Collagen Remodeling of Strattice™ Firm in a Nonhuman Primate Model of Abdominal Wall Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomaterials
2.2. Study Design
2.3. Animals
2.4. Primate Abdominal Wall Repair Model
2.5. Tissue Cytokine Assay
2.6. Serum IgG Antibody ELISA
2.7. Histology/Immunohistochemistry
2.7.1. MMP-1 and TIMP-1 IHC Image Analysis
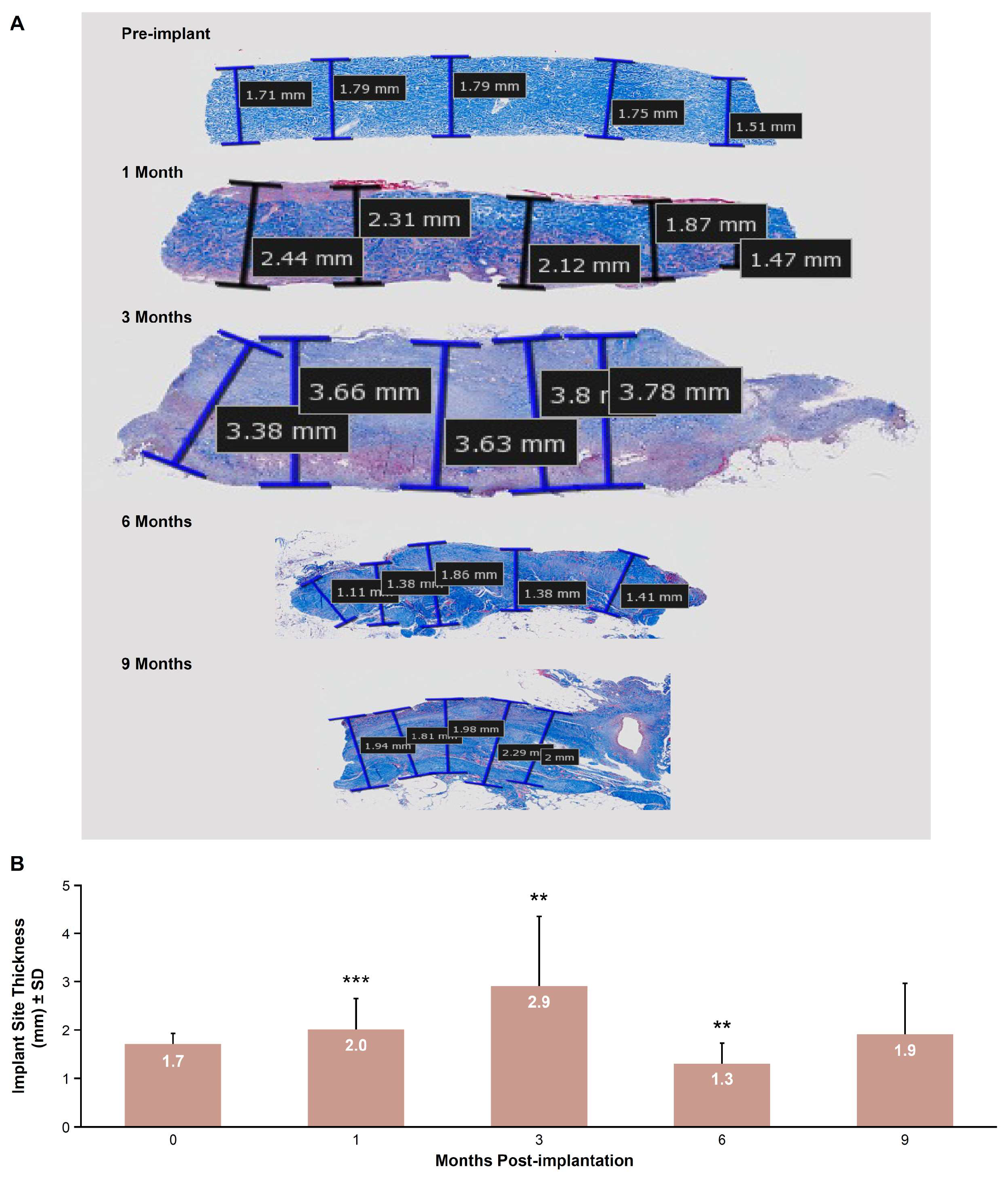
2.7.2. Implant Site Morphometry
2.8. Statistical Analyses
3. Results
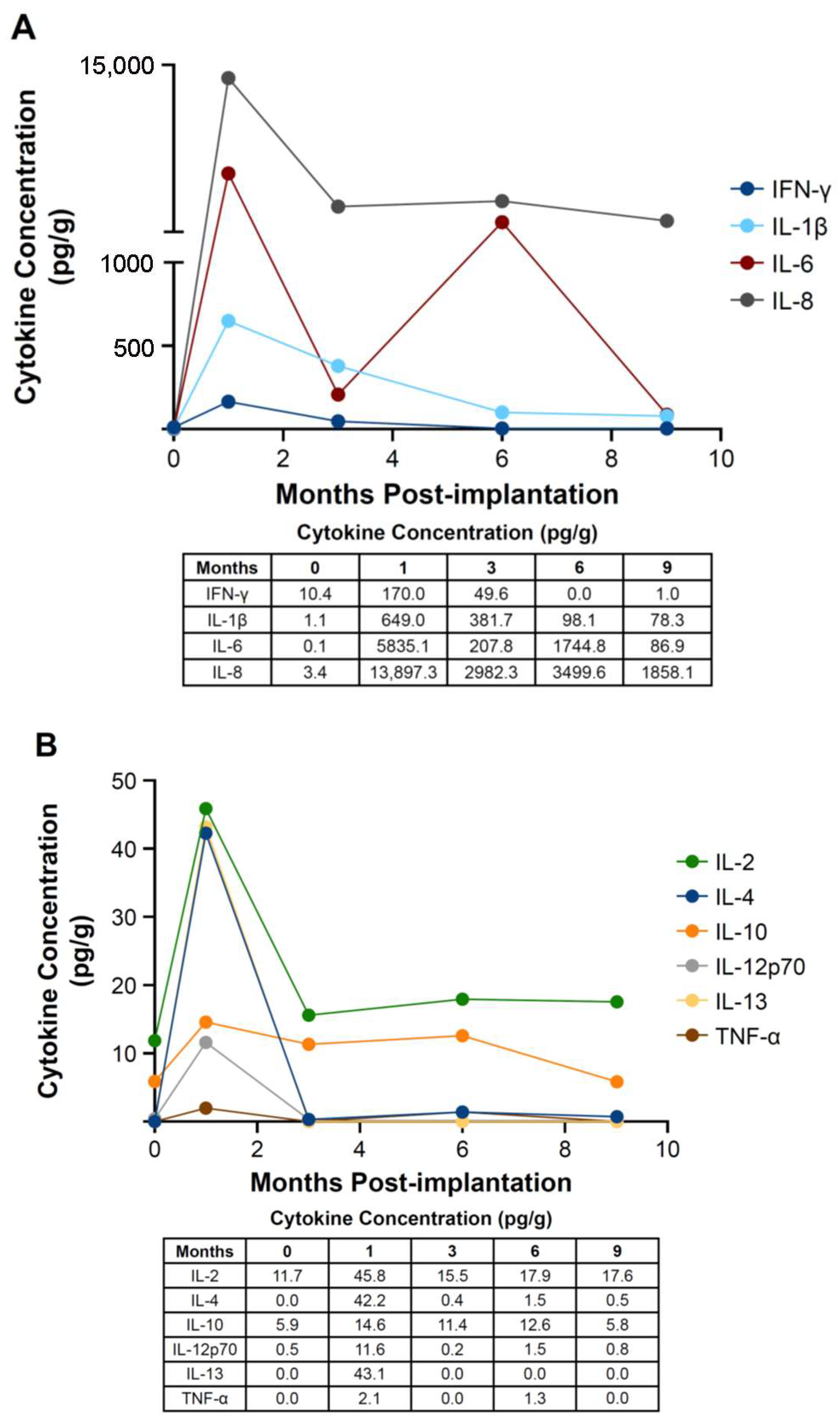
3.1. Tissue Cytokine Assay
3.2. Serum IgG ELISA
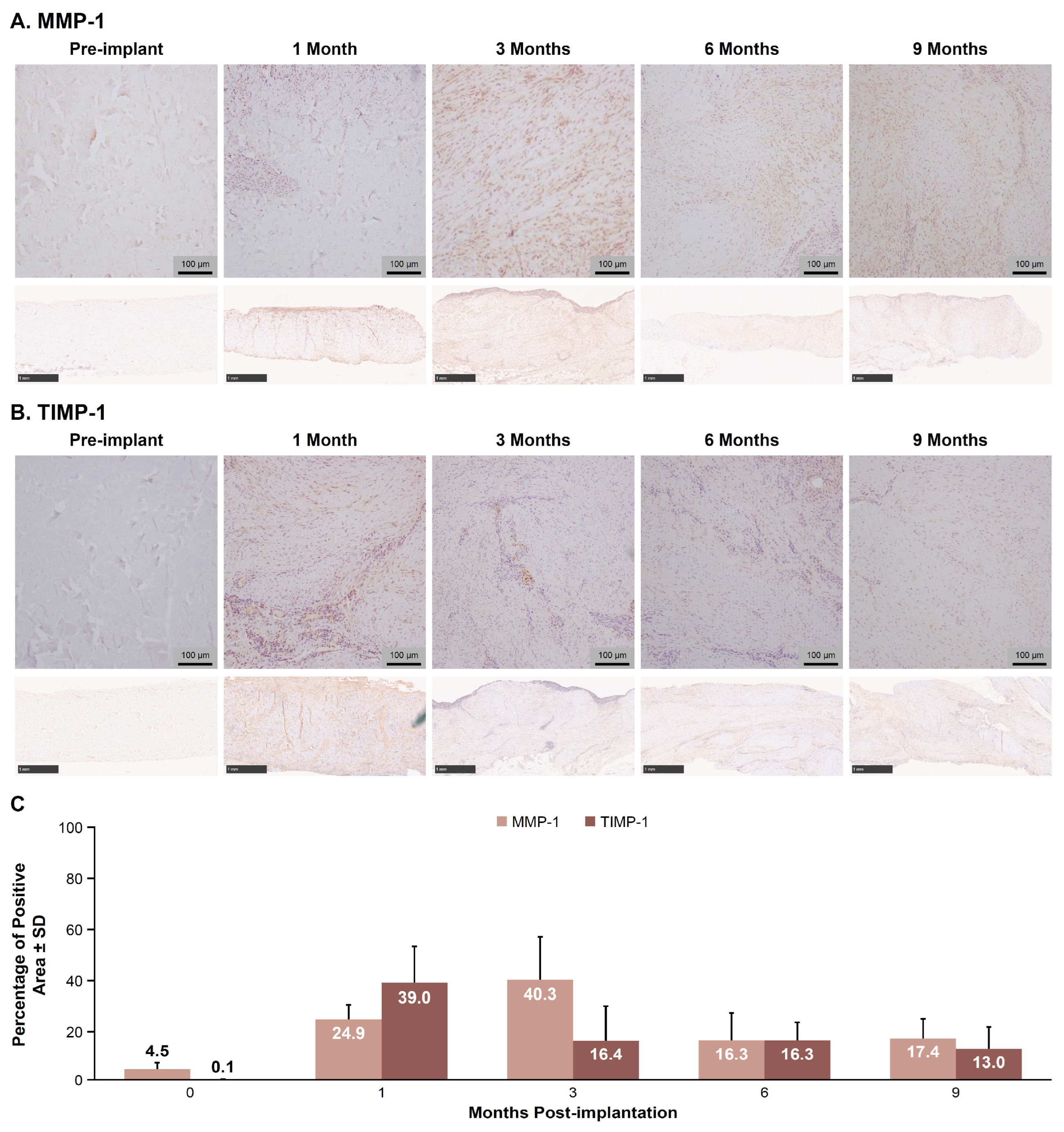
3.3. MMP-1 and TIMP-1 IHC Image Analysis
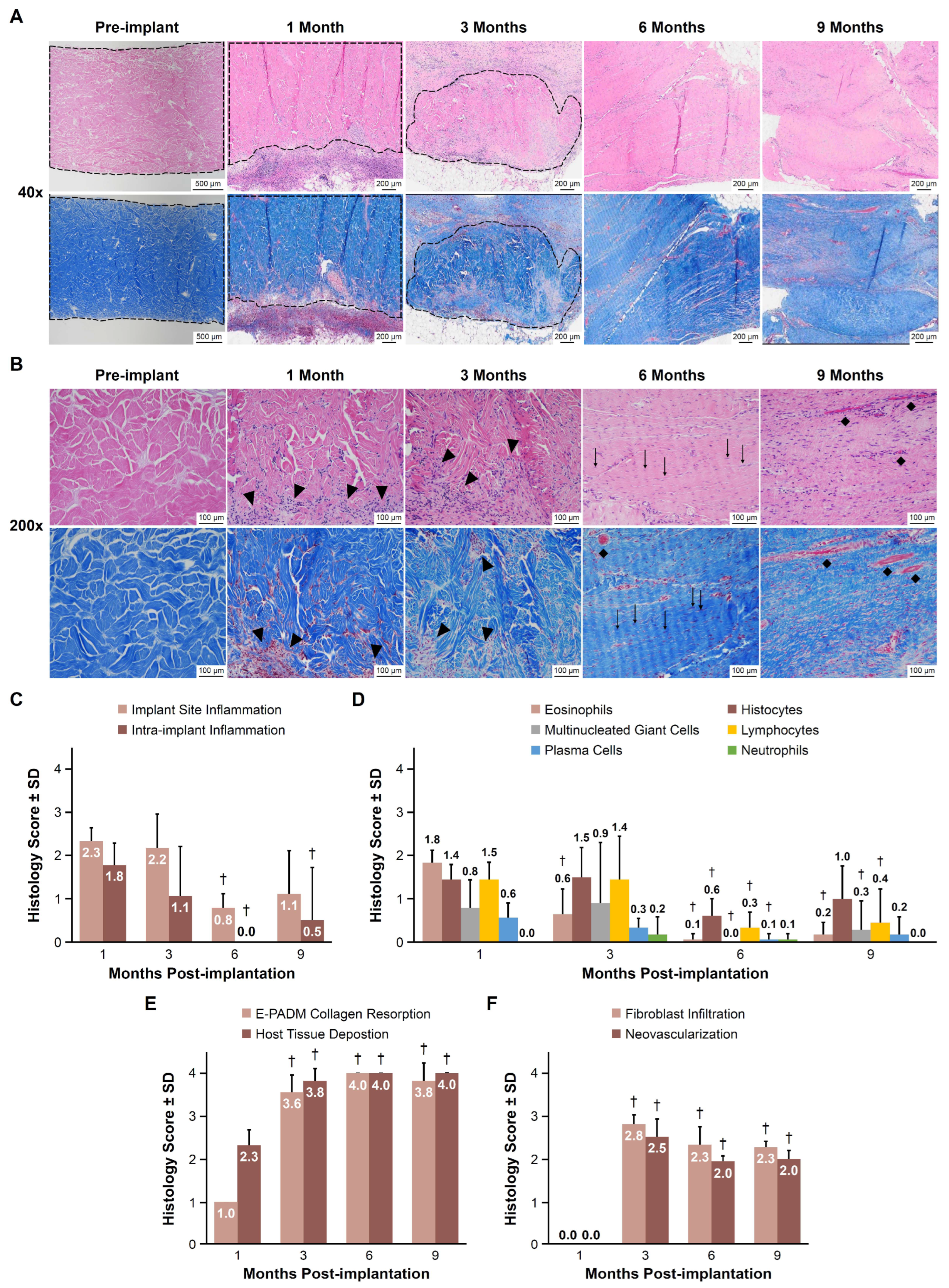
3.4. Histology Scoring
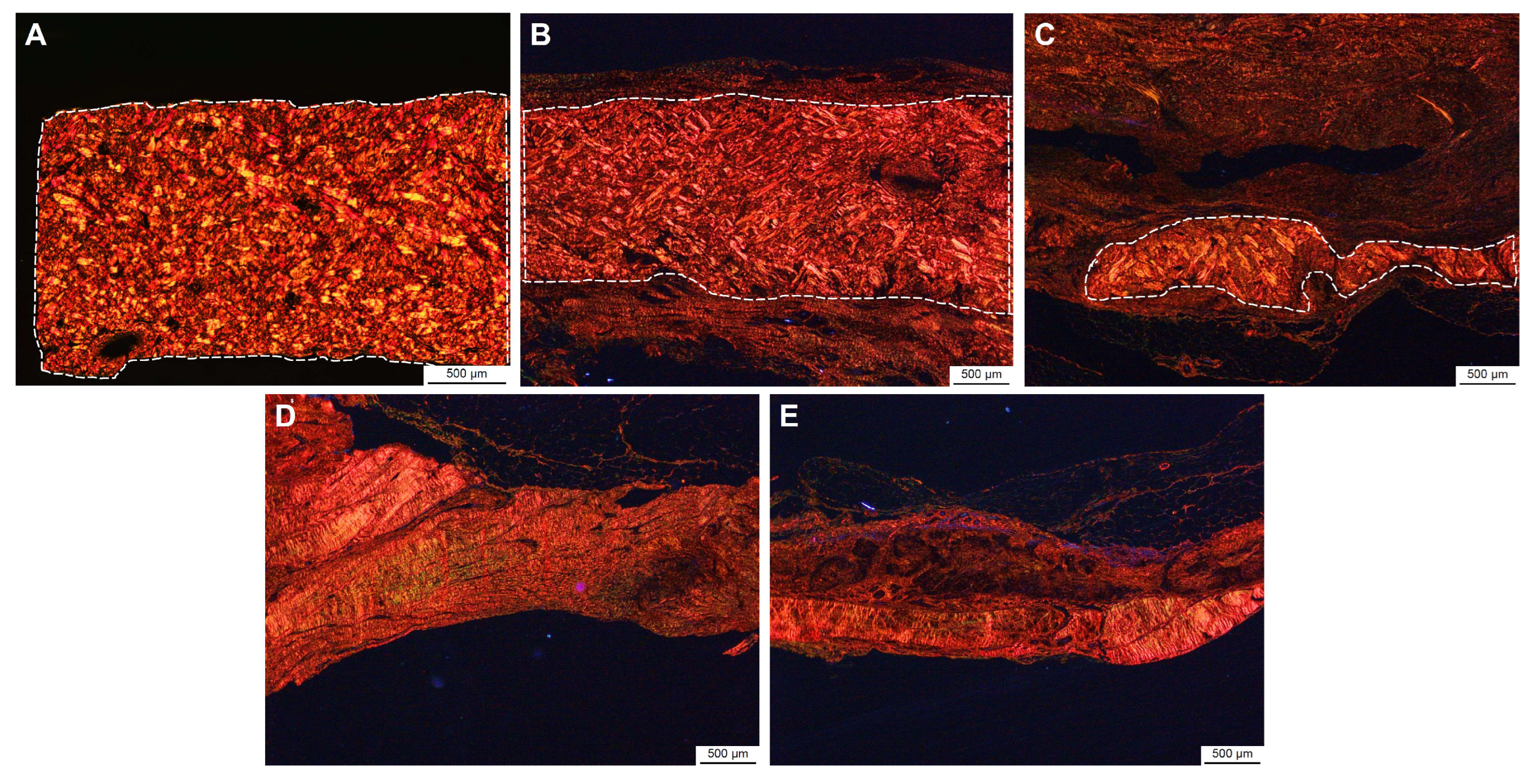
