Umbilical Cord Tensile Strength Under Varying Strain Rates
Abstract
1. Introduction
2. Methods
2.1. Data Collection and Patient Selection
2.2. Biomechanical Tests
2.3. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CS | Cesarean section |
| ECM | Extracellular matrix |
| GAG | Glycosaminoglycan |
| GS | Ground substance |
| MSC | Mesenchymal stem cell |
| PAI | Polyamideimide |
| PMMA | Polymethylmethacrylate |
| SEF | Strain energy |
| SLS | Standard Linear Solid |
| UC | Umbilical cord |
| WJ | Wharton’s jelly |
References
- Ferguson, V.L.; Dodson, R.B. Bioengineering aspects of the umbilical cord. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 144 (Suppl. 1), S108–S113. [Google Scholar] [CrossRef] [PubMed]
- Marino, L.; Castaldi, M.A.; Rosamilio, R.; Ragni, E.; Vitolo, R.; Fulgione, C.; Castaldi, S.G.; Serio, B.; Bianco, R.; Guida, M.; et al. Mesenchymal Stem Cells from the Wharton’s Jelly of the Human Umbilical Cord: Biological Properties and Therapeutic Potential. Int. J. Stem Cells 2019, 12, 218–226. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McElreavey, K.D.; Irvine, A.I.; Ennis, K.T.; McLean, W.H. Isolation, culture and characterisation of fibroblast-like cells derived from the Wharton’s jelly portion of human umbilical cord. Biochem. Soc. Trans. 1991, 19, 29S. [Google Scholar] [CrossRef] [PubMed]
- Isaković, J.; Šerer, K.; Barišić, B.; Mitrečić, D. Mesenchymal stem cell therapy for neurological disorders: The light or the dark side of the force? Front. Bioeng. Biotechnol. 2023, 11, 1139359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Du, T.; Zou, X.; Cheng, J.; Wu, S.; Zhong, L.; Ju, G.; Zhu, J.; Liu, G.; Zhu, Y.; Xia, S. Human Wharton’s jelly-derived mesenchymal stromal cells reduce renal fibrosis through induction of native and foreign hepatocyte growth factor synthesis in injured tubular epithelial cells. Stem Cell Res. Ther. 2013, 4, 59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vats, A.; Chaturvedi, P. The Regenerative Power of Stem Cells: Treating Bleomycin-Induced Lung Fibrosis. Stem Cells Cloning 2023, 16, 43–59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lamparelli, E.P.; Ciardulli, M.C.; Giudice, V.; Scala, P.; Vitolo, R.; Dale, T.P.; Selleri, C.; Forsyth, N.R.; Maffulli, N.; Della Porta, G. 3D in-vitro cultures of human bone marrow and Wharton’s jelly derived mesenchymal stromal cells show high chondrogenic potential. Front. Bioeng. Biotechnol. 