Chemical, Mechanical and Tribological Effects of Artificially Aging up to 6 Weeks on Virgin and Crosslinked UHMWPE Evaluated for a TKR Design
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
6. Disclosure Policy
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdelaal, M.S.; Restrepo, C.; Sharkey, P.F. Global Perspectives on Arthroplasty of Hip and Knee Joints. Orthop. Clin. N. Am. 2020, 51, 169–176. [Google Scholar] [CrossRef] [PubMed]
- OECD. Hip and knee replacement. In Health at a Glance 2021; OECD Indicators; OECD Publishing: Paris, France, 2021. [Google Scholar]
- Bracco, P.; Bellare, A.; Bistolfi, A.; Affatato, S. Ultra-High Molecular Weight Polyethylene: Influence of the Chemical, Physical and Mechanical Properties on the Wear Behavior. A Review. Materials 2017, 10, 791. [Google Scholar] [CrossRef] [PubMed]
- Lum, Z.C.; Shieh, A.K.; Dorr, L.D. Why total knees fail-A modern perspective review. World J. Orthop. 2018, 9, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Lau, E.; Ong, K.; Zhao, K.; Kelly, M.; Bozic, K.J. Future young patient demand for primary and revision joint replacement: National projections from 2010 to 2030. Clin. Orthop. Relat. Res. 2009, 467, 2606–2612. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Hosoba, M.; Miura, S.; Mochizuki, T. Effects of knee simulator control method and radiation dose on UHMWPE wear rate, and relationship between wear rate and clinical revision rate in National Joint Registry. J. Mech. Behav. Biomed. Mater. 2019, 90, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Weinstein, A.M.; Rome, B.N.; Reichmann, W.M.; Collins, J.E.; Burbine, S.A.; Thornhill, T.S.; Wright, J.; Katz, J.N.; Losina, E. Estimating the burden of total knee replacement in the United States. J. Bone Jt. Surg. Am. 2013, 95, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Asher, D.P.; Wright, J.L.; Hall, D.J.; Lundberg, H.J.; Van Citters, D.W.; Jacobs, J.J.; Levine, B.R.; Pourzal, R. Is Wear Still a Concern in Total Knee Arthroplasty With Contemporary Conventional and Highly Crosslinked Polyethylene Tibial Inserts in the mid- to Long-Term? Arthroplast. Today 2024, 30, 101550. [Google Scholar] [CrossRef] [PubMed]
- Robertsson, O.; W-Dahl, A.; Lidgren, L.; Sundberg, M. Swedish Knee Arthroplasty Register: Annual Report 2019. 2019. Available online: http://www.myknee.se/pdf/SVK_2019_1.0_Eng.pdf (accessed on 28 August 2024).
- Graves, S. 20th Annual Report 2019: Australian Orthopaedic Association National Joint Replacement Registry. Hip, Knee & Shoulder Arthroplasty September 1999–December 2018. 2019. Available online: https://aoanjrr.sahmri.com/documents/10180/668596/Hip%2C+Knee+%26+Shoulder+Arthroplasty/c287d2a3-22df-a3bb-37a2-91e6c00bfcf0 (accessed on 29 August 2024).
- Robinson, R.P. The Early Innovators of Today’s Resurfacing Condylar Knees. J. Arthroplast. 2005, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Muratoglu, O.K.; Bragdon, C.R.; O’Connor, D.O.; Jasty, M.; Harris, W.H. A novel method of cross-linking ultra-high-molecular-weight polyethylene to improve wear, reduce oxidation, and retain mechanical properties. Recipient of the 1999 HAP Paul Award. J. Arthroplast. 2001, 16, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Currier, B.H.; Van Citters, D.W.; Currier, J.H.; Carlson, E.M.; Tibbo, M.E.; Collier, J.P. In vivo oxidation in retrieved highly crosslinked tibial inserts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Kump, J.; Teeter, M.G.; Matheson, J.; Klassen, R.; Lanting, B.A.; Decker, M.M. The impact of free-radical stabilization techniques on in vivo subsurface mechanical properties in highly cross-linked polyethylene acetabular liners. J. Orthop. Res. 2023, 41, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Regis, M.; Bracco, P.; Giorgini, L.; Fusi, S.; Dalla Pria, P.; Costa, L.; Schmid, C. Correlation between in vivo stresses and oxidation of UHMWPE in total hip arthroplasty. J. Mater. Sci. Mater. Med. 2014, 25, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Muratoglu, O.K.; Bragdon, C.R.; O’Connor, D.O.; Jasty, M.; Harris, W.H.; Gul, R.; McGarry, F. Unified wear model for highly crosslinked ultra-high molecular weight polyethylenes (UHMWPE). Biomaterials 1999, 20, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Bistolfi, A.; Giustra, F.; Bosco, F.; Sabatini, L.; Aprato, A.; Bracco, P.; Bellare, A. Ultra-high molecular weight polyethylene (UHMWPE) for hip and knee arthroplasty: The present and the future. J. Orthop. 2021, 25, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Currier, B.H.; Jevsevar, K.C.; Van Citters, D.W. Oxidation in Retrieved, Never-Irradiated UHMWPE Bearings: What Can We Learn About in Vivo Oxidation? J. Bone Jt. Surg. Am. 2023, 105, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Spece, H.; Yarbrough, R.; Kurtz, S.M. A Review of Early In vivo Performance of Antioxidant Stabilized Polyethylene for Total Knee Arthroplasty. J. Arthroplast. 2023, 9, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Ponzio, D.Y.; Weitzler, L.; deMeireles, A.; Esposito, C.I.; Wright, T.M.; Padgett, D.E. Antioxidant-stabilized highly crosslinked polyethylene in total knee arthroplasty: A retrieval analysis. Bone Jt. J. 2018, 100-B, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.; Ries, M.D.; Pruitt, L. Effect of processing, sterilization and crosslinking on UHMWPE fatigue fracture and fatigue wear mechanisms in joint arthroplasty. J. Mech. Behav. Biomed. Mater. 2016, 53, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Currier, B.H.; Currier, J.H.; Holdcroft, L.A.; Van Citters, D.W. Effectiveness of anti-oxidant polyethylene: What early retrievals can tell us. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.; Neils, A.L.; Rowell, S.L.; Lozynsky, A.J.; Muratoglu, O.K. Increasing irradiation temperature maximizes vitamin E grafting and wear resistance of ultrahigh molecular weight polyethylene. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 436–440. [Google Scholar] [CrossRef] [PubMed]
- García-Rey, E.; Cruz-Pardos, A.; Saldaña, L. New polyethylenes in total hip arthroplasty a 20- to 22-year follow-up study. Bone Jt. J. 2022, 104-B, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Merfort, R.; Maffulli, N.; Hofmann, U.K.; Hildebrand, F.; Simeone, F.; Eschweiler, J.; Migliorini, F. Head, acetabular liner composition, and rate of revision and wear in total hip arthroplasty: A Bayesian network meta-analysis. Sci. Rep. 2023, 13, 20327. [Google Scholar] [CrossRef] [PubMed]
- Muratoglu, O.K.; Wannomae, K.K.; Rowell, S.L.; Micheli, B.R.; Malchau, H. Ex vivo stability loss of irradiated and melted ultra-high molecular weight polyethylene. J. Bone Jt. Surg. Am. 2010, 92, 2809–2816. [Google Scholar] [CrossRef] [PubMed]
- Rowell, S.L.; Reyes, C.R.; Malchau, H.; Muratoglu, O.K. In Vivo Oxidative Stability Changes of Highly Cross-Linked Polyethylene Bearings: An Ex Vivo Investigation. J. Arthroplast. 2015, 30, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.; Ghali, B.W.; Neils, A.; Muratoglu, O.K. A new mechanism of oxidation in ultrahigh molecular weight polyethylene caused by squalene absorption. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Kop, A.M.; Pabbruwe, M.B.; Keogh, C.; Swarts, E. Oxidation of Second Generation Sequentially Irradiated and Annealed Highly Cross-Linked X3™ Polyethylene Tibial Bearings. J. Arthroplast. 2015, 30, 1842–1846. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Esposito, C.I.; Burket, J.C.; Wright, T.M. Crosslink Density Is Reduced and Oxidation Is Increased in Retrieved Highly Crosslinked Polyethylene TKA Tibial Inserts. Clin. Orthop. Relat. Res. 2017, 475, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Reinitz, S.D.; Currier, B.H.; Levine, R.A.; Van Citters, D.W. Crosslink density, oxidation and chain scission in retrieved, highly cross-linked UHMWPE tibial bearings. Biomaterials 2014, 35, 4436–4440. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Park, J.-W.; Jang, Y.-S.; Kim, E.-J. Is Highly Cross-Linked Polyethylene Safe for Use in High-Flexion Posterior Stabilized Total Knee Arthroplasty? J. Arthroplast. 2023, 38, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Prentice, H.A. Long-term revision risk for highly crosslinked versus conventional polyethylene in total knee arthroplasty. In Proceedings of the 2024 Implant Polymers: UHMWPE & PEEK conference, Barcelona, Spain, 20–21 June 2024. [Google Scholar]
- Bistolfi, A.; Giustra, F.; Bosco, F.; Faccenda, C.; Viotto, M.; Sabatini, L.; Berchialla, P.; Sciannameo, V.; Graziano, E.; Massè, A. Comparable results between crosslinked polyethylene and conventional ultra-high molecular weight polyethylene implanted in total knee arthroplasty: Systematic review and meta-analysis of randomised clinical trials, Knee surgery, sports traumatology. Arthrosc. Off. J. ESSKA 2022, 30, 3120–3130. [Google Scholar] [CrossRef] [PubMed]
- Gkiatas, I.; Karasavvidis, T.; Sharma, A.K.; Xiang, W.; Malahias, M.-A.; Chalmers, B.P.; Sculco, P.K. Highly cross-linked polyethylene in primary total knee arthroplasty is associated with a lower rate of revision for aseptic loosening: A meta-analysis of 962,467 cases. Arch. Orthop. Trauma Surg. 2022, 142, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Revell, P.A.; al-Saffar, N.; Kobayashi, A. Biological reaction to debris in relation to joint prostheses. Proc. Inst. Mech. Eng. H 1997, 211, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Ollivere, B.; Wimhurst, J.A.; Clark, I.M.; Donell, S.T. Current concepts in osteolysis. J. Bone Jt. Surg. Br. 2012, 94, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Sadoghi, P.; Liebensteiner, M.; Agreiter, M.; Leithner, A.; Böhler, N.; Labek, G. Revision surgery after total joint arthroplasty: A complication-based analysis using worldwide arthroplasty registers. J. Arthroplast. 2013, 28, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, P.F.; Lichstein, P.M.; Shen, C.; Tokarski, A.T.; Parvizi, J. Why are total knee arthroplasties failing today--has anything changed after 10 years? J. Arthroplast. 2014, 29, 1774–1778. [Google Scholar] [CrossRef] [PubMed]
- Dalury, D.F.; Pomeroy, D.L.; Gorab, R.S.; Adams, M.J. Why are total knee arthroplasties being revised? J. Arthroplast. 2013, 28, 120–121. [Google Scholar] [CrossRef] [PubMed]
- Sobieraj, M.C.; Rimnac, C.M. Ultra high molecular weight polyethylene: Mechanics, morphology, and clinical behavior. J. Mech. Behav. Biomed. Mater. 2009, 2, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Del Brach Prever, E.M.; Bistolfi, A.; Bracco, P.; Costa, L. UHMWPE for arthroplasty: Past or future? J. Orthop. Traumatol. 2009, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lerf, R.; Zurbrügg, D.; Delfosse, D. Use of vitamin E to protect cross-linked UHMWPE from oxidation. Biomaterials 2010, 31, 3643–3648. [Google Scholar] [CrossRef] [PubMed]
- Baena, J.; Wu, J.; Peng, Z. Wear Performance of UHMWPE and Reinforced UHMWPE Composites in Arthroplasty Applications: A Review. Lubricants 2015, 3, 413–436. [Google Scholar] [CrossRef]
- Bracco, P.; Oral, E. Vitamin E-stabilized UHMWPE for total joint implants: A review. Clin. Orthop. Relat. Res. 2011, 469, 2286–2293. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.; Neils, A.L.; Doshi, B.N.; Fu, J.; Muratoglu, O.K. Effects of simulated oxidation on the in vitro wear and mechanical properties of irradiated and melted highly crosslinked UHMWPE. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Grupp, T.M.; Holderied, M.; Mulliez, M.A.; Streller, R.; Jäger, M.; Blömer, W.; Utzschneider, S. Biotribology of a vitamin E-stabilized polyethylene for hip arthroplasty—Influence of artificial ageing and third-body particles on wear. Acta Biomater. 2014, 10, 3068–3078. [Google Scholar] [CrossRef] [PubMed]
- ASTM F2003; Standard Practice for Accelerated Aging of Ultra-High Molecular Weight Polyethylene After Gamma Irradiation in Air. ASTM International: Conshohocken, PA, USA, 2015.
