Fermentative Synthesis of Gluconic and Xylonic Acids from Hydrolyzed Palm Fronds Using Gluconobacter oxydans
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and OPF Hydrolysate Preparation
2.2. Strain and Media
2.3. Gluconic Acid and Xylonic Acid Fermentation
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results and Discussions
3.1. Effect of Inoculum and Speed Agitation on Gluconobacter oxydans Growth
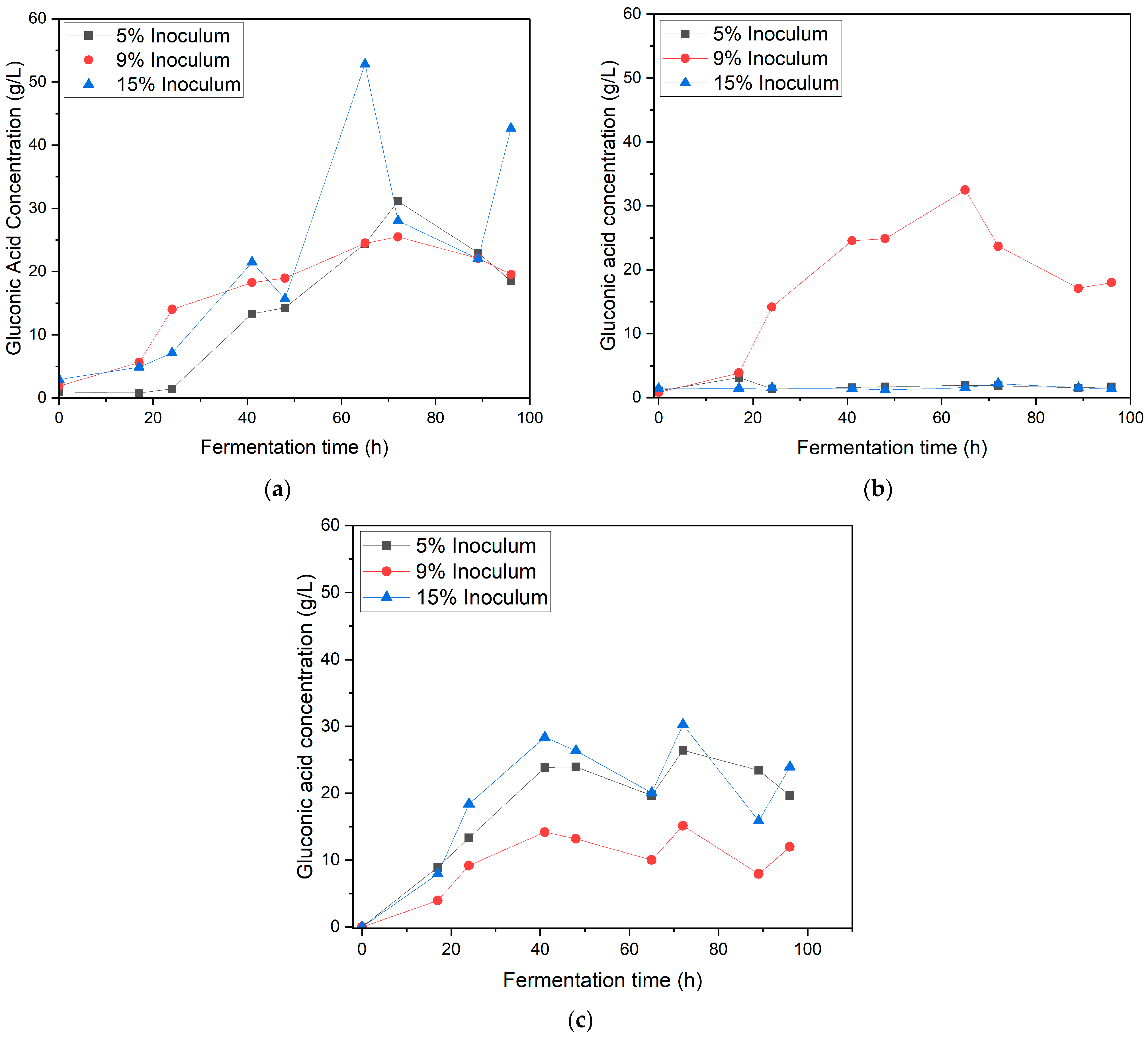
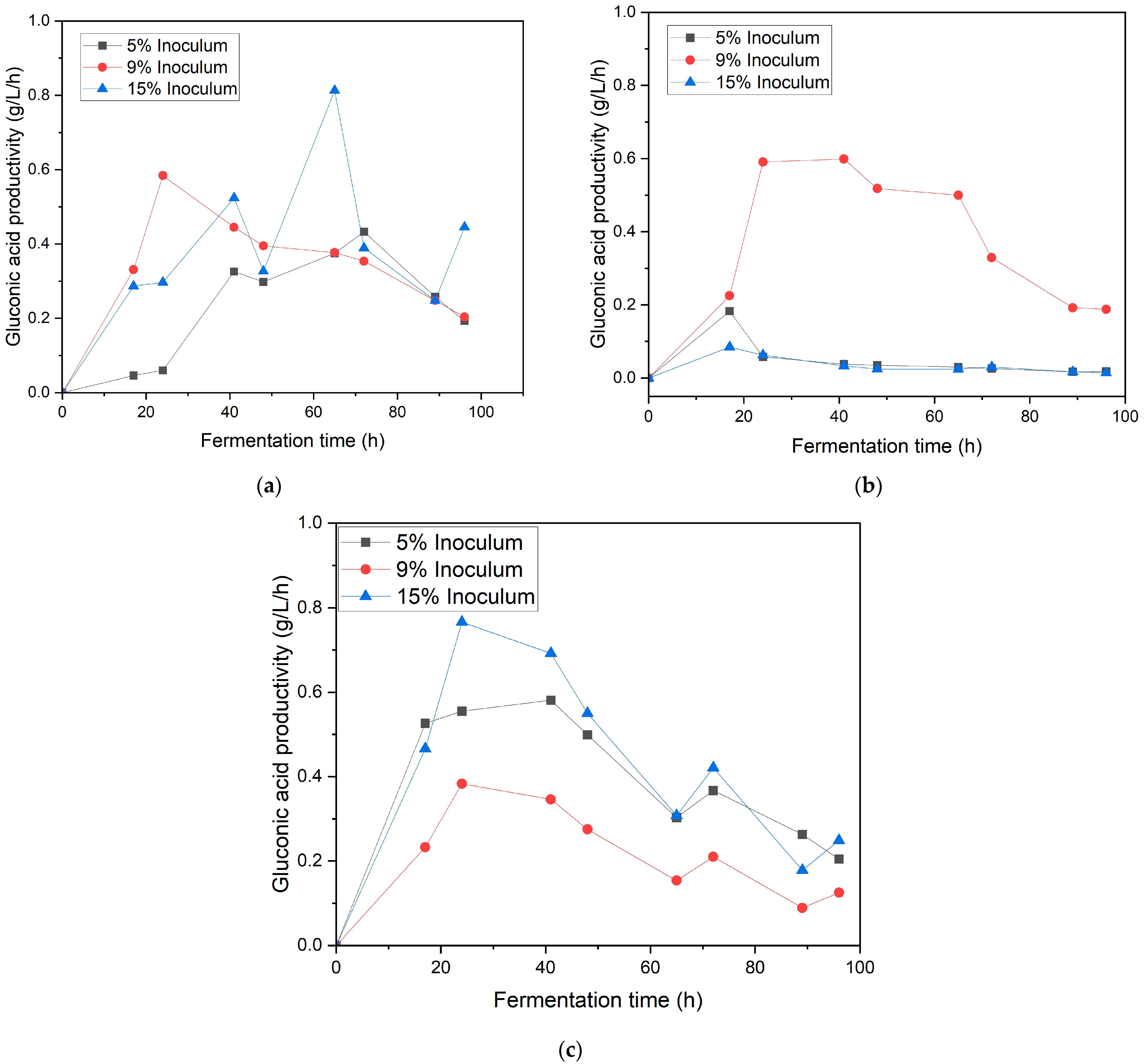
3.2. Effect of Inoculum Ratio and Speed Agitation on Gluconic Acid Production in Synthetic Medium
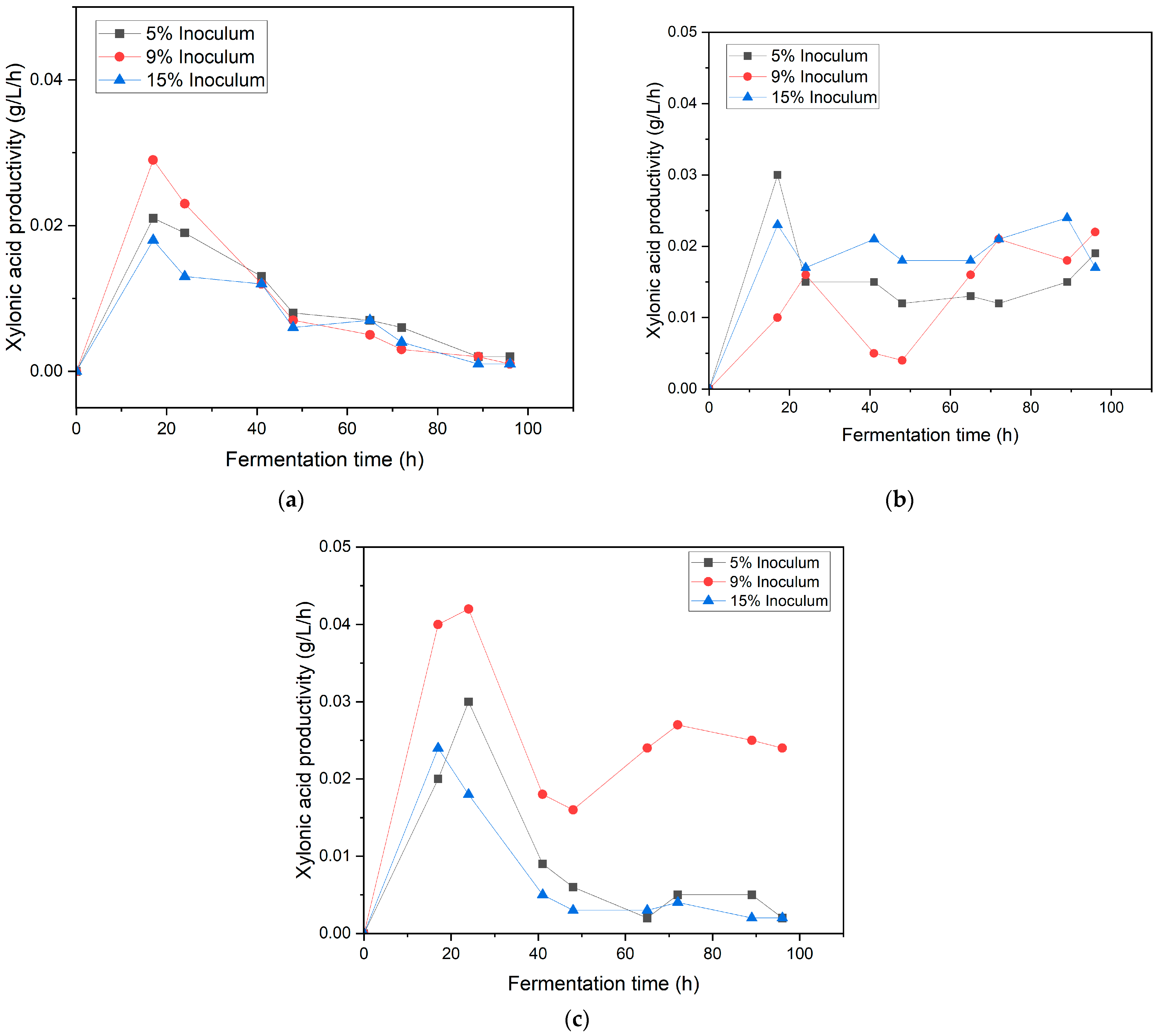
3.3. Effect of Inoculum Ratio and Speed Agitation on Xylonic Acid Production in Synthetic Medium
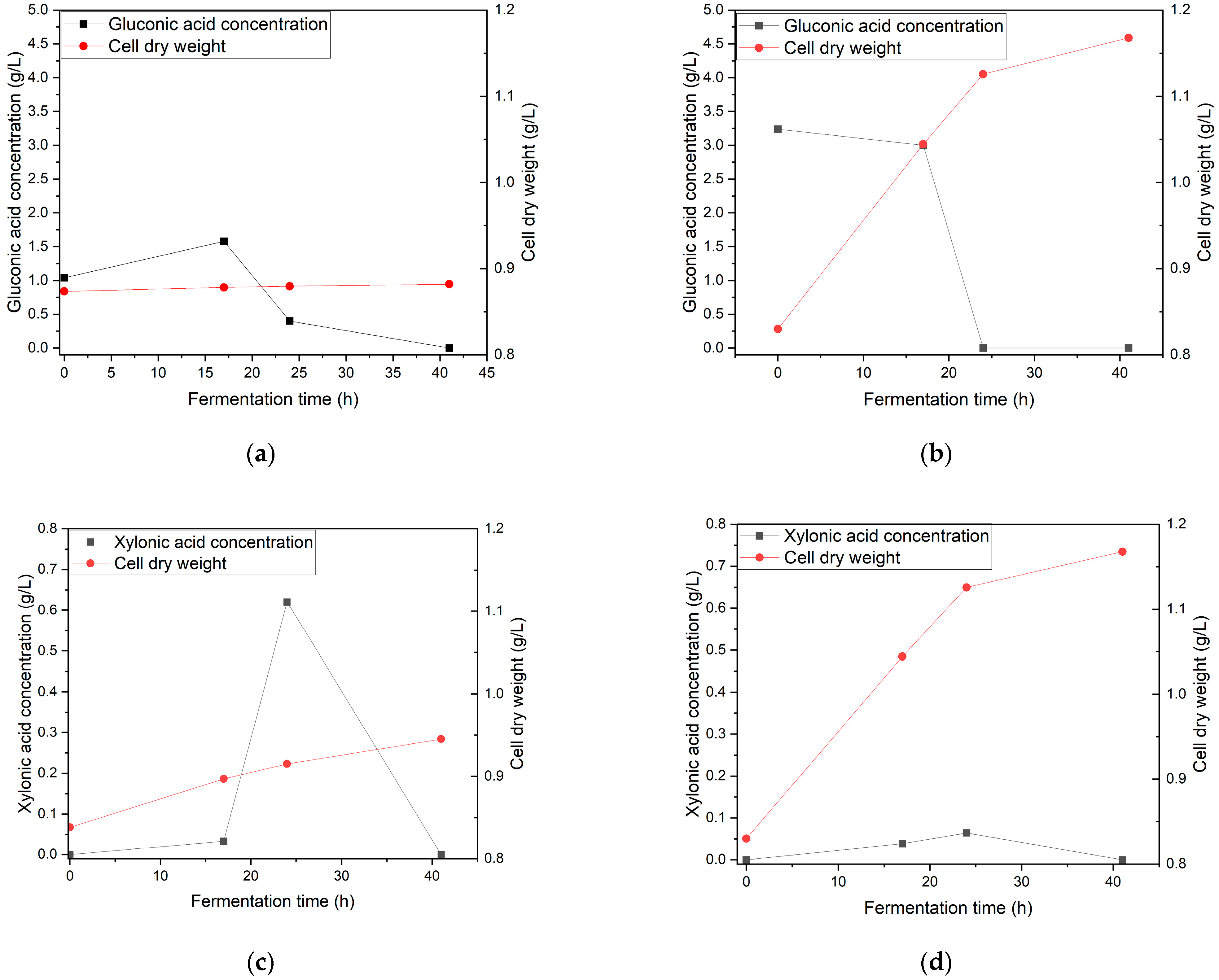
3.4. Effect of Inoculum and Speed Agitation on the Growth of Gluconobacter oxydans and the Production of Gluconic Acid and Xylonic Acid in Oil Palm Fronds Hydrolysate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okarda, B.; Purnomo, H.; Juniyanti, L.; Kusumadewi, S.D.; Nadhira, S. Indonesian Palm Oil towards Sustainability: A System Dynamic Approach. IOP Conf. Ser. Earth Environ. Sci. 2024, 1379, 012037. [Google Scholar] [CrossRef]
- Hong, L.S.; Ibrahim, D.; Omar, I.C. Oil Palm Frond for the Production of Bioethanol. Int. J. Biochem. Biotechnol. 2012, 1, 7–11. [Google Scholar]