2022, 10, 986310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scheers, I.; Lombard, C.; Najimi, M.; Sokal, E.M. Cell therapy for the treatment of metabolic liver disease: An update on the umbilical cord derived stem cells candidates. Open Tissue Eng. Regen. Med. J. 2011, 4, 48–53. [Google Scholar] [CrossRef]
- Tamura, M.; Kawabata, A.; Ohta, N.; Uppalapati, L.; Becker, K.G.; Troyer, D. Wharton’s jelly stem cells as agents for cancer therapy. Open Tissue Eng. Regen. Med. J. 2011, 4, 39–47. [Google Scholar] [CrossRef]
- Brunelli, R.; De Spirito, M.; Giancotti, A.; Palmieri, V.; Parasassi, T.; Di Mascio, D.; Flammini, G.; D’Ambrosio, V.; Monti, M.; Boccaccio, A.; et al. The biomechanics of the umbilical cord Wharton Jelly: Roles in hemodynamic proficiency and resistance to compression. J. Mech. Behav. Biomed. Mater. 2019, 100, 103377. [Google Scholar] [CrossRef] [PubMed]
- Gervaso, F.; Boschetti, F.; Pennati, G. Evaluation of the Wharton׳s jelly poroelastic parameters through compressive tests on placental and foetal ends of human umbilical cords. J. Mech. Behav. Biomed. Mater. 2014, 35, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Sherer, D.M.; Al-Haddad, S.; Cheng, R.; Dalloul, M. Current Perspectives of Prenatal Sonography of Umbilical Cord Morphology. Int. J. Womens Health 2021, 13, 939–971. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baldit, A.; Dubus, M.; Sergheraert, J.; Kerdjoudj, H.; Mauprivez, C.; Rahouadj, R. Biomechanical tensile behavior of human Wharton’s jelly. J. Mech. Behav. Biomed. Mater. 2022, 126, 104981. [Google Scholar] [CrossRef] [PubMed]