3.5. Implant Site Morphometry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ELISA | enzyme-linked immunosorbent assay |
| E-PADM | electron-beam terminally sterilized porcine-derived acellular dermal matrix |
| IgG | immunoglobulin G |
| IHC | immunohistochemistry |
| IM | intramuscular |
References
- See, C.W.; Kim, T.; Zhu, D. Hernia mesh and hernia repair: A review. Eng. Regen. 2020, 1, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Nahabedian, M.Y.; Sosin, M.; Bhanot, P. A current review of biologic meshes in abdominal wall reconstruction. Plast. Reconstr. Surg. 2018, 142, 74S–81S. [Google Scholar] [CrossRef] [PubMed]
- King, V.A.; Vishwanath, N.; Sobti, N.; Rao, V.; Mehrzad, R.; Crozier, J.; Breuing, K.H. An evaluation of the relative safety of Artia porcine acellular dermal matrix in the setting of implant-based breast reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2023, 86, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, W.A., 4th; Hammer, J.; Luo, L.; Schumacher, A. Acellular dermal matrices in breast reconstruction: CARE trial 5-year outcomes data for more than 9500 patients. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4258. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.; Cho, Y.S.; Seo, C.H.; Lee, B.C.; Ko, J.H.; Kim, D.; Hur, J.; Chun, W.; Kim, J.H. The use of AlloDerm on major burn patients: AlloDerm prevents post-burn joint contracture. Burns 2010, 36, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Birindelli, A.; Sartelli, M.; Di Saverio, S.; Coccolini, F.; Ansaloni, L.; van