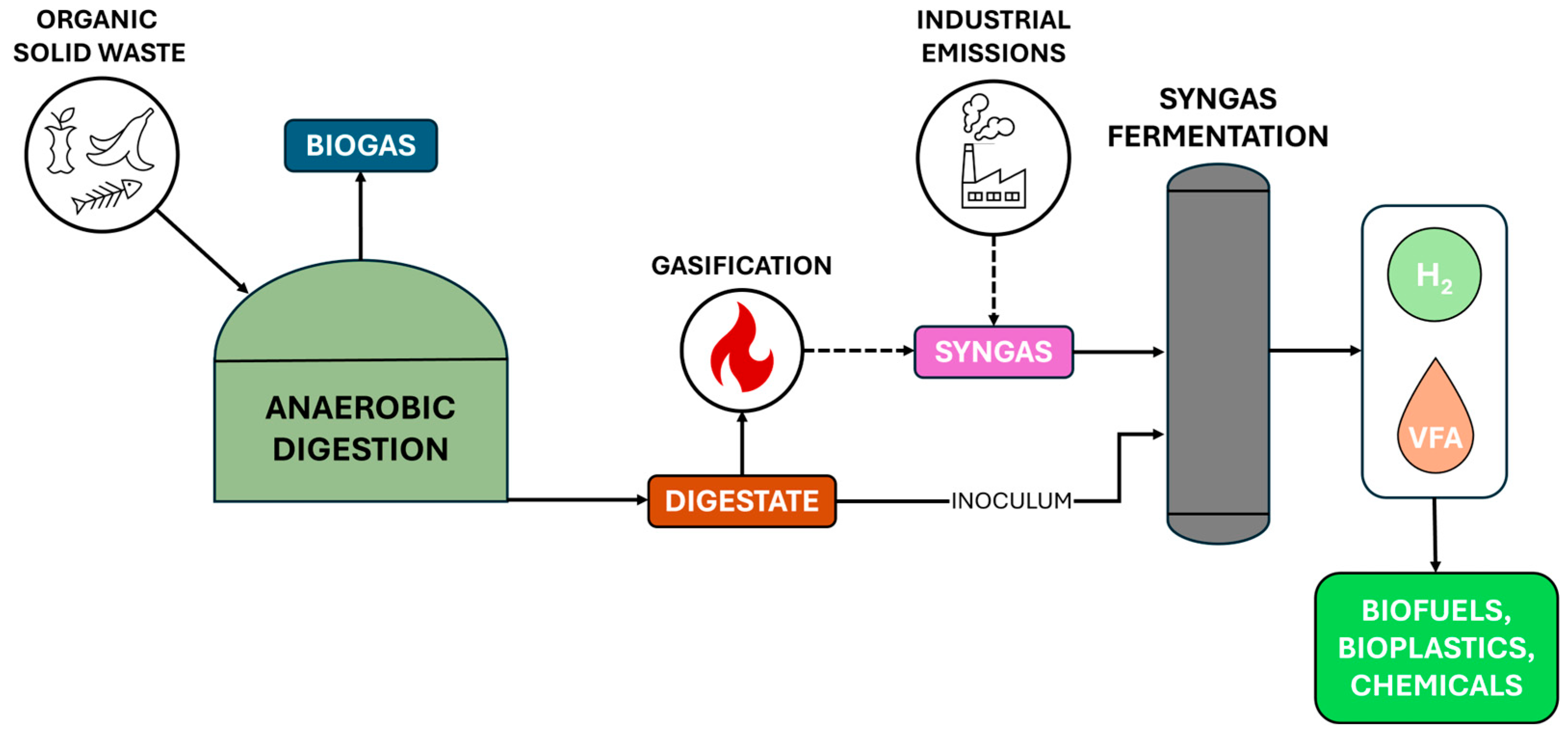
3. Syngas Fermentation: A Gateway for Intermediate Products Valorisation
4. Microbial “Decision-Making”
4.1. Overview of the Metabolic Pathways for Hydrogen and VFA Production
4.1.1. Biological Water–Gas Shift Reaction
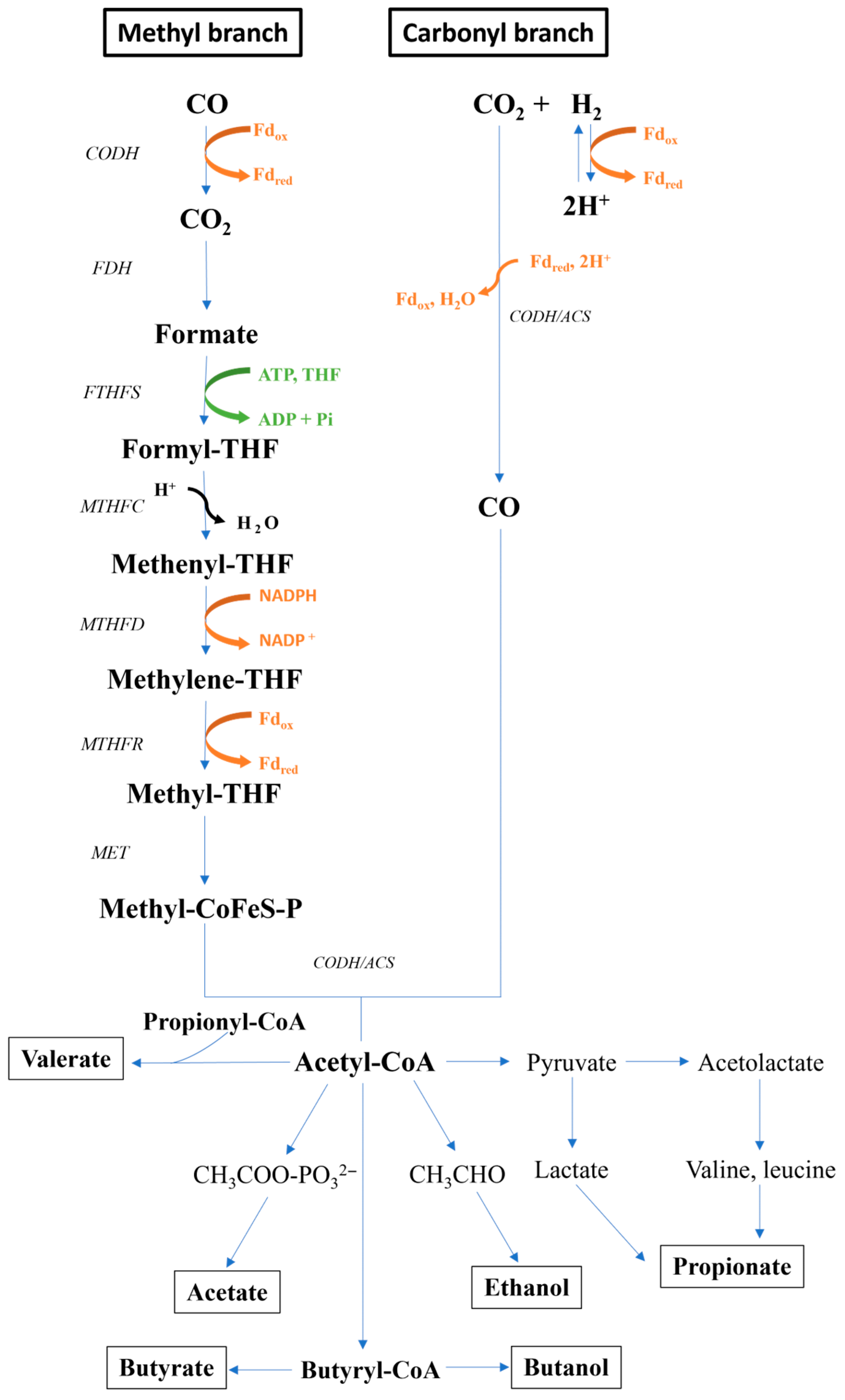
4.1.2. Wood–Ljungdahl Pathway
4.2. Thermodynamics
4.3. Kinetics
4.4. Environmental Factors and Their Impact
4.4.1. Gas Composition
4.4.2. pH and Temperature
4.4.3. Culture Media Components
4.4.4. Hydraulic Retention Time, Gas Flow Rate, and Cell Density
5. Current Technologies and Challenges for Implementation
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wainaina, S.; Awasthi, M.K.; Sarsaiya, S.; Chen, H.; Singh, E.; Kumar, A.; Ravindran, B.; Awasthi, S.K.; Liu, T.; Duan, Y.; et al. Resource recovery and circular economy from organic solid waste using aerobic and anaerobic digestion technologies. Bioresour. Technol. 2020, 301, 122778. [Google Scholar] [CrossRef]
- Muthu, P.; Muniappan, G.; Jeyakumar, R.B. Efficacious Utilization of Food Waste for Bioenergy Generation through the Anaerobic Digestion Method. Processes 2023, 11, 702. [Google Scholar] [CrossRef]
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Varjani, S.; Khanal, S.K.; Wong, J.; Awasthi, M.K.; et al. Microbiological insights into anaerobic digestion for biogas, hydrogen or volatile fatty acids (VFAs): A review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef] [PubMed]
- Millati, R.; Wikandari, R.; Ariyanto, T.; Hasniah, N.; Taherzadeh, M.J. Anaerobic digestion biorefinery for circular bioeconomy development. Bioresour. Technol. Rep. 2023, 21, 101315. [Google Scholar] [CrossRef]
- Wang, W.; Chang, J.S.; Lee, D.J. Anaerobic digestate valorization beyond agricultural application: Current status and prospects. Bioresour. Technol. 2023, 373, 128742. [Google Scholar] [CrossRef]
- Barz, M.; Wesenfeld, H.; Laß-Seyoum, A.; Meibohm, A.; Knist, S. Increasing Flexibility of Biogas Plants Through the Application of Innovative Concepts. In Bio#Futures; Springer: Berlin/Heidelberg, Germany, 2021; pp. 435–459. [Google Scholar]
- Shinde, R.; Hackula, A.; Marycz, M.; Bose, A.; O’Shea, R.; Barth, S.; Murphy, J.D.; Wall, D.M. Dynamic anaerobic digestion-based biorefineries for on-demand renewable energy and bioproducts in a circular bioeconomy. Trends Biotechnol. 2025, 43, 1140–1165. [Google Scholar] [CrossRef]
- Andreides, D.; Fliegerova, K.O.; Pokorna, D.; Zabranska, J. Biological conversion of carbon monoxide and hydrogen by anaerobic culture: Prospect of anaerobic digestion and thermochemical processes combination. Biotechnol. Adv. 2022, 58, 107886. [Google Scholar] [CrossRef]
- Neto, A.S.; Wainaina, S.; Chandolias, K.; Piatek, P.; Taherzadeh, M.J. Exploring the Potential of Syngas Fermentation for Recovery of High-Value Resources: A Comprehensive Review. Curr. Pollut. Rep. 2024, 11, 7. [Google Scholar] [CrossRef]
- Grimalt-Alemany, A.; Skiadas, I.V.; Gavala, H.N. Syngas biomethanation: State-of-the-art review and perspectives. Biofuels, Bioprod. Biorefining 2017, 12, 139–158. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Peng, Y.; Huang, W.; Liu, J.; Mironov, V.; Zhang, S. Deeper insights into the effects of substrate to inoculum ratio selection on the relationship of kinetic parameters, microbial communities, and key metabolic pathways during the anaerobic digestion of food waste. Water Res. 2022, 217, 118440. [Google Scholar] [CrossRef]