- Fernando, D.M.G.; Gamage, S.M.K.; Ranmohottige, S.; Weerakkody, I.; Abeyruwan, H.; Parakrama, H. Relationship between the tensile strengths and diameters of human umbilical cords. J. Forensic Leg. Med. 2018, 56, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Navidbakhsh, M. A comparative study on the uniaxial mechanical properties of the umbilical vein and umbilical artery using different stress-strain definitions. Australas. Phys. Eng. Sci. Med. 2014, 37, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Tantius, B.; Rothschild, M.A.; Valter, M.; Michael, J.; Banaschak, S. Experimental studies on the tensile properties of human umbilical cords. Forensic Sci. Int. 2014, 236, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.G.; Ghosh, S.N.; Gupta, A.B. Tensile properties of human umbilical cord. Indian. J. Med. Res. 1984, 79, 538–541. [Google Scholar] [PubMed]
- Sánchez Gutiérrez, J.F.; Olaya-C, M.; Franco, J.A.; Guevara, J.; Garzón-Alvarado, D.A.; Gutiérrez Gómez, M.L. Effect of umbilical cord length on early fetal biomechanics. Comput. Methods Biomech. Biomed. Eng. 2021, 24, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Pennati, G.; Laganà, K.; Gervaso, F.; Rigano, S.; Ferrazzi, E. How Do Cord Compressions Affect the Umbilical Venous Flow Resistance? An In Vitro Investigation of the Biomechanical Mechanisms. Cardiovasc. Eng. Technol. 2013, 4, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Dado, G.M.; Dobrin, P.B.; Mrkvicka, R.S. Venous flow through coiled and noncoiled umbilical cords. Effects of external compression, twisting and longitudinal stretching. J. Reprod. Med. 1997, 42, 576–580. [Google Scholar] [PubMed]
- Georgiou, H.M.; Rice, G.E.; Walker, S.P.; Wein, P.; Gude, N.M.; Permezel, M. The effect of vascular coiling on venous perfusion during experimental umbilical cord encirclement. Am. J. Obstet. Gynecol. 2001, 184, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Pennati, G. Biomechanical properties of the human umbilical cord. Biorheology 2001, 38, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Crichton, J.L. Tensile strength of the umbilical cord. Am. J. Obstet. Gynecol. 1973, 115, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Spann, W.; Englert, H.M. Experimentelle Untersuchungen der Zerreissfestigkeit der Nabelschnur [Experimental studies on the laceration resistance of the umbilical cord]. Dtsch. Z. Gesamte Gerichtl Med. 1967, 59, 196–200. (In German) [Google Scholar] [PubMed]