Ramshorst, G.H.; Campanelli, G.; Khokha, V.; Moore, E.E.; Peitzman, A.; et al. 2017 Update of the WSES guidelines for emergency repair of complicated abdominal wall hernias. World J. Emerg. Surg. 2017, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.; McQuillan, D.; Sandor, M.; Wan, H.; Lombardi, J.; Bachrach, N.; Harper, J.; Xu, H. Retention of structural and biochemical integrity in a biological mesh supports tissue remodeling in a primate abdominal wall model. Regen. Med. 2009, 4, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in wound healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Novitsky, Y.W.; Rosen, M.J. The biology of biologics: Basic science and clinical concepts. Plast. Reconstr. Surg. 2012, 130, 9S–17S. [Google Scholar] [CrossRef] [PubMed]
- Orenstein, S.B.; Qiao, Y.; Klueh, U.; Kreutzer, D.L.; Novitsky, Y.W. Activation of human mononuclear cells by porcine biologic meshes in vitro. Hernia 2010, 14, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Novitsky, Y.W.; Orenstein, S.B.; Kreutzer, D.L. Comparative analysis of histopathologic responses to implanted porcine biologic meshes. Hernia 2014, 18, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wan, H.; Sandor, M.; Qi, S.; Ervin, F.; Harper, J.R.; Silverman, R.P.; McQuillan, D.J. Host response to human acellular dermal matrix transplantation in a primate model of abdominal wall repair. Tissue Eng. Part A 2008, 14, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Cramer, M.C.; Badylak, S.F. Extracellular matrix-based biomaterials and their influence upon cell behavior. Ann. Biomed. Eng. 2020, 48, 2132–2153. [Google Scholar] [CrossRef] [PubMed]
- Sandor, M.; Xu, H.; Connor, J.; Lombardi, J.; Harper, J.R.; Silverman, R.P.; McQuillan, D.J. Host response to implanted porcine-derived biologic materials in a primate model of abdominal wall repair. Tissue Eng. Part A 2008, 14, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Strattice [Instruction for Use]; LifeCell Corporation: Branchburg, NJ, USA, 2021.
- Strattice Portfolio Brochure Europe. 2016. Available online: https://www.gdmedical.nl/wp-content/uploads/2018/05/STRATTICE_Portfolio_Brochure.pdf (accessed on 14 December 2020).
- Strattice Reconstructive Tissue Matrix Product Offerings. 2018. Available online: http://hcp.stratticetissuematrix.com/en/products (accessed on 14 December 2020).