- Baleeiro, F.C.F.; Kleinsteuber, S.; Neumann, A.; Strauber, H. Syngas-aided anaerobic fermentation for medium-chain carboxylate and alcohol production: The case for microbial communities. Appl. Microbiol. Biotechnol. 2019, 103, 8689–8709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, C.; Yuan, Z.; Wang, R.; Angelidaki, I.; Zhu, G. Syntrophy mechanism, microbial population, and process optimization for volatile fatty acids metabolism in anaerobic digestion. Chem. Eng. J. 2023, 452, 139137. [Google Scholar] [CrossRef]
- Lohani, S.P.; Havukainen, J. Anaerobic Digestion: Factors Affecting Anaerobic Digestion Process. In Waste Bioremediation; Energy, Environment, and Sustainability; Varjani, S.J., Gnansounou, E., Baskar, G., Pant, D., Zakaria, Z.A., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2018; pp. 343–359. [Google Scholar]
- Pan, X.; Zhao, L.; Li, C.; Angelidaki, I.; Lv, N.; Ning, J.; Cai, G.; Zhu, G. Deep insights into the network of acetate metabolism in anaerobic digestion: Focusing on syntrophic acetate oxidation and homoacetogenesis. Water Res. 2021, 190, 116774. [Google Scholar] [CrossRef] [PubMed]
- Nwokolo, N.; Mukumba, P.; Obileke, K.; Enebe, M. Waste to Energy: A Focus on the Impact of Substrate Type in Biogas Production. Processes 2020, 8, 1224. [Google Scholar] [CrossRef]
- Vázquez-Fernández, A.; Suárez-Ojeda, M.E.; Carrera, J. Review about bioproduction of Volatile Fatty Acids from wastes and wastewaters: Influence of operating conditions and organic composition of the substrate. J. Environ. Chem. Eng. 2022, 10, 107917. [Google Scholar] [CrossRef]
- Wainaina, S.; Awasthi, M.K.; Horváth, I.S.; Taherzadeh, M.J. Anaerobic digestion of food waste to volatile fatty acids and hydrogen at high organic loading rates in immersed membrane bioreactors. Renew. Energy 2020, 152, 1140–1148. [Google Scholar] [CrossRef]
- Lukitawesa; Patinvoh, R.J.; Millati, R.; Sarvari-Horvath, I.; Taherzadeh, M.J. Factors influencing volatile fatty acids production from food wastes via anaerobic digestion. Bioengineered 2020, 11, 39–52. [Google Scholar] [CrossRef]
- Khatami, K.; Atasoy, M.; Ludtke, M.; Baresel, C.; Eyice, O.; Cetecioglu, Z. Bioconversion of food waste to volatile fatty acids: Impact of microbial community, pH and retention time. Chemosphere 2021, 275, 129981. [Google Scholar] [CrossRef]
- Robazza, A.; Raya, I.G.A.; Baleeiro, F.C.F.; Kleinsteuber, S.; Neumann, A. Acetate Shock Loads Enhance CO Uptake Rates of Anaerobic Microbiomes. Microb. Biotechnol. 2024, 17, e70063. [Google Scholar] [CrossRef]
- Quintela, C.; Alexe, I.G.; Nygard, Y.; Olsson, L.; Skiadas, I.V.; Gavala, H.N. Influence of Hydrogen and Ethanol Addition in Methanogen-Free Mixed Culture Syngas Fermentations in Trickle Bed Reactors. Molecules 2024, 29, 5653. [Google Scholar] [CrossRef]
- Esquivel-Elizondo, S.; Delgado, A.G.; Krajmalnik-Brown, R. Evolution of microbial communities growing with carbon monoxide, hydrogen, and carbon dioxide. FEMS Microbiol. Ecol. 2017, 93, fix076. [Google Scholar] [CrossRef]
- Guiot, S.R.; Cimpoia, R.; Carayon, G. Potential of wastewater-treating anaerobic granules for biomethanation of synthesis gas. Environ. Sci. Technol. 2011, 45, 2006–2012. [Google Scholar] [CrossRef]
- Alves, J.I.; Stams, A.J.; Plugge, C.M.; Alves, M.M.; Sousa, D.Z. Enrichment of anaerobic syngas-converting bacteria from thermophilic bioreactor sludge. FEMS Microbiol. Ecol. 2013, 86, 590–597. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, J.; Han, S.; Zhang, S.; Luo, G. Selective conversion of carbon monoxide to hydrogen by anaerobic mixed culture. Bioresour. Technol. 2016, 202, 1–7. [Google Scholar] [CrossRef]
- Neto, A.S.; Wainaina, S.; Chandolias, K.; Piatek, P.; Taherzadeh, M.J. Syngas fermentation for hydrogen and volatile fatty acids production: Effect of inoculum source, pretreatment, and environmental parameters using natural microbial consortia. Bioresour. Technol. Rep. 2025, 30, 102109. [Google Scholar] [CrossRef]