- Langer, H. Zug an der Nabelschnur [The pull on the umbilical cord]. Dtsch. Med. Wochenschr. 1968, 93, 246. (In German) [Google Scholar] [CrossRef] [PubMed]
- Pettersson, K.; Yousaf, K.; Ranstam, J.; Westgren, M.; Ajne, G. Predictive value of traction force measurement in vacuum extraction: Development of a multivariate prognostic model. PLoS ONE 2017, 12, e0171938. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Vacca, A. Vacuum-assisted delivery: An analysis of traction force and maternal and neonatal outcomes. Aust. N. Z. J. Obstet. Gynaecol. 2006, 46, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, K.; Ajne, J.; Yousaf, K.; Sturm, D.; Westgren, M.; Ajne, G. Traction force during vacuum extraction: A prospective observational study. BJOG 2015, 122, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- ACOG. Practice Bulletin No. 154: Operative Vaginal Delivery. Obstet. Gynecol. 2015, 126, e56–e65. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, G.A. Biomechanics of Soft Tissue. In Handbook of Material Behaviour—Nonlinear Models and Properties; Composite Media, Ed.; Academic Press: Boston, MA, USA, 2001; Volume III, Multiphysics Behaviors; Chapter 10; pp. 1049–1063. [Google Scholar]
- Holzapfel, G.A.; Ogden, R.W.; Sherifova, S. On fibre dispersion modelling of soft biological tissues: A review. Proc. Math. Phys. Eng. Sci. 2019, 475, 20180736. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tai, Y.; Woods, E.L.; Dally, J.; Kong, D.; Steadman, R.; Moseley, R.; Midgley, A.C. Myofibroblasts: Function, Formation, and Scope of Molecular Therapies for Skin Fibrosis. Biomolecules 2021, 11, 1095. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takechi, K.; Kuwabara, Y.; Mizuno, M. Ultrastructural and immunohistochemical studies of Wharton’s jelly umbilical cord cells. Placenta 1993, 14, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Sobolewski, K.; Bańkowski, E.; Chyczewski, L.; Jaworski, S. Collagen and glycosaminoglycans of Wharton’s jelly. Biol. Neonate 1997, 71, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Pisárčik, M.; Bakoš, D.; Čeppan, M. Non-Newtonian properties of hyaluronic acid aqueous solution. Colloids Surf. A Physicochem. Eng. Aspects 1995, 97, 197–202. [Google Scholar] [CrossRef]