- Essner, A.; Herrera, L.; Hughes, P.; Kester, M. The influence of material and design on total knee replacement wear. J. Knee Surg. 2011, 24, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Schwiesau, J.; Fritz, B.; Kutzner, I.; Bergmann, G.; Grupp, T.M. CR TKA UHMWPE wear tested after artificial aging of the vitamin E treated gliding component by simulating daily patient activities. BioMed Res. Int. 2014, 2014, 567374. [Google Scholar] [CrossRef] [PubMed]
- Puente Reyna, A.L.; Fritz, B.; Schwiesau, J.; Schilling, C.; Summer, B.; Thomas, P.; Grupp, T.M. Metal ion release barrier function and biotribological evaluation of a zirconium nitride multilayer coated knee implant under highly demanding activities wear simulation. J. Biomech. 2018, 79, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Hanes, M. The Effects of Load Soak Control on the Wear of UHMWPE at Various Hydration Levels in a Joint Simulation Study: In Wear of Articulating Surfaces. Underst. Jt. Simul. 2006, 3, 102. [Google Scholar]
- Schwiesau, J.; Schilling, C.; Kaddick, C.; Utzschneider, S.; Jansson, V.; Fritz, B.; Blömer, W.; Grupp, T.M. Definition and evaluation of testing scenarios for knee wear simulation under conditions of highly demanding daily activities. Med. Eng. Phys. 2013, 35, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Schwiesau, J.; Schilling, C.; Utzschneider, S.; Jansson, V.; Fritz, B.; Blömer, W.; Grupp, T.M. Knee wear simulation under conditions of highly demanding daily activities—influence on an unicompartmental fixed bearing knee design. Med. Eng. Phys. 2013, 35, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Popoola, O.O.; Yao, J.Q.; Johnson, T.S.; Blanchard, C.R. Wear, delamination, and fatigue resistance of melt-annealed highly crosslinked UHMWPE cruciate-retaining knee inserts under activities of daily living. J. Orthop. Res. 2010, 28, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Reinders, J.; Sonntag, R.; Vot, L.; Gibney, C.; Nowack, M.; Kretzer, J.P. Wear testing of moderate activities of daily living using in vivo measured knee joint loading. PLoS ONE 2015, 10, e0123155. [Google Scholar] [CrossRef] [PubMed]
- Jaber, S.A.; Taddei, P.; Tozzi, S.; Sudanese, A.; Affatato, S. In vitro effects on mobile polyethylene insert under highly demanding daily activities: Stair climbing. Int. Orthop. 2015, 39, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Grupp, T.M.; Fritz, B.; Kutzner, I.; Schilling, C.; Bergmann, G.; Schwiesau, J. Vitamin E stabilised polyethylene for total knee arthroplasty evaluated under highly demanding activities wear simulation. Acta Biomater. 2017, 48, 415–422. [Google Scholar] [CrossRef] [PubMed]
- ISO, 5832–4:2014; Implants for Surgery—Metallic Materials—Part 4: Cobalt-Chromium-Molybdenum Casting Alloy. International Organization for Standardization: Geneva, Switzerland, 2014.
- Wolf, C.; Krivec, T.; Blassnig, J.; Lederer, K.; Schneider, W. Examination of the suitability of alpha-tocopherol as a stabilizer for ultra-high molecular weight polyethylene used for articulating surfaces in joint endoprostheses. J. Mater. Sci. Mater. Med. 2002, 13, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Schwiesau, J.; Fritz, B.; Bergmann, G.; Puente Reyna, A.L.; Schilling, C.; Grupp, T.M. Influence of radiation conditions on the wear behaviour of Vitamin E treated UHMWPE gliding components for total knee arthroplasty after extended artificial aging and simulated daily patient activities. J. Mech. Behav. Biomed. Mater. 2021, 122, 104652. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.; Greenbaum, E.S.; Malhi, A.S.; Harris, W.H.; Muratoglu, O.K. Characterization of irradiated blends of α-tocopherol and UHMWPE // Characterization of irradiated blends of alpha-tocopherol and UHMWPE. Biomaterials 2005, 26, 6657–6663. [Google Scholar] [CrossRef] [PubMed]
- Edidin, A.A.; Herr, M.P.; Villarraga, M.L.; Muth, J.; Yau, S.S.; Kurtz, S.M. Accelerated aging studies of UHMWPE. I. Effect of resin, processing, and radiation environment on resistance to mechanical degradation. J. Biomed. Mater. Res. 2002, 61, 312–322. [Google Scholar] [CrossRef] [PubMed]
- ASTM F2977; Standard Test Method for Small Punch Testing of Polymeric Biomaterials Used in Surgical Implants. ASTM International: West Conshohocken, PA, USA, 2013.
- ISO, 14243–1:2009; Implants for Surgery—Wear of Total Knee-Joint Prostheses—Part 1: Loading and Displacement Parameters for Wear-Testing Machines with Load Control and Corresponding Environmental Conditions for Test. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 5834-4:2025; Implants for Surgery—Ultra-High-Molecular-Weight Polyethylene—Part 4: Oxidation Index Measurement Method. International Organization for Standardization: Geneva, Switzerland, 2025.
- ASTM F2214; Standard Test Method for In Situ Determination of Network Parameters of Crosslinked Ultra High Molecular Weight Polyethylene (UHMWPE). ASTM International: West Conshohocken, PA, USA, 2023.
- Affatato, S.; Bracco, P.; Costa, L.; Villa, T.; Quaglini, V.; Toni, A. In vitro wear performance of standard, crosslinked, and vitamin-E-blended UHMWPE. J. Biomed. Mater. Res. A 2012, 100, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.; Ghali, B.W.; Rowell, S.L.; Micheli, B.R.; Lozynsky, A.J.; Muratoglu, O.K. A surface crosslinked UHMWPE stabilized by vitamin E with low wear and high fatigue strength. Biomaterials 2010, 31, 7051–7060. [Google Scholar] [CrossRef] [PubMed]
- Reinitz, S.D.; Currier, B.H.; Levine, R.A.C.; Collier, J.P.; Van Citters, D.W. Oxidation and other property changes of a remelted highly crosslinked UHMWPE in retrieved tibial bearings. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Mulliez, M.A.; Schilling, C.; Grupp, T.M. Influence of Irradiation Temperature on Oxidative and Network Properties of X-Ray Cross-Linked Vitamin E Stabilized UHMWPE for Hip Arthroplasty. BioMed Res. Int. 2020, 2020, 2568428. [Google Scholar] [CrossRef] [PubMed]
- McEwen, H.M.J.; Barnett, P.I.; Bell, C.J.; Farrar, R.; Auger, D.D.; Stone, M.H.; Fisher, J. The influence of design, materials and kinematics on the in vitro wear of total knee replacements. J. Biomech. 2005, 38, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Morlock, M.; Schneider, E.; Bluhm, A.; Vollmer, M.; Bergmann, G.; Müller, V.; Honl, M. Duration and frequency of every day activities in total hip patients. J. Biomech. 2001, 34, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Orozco, D.A.; Wimmer, M.A. The Impact of Daily Physical Activities on TKR Wear: Einfluß der Tagesaktivität auf den Verschleiß von Knieendoprothesen. Biomaterialien 2010, 11, 107. [Google Scholar]
- ISO 14243–2:2009; Implants for Surgery—Wear of Total Knee-Joint Prostheses—Part 2: Methods of Measurement. International Organization for Standardization: Geneva, Switzerland, 2009.
- Harman, M.; Cristofolini, L.; Erani, P.; Stea, S.; Viceconti, M. A pictographic atlas for classifying damage modes on polyethylene bearings. J. Mater. Sci. Mater. Med. 2011, 22, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, S.; Klapperich, C.; Short, J.; Jani, S.; Ries, M.; Pruitt, L. Comparison of three joint simulator wear debris isolation techniques: Acid digestion, base digestion, and enzyme cleavage. J. Biomed. Mater. Res. 2001, 56, 245–249. [Google Scholar] [CrossRef] [PubMed]
- ASTM F1877; Standard Practice for Characterization of Particles. ASTM International: West Conshohocken, PA, USA, 2016.