- Jojoa-Unigarro, G.D.; González-Martínez, S. Kinetic analysis of the methanization of the byproducts from OFMSW fermentation. J. Chem. Technol. Biotechnol. 2021, 97, 1555–1566. [Google Scholar] [CrossRef]
- Sinharoy, A.; Pakshirajan, K. Methane free biohydrogen production from carbon monoxide using a continuously operated moving bed biofilm reactor. Int. J. Hydrogen Energy 2021, 46, 306–313. [Google Scholar] [CrossRef]
- Techtmann, S.M.; Colman, A.S.; Robb, F.T. ‘That which does not kill us only makes us stronger’: The role of carbon monoxide in thermophilic microbial consortia. Environ. Microbiol. 2009, 11, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Robb, F.T.; Techtmann, S.M. Life on the fringe: Microbial adaptation to growth on carbon monoxide. F1000Res 2018, 7, 1981. [Google Scholar] [CrossRef]
- Omae, K.; Fukuyama, Y.; Yasuda, H.; Mise, K.; Yoshida, T.; Sako, Y. Diversity and distribution of thermophilic hydrogenogenic carboxydotrophs revealed by microbial community analysis in sediments from multiple hydrothermal environments in Japan. Arch. Microbiol. 2019, 201, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Techtmann, S.M.; Lebedinsky, A.V.; Colman, A.S.; Sokolova, T.G.; Woyke, T.; Goodwin, L.; Robb, F.T. Evidence for horizontal gene transfer of anaerobic carbon monoxide dehydrogenases. Front. Microbiol. 2012, 3, 132. [Google Scholar] [CrossRef]
- Diender, M.; Stams, A.J.; Sousa, D.Z. Pathways and Bioenergetics of Anaerobic Carbon Monoxide Fermentation. Front. Microbiol. 2015, 6, 1275. [Google Scholar] [CrossRef]
- Henstra, A.M.; Sipma, J.; Rinzema, A.; Stams, A.J. Microbiology of synthesis gas fermentation for biofuel production. Curr. Opin. Biotechnol. 2007, 18, 200–206. [Google Scholar] [CrossRef]
- Bengelsdorf, F.R.; Straub, M.; Durre, P. Bacterial synthesis gas (syngas) fermentation. Environ. Technol. 2013, 34, 1639–1651. [Google Scholar] [CrossRef]
- Sahoo, J.; Patil, P.; Verma, A.; Lodh, A.; Khanna, N.; Prasad, R.; Pandit, S.; Fosso-Kankeu, E. Biochemical Aspects of Syngas Fermentation. In Recent Developments in Microbial Technologies; Environmental and Microbial Biotechnology; Springer: Singapore, 2021; pp. 395–424. [Google Scholar]
- Ciliberti, C.; Biundo, A.; Albergo, R.; Agrimi, G.; Braccio, G.; de Bari, I.; Pisano, I. Syngas Derived from Lignocellulosic Biomass Gasification as an Alternative Resource for Innovative Bioprocesses. Processes 2020, 8, 1567. [Google Scholar] [CrossRef]
- Latif, H.; Zeidan, A.A.; Nielsen, A.T.; Zengler, K. Trash to treasure: Production of biofuels and commodity chemicals via syngas fermenting microorganisms. Curr. Opin. Biotechnol. 2014, 27, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, P.A.; Münck, E.; Ragsdale, S.W. CO dehydrogenase from Clostridium thermoaceticum. EPR and electrochemical studies in CO2 and argon atmospheres. J. Biol. Chem. 1990, 265, 3873–3879. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta 2008, 1784, 1873–1898. [Google Scholar] [CrossRef]
- Dong, W.; Yang, Y.; Liu, C.; Zhang, J.; Pan, J.; Luo, L.; Wu, G.; Awasthi, M.K.; Yan, B. Caproic acid production from anaerobic fermentation of organic waste—Pathways and microbial perspective. Renew. Sustain. Energy Rev. 2023, 175, 113181. [Google Scholar] [CrossRef]
- He, Y.; Kennes, C.; Lens, P.N.L. Enhanced solventogenesis in syngas bioconversion: Role of process parameters and thermodynamics. Chemosphere 2022, 299, 134425. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, R.; McCubbin, T.; Navone, L.; Stowers, C.; Nielsen, L.; Marcellin, E. Microbial Propionic Acid Production. Fermentation 2017, 3, 21. [Google Scholar] [CrossRef]