- Subashini, G.; Anitha, C.; Gopinath, G.; Ramyathangam, K. A Longitudinal Analytical Study on Umbilical Cord Coiling Index as a Predictor of Pregnancy Outcome. Cureus 2023, 15, e35680. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dubos, C.; Delanaud, S.; Brenac, W.; Chahin Yassin, F.; Carpentier, M.; Tourneux, P. The newborn infant’s thermal environment in the delivery room when skin-to-skin care has to be interrupted. J. Matern. Fetal Neonatal Med. 2022, 35, 3707–3713. [Google Scholar] [CrossRef] [PubMed]
- Laptook, A.R.; Watkinson, M. Temperature management in the delivery room. Semin. Fetal Neonatal Med. 2008, 13, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.; Di Martino, D.D.; Ferrazzi, E.M.; Stampalija, T. Umbilical vein blood flow: State-of-the-art. J. Clin. Ultrasound 2023, 51, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.H. Silent Risk: Issues About the Human Umbilical Cord, 2nd ed.; Xlibris Corporation: Bloomington, IN, USA, 2014; 238p, ISBN 1499039115/9781499039115. [Google Scholar]
- Richeton, J.; Ahzi, S.; Vecchio, K.S.; Jiang, F.C.; Adharapurapu, R.R. Influence of temperature and strain rate on the mechanical behavior of three amorphous polymers: Characterization and modeling of the compressive yield stress. Int. J. Solids Struct. 2006, 43, 2318–2335. [Google Scholar] [CrossRef]
- Holzapfel, G.A.; Gasser, T.C.; Ogden, R.W.; New, A. Constitutive framework for arterial wall mechanics and a comparative study of material models. J. Elast. 2000, 61, 1–48. [Google Scholar] [CrossRef]
- Marino, L.; Castaldi, M.A.; Fulgione, C.; Castaldi, S.G.; Manzo, P.; Giudice, V.; Picone, F.; Campitiello, M.R.; Polichetti, M.; Guida, M.; et al. Reduced Proliferative Potential with Conserved Stem/Stromal Phenotype of Human Umbilical Cord Mesenchymal Stem Cells in Placental Syndromes: A Prospective Cohort Study. Clin. Exp. Obstet. Gynecol. 2023, 50, 196. [Google Scholar] [CrossRef]