- Maluin, F.N.; Hussein, M.Z.; Idris, A.S. An Overview of the Oil Palm Industry: Challenges and Some Emerging Opportunities for Nanotechnology Development. Agronomy 2020, 10, 356. [Google Scholar] [CrossRef]
- Verma, A.K.; Chettri, D.; Verma, A.K.; Selvaraj, M.; Assiri, M.A. Bioprospecting Lignin for Biorefinery: Emerging Innovations and Strategies in Microbial Technology. Biomass Bioenergy 2024, 181, 107052. [Google Scholar] [CrossRef]
- Harahap, B.M. Degradation Techniques of Hemicellulose Fraction from Biomass Feedstock for Optimum Xylose Production: A Review. J. Trop. Agric. Eng. Biosyst. J. Keteknikan Pertan. Trop. Dan Biosist. 2020, 8, 107–124. [Google Scholar] [CrossRef]
- Dai, L.; Lian, Z.; Zhang, R.; Nawaz, A.; ul Haq, I.; Zhou, X.; Xu, Y. Multi-Strategy in Production of High Titer Gluconic Acid by the Fermentation of Concentrated Cellulosic Hydrolysate with Gluconobacter oxydans. Ind. Crops Prod. 2022, 189, 115748. [Google Scholar] [CrossRef]
- Pal, P.; Kumar, R.; Banerjee, S. Manufacture of Gluconic Acid: A Review towards Process Intensification for Green Production. Chem. Eng. Process. Process Intensif. 2016, 104, 160–171. [Google Scholar] [CrossRef]
- Toivari, M.; Vehkomäki, M.-L.; Nygård, Y.; Penttilä, M.; Ruohonen, L.; Wiebe, M.G. Low PH D-Xylonate Production with Pichia kudriavzevii. Bioresour. Technol. 2013, 133, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, G.; Zhang, J.; Bao, J. Fermentative Production of High Titer Gluconic and Xylonic Acids from Corn Stover Feedstock by Gluconobacter oxydans and Techno-Economic Analysis. Bioresour. Technol. 