- Kim, H.; Jeon, B.S.; Sang, B.I. An Efficient New Process for the Selective Production of Odd-Chain Carboxylic Acids by Simple Carbon Elongation Using Megasphaera hexanoica. Sci. Rep. 2019, 9, 11999. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Bethke, C.M. The thermodynamics and kinetics of microbial metabolism. Am. J. Sci. 2007, 307, 643–677. [Google Scholar] [CrossRef]
- Buckel, W.; Thauer, R.K. Energy conservation via electron bifurcating ferredoxin reduction and proton/Na+ translocating ferredoxin oxidation. Biochim. Biophys. Acta 2013, 1827, 94–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-N.; Sun, G.-X.; Zhu, Y.-G. Thermodynamic energy of anaerobic microbial redox reactions couples elemental biogeochemical cycles. J. Soils Sediments 2017, 17, 2831–2846. [Google Scholar] [CrossRef]
- Schoelmerich, M.C.; Muller, V. Energy-converting hydrogenases: The link between H2 metabolism and energy conservation. Cell Mol. Life Sci. 2020, 77, 1461–1481. [Google Scholar] [CrossRef]
- Henstra, A.M.; Stams, A.J. Deep Conversion of Carbon Monoxide to Hydrogen and Formation of Acetate by the Anaerobic Thermophile Carboxydothermus hydrogenoformans. Int. J. Microbiol. 2011, 2011, 641582. [Google Scholar] [CrossRef]
- Junicke, H.; van Loosdrecht, M.C.; Kleerebezem, R. Kinetic and thermodynamic control of butyrate conversion in non-defined methanogenic communities. Appl. Microbiol. Biotechnol. 2016, 100, 915–925. [Google Scholar] [CrossRef]
- Hu, P.; Bowen, S.H.; Lewis, R.S. A thermodynamic analysis of electron production during syngas fermentation. Bioresour. Technol. 2011, 102, 8071–8076. [Google Scholar] [CrossRef]
- Phillips, J.; Huhnke, R.; Atiyeh, H. Syngas Fermentation: A Microbial Conversion Process of Gaseous Substrates to Various Products. Fermentation 2017, 3, 28. [Google Scholar] [CrossRef]
- Allaart, M.T.; Korkontzelos, C.; Sousa, D.Z.; Kleerebezem, R. A novel experimental method to determine substrate uptake kinetics of gaseous substrates applied to the carbon monoxide-fermenting Clostridium autoethanogenum. Biotechnol. Bioeng. 2024, 121, 1325–1335. [Google Scholar] [CrossRef]
- Mayer, A.; Schadler, T.; Trunz, S.; Stelzer, T.; Weuster-Botz, D. Carbon monoxide conversion with Clostridium aceticum. Biotechnol. Bioeng. 2018, 115, 2740–2750. [Google Scholar] [CrossRef]
- Moreira, J.P.C.; Domingues, L.; Alves, J.I. Metabolic Versatility of acetogens in syngas Fermentation: Responding to varying CO availability. Bioresour. Technol. 2025, 417, 131823. [Google Scholar] [CrossRef]
- Zhao, Y.; Cimpoia, R.; Liu, Z.; Guiot, S.R. Kinetics of CO conversion into H2 by Carboxydothermus hydrogenoformans. Appl. Microbiol. Biotechnol. 2011, 91, 1677–1684. [Google Scholar] [CrossRef]
- Grimalt-Alemany, A.; Asimakopoulos, K.; Skiadas, I.V.; Gavala, H.N. Modeling of syngas biomethanation and catabolic route control in mesophilic and thermophilic mixed microbial consortia. Appl. Energy 2020, 262, 114502. [Google Scholar] [CrossRef]
- Ako, O.Y.; Kitamura, Y.; Intabon, K.; Satake, T. Steady state characteristics of acclimated hydrogenotrophic methanogens on inorganic substrate in continuous chemostat reactors. Bioresour. Technol. 2008, 99, 6305–6310. [Google Scholar] [CrossRef]