- Kurtz, S.M.; Pruitt, L.A.; Jewett, C.W.; Foulds, J.R.; Edidin, A.A. Radiation and chemical crosslinking promote strain hardening behavior and molecular alignment in ultra high molecular weight polyethylene during multi-axial loading conditions. Biomaterials 1999, 20, 1449–1462. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.J.; Currier, B.H.; Mayor, M.B.; Collier, J.P. Gamma-irradiation sterilization in an inert environment: A partial solution. Clin. Orthop. Relat. Res. 2012, 470, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.J.; Walker, P.S.; Abeysundera, M.R.; Simmons, J.M.; King, P.M.; Blunn, G.W. Effect of oxidation on delamination of ultrahigh-molecular-weight polyethylene tibial components. J. Arthroplast. 1998, 13, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Premnath, V.; Harris, W.H.; Jasty, M.; Merrill, E.W. Gamma sterilization of UHMWPE articular implants: An analysis of the oxidation problem. Biomaterials 1996, 17, 1741–1753. [Google Scholar] [CrossRef] [PubMed]
- Currier, B.H.; Currier, J.H.; Mayor, M.B.; Lyford, K.A.; Van Citters, D.W.; Collier, J.P. In vivo oxidation of gamma-barrier-sterilized ultra-high-molecular-weight polyethylene bearings. J. Arthroplast. 2007, 22, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Spece, H.; Schachtner, J.T.; MacDonald, D.W.; Klein, G.R.; Mont, M.A.; Lee, G.-C.; Kurtz, S.M. Reasons for Revision, Oxidation, and Damage Mechanisms of Retrieved Vitamin E-Stabilized Highly Crosslinked Polyethylene in Total Knee Arthroplasty. J. Arthroplast. 2019, 34, 3088–3093. [Google Scholar] [CrossRef] [PubMed]
- Fung, M.; Bowsher, J.G.; Van Citters, D.W. Variation of mechanical properties and oxidation with radiation dose and source in highly crosslinked remelted UHMWPE. J. Mech. Behav. Biomed. Mater. 2018, 82, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Medel, F.; Kurtz, S.; MacDonald, D.; Pascual, F.J.; Puértolas, J.A. Does cyclic stress play a role in highly crosslinked polyethylene oxidation? Clin. Orthop. Relat. Res. 2015, 473, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Rowell, S.L.; Reyes, C.R.; Hopper, R.H.; Engh, C.A.; Muratoglu, O.K. Do total hip arthroplasty polyethylene liners without free radicals oxidize in vivo or ex vivo? J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Muratoglu, O.K.; Evans, M.; Edidin, A.A. Advances in the processing, sterilization, and crosslinking of ultra-high molecular weight polyethylene for total joint arthroplasty. Biomaterials 1999, 20, 1659–1688. [Google Scholar] [CrossRef] [PubMed]
- Edidin, A.A.; Villarraga, M.L.; Herr, M.P.; Muth, J.; Yau, S.S.; Kurtz, S.M. Accelerated aging studies of UHMWPE. II. Virgin UHMWPE is not immune to oxidative degradation J. Biomed. Mater. Res. 2002, 61, 323–329. [Google Scholar] [CrossRef]
- Costa, L.; Luda, M.P.; Trossarelli, L.; Del Brach Prever, E.M.; Crova, M.; Gallinaro, P. Oxidation in orthopaedic UHMWPE sterilized by gamma-radiation and ethylene oxide. Biomaterials 1998, 19, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Jacobson, K.; Bracco, P.; Del Brach Prever, E.M. Oxidation of orthopaedic UHMWPE. Biomaterials 2002, 23, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- ASTM F2102; Guide for Evaluating the Extent of Oxidation in Polyethylene Fabricated Forms Intended for Surgical Implants. ASTM International: West Conshohocken, PA, USA, 2017.
- MacDonald, D.; Hanzlik, J.; Sharkey, P.; Parvizi, J.; Kurtz, S.M. In vivo oxidation and surface damage in retrieved ethylene oxide-sterilized total knee arthroplasties. Clin. Orthop. Relat. Res. 2012, 470, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, D.; Sakona, A.; Ianuzzi, A.; Rimnac, C.M.; Kurtz, S.M. Do first-generation highly crosslinked polyethylenes oxidize in vivo? Clin. Orthop. Relat. Res. 2011, 469, 2278–2285. [Google Scholar] [CrossRef] [PubMed]
- Williams, I.R.; Mayor, M.B.; Collier, J.P. The impact of sterilization method on wear in knee arthroplasty. Clin. Orthop. Relat. Res. 1998, 356, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Oonishi, H.; Kim, S.C.; Kyomoto, M.; Masuda, S.; Asano, T.; Clarke, I.C. Change in UHMWPE properties of retrieved ceramic total knee prosthesis in clinical use for 23 years. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 74, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Parth, M.; Aust, N.; Lederer, K. Studies on the effect of electron beam radiation on the molecular structure of ultra-high molecular weight polyethylene under the influence of alpha-tocopherol with respect to its application in medical implants. J. Mater. Sci. Mater. Med. 2002, 13, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Luda, M.P.; Trossarelli, L.; Del Brach Prever, E.M.; Crova, M.; Gallinaro, P. In vivo UHMWPE biodegradation of retrieved prosthesis. Biomaterials 1998, 19, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Dumbleton, J.; Siskey, R.S.; Wang, A.; Manley, M. Trace concentrations of vitamin E protect radiation crosslinked UHMWPE from oxidative degradation. J. Biomed. Mater. Res. A 2009, 90, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Lambert, B.; Neut, D.; van der Veen, H.C.; Bulstra, S.K. Effects of vitamin E incorporation in polyethylene on oxidative degradation, wear rates, immune response, and infections in total joint arthroplasty: A review of the current literature. Int. Orthop. 2019, 43, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Rowell, S.L.; Oral, E.; Muratoglu, O.K. Comparative oxidative stability of α-tocopherol blended and diffused UHMWPEs at 3 years of real-time aging. J. Orthop. Res. 2011, 29, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Currier, B.H.; Van Citters, D.W. A Novel Technique for Assessing Antioxidant Concentration in Retrieved UHMWPE. Clin. Orthop. Relat. Res. 2017, 475, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Rowell, S.L.; Muratoglu, O.K. Investigation of surgically retrieved, vitamin E-stabilized, crosslinked UHMWPE implants after short-term in vivo service. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Muratoglu, O.K.; Harris, W.H. Identification and quantification of irradiation in UHMWPE through trans-vinylene yield. J. Biomed. Mater. Res. 2001, 56, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Rezhdo, A.; Reinitz, S.D.; Currier, J.H.; Currier, B.H.; Collier, J.P.; Van Citters, D.W. Correlation Between Small Punch Test and Thin Section Tensile Testing. In Proceedings of the Annual Meeting Orthopaedic Research Society (ORS), New Orleans, LA, USA, 15–18 March 2014. Poster No: 0975. [Google Scholar]
- Kurtz, S.M.; Foulds, J.R.; Jewett, C.W.; Srivastav, S.; Edidin, A.A. Validation of a small punch testing technique to characterize the mechanical behaviour of ultra-high-molecular-weight polyethylene. Biomaterials 1997, 18, 1659–1663. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Jewett, C.W.; Foulds, J.R.; Edidin, A.A. A miniature specimen mechanical testing technique scaled to articulating surface of polyethylene components for total joint arthroplasty. J. Biomed. Mater. Res. 1999, 48, 75–81. [Google Scholar] [CrossRef]
- Mulliez, M.A.; Schilling, C.; Grupp, T.M. Equivalent mechanical properties of X-ray and E-beam cross-linked vitamin E blended ultrahigh molecular weight polyethylene. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2131–2140. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, R.; Moscatelli, M.; Giordano, C.; Siccardi, F.; Cigada, A. Influence of heat treatment on structural, mechanical and wear properties of crosslinked UHMWPE. J. Appl. Biomater. Biomech. 2004, 2, 20–28. [Google Scholar] [PubMed]
- Haider, H.; Walker, P.S.; Bell, C.J.; Blunn, G.W. On the ‘Real’ Input of Prescribed Force-Control Conditions in TKR Simulation and Wear Testing. In Proceedings of the Sixth World Biomaterials Congress, Kamuela, HI, USA, 15–20 May 2000; Available online: https://www.researchgate.net/publication/283463731_On_the_‘real’_input_of_prescribed_force-control_conditions_in_TKR_simulation_and_wear_testing (accessed on 8 June 2025).
- Akagi, M.; Asano, T.; Clarke, I.C.; Niiyama, N.; Kyomoto, M.; Nakamura, T.; Hamanishi, C. Wear and toughness of crosslinked polyethylene for total knee replacements: A study using a simulator and small-punch testing. J. Orthop. Res. 2006, 24, 2021–2027. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Akagi, M.; Clarke, I.C.; Masuda, S.; Ishii, T.; Nakamura, T. Dose effects of cross-linking polyethylene for total knee arthroplasty on wear performance and mechanical properties. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 83, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Tsao, A.; Mintz, L.; McRae, C.R.; Stulberg, S.D.; Wright, T. Failure of the porous-coated anatomic prosthesis in total knee arthroplasty due to severe polyethylene wear. J. Bone Jt. Surg. Am. 1993, 75, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Bloebaum, R.D.; Nelson, K.; Dorr, L.D.; Hofmann, A.A.; Lyman, D.J. Investigation of early surface delamination observed in retrieved heat-pressed tibial inserts. Clin. Orthop. Relat. Res. 1991, 269, 120–127. [Google Scholar] [CrossRef]
- Collier, J.P.; Mayor, M.B.; McNamara, J.L.; Surprenant, V.A.; Jensen, R.E. Analysis of the failure of 122 polyethylene inserts from uncemented tibial knee components. Clin. Orthop. Relat. Res. 1991, 273, 232–242. [Google Scholar] [CrossRef]