- Xu, H.; Wan, H.; Zuo, W.; Sun, W.; Owens, R.T.; Harper, J.R.; Ayares, D.L.; McQuillan, D.J. A porcine-derived acellular dermal scaffold that supports soft tissue regeneration: Removal of terminal galactose-alpha-(1,3)-galactose and retention of matrix structure. Tissue Eng. Part A 2009, 15, 1807–1819. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.Q.; Xu, H.; Sandor, M.; Lombardi, J. Process-induced extracellular matrix alterations affect the mechanisms of soft tissue repair and regeneration. J. Tissue Eng. 2013, 4, 2041731413505305. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, G.A.; Delossantos, A.I.; Rodriguez, N.L.; Patel, P.; Franz, M.G.; Wagner, C.T. Porcine incisional hernia model: Evaluation of biologically derived intact extracellular matrix repairs. J. Tissue Eng. 2013, 4, 2041731413508771. [Google Scholar] [CrossRef] [PubMed]
- Stec, E.; Lombardi, J.; Augustin, J.; Sandor, M. Acellular dermal matrix susceptibility to collagen digestion: Effect on mechanics and host response. Tissue Eng. Part A 2023, 29, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, J.; Stec, E.; Edwards, M.; Connell, T.; Sandor, M. Comparison of mechanical properties and host tissue response to OviTex™ and Strattice™ surgical meshes. Hernia 2023, 27, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Stefanelli, V.; Lombardi, J.; Ferrer, J.; Gardocki-Sandor, M. Vascularization of human acellular dermal matrices: A comparative study in a nonhuman primate model. Tissue Eng. Part A 2025, 31, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Sandor, M.; Singh, D.; Silverman, R.P.; Xu, H.; De Deyne, P.G. Comparative host response of 2 human acellular dermal matrices in a primate implant model. Eplasty 2014, 14, 52–64. [Google Scholar]
- Rich, L.; Whittaker, P. Collagen and picrosirius red staining: Polarized light assessment of fibrillar hue and spatial distribution. Braz. J. Morphol. Sci. 2005, 22, 97–104. [Google Scholar]
- King, F.A.; Yarbrough, C.J.; Anderson, D.C.; Gordon, T.P.; Gould, K.G. Primates. Science 1988, 240, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.Z.; Stevenson, A.W.; Prêle, C.M.; Fear, M.W.; Wood, F.M. The role of IL-6 in skin fibrosis and cutaneous wound healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.Q.; Kondo, T.; Ishida, Y.; Takayasu, T.; Mukaida, N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J. Leukoc. Biol. 2003, 73, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of wound healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef]
- Morris, A.H.; Stamer, D.K.; Kyriakides, T.R. The host response to naturally-derived extracellular matrix biomaterials. Semin. Immunol. 2017, 29, 72–91. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.R.; Deeken, C.R.; Martindale, R.G.; Rosen, M.J. Evaluation of a fully absorbable poly-4-hydroxybutyrate/absorbable barrier composite mesh in a porcine model of ventral hernia repair. Surg. Endosc. 2016, 30, 3691–3701. [Google Scholar] [CrossRef] [PubMed]
- Luijendijk, R.W.; Hop, W.C.; van den Tol, M.P.; de Lange, D.C.; Braaksma, M.M.; IJzermans, J.N.; Boelhouwer, R.U.; de Vries, B.C.; Salu, M.K.; Wereldsma, J.C.; et al. A comparison of suture repair with mesh repair for incisional hernia. N. Engl. J. Med. 2000, 343, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.W.; Primus, F.; Young, C.; Carter, J.T.; Lin, M.; Mukhtar, R.A.; Yeh, B.; Allen, I.E.; Freise, C.; Kim, E.; et al. Preventing recurrence in clean and contaminated hernias using biologic versus synthetic mesh in ventral hernia repair: The PRICE randomized clinical trial. Ann. Surg. 2021, 273, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Katzen, M.; Ayuso, S.A.; Sacco, J.; Ku, D.; Scarola, G.T.; Kercher, K.W.; Colavita, P.D.; Augenstein, V.A.; Heniford, B.T. Outcomes of biologic versus synthetic mesh in CDC class 3 and 4 open abdominal wall reconstruction. Surg. Endosc. 2023, 37, 3073–3083. [Google Scholar] [CrossRef] [PubMed]