- Pan, X.; Angelidaki, I.; Alvarado-Morales, M.; Liu, H.; Liu, Y.; Huang, X.; Zhu, G. Methane production from formate, acetate and H2/CO2; focusing on kinetics and microbial characterization. Bioresour. Technol. 2016, 218, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Grimalt-Alemany, A.; Lezyk, M.; Lange, L.; Skiadas, I.V.; Gavala, H.N. Enrichment of syngas-converting mixed microbial consortia for ethanol production and thermodynamics-based design of enrichment strategies. Biotechnol. Biofuels 2018, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Lanzillo, F.; Ruggiero, G.; Raganati, F.; Russo, M.E.; Marzocchella, A. Batch Syngas Fermentation by Clostridium carboxidivorans for Production of Acids and Alcohols. Processes 2020, 8, 1075. [Google Scholar] [CrossRef]
- Ruggiero, G.; Lanzillo, F.; Raganati, F.; Russo, M.E.; Salatino, P.; Marzocchella, A. Bioreactor modelling for syngas fermentation: Kinetic characterization. Food Bioprod. Process. 2022, 134, 1–18. [Google Scholar] [CrossRef]
- Ragsdale, S.W. Life with carbon monoxide. Crit. Rev. Biochem. Mol. Biol. 2004, 39, 165–195. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.A.; Fantom, N.; Martin-Pozas, T.; Aguila, P.; King, G.M.; Hernandez, M. Carbon monoxide-oxidising Pseudomonadota on volcanic deposits. Environ. Microbiome 2025, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Elizondo, S.; Maldonado, J.; Krajmalnik-Brown, R. Anaerobic carbon monoxide metabolism by Pleomorphomonas carboxyditropha sp. nov., a new mesophilic hydrogenogenic carboxydotroph. FEMS Microbiol. Ecol. 2018, 94, fiy056. [Google Scholar] [CrossRef] [PubMed]
- Majstorović, A.; Babić, V.; Todić, M. Carbon monoxide in the process of uncontrolled combustion—Occurrence, hazards and first aid. J. Phys. Conf. Ser. 2020, 1426, 012008. [Google Scholar] [CrossRef]
- Sun, X.; Atiyeh, H.K.; Huhnke, R.L.; Tanner, R.S. Syngas fermentation process development for production of biofuels and chemicals: A review. Bioresour. Technol. Rep. 2019, 7, 100279. [Google Scholar] [CrossRef]
- Lubitz, W.; Ogata, H.; Rudiger, O.; Reijerse, E. Hydrogenases. Chem. Rev. 2014, 114, 4081–4148. [Google Scholar] [CrossRef]
- Liebgott, P.P.; Leroux, F.; Burlat, B.; Dementin, S.; Baffert, C.; Lautier, T.; Fourmond, V.; Ceccaldi, P.; Cavazza, C.; Meynial-Salles, I.; et al. Relating diffusion along the substrate tunnel and oxygen sensitivity in hydrogenase. Nat. Chem. Biol. 2010, 6, 63–70. [Google Scholar] [CrossRef]
- Fourmond, V.; Baffert, C.; Sybirna, K.; Dementin, S.; Abou-Hamdan, A.; Meynial-Salles, I.; Soucaille, P.; Bottin, H.; Leger, C. The mechanism of inhibition by H2 of H2-evolution by hydrogenases. Chem. Commun 2013, 49, 6840–6842. [Google Scholar] [CrossRef]
- Goldet, G.; Brandmayr, C.; Stripp, S.T.; Happe, T.; Cavazza, C.; Fontecilla-Camps, J.C.; Armstrong, F.A. Electrochemical kinetic investigations of the reactions of [FeFe]-hydrogenases with carbon monoxide and oxygen: Comparing the importance of gas tunnels and active-site electronic/redox effects. J. Am. Chem. Soc. 2009, 131, 14979–14989. [Google Scholar] [CrossRef]
- Garcia-Casado, S.; Munoz, R.; Lebrero, R. Enrichment of a mixed syngas-converting culture for volatile fatty acids and methane production. Bioresour. Technol. 2024, 400, 130646. [Google Scholar] [CrossRef]
- Esquivel-Elizondo, S.; Delgado, A.G.; Rittmann, B.E.; Krajmalnik-Brown, R. The effects of CO2 and H2 on CO metabolism by pure and mixed microbial cultures. Biotechnol. Biofuels 2017, 10, 220. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Krikeli, M.; Gavala, H.N.; Skiadas, I.V. Modeling and simulation of biological CO conversion in trickle bed reactor and comparison with CSTR. Biochem. Eng. J. 2025, 222, 109819. [Google Scholar] [CrossRef]
- Silverstein, T.P. The Proton in Biochemistry: Impacts on Bioenergetics, Biophysical Chemistry, and Bioorganic Chemistry. Front. Mol. Biosci. 2021, 8, 764099. [Google Scholar] [CrossRef] [PubMed]