- Oonishi, H.; Ueno, M.; Kim, S.C.; Oonishi, H.; Iwamoto, M.; Kyomoto, M. Ceramic versus cobalt-chrome femoral components; wear of polyethylene insert in total knee prosthesis. J. Arthroplast. 2009, 24, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Massin, P. How does total knee replacement technique influence polyethylene wear? Orthop. Traumatol. Surg. Res. 2017, 103, S21–S27. [Google Scholar] [CrossRef] [PubMed]
- Manescu Paltanea, V.; Antoniac, I.; Antoniac, A.; Paltanea, G.; Miculescu, M.; Bita, A.-I.; Laptoiu, S.; Niculescu, M.; Stere, A.; Paun, C.; et al. Failure Analysis of Ultra-High Molecular Weight Polyethylene Tibial Insert in Total Knee Arthroplasty. Materials 2022, 15, 7102. [Google Scholar] [CrossRef] [PubMed]
- Teeter, M.G.; Yuan, X.; Naudie, D.D.R.; Holdsworth, D.W. Technique to quantify subsurface cracks in retrieved polyethylene components using micro-CT. J. Long Term Eff. Med. Implant. 2010, 20, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Won, C.H.; Rohatgi, S.; Kraay, M.J.; Goldberg, V.M.; Rimnac, C.M. Effect of resin type and manufacturing method on wear of polyethylene tibial components. Clin. Orthop. Relat. Res. 2000, 376, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Valič, M.; Milošev, I.; Levašič, V.; Blas, M.; Podovšovnik, E.; Koren, J.; Trebše, R. Linear and Volumetric Polyethylene Wear Patterns after Primary Cruciate-Retaining Total Knee Arthroplasty Failure: An Analysis Using Optical Scanning and Computer-Aided Design Models. Materials 2024, 17, 5007. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Lee, G.Y.; Steklov, N.; Colwell, C.W.; Ezzet, K.A.; D’Lima, D.D. Effect of tibial component varus on wear in total knee arthroplasty. Knee 2012, 19, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, C.B.; Bhutani, P.; Wimmer, M.A. Relationship of surface damage appearance and volumetric wear in retrieved TKR polyethylene liners. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, A.S.; Bailey, H.O.; Hwang, D.-S.; Cha, C.W.; Eror, N.G.; Rubash, H.E. Quantitative analysis of ultrahigh molecular weight polyethylene (UHMWPE) wear debris associated with total knee replacements. J. Biomed. Mater. Res. 2000, 53, 100–110. [Google Scholar] [CrossRef]
- Cyndari, K.I.; Goodheart, J.R.; Miller, M.A.; Oest, M.E.; Damron, T.A.; Mann, K.A. Peri-Implant Distribution of Polyethylene Debris in Postmortem-Retrieved Knee Arthroplasties: Can Polyethylene Debris Explain Loss of Cement-Bone Interlock in Successful Total Knee Arthroplasties? J. Arthroplast. 2017, 32, 2289–2300. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.D.; Hillberry, B.M.; Heck, D.A. Component wear of total knee prostheses using Ti-6A1-4V, titanium nitride coated Ti-6A1-4V, and cobalt-chromium-molybdenum femoral components. J. Biomed. Mater. Res. 1988, 22, 887–903. [Google Scholar] [CrossRef] [PubMed]
- Blunn, G.W.; Walker, P.S.; Joshi, A.; Hardinge, K. The dominance of cyclic sliding in producing wear in total knee replacements. Clin. Orthop. Relat. Res. 1991, 273, 253–260. [Google Scholar] [CrossRef]
- White, S.E.; Whiteside, L.A.; McCarthy, D.S.; Anthony, M.; Poggie, R.A. Simulated knee wear with cobalt chromium and oxidized zirconium knee femoral components. Clin. Orthop. Relat. Res. 1994, 309, 176–184. [Google Scholar]
- Walker, P.S.; Blunn, G.W.; Perry, J.P.; Bell, C.J.; Sathasivam, S.; Andriacchi, T.P.; Paul, J.P.; Haider, H.; Campbell, P.A. Methodology for long-term wear testing of total knee replacements. Clin. Orthop. Relat. Res. 2000, 372, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Deluzio, K.J.; O’Connor, D.O.; Bragdon, C.R.; Muratoglu, O.K.; O’Flynn, H.; Rubash, H.; Jasty, M.; Wyss, U.P.; Harris, W.H. Development of an in Vitro Knee Delamination Model in a Knee Simulator with Physiologie Load and Motion. In Proceedings of the 46th Annual Meeting, Orthopaedic Research Society, Orlando, FL, USA, 12–15 March 2000. [Google Scholar]
- Muratoglu, O.K.; Bragdon, C.R.; O’Connor, D.; Perinchief, R.; Travers, J.; Jasty, M.; Rubash, H.E.; Harris, W.H. Markedly Improved Adhesive Wear and Delamination Resistance with a Highly Crosslinked Uhmwpe for use in Total Knee Arthroplasty. In Proceedings of the 27th Annual Meeting of the Society for Biomaterials in Conjunction with the 33rd International Biomaterials Symposium, Seattle, WA, USA, 27 April 2027. [Google Scholar]
- Hermida, J.C.; Fischler, A.; Colwell, C.W.; D’Lima, D.D. The effect of oxidative aging on the wear performance of highly crosslinked polyethylene knee inserts under conditions of severe malalignment. J. Orthop. Res. 2008, 26, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Saikko, V. Effect of shelf versus accelerated aging of UHMWPE on delamination in knee wear simulation. Tribol. Int. 2014, 73, 10–16. [Google Scholar] [CrossRef]