2016, 219, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Latifah, I.; Sudaryati, H.P. Pembuatan Asam Glukonat Dari Glukosa Dengan Proses Fermentasi Menggunakan Aspergillus niger. J. Teknol. Pangan 2013, 4, 2. [Google Scholar]
- Jiang, Y.; Liu, K.; Zhang, H.; Wang, Y.; Yuan, Q.; Su, N.; Bao, J.; Fang, X. Gluconic Acid Production from Potato Waste by Gluconobacter Oxidans Using Sequential Hydrolysis and Fermentation. ACS Sustain. Chem. Eng. 2017, 5, 6116–6123. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X.; Huang, L.; Cao, R.; Xu, Y. Efficient Coproduction of Gluconic Acid and Xylonic Acid from Lignocellulosic Hydrolysate by Zn (II)-Selective Inhibition on Whole-Cell Catalysis by Gluconobacter oxydans. Bioresour. Technol. 2017, 243, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Lu, L. Gluconic and Xylonic Acid Production from Lignocellulosic Biomass by Gluconobacter oxydans. Master’s Thesis, Auburn University, Auburn, AL, USA, 2013. [Google Scholar]
- Sahlan, M.; Hermawan, M.E.; Hidayatullah, I.M.; Kartohardjono, S.; Arevin, A.R.; Hermansyah, H. Sodium Gluconate Synthesis from Oil Palm Frond: Optimization of Neutralisation and Purity Enhancement through Low-Pressure Nanofiltration. Results Eng. 2024, 23, 102367. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3-B. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, X.; Xu, Y. Improvement of Fermentation Performance of Gluconobacter oxydans by Combination of Enhanced Oxygen Mass Transfer in Compressed-Oxygen-Supplied Sealed System and Cell-Recycle Technique. Bioresour. Technol. 2017, 244, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; He, T.; Zhou, X.; Xu, Y.; Gu, X. Influence of Oxygen Transfer and Uptake Rates on Xylonic Acid Production from Xylose by Gluconobacter oxydans. Biochem. Eng. J. 2021, 176, 108192. [Google Scholar] [CrossRef]
- Li, R.; Xu, Y.; Chen, J.; Wang, F.; Zou, C.; Yin, J. Enhancing the Proportion of Gluconic Acid with a Microbial Community Reconstruction Method to Improve the Taste Quality of Kombucha. LWT 2022, 155, 112937. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Yang, Z.; Chen, G.; Liang, Z.; Zeng, W. Enhanced Production of Poly-γ-Glutamic Acid by Bacillus Subtilis Using Stage-Controlled Fermentation and Viscosity Reduction Strategy. Appl. Biochem. Biotechnol. 2024, 196, 1527–1543. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Xu, C.; Ding, C.; Liu, X.; Gu, X. Optimization of Specific Productivity for Xylonic Acid Production by Gluconobacter oxydans Using Response Surface Methodology. Front. Bioeng. Biotechnol. 2021, 9, 729988. [Google Scholar] [CrossRef] [PubMed]
- Rodzri, N.A.M.; Zain, W.S.W.M.; Hanapiah, R.M.A.; Samah, R.A.; Illias, R.M. Effect of Physical Parameters on Production of ᴅ-Xylonic Acid Using Recombinant E. Coli BL21 (DE3). Mater. Today Proc. 2019, 19, 1247–1254. [Google Scholar] [CrossRef]