- Harahap, B.M.; Ahring, B.K. Acetate Production from Syngas Produced from Lignocellulosic Biomass Materials along with Gaseous Fermentation of the Syngas: A Review. Microorganisms 2023, 11, 995. [Google Scholar] [CrossRef]
- Mohammadi, M.; Younesi, H.; Najafpour, G.; Mohamed, A.R. Sustainable ethanol fermentation from synthesis gas by Clostridium ljungdahlii in a continuous stirred tank bioreactor. J. Chem. Technol. Biotechnol. 2012, 87, 837–843. [Google Scholar] [CrossRef]
- Katakojwala, R.; Tharak, A.; Sarkar, O.; Venkata Mohan, S. Design and evaluation of gas fermentation systems for CO2 reduction to C2 and C4 fatty acids: Non-genetic metabolic regulation with pressure, pH and reaction time. Bioresour. Technol. 2022, 351, 126937. [Google Scholar] [CrossRef]
- Park, G.W.; Moon, M.; Park, J.H.; Jo, J.H.; Kim, H.J.; Lee, J.Y.; Lee, H.S.; Lee, J.P.; Lee, S.; Lee, S.Y.; et al. Improving hydrogen production by pH adjustment in pressurized gas fermentation. Bioresour. Technol. 2022, 346, 126605. [Google Scholar] [CrossRef]
- Andreides, D.; Lopez Marin, M.A.; Zabranska, J. Selective syngas fermentation to acetate under acidic and psychrophilic conditions using mixed anaerobic culture. Bioresour. Technol. 2024, 394, 130235. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Li, H.; Shi, B.; Xu, Y.; Liu, J.; Zhang, D.; Tang, J.; Hou, P. Effect of temperature on fermentative VFAs production from waste sludge stimulated by riboflavin and the shifts of microbial community. Water Sci. Technol. 2022, 85, 1191–1201. [Google Scholar] [CrossRef]
- Liu, C.; Luo, G.; Wang, W.; He, Y.; Zhang, R.; Liu, G. The effects of pH and temperature on the acetate production and microbial community compositions by syngas fermentation. Fuel 2018, 224, 537–544. [Google Scholar] [CrossRef]
- Xu, S.; Fu, B.; Zhang, L.; Liu, H. Bioconversion of H2/CO2 by acetogen enriched cultures for acetate and ethanol production: The impact of pH. World J. Microbiol. Biotechnol. 2015, 31, 941–950. [Google Scholar] [CrossRef]
- Nam, C.W.; Jung, K.A.; Park, J.M. Biological carbon monoxide conversion to acetate production by mixed culture. Bioresour. Technol. 2016, 211, 478–485. [Google Scholar] [CrossRef]
- Kim, M.S.; Moon, M.; Fitriana, H.N.; Lee, J.S.; Na, J.G.; Park, G.W. Pressurized cultivation strategies for improved microbial hydrogen production by Thermococcus onnurineus NA1. Bioprocess Biosyst. Eng. 2020, 43, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Brown, R.; Wen, Z. Syngas fermentation of Clostridium carboxidivoran P7 in a hollow fiber membrane biofilm reactor: Evaluating the mass transfer coefficient and ethanol production performance. Biochem. Eng. J. 2014, 85, 21–29. [Google Scholar] [CrossRef]
- Dutta, S.; Gavala, H.N.; Skiadas, I.V. Scaling up Trickle Bed Reactor for Gas Fermentation Technology: The Effect of Temperature and Reactor Characteristics on Mass Transfer. Fermentation 2024, 10, 623. [Google Scholar] [CrossRef]
- Saxena, J.; Tanner, R.S. Optimization of a corn steep medium for production of ethanol from synthesis gas fermentation by Clostridium ragsdalei. World J. Microbiol. Biotechnol. 2012, 28, 1553–1561. [Google Scholar] [CrossRef]
- García-Depraect, O.; Mena-Navarro, V.; Muñoz, R.; Rene, E.R.; León-Becerril, E. Effect of nitrogen and iron supplementation on the process performance and microbial community structure of a hydrogen-producing reactor continuously fed with tequila vinasse. Fuel 2023, 334, 126736. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; O-Thong, S.; Yang, Z.; Zhang, S.; Liu, G.; Luo, G. Microbial insights of enhanced anaerobic conversion of syngas into volatile fatty acids by co-fermentation with carbohydrate-rich synthetic wastewater. Biotechnol. Biofuels 2020, 13, 53. [Google Scholar] [CrossRef]