- Ungethüm, M.; Stallforth, H. Der Einfluss der Oberflächenrauhigkeit und der Form der Gleitflächen auf das tribologische Verhalten von Kniegelenkendroprothesen. Biomed. Tech. 1982, 27, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Burnett, R.S.J.; Biggerstaff, S.; Currier, B.H.; Collier, J.P.; Barrack, R.L. Unilateral tibial polyethylene liner failure in bilateral total knee arthroplasty--bilateral retrieval analysis at 8 years. J. Arthroplast. 2007, 22, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Derr, T.; MacDonald, D.W.; Malkani, A.L.; Mont, M.A.; Piuzzi, N.S.; Kurtz, S.M. Oxidation and Damage Mechanisms of 2nd Generation Highly Cross-Linked Polyethylene Tibial Inserts. J. Arthroplast. 2024, 39, 3084–3091. [Google Scholar] [CrossRef] [PubMed]
- Reinitz, S.D.; Currier, B.H.; Van Citters, D.W.; Levine, R.A.; Collier, J.P. Oxidation and other property changes of retrieved sequentially annealed UHMWPE acetabular and tibial bearings. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Tateiwa, T.; Takahashi, Y. Vitamin E-stabilized highly crosslinked polyethylenes: The role and effectiveness in total hip arthroplasty. J. Orthop. Sci. 2017, 22, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Bitsch, R.G.; Loidolt, T.; Heisel, C.; Ball, S.; Schmalzried, T.P. Reduction of osteolysis with use of Marathon cross-linked polyethylene. A concise follow-up, at a minimum of five years, of a previous report. J. Bone Jt. Surg. Am. 2008, 90, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Grupp, T.M.; Kaddick, C.; Rieker, C.; Kretzer, J.P.; Fisher, J. Medical & Scientific Research Requirements for the Clinical Introduction of Artificial Joint Arthroplasty Devices: Biotribological Methodologies in Wear Simulation and Wear Debris Characterisation in Hip and Knee Arthroplasty—Review and Introduction of Thresholds for Standard Testing. RESEARCH TOPIC 7. EFORT European Consensus Book. Book Page 60. 2023. Available online: https://www.efort.org/consensus-book/ (accessed on 8 June 2025).
- Engh, C.A.; Zimmerman, R.L.; Hopper, R.H.; Engh, G.A. Can microcomputed tomography measure retrieved polyethylene wear? Comparing fixed-bearing and rotating-platform knees. Clin. Orthop. Relat. Res. 2013, 471, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Currier, J.H.; Currier, B.H.; Abdel, M.P.; Berry, D.J.; Titus, A.J.; Van Citters, D.W. What factors drive polyethylene wear in total knee arthroplasty? results of a large retrieval series. Bone Jt. J. 2021, 103-B, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Archibeck, M.J.; Jacobs, J.J.; Roebuck, K.A.; Glant, T.T. The Basic Science of Periprosthetic Osteolysis. J. Bone Jt. Surg. 2000, 82, 1478. [Google Scholar] [CrossRef]
- Catelas, I.; Wimmer, M.A.; Utzschneider, S. Polyethylene and metal wear particles: Characteristics and biological effects. Semin. Immunopathol. 2011, 33, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Wooley, P.H. How has the introduction of new bearing surfaces altered the biological reactions to byproducts of wear and modularity? Clin. Orthop. Relat. Res. 2014, 472, 3699–3708. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, B.W.; Hallab, N.J.; Hu, N.; McAfee, P.C. Epidural application of spinal instrumentation particulate wear debris: A comprehensive evaluation of neurotoxicity using an in vivo animal model. J. Neurosurg. Spine 2013, 19, 336–350. [Google Scholar] [CrossRef] [PubMed]
- Utzschneider, S.; Lorber, V.; Dedic, M.; Paulus, A.C.; Schröder, C.; Gottschalk, O.; Schmitt-Sody, M.; Jansson, V. Biological activity and migration of wear particles in the knee joint: An in vivo comparison of six different polyethylene materials. J. Mater. Sci. Mater. Med. 2014, 25, 1599–1612. [Google Scholar] [CrossRef] [PubMed]
- Green, T.R.; Fisher, J.; Stone, M.; Wroblewski, B.M.; Ingham, E. Polyethylene particles of a ‘critical size’ are necessary for the induction of cytokines by macrophages in vitro. Biomaterials 1998, 19, 2297–2302. [Google Scholar] [CrossRef] [PubMed]
- Savio, J.A.; Overcamp, L.M.; Black, J. Size and shape of biomaterial wear debris. Clin. Mater. 1994, 15, 101–147. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, A.S.; Jacobs, J.J.; Black, J.; Galante, J.O.; Glant, T.T. Human monocyte response to particulate biomaterials generated in vivo and in vitro. J. Orthop. Res. 1995, 13, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.B.; Besong, A.A.; Green, T.R.; Stone, M.H.; Wroblewski, B.M.; Fisher, J.; Ingham, E. Evaluation of the response of primary human peripheral blood mononuclear phagocytes to challenge within vitro generated clinically relevant UHMWPE particles of known size and dose. J. Biomed. Mater. Res. 2000, 52, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Benz, E.B.; Federman, M.; Godleski, J.J.; Bierbaum, B.E.; Thornhill, T.S.; Spector, M. Transmission electron microscopy of intracellular particles of polyethylene from joint replacement prostheses: Size distribution and cellular response. Biomaterials 2001, 22, 2835–2842. [Google Scholar] [CrossRef] [PubMed]