- Ma, Q.; Yi, J.; Tang, Y.; Geng, Z.; Zhang, C.; Sun, W.; Liu, Z.; Xiong, W.; Wu, H.; Xie, X. Co-Utilization of Carbon Sources in Microorganisms for the Bioproduction of Chemicals. Biotechnol. Adv. 2024, 73, 108380. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Lian, Z.; Fu, Y.; Zhou, X.; Xu, Y.; Zhou, X.; Kuznetsov, B.N.; Jiang, K. Low PH Stress Enhances Gluconic Acid Accumulation with Enzymatic Hydrolysate as Feedstock Using Gluconobacter oxydans. Fermentation 2023, 9, 278. [Google Scholar] [CrossRef]
- Olijve, W.; Kok, J.J. Analysis of Growth of Gluconobacter oxydans in Glucose Containing Media. Arch. Microbiol. 1979, 121, 283–290. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Xiao, J.; Zhou, X.; Xu, Y. Osmotic Stress Tolerance and Transcriptome Analysis of Gluconobacter oxydans to Extra-High Titers of Glucose. Front. Microbiol. 2022, 13, 977024. [Google Scholar] [CrossRef] [PubMed]
- Stasiak-Różańska, L.; Błażejak, S.; Gientka, I. Effect of Glycerol and Dihydroxyacetone Concentrations in the Culture Medium on the Growth of Acetic Acid Bacteria, Gluconobacter oxydans ATCC 621. Eur. Food Res. Technol. 2014, 239, 453–461. [Google Scholar] [CrossRef]
- Jiang, W.; Dai, L.; Tan, X.; Zhou, X.; Xu, Y. Screening of Gluconobacter oxydans in Xylonic Acid Fermentation for Tolerance of the Inhibitors Formed Dilute Acid Pretreatment. Bioprocess Biosyst. Eng. 2023, 46, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ding, C.; He, T.; Zhu, Y.; Sun, L.; Xu, C.; Gu, X. Kinetic Modeling of Xylonic Acid Production by Gluconobacter oxydans: Effects of Hydrodynamic Conditions. Bioprocess Biosyst. Eng. 2023, 46, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Yang, X.; Gan, T.; Zhou, W.; Lin, J.; Wei, D. High Cell Density Fermentation of Gluconobacter oxydans DSM 2003 for Glycolic Acid Production. J. Ind. Microbiol. Biotechnol. 2009, 36, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Battling, S.; Pastoors, J.; Deitert, A.; Götzen, T.; Hartmann, L.; Schröder, E.; Yordanov, S.; Büchs, J. Development of a Novel Defined Minimal Medium for Gluconobacter oxydans 621H by Systematic Investigation of Metabolic Demands. J. Biol. Eng. 2022, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Battling, S.; Wohlers, K.; Igwe, C.; Kranz, A.; Pesch, M.; Wirtz, A.; Baumgart, M.; Büchs, J.; Bott, M. Novel Plasmid-Free Gluconobacter oxydans Strains for Production of the Natural Sweetener 5-Ketofructose. Microb. Cell Fact. 2020, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Lian, Z.; Dai, L.; Zhang, R.; Liu, Y.; Zhou, X.; Xu, Y. Efficient Aerobic Fermentation of Gluconic Acid by High Tension Oxygen Supply Strategy with Reusable Gluconobacter oxydans HG19 Cells. Bioprocess Biosyst. Eng. 2022, 45, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.A.R.; Oliveira, S.S.d.S.; Lima, S.F.; Nascimento, R.P.D.; Baptista, A.R.d.S.; Fiaux, S.B. The Industrial Versatility of Gluconobacter oxydans: Current Applications and Future Perspectives. World J. Microbiol. Biotechnol. 