- Sun, X.; Atiyeh, H.K.; Kumar, A.; Zhang, H. Enhanced ethanol production by Clostridium ragsdalei from syngas by incorporating biochar in the fermentation medium. Bioresour. Technol. 2018, 247, 291–301. [Google Scholar] [CrossRef]
- Phillips, J.R.; Atiyeh, H.K.; Tanner, R.S.; Torres, J.R.; Saxena, J.; Wilkins, M.R.; Huhnke, R.L. Butanol and hexanol production in Clostridium carboxidivorans syngas fermentation: Medium development and culture techniques. Bioresour. Technol. 2015, 190, 114–121. [Google Scholar] [CrossRef]
- Saxena, J.; Tanner, R.S. Effect of trace metals on ethanol production from synthesis gas by the ethanologenic acetogen, Clostridium ragsdalei. J. Ind. Microbiol. Biotechnol. 2011, 38, 513–521. [Google Scholar] [CrossRef]
- Modjewski, L.D.; Karavaeva, V.; Mrnjavac, N.; Knopp, M.; Martin, W.F.; Sousa, F.L. Evidence for corrin biosynthesis in the last universal common ancestor. FEBS J. 2025, 292, 827–850. [Google Scholar] [CrossRef]
- Han, Y.F.; Xie, B.T.; Wu, G.X.; Guo, Y.Q.; Li, D.M.; Huang, Z.Y. Combination of Trace Metal to Improve Solventogenesis of Clostridium carboxidivorans P7 in Syngas Fermentation. Front. Microbiol. 2020, 11, 577266. [Google Scholar] [CrossRef] [PubMed]
- Chandolias, K.; Wainaina, S.; Niklasson, C.; Taherzadeh, M.J. Effects of Heavy Metals and pH on the Conversion of Biomass to Hydrogen via Syngas Fermentation. BioResources 2018, 13, 4455–4469. [Google Scholar] [CrossRef]
- Karadag, D.; Puhakka, J.A. Enhancement of anaerobic hydrogen production by iron and nickel. Int. J. Hydrogen Energy 2010, 35, 8554–8560. [Google Scholar] [CrossRef]
- Bayar, B.; Veiga, M.C.; Kennes, C. Bioproduction of acetic acid from carbon dioxide as single substrate and zero valent iron (ZVI) by clostridia. J. CO2 Util. 2022, 58, 101915. [Google Scholar] [CrossRef]
- Dahiya, S.; Lakshminarayanan, S.; Mohan, S.V. Steering acidogenesis towards selective propionic acid production using co-factors and evaluating environmental sustainability. Chem. Eng. J. 2020, 379, 122135. [Google Scholar] [CrossRef]
- Thi, H.N.; Park, S.; Li, H.; Kim, Y.-K. Medium Compositions for the Improvement of Productivity in Syngas Fermentation with Clostridium autoethanogenum. Biotechnol. Bioprocess Eng. 2020, 25, 493–501. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Yu, S.J.; Zhang, F.; Xia, X.Y.; Zeng, R.J. Enhancement of acetate productivity in a thermophilic (55 °C) hollow-fiber membrane biofilm reactor with mixed culture syngas (H2/CO2) fermentation. Appl. Microbiol. Biotechnol. 2017, 101, 2619–2627. [Google Scholar] [CrossRef]
- Martins, I.; Surra, E.; Ventura, M.; Lapa, N. BioH2 from Dark Fermentation of OFMSW: Effect of the Hydraulic Retention Time and Organic Loading Rate. Appl. Sci. 2022, 12, 4240. [Google Scholar] [CrossRef]
- Asimakopoulos, K.; Kaufmann-Elfang, M.; Lundholm-Høffner, C.; Rasmussen, N.B.K.; Grimalt-Alemany, A.; Gavala, H.N.; Skiadas, I.V. Scale up study of a thermophilic trickle bed reactor performing syngas biomethanation. Appl. Energy 2021, 290, 116771. [Google Scholar] [CrossRef]
- Shen, Y.; Brown, R.; Wen, Z. Enhancing mass transfer and ethanol production in syngas fermentation of Clostridium carboxidivorans P7 through a monolithic biofilm reactor. Appl. Energy 2014, 136, 68–76. [Google Scholar] [CrossRef]
- Perret, L.; Boukis, N.; Sauer, J. Influence of Increased Cell Densities on Product Ratio and Productivity in Syngas Fermentation. Ind. Eng. Chem. Res. 2023, 62, 13799–13810. [Google Scholar] [CrossRef]
- SynoProtein. Syngas Becomes a Raw Material for Fish Feed. Available online: https://synoprotein.eu/syngas-becomes-a-raw-material-for-fish-feed/ (accessed on 26 June 2025).