- Minoda, Y.; Kobayashi, A.; Iwaki, H.; Miyaguchi, M.; Kadoya, Y.; Ohashi, H.; Takaoka, K. Polyethylene wear particle generation in vivo in an alumina medial pivot total knee prosthesis. Biomaterials 2005, 26, 6034–6040. [Google Scholar] [CrossRef] [PubMed]
- Minoda, Y.; Kobayashi, A.; Iwaki, H.; Miyaguchi, M.; Kadoya, Y.; Ohashi, H.; Takaoka, K. Characteristics of polyethylene wear particles isolated from synovial fluid after mobile-bearing and posterior-stabilized total knee arthroplasties. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 71, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Minoda, Y.; Kobayashi, A.; Iwaki, H.; Miyaguchi, M.; Kadoya, Y.; Ohashi, H.; Yamano, Y.; Takaoka, K. Polyethylene wear particles in synovial fluid after total knee arthroplasty. Clin. Orthop. Relat. Res. 2003, 410, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Minoda, Y.; Hata, K.; Iwaki, H.; Ikebuchi, M.; Hashimoto, Y.; Inori, F.; Nakamura, H. No difference in in vivo polyethylene wear particles between oxidized zirconium and cobalt-chromium femoral component in total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Minoda, Y.; Hata, K.; Goto, K.; Itohara, T.; Nakamura, H. Sequentially annealed highly cross-linked polyethylene reduced in vivo wear particle generation in total knee arthroplasty. J. Orthop. Surg. 2017, 25, 2309499017718909. [Google Scholar] [CrossRef] [PubMed]
- Hinarejos, P.; Piñol, I.; Torres, A.; Prats, E.; Gil-Gómez, G.; Puig-Verdie, L. Highly crosslinked polyethylene does not reduce the wear in total knee arthroplasty: In vivo study of particles in synovial fluid. J. Arthroplast. 2013, 28, 1333–1337. [Google Scholar] [CrossRef] [PubMed]
- Iwakiri, K.; Minoda, Y.; Kobayashi, A.; Sugama, R.; Iwaki, H.; Inori, F.; Hashimoto, Y.; Ohashi, H.; Ohta, Y.; Fukunaga, K.; et al. In vivo comparison of wear particles between highly crosslinked polyethylene and conventional polyethylene in the same design of total knee arthroplasties. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Orita, K.; Minoda, Y.; Sugama, R.; Ohta, Y.; Ueyama, H.; Takemura, S.; Nakamura, H. Vitamin E-infused highly cross-linked polyethylene did not reduce the number of in vivo wear particles in total knee arthroplasty. Bone Jt. J. 2020, 102-B, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Kim, J.-S.; Huh, W.; Lee, K.-H. Weight of polyethylene wear particles is similar in TKAs with oxidized zirconium and cobalt-chrome prostheses. Clin. Orthop. Relat. Res. 2010, 468, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Bladen, C.L.; Teramura, S.; Russell, S.L.; Fujiwara, K.; Fisher, J.; Ingham, E.; Tomita, N.; Tipper, J.L. Analysis of wear; wear particles, and reduced inflammatory potential of vitamin E ultrahigh-molecular-weight polyethylene for use in total joint replacement. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Galliera, E.; Ragone, V.; Marazzi, M.G.; Selmin, F.; Banci, L.; Corsi Romanelli, M.M. Vitamin E-stabilized UHMWPE: Biological response on human osteoblasts to wear debris. Clin. Chim. Acta 2018, 486, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Massaccesi, L.; Ragone, V.; Papini, N.; Goi, G.; Corsi Romanelli, M.M.; Galliera, E. Effects of Vitamin E-Stabilized Ultra High Molecular Weight Polyethylene on Oxidative Stress Response and Osteoimmunological Response in Human Osteoblast. Front. Endocrinol. 2019, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Bichara, D.A.; Suhardi, J.; Sheng, P.; Muratoglu, O.K. Effects of vitamin E-diffused highly cross-linked UHMWPE particles on inflammation, apoptosis and immune response against S. aureus. Biomaterials 2017, 143, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Lu, Y.-C.; Chang, T.-K.; Hsiao, I.-L.; Su, Y.-C.; Yeh, S.-T.; Fang, H.-W.; Huang, C.-H. In vivo biological response to highly cross-linked and vitamin e-doped polyethylene—A particle-Induced osteolysis animal study. J. Biomed. Mater. Res. 2016, 104, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Bichara, D.A.; Malchau, E.; Sillesen, N.H.; Cakmak, S.; Nielsen, G.P.; Muratoglu, O.K. Vitamin E-diffused highly cross-linked UHMWPE particles induce less osteolysis compared to highly cross-linked virgin UHMWPE particles in vivo. J. Arthroplast. 2014, 29, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Neuerburg, C.; Loer, T.; Mittlmeier, L.; Polan, C.; Farkas, Z.; Holdt, L.M.; Utzschneider, S.; Schwiesau, J.; Grupp, T.M.; Böcker, W.; et al. Impact of vitamin E-blended UHMWPE wear particles on the osseous microenvironment in polyethylene particle-induced osteolysis. Int. J. Mol. Med. 2016, 38, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