2022, 38, 134. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidayatullah, I.M.; Maritza, D.A.; Yohda, M.; Sahlan, M.; Kusmayadi, A.; Leong, Y.K.; Hermansyah, H. Fermentative Synthesis of Gluconic and Xylonic Acids from Hydrolyzed Palm Fronds Using Gluconobacter oxydans. Bioengineering 2025, 12, 801. https://doi.org/10.3390/bioengineering12080801
Hidayatullah IM, Maritza DA, Yohda M, Sahlan M, Kusmayadi A, Leong YK, Hermansyah H. Fermentative Synthesis of Gluconic and Xylonic Acids from Hydrolyzed Palm Fronds Using Gluconobacter oxydans. Bioengineering. 2025; 12(8):801. https://doi.org/10.3390/bioengineering12080801
Chicago/Turabian StyleHidayatullah, Ibnu Maulana, Dhea Annora Maritza, Masafumi Yohda, Muhammad Sahlan, Adi Kusmayadi, Yoong Kit Leong, and Heri Hermansyah. 2025. "Fermentative Synthesis of Gluconic and Xylonic Acids from Hydrolyzed Palm Fronds Using Gluconobacter oxydans" Bioengineering 12, no. 8: 801. https://doi.org/10.3390/bioengineering12080801
APA StyleHidayatullah, I. M., Maritza, D. A., Yohda, M., Sahlan, M., Kusmayadi, A., Leong, Y. K., & Hermansyah, H. (2025). Fermentative Synthesis of Gluconic and Xylonic Acids from Hydrolyzed Palm Fronds Using Gluconobacter oxydans. Bioengineering, 12(8), 801. https://doi.org/10.3390/bioengineering12080801