- CO2SMOS. CO2SMOS Project: Our Routes for a Better Future. Available online: https://co2smos.eu/co2smos-project/#processo_cosmos (accessed on 26 June 2025).
- Puiman, L.; Bokelmann, C.; Simpson, S.D.; Spormann, A.M.; Takors, R. Dos and don’ts for scaling up gas fermentations. Curr. Opin. Biotechnol. 2025, 93, 103294. [Google Scholar] [CrossRef]
- Wright, A. ArcelorMittal Scraps €1.3bn Low-Carbon Steel Plans; Considers Closing Steelanol Plant. Available online: https://www.gasworld.com/story/arcelormittal-scraps-e1-3bn-low-carbon-steel-plans-considers-closing-steelanol-plant/2160610.article/ (accessed on 26 June 2025).


| Strain | Maximum CO Tolerance (mbar) | Reference |
|---|---|---|
| C. aceticum | 5.4 | [55] |
| C. autoethanogenum | ~ 600 | [54] |
| C. hydrogenoformans | ~ 1000 | [57] |
| C. carboxidivorans | ~ 1115 | [62] |
| Inoculum | pH | Temperature (°C) | Products | Reference |
|---|---|---|---|---|
| Enriched anaerobic sludge | 8.5 | 28 | VFA | [79] |
| Thermococcus onnurineus | 6.5 | 80 | H2 | [80] |
| Anaerobic sludge | 4.5 | 20 | Acetate | [81] |
| Waste-activated sludge | 7.5 | 35 | VFA | [82] |
| Sewage sludge | 9.0 | 20/37 | Acetate | [83] |
| Cow manure | 7.0 | 37 | Acetate | [84] |
| Domestic wastewater sludge | 7.18 | 37 | Acetate | [85] |
| Clostridium carboxidivorans | - | 35 | Ethanol and VFA | [62] |
| Thermococcus onnurineus NA1 | 6.5 | 80 | H2 | [86] |
| Clostridium carboxidivorans P7 | 6.0 | - | Ethanol and acetic acid | [87] |
| Anaerobic sludge | 6.0/9.0 | 65 | H2 and VFA | [27] |
| Trace metal | Impact on | Reference |
|---|---|---|
| Ni2+ | HYD, cell growth | [69,94] |
| Fe2+ | HYD | [69] |
| Co2+ | CoFeS-P, ACS | [95] |
| Mo | Cell growth, FDH | [96] |
| Cu | MTFS | [97] |
| Zn | HYD, MTFS, FDH | [97] |
| Mn | HYD, MTFS | [97] |
| Inoculum | HRT (day) | Gas Flow (vvm) | Cell Density | Main End-Product | Reference |
|---|---|---|---|---|---|
| Anaerobic sludge | 2.5 | - | - | VFA | [102] |
| Anaerobic sludge | 5 | - | 18 g VSs/ L/ d | H2 | [103] |
| Thermophilic mixed microbial consortium | 8 | ~0.05 | - | CH4 | [104] |
| C. carboxidivorans strain P7 | ~2 | ~0.04 | - | Ethanol | [105] |
| Anaerobic granular sludge | - | - | 20–30 g VSSs/ L | H2 | [27] |
| C. ljungdahlii | ~1.4 | ~0.04 | 3.15 g/ L | Ethanol | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neto, A.d.S.; Taherzadeh, M.J. Guiding Microbial Crossroads: Syngas-Driven Valorisation of Anaerobic-Digestion Intermediates into Bio-Hydrogen and Volatile Fatty Acids. Bioengineering 2025, 12, 816. https://doi.org/10.3390/bioengineering12080816
Neto AdS, Taherzadeh MJ. Guiding Microbial Crossroads: Syngas-Driven Valorisation of Anaerobic-Digestion Intermediates into Bio-Hydrogen and Volatile Fatty Acids. Bioengineering. 2025; 12(8):816. https://doi.org/10.3390/bioengineering12080816
Chicago/Turabian StyleNeto, Alvaro dos Santos, and Mohammad J. Taherzadeh. 2025. "Guiding Microbial Crossroads: Syngas-Driven Valorisation of Anaerobic-Digestion Intermediates into Bio-Hydrogen and Volatile Fatty Acids" Bioengineering 12, no. 8: 816. https://doi.org/10.3390/bioengineering12080816
APA StyleNeto, A. d. S., & Taherzadeh, M. J. (2025). Guiding Microbial Crossroads: Syngas-Driven Valorisation of Anaerobic-Digestion Intermediates into Bio-Hydrogen and Volatile Fatty Acids. Bioengineering, 12(8), 816. https://doi.org/10.3390/bioengineering12080816