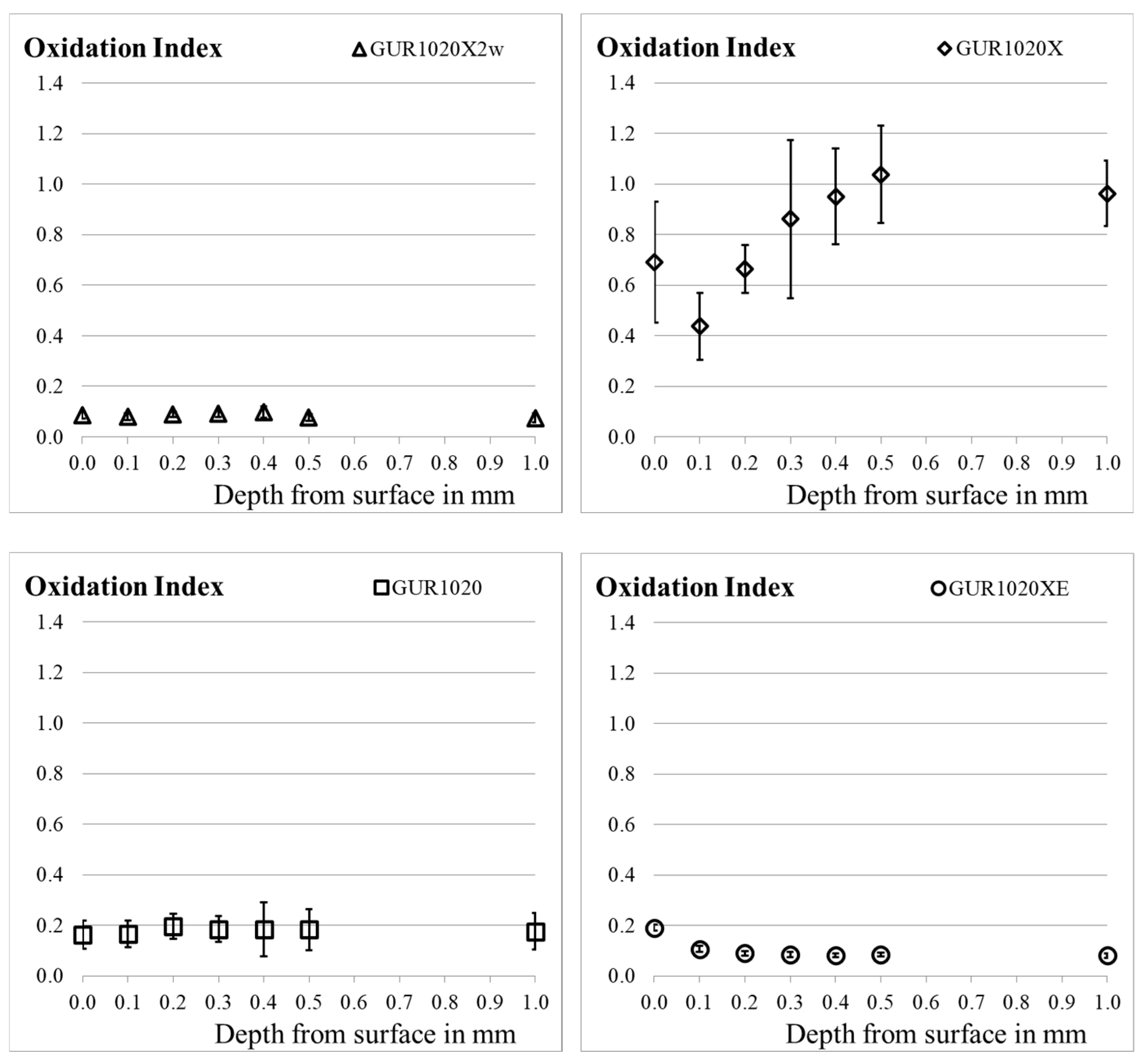
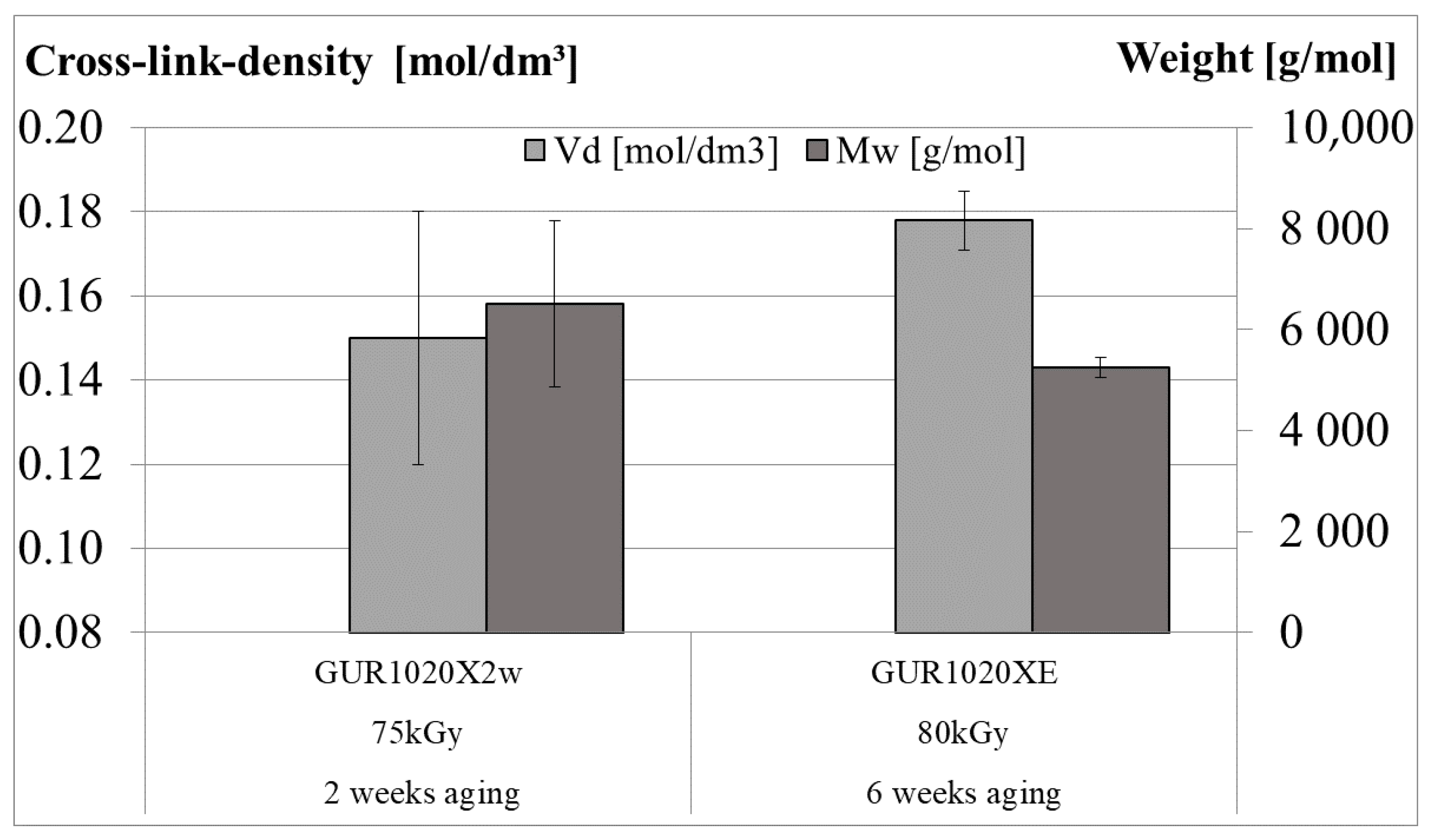

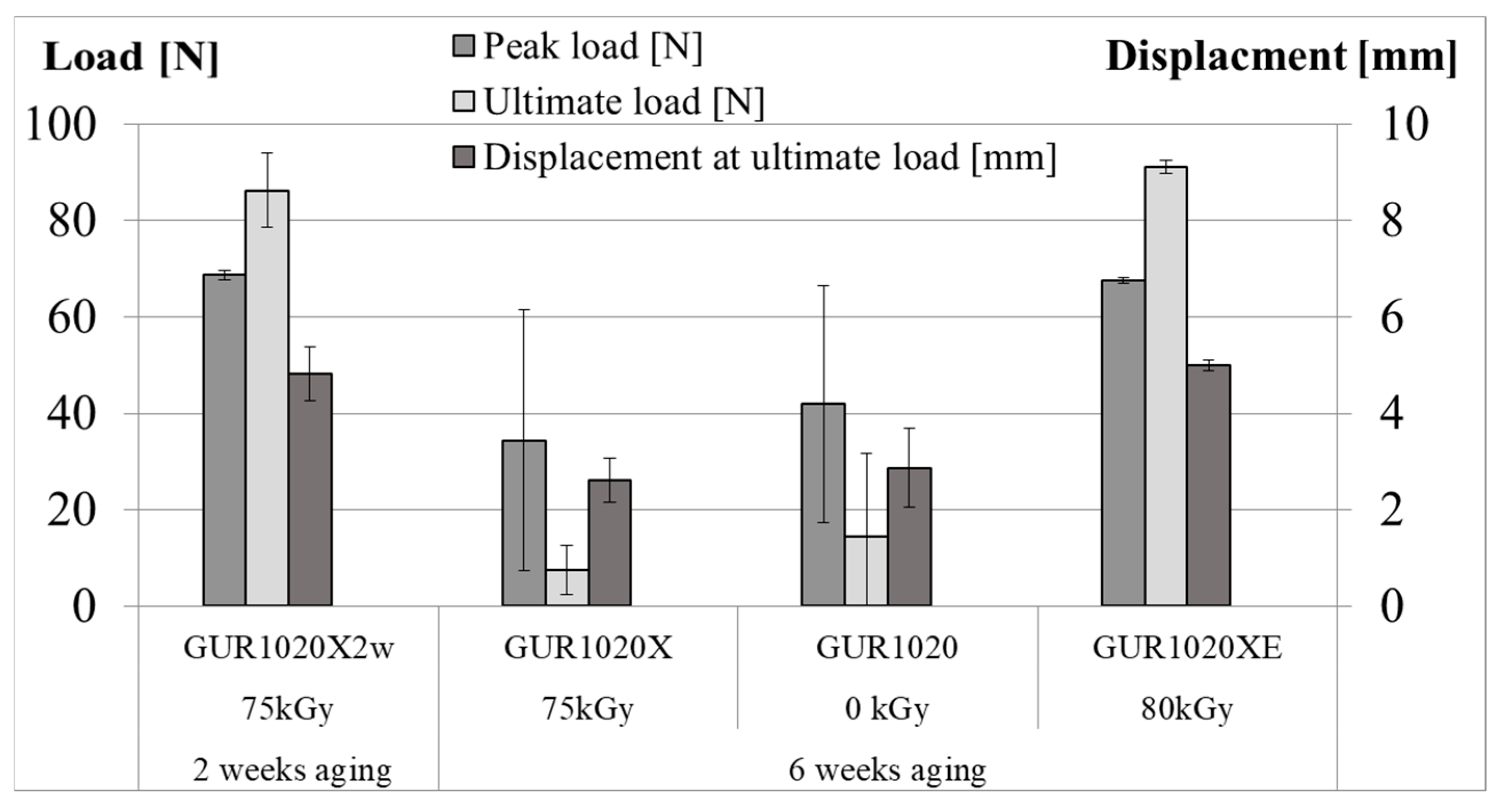

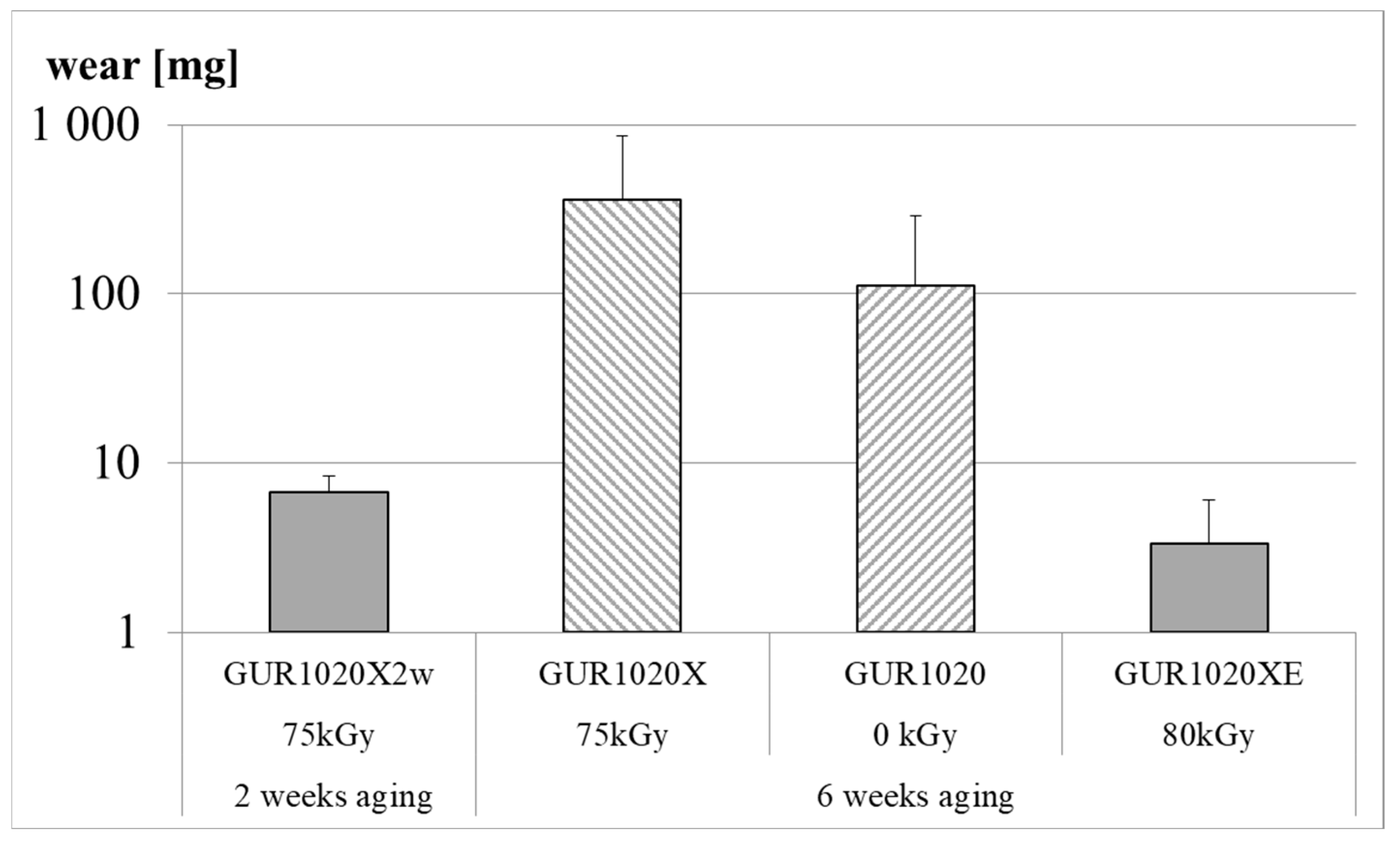
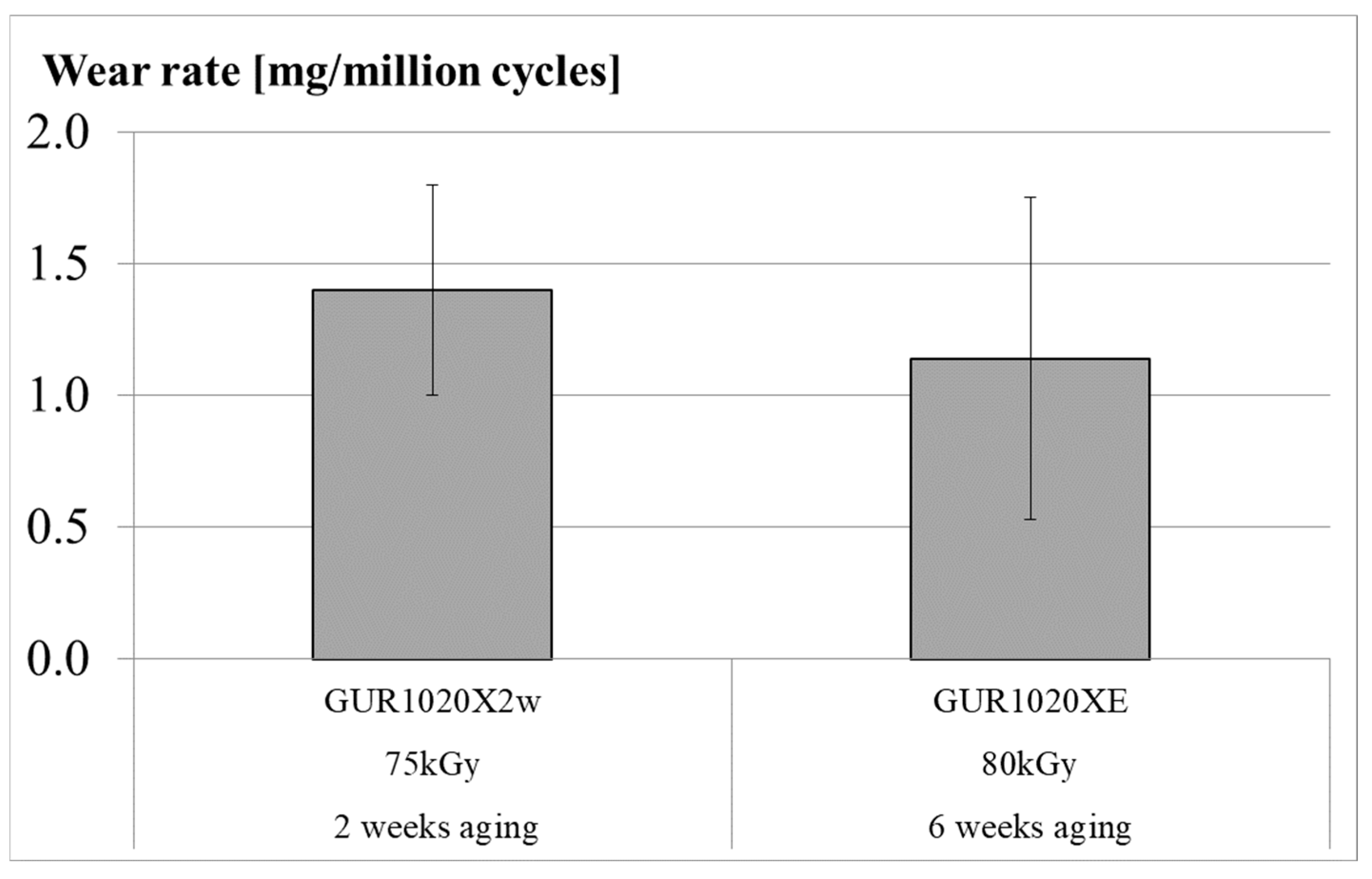
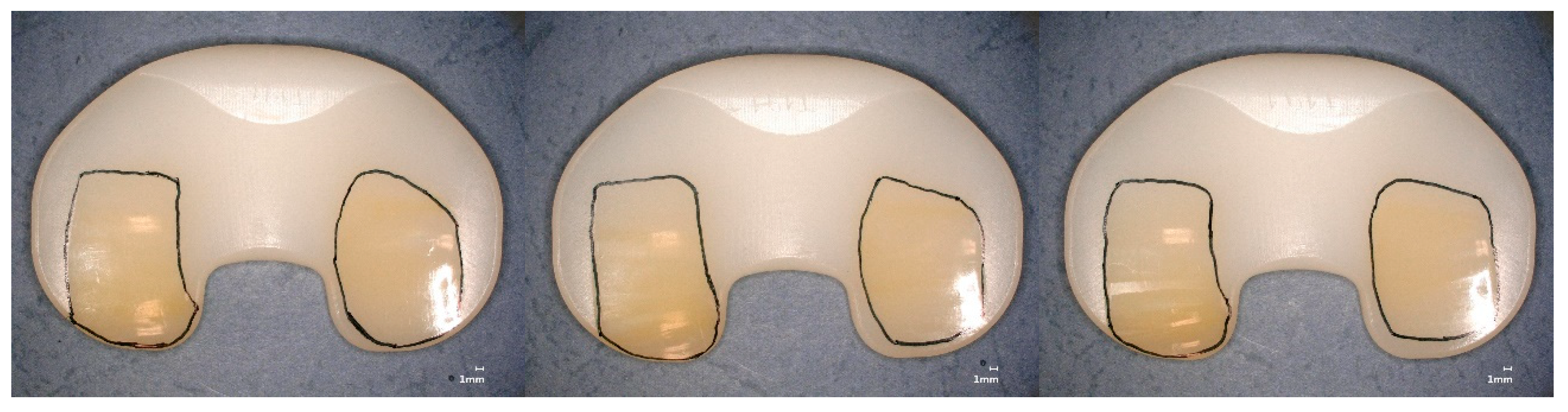
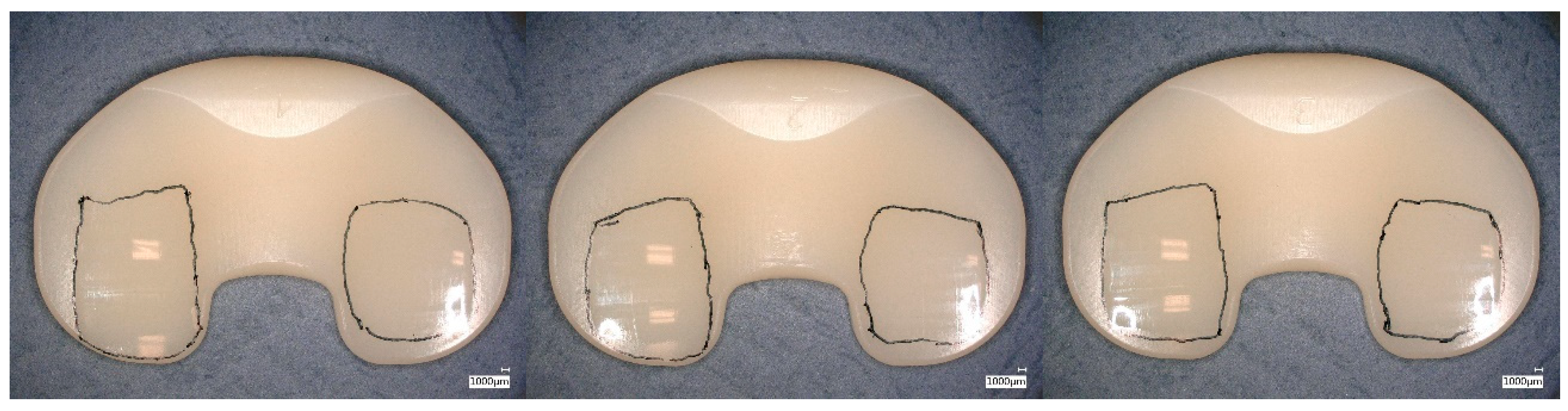
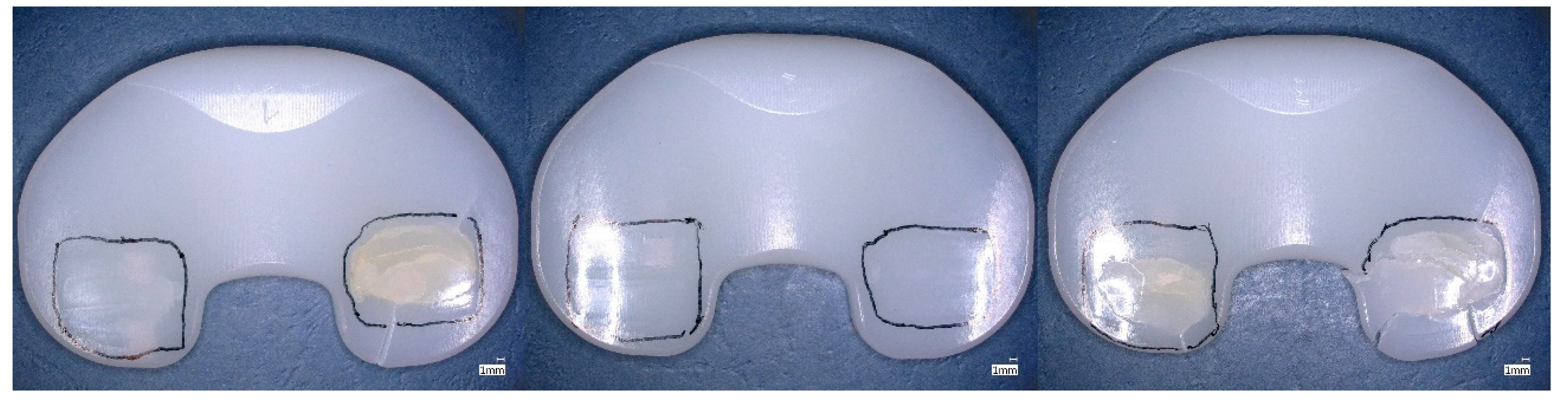

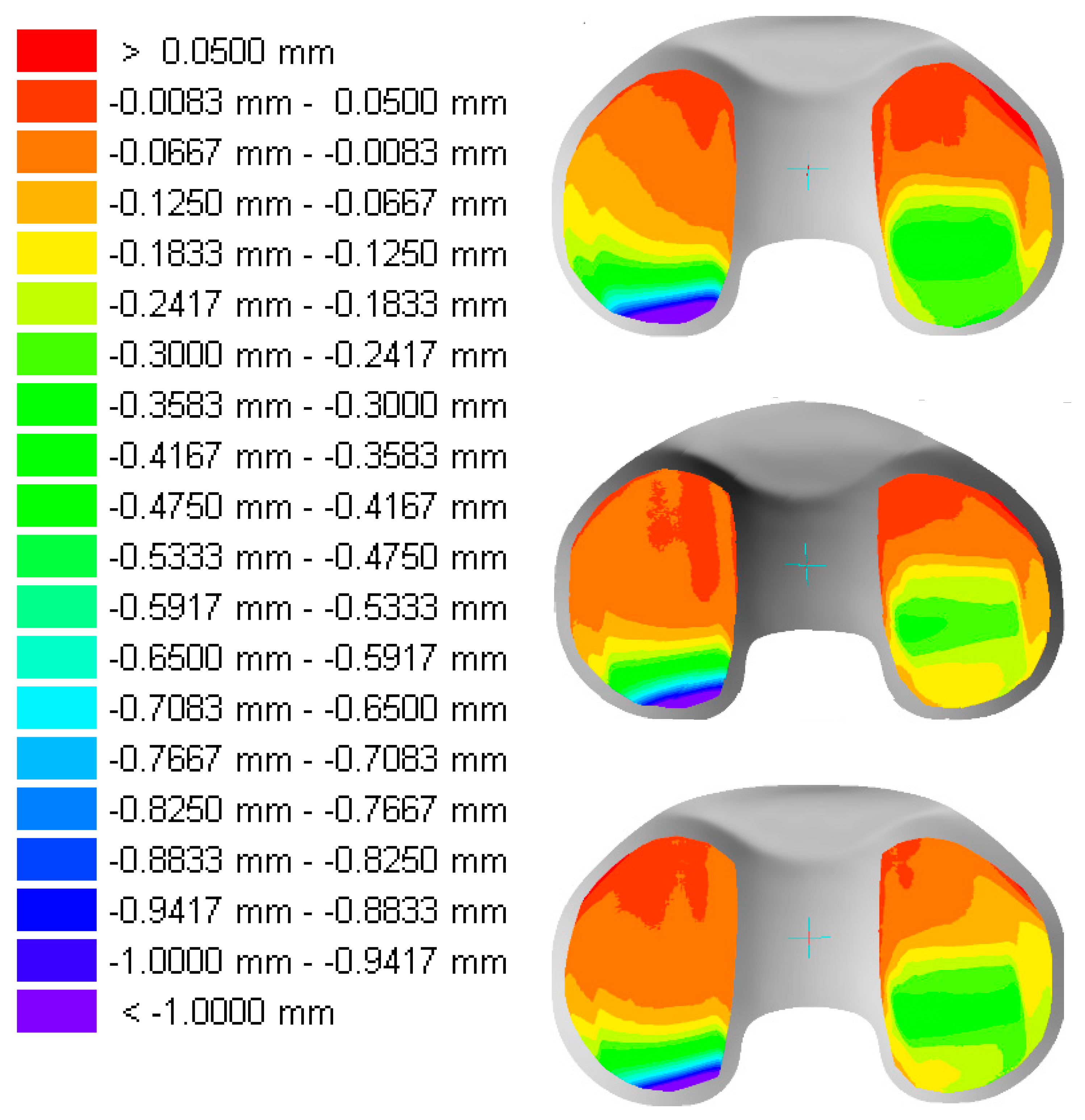
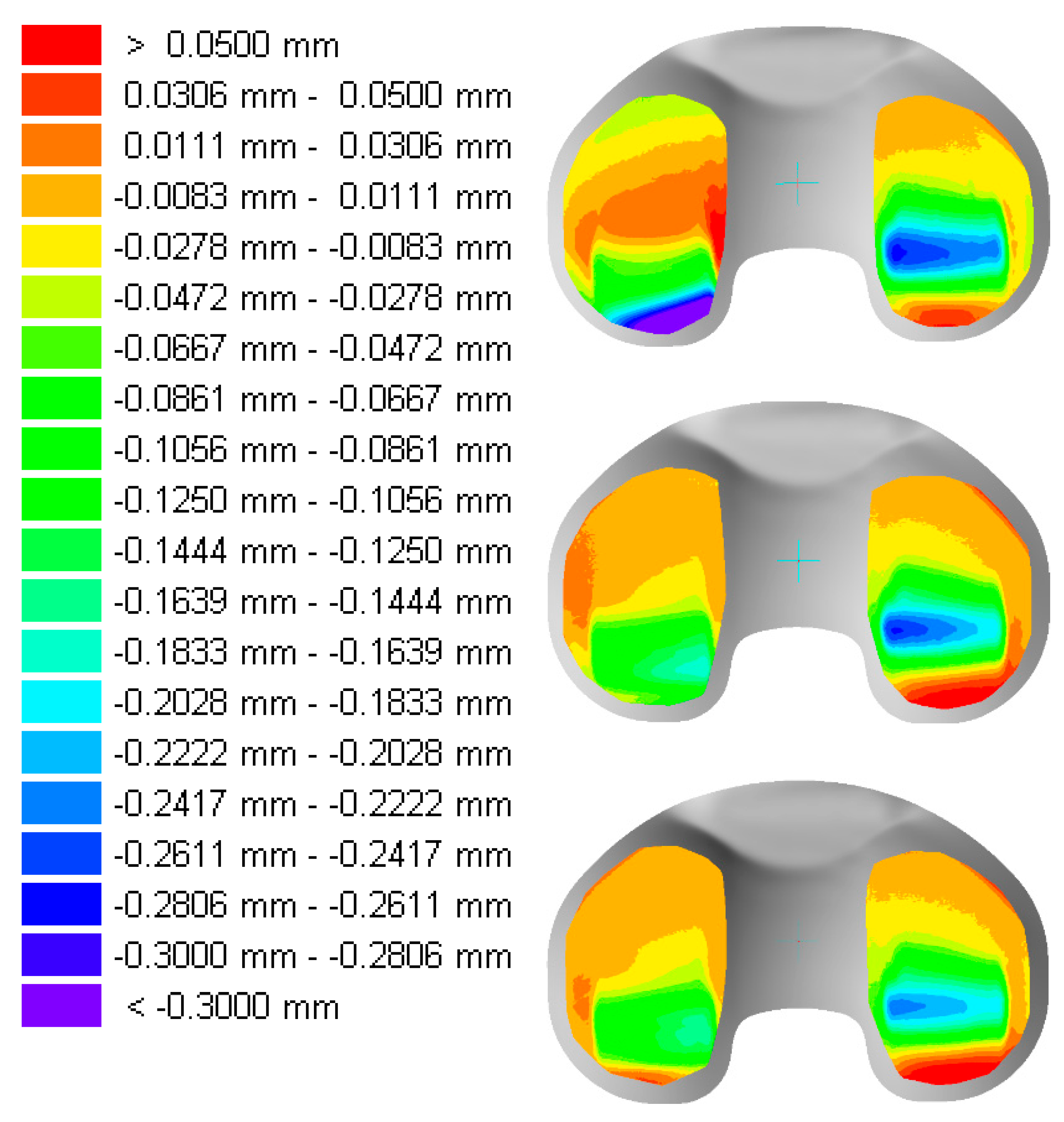
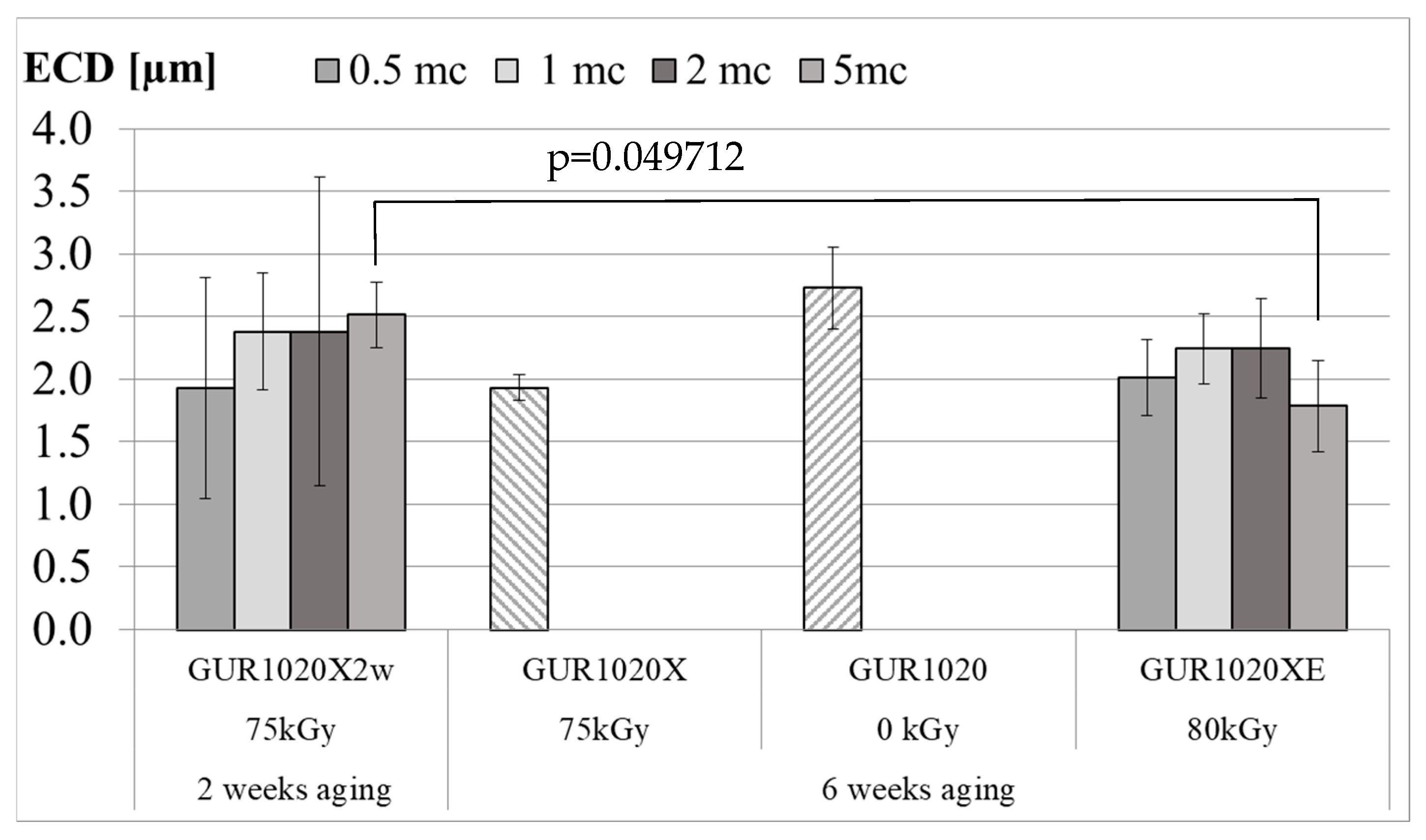
| Material | Irradiation Dose | Irradiation Type | Aging Time | Specimen Description |
|---|---|---|---|---|
| GUR1020X | 75 kGy | Gamma | 2 weeks | GUR1020X2w |
| GUR1020X | 75 kGy | Gamma | 6 weeks | GUR1020X |
| GUR1020 | 0 | --- | 6 weeks | GUR1020 |
| GUR1020XE | 80 kGy | E-beam | 6 weeks | GUR1020XE |
| Cutting Depth [mm] | Material | GUR1020X2w | GUR1020X | GUR1020 |
|---|---|---|---|---|
| 0.0 | GUR1020X | 0.000000 | ||
| GUR1020 | 0.233113 | 0.001802 | ||
| GUR1020XE | 0.002788 | 0.175317 | 0.908496 | |
| 0.1 | GUR1020X | 0.000000 | ||
| GUR1020 | 0.001240 | 0.267626 | ||
| GUR1020XE | 0.244172 | 0.001441 | 0.576584 | |
| 0.2 | GUR1020X | 0.000014 | ||
| GUR1020 | 0.029567 | 0.334696 | ||
| GUR1020XE | 1.000000 | 0.000008 | 0.020569 | |
| 0.3 | GUR1020X | 0.000060 | ||
| GUR1020 | 0.073330 | 0.334696 | ||
| GUR1020XE | 1.000000 | 0.000002 | 0.007362 | |
| 0.4 | GUR1020X | 0.000783 | ||
| GUR1020 | 1.000000 | 0.037405 | ||
| GUR1020XE | 1.000000 | 0.000005 | 0.175317 | |
| 0.5 | GUR1020X | 0.000002 | ||
| GUR1020 | 0.011665 | 0.292953 | ||
| GUR1020XE | 1.000000 | 0.000055 | 0.081654 | |
| 1.0 | GUR1020X | 0.000003 | ||
| GUR1020 | 0.019339 | 0.222483 | ||
| GUR1020XE | 1.000000 | 0.000078 | 0.137023 |
| Cutting Depth [mm] ↓ → | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 1.0 | |
|---|---|---|---|---|---|---|---|
| GUR1020X | 0.0 | 0.236339 | 0.999980 | 0.705326 | 0.212038 | 0.025989 | 0.163247 |
| 0.1 | 0.374179 | 0.002347 | 0.000096 | 0.000003 | 0.000058 | ||
| 0.2 | 0.536987 | 0.120274 | 0.011614 | 0.088948 | |||
| 0.3 | 0.984783 | 0.675876 | 0.968948 | ||||
| 0.4 | 0.984510 | 1.000000 | |||||
| 0.5 | 0.993461 | ||||||
| GUR1020XE | 0.0 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 |
| 0.1 | 0.045180 | 0.001683 | 0.000264 | 0.002330 | 0.000087 | ||
| 0.2 | 0.958683 | 0.774330 | 0.973910 | 0.602857 | |||
| 0.3 | 0.999201 | 1.000000 | 0.990175 | ||||
| 0.4 | 0.997912 | 0.999970 | |||||
| 0.5 | 0.982393 | ||||||
| Significant for p < 0.05 | GUR1020X | GUR1020 | GUR1020XE | |
|---|---|---|---|---|
| Work to failure [mJ] | GUR1020X2w | 0.000017 | 0.000079 | 0.901156 |
| GUR1020X | 0.901065 | 0.000079 | ||
| GUR1020 | 0.000393 | |||
| Peak load [N] | GUR1020X2w | 0.034103 | 0.126024 | 0.999652 |
| GUR1020X | 0.917506 | 0.042128 | ||
| GUR1020 | 0.151105 | |||
| Ultimate load [N] | GUR1020X2w | 0.000000 | 0.000000 | 0.869565 |
| GUR1020X | 0.688361 | 0.000000 | ||
| GUR1020 | 0.000000 | |||
| Displacement at ultimate load [mm] | GUR1020X2w | 0.000014 | 0.000077 | 0.961666 |
| GUR1020X | 0.87641 | 0.000005 | ||
| GUR1020 | 0.000026 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwiesau, J.; Fritz, B.; Bracco, P.; Bergmann, G.; Puente Reyna, A.L.; Schilling, C.; Grupp, T.M. Chemical, Mechanical and Tribological Effects of Artificially Aging up to 6 Weeks on Virgin and Crosslinked UHMWPE Evaluated for a TKR Design. Bioengineering 2025, 12, 793. https://doi.org/10.3390/bioengineering12080793
Schwiesau J, Fritz B, Bracco P, Bergmann G, Puente Reyna AL, Schilling C, Grupp TM. Chemical, Mechanical and Tribological Effects of Artificially Aging up to 6 Weeks on Virgin and Crosslinked UHMWPE Evaluated for a TKR Design. Bioengineering. 2025; 12(8):793. https://doi.org/10.3390/bioengineering12080793
Chicago/Turabian StyleSchwiesau, Jens, Bernhard Fritz, Pierangiola Bracco, Georg Bergmann, Ana Laura Puente Reyna, Christoph Schilling, and Thomas M. Grupp. 2025. "Chemical, Mechanical and Tribological Effects of Artificially Aging up to 6 Weeks on Virgin and Crosslinked UHMWPE Evaluated for a TKR Design" Bioengineering 12, no. 8: 793. https://doi.org/10.3390/bioengineering12080793
APA StyleSchwiesau, J., Fritz, B., Bracco, P., Bergmann, G., Puente Reyna, A. L., Schilling, C., & Grupp, T. M. (2025). Chemical, Mechanical and Tribological Effects of Artificially Aging up to 6 Weeks on Virgin and Crosslinked UHMWPE Evaluated for a TKR Design. Bioengineering, 12(8), 793. https://doi.org/10.3390/bioengineering12080793