- Sandor, M.; Scott, N.; Edwards, M.; Qi, S.; De Deyne, P.G. In vitro and in vivo characterization of a fully resorbable and composite surgical mesh. Bioact. Compat. 2014, 29, 121–136. [Google Scholar] [CrossRef]
- Vogels, R.R.M.; Kaufmann, R.; van den Hil, L.C.L.; van Steensel, S.; Schreinemacher, M.H.F.; Lange, J.F.; Bouvy, N.D. Critical overview of all available animal models for abdominal wall hernia research. Hernia 2017, 21, 667–675. [Google Scholar] [CrossRef] [PubMed]

| Evaluation | Scoring Criteria |
|---|---|
| Inflammation/inflammatory cells | 0 = Absent 1 = Rare, minimal, 1–5/per high-power field (40×) 2 = Mild, 5–10/per high-power field (40×) 3 = Heavy infiltrate with preservation of local architecture 4 = Packed, with effacement of regional architecture |
| Collagen resorption | 0 = Original E-PADM intact, borders clearly demarcated (0% resorbed) 1 = E-PADM minimally resorbed (<25%), with some separation by host tissue/infiltrates 2 = E-PADM notably resorbed (≈25–75%), difficult to distinguish scaffold from host tissue 3 = E-PADM markedly resorbed (≈>75%), difficult to distinguish scaffold from host tissue 4 = No evidence of E-PADM remaining, ≈100% resorbed |
| Host tissue deposition | 0 = Absent 1 = Host tissue restricted to periphery of E-PADM 2 = Host tissue present within the E-PADM interstitium, not extending to center 3 = Host tissue; present throughout E-PADM, including center 4 = Host tissue diffusely expands throughout E-PADM |
| Fibroblast infiltration | 0 = Absent (i.e., fibrocytes present but no fibroblasts, typical of quiescent native tissue) 1 = Minimal, rare fibroblasts present within connective tissue 2 = Mild, multifocal presence, fibroblasts constitute minority of connective tissue 3 = Moderate, diffuse presence, fibroblasts are notable component of connective tissue 4 = Marked, diffuse presence, fibroblasts predominate connective tissue response |
| Neovascularization | 0 = Absent 1 = Minimal capillary proliferation, focal, 1–3 buds/area 2 = Clusters of 4–7 capillaries with supporting fibroblastic structures 3 = Broad band of capillaries, or larger vessels (arteries/veins) with supporting structures 4 = Extensive bands of vessels with supporting fibroblastic structures |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolden, K.; Lombardi, J.; Kabaria, N.; Stec, E.; Gardocki-Sandor, M. Collagen Remodeling of Strattice™ Firm in a Nonhuman Primate Model of Abdominal Wall Repair. Bioengineering 2025, 12, 796. https://doi.org/10.3390/bioengineering12080796
Bolden K, Lombardi J, Kabaria N, Stec E, Gardocki-Sandor M. Collagen Remodeling of Strattice™ Firm in a Nonhuman Primate Model of Abdominal Wall Repair. Bioengineering. 2025; 12(8):796. https://doi.org/10.3390/bioengineering12080796
Chicago/Turabian StyleBolden, Kelly, Jared Lombardi, Nimesh Kabaria, Eric Stec, and Maryellen Gardocki-Sandor. 2025. "Collagen Remodeling of Strattice™ Firm in a Nonhuman Primate Model of Abdominal Wall Repair" Bioengineering 12, no. 8: 796. https://doi.org/10.3390/bioengineering12080796
APA StyleBolden, K., Lombardi, J., Kabaria, N., Stec, E., & Gardocki-Sandor, M. (2025). Collagen Remodeling of Strattice™ Firm in a Nonhuman Primate Model of Abdominal Wall Repair. Bioengineering, 12(8), 796. https://doi.org/10.3390/bioengineering12080796