- Cowman, M.K.; Schmidt, T.A.; Raghavan, P.; Stecco, A. Viscoelastic Properties of Hyaluronan in Physiological Conditions. F1000Research 2015, 4, 622. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, X.; Yu, D.; Barrera, O. Chapter Three—Mechanics constitutive models for viscoelastic solid materials: Development and a critical review. In Advances in Applied Mechanics; Bordas, S.P.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 56, pp. 189–321. [Google Scholar]
- Epaarachchi, J.A. Chapter 17—The effect of viscoelasticity on fatigue behaviour of polymer matrix composites. In Woodhead Publishing Series in Composites Science and Engineering, Creep and Fatigue in Polymer Matrix Composites; Guedes, R.M., Ed.; Woodhead Publishing: England, UK, 2011; pp. 492–513. [Google Scholar]
- Ouis, D. Characterization of polymers by means of a standard viscoelastic model and fractional derivate calculus. Int. J. Polym. Mater. Polym. Biomater. 2004, 53, 633–644. [Google Scholar] [CrossRef]

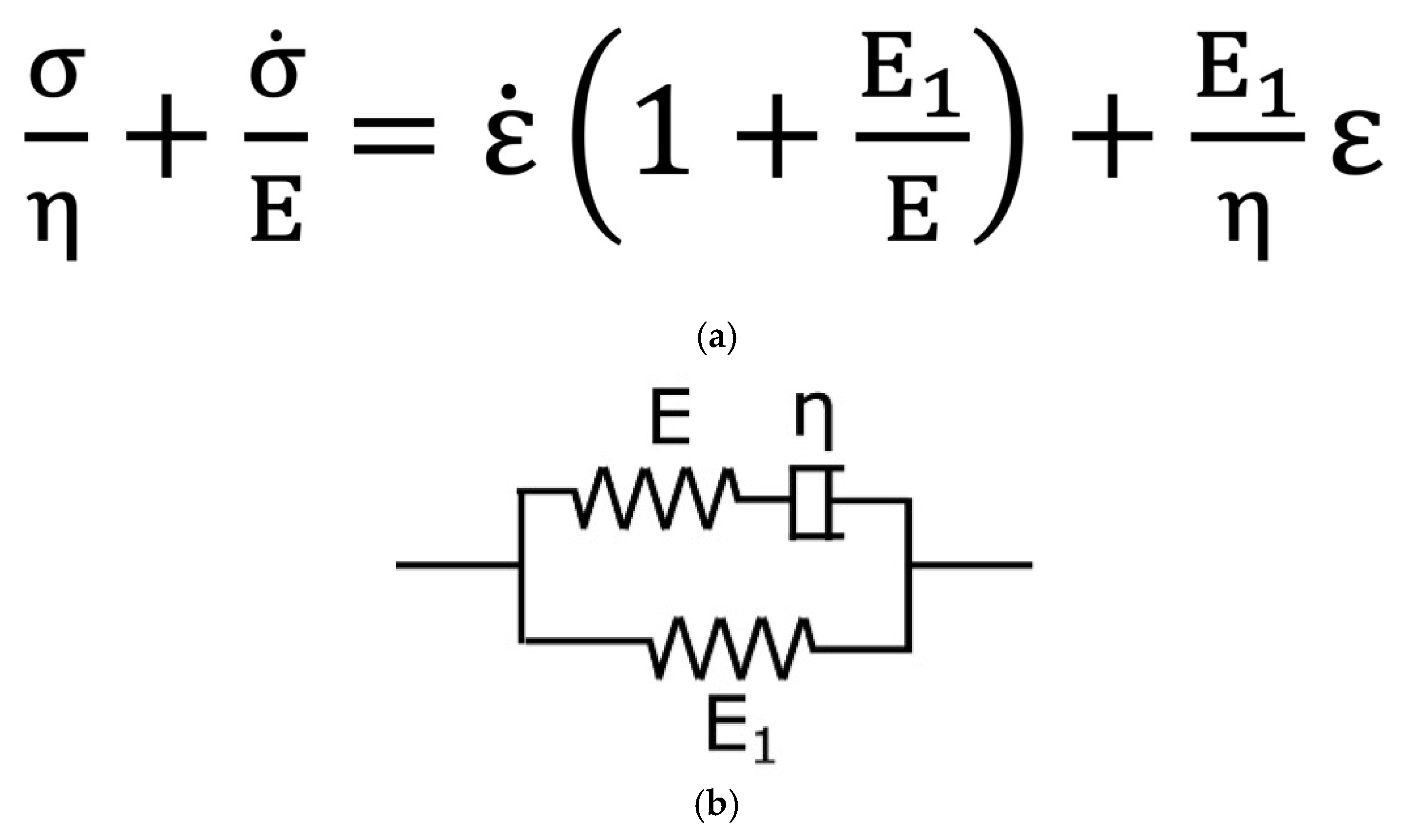
| (n = 5) | |
|---|---|
| Age | 36 ± 1.2 [32–39] |
| Height (m) | 1.62 ± 0.02 [1.56–1.67] |
| Weight (kg) | 91.8 ± 5.5 [76.4–107.1] |
| BMI (kg/m2) | 34.8 ± 1.5 [30.7–38.8] |
| Previous surgery | 40% |
| Parity | 0.8 ± 0.3 [0–1.8] |
| Gestational age | 37 ± 4 [35.5–38.1] |
| Neonatal weight (grams) | 3106 ± 140 [2716–3495] |
| Biomechanical Parameters § | Group A (0.2 1/min) (n = 10) | Group B (0.3 1/min) (n = 7) | Group C (0.4 1/min) (n = 12) | ANOVA Values | |
|---|---|---|---|---|---|
| p (*) | F (**) | ||||
| Strain Rate, 1/min | 0.20 | 0.30 | 0.40 | - | - |
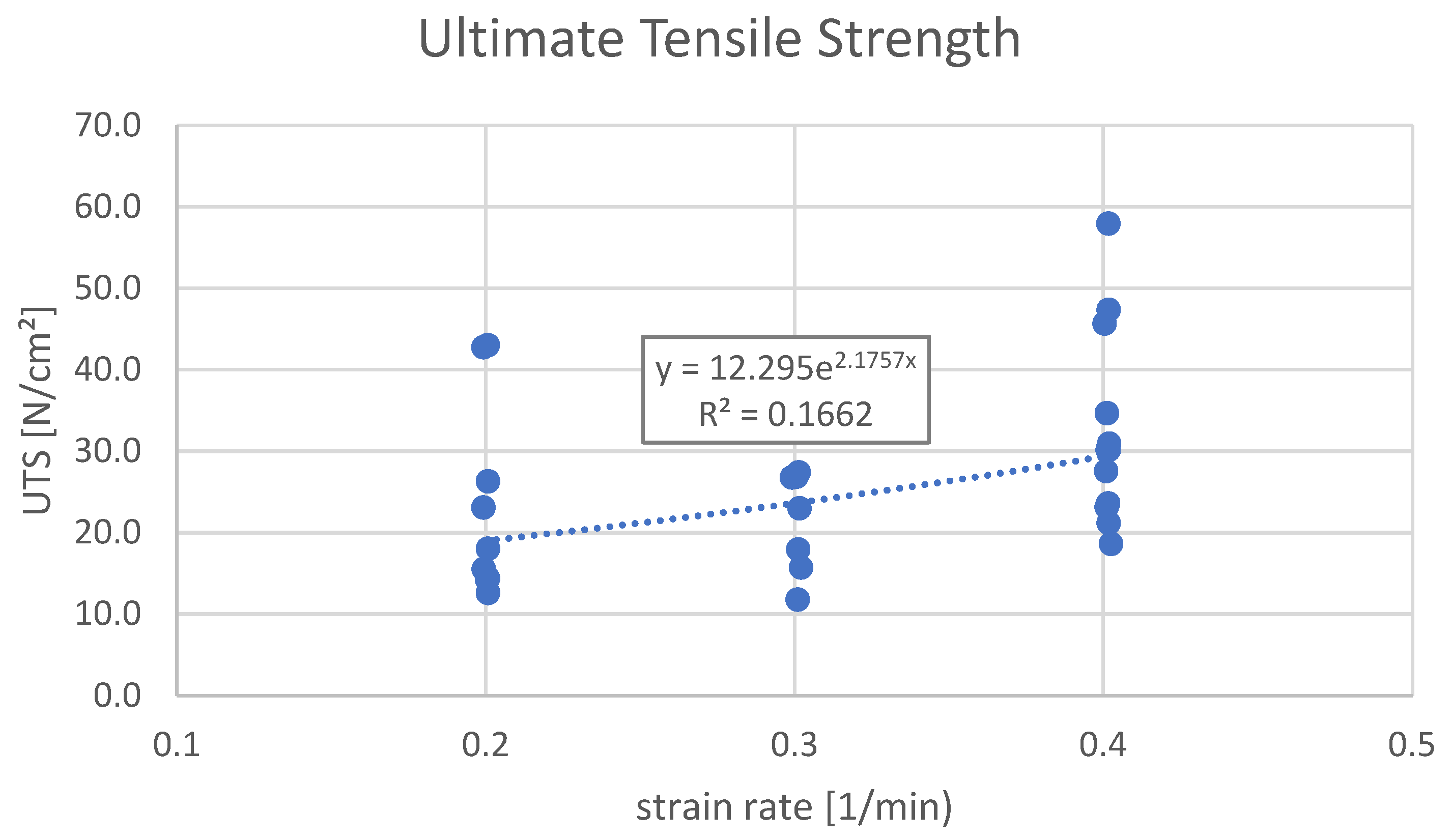
| Ultimate Tensile Strength (UTS) vs. Strain Rate, N/cm2 | 0.047 | 3.439 | |||
| AVG value | 22.2 | 21.3 | 32.6 | ||
| MIN, MAX values | 12.6, 43.0 | 11.8, 27.4 | 18.6, 57.9 | ||
| Elongation at UTS (Elo@UTS) vs. Strain Rate, % | 0.103 | 2.483 | |||
| AVG value | 34 | 17 | 22 | ||
| MIN, MAX values | 12, 89 | 10, 28 | 5, 58 | ||
| Elongation at Break (Elo@Break) vs. Strain Rate, % | 0.066 | 3.032 | |||
| AVG value | 55 | 35 | 36 | ||
| MIN, MAX values | 23, 103 | 21, 55 | 11, 76 | ||
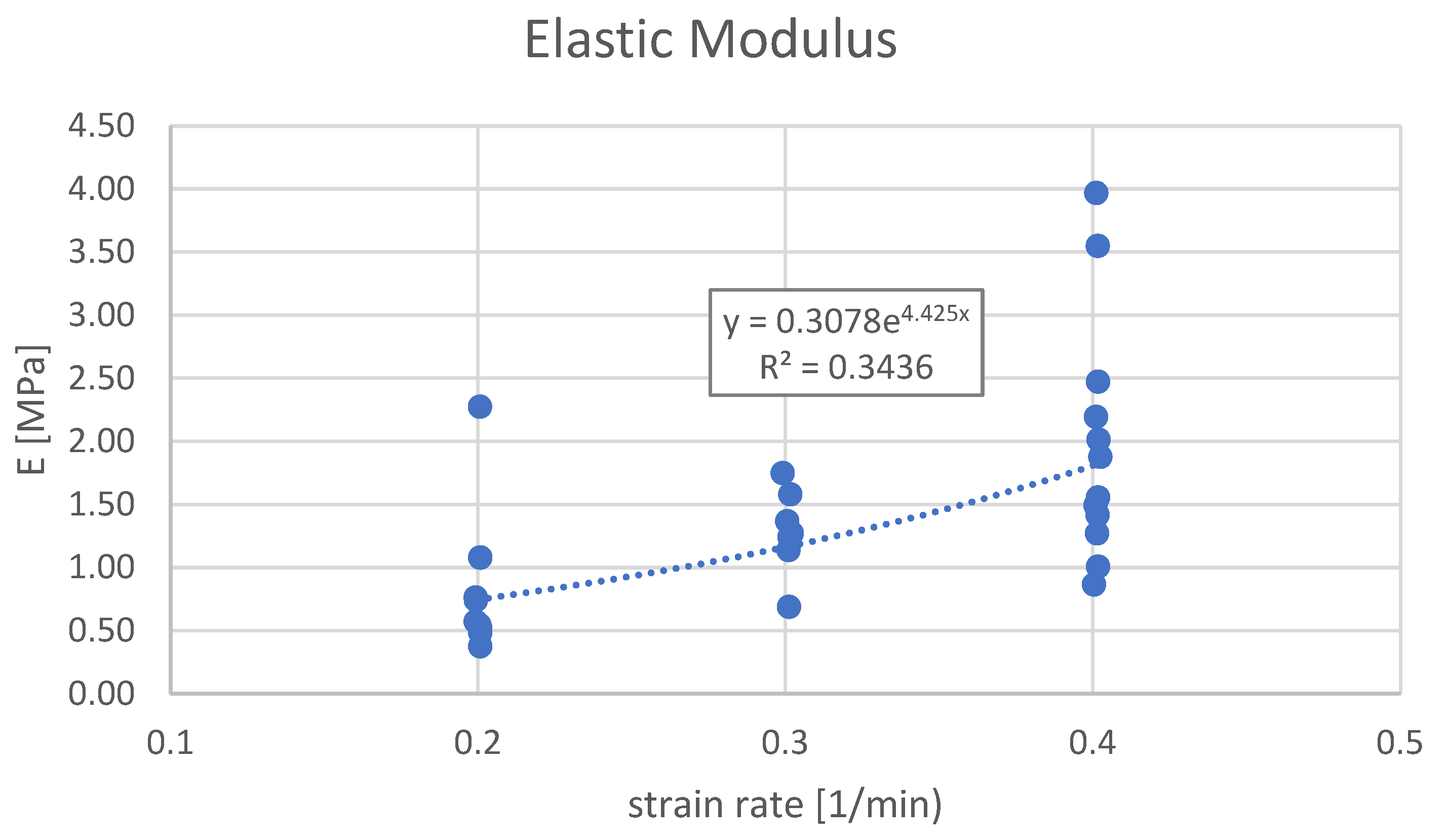
| Elastic Modulus (E) vs. Strain Rate, MPa | 0.004 | 6.800 | |||
| AVG value | 0.84 | 1.29 | 1.97 | ||
| MIN, MAX values | 0.37, 2.287 | 0.69, 1.75 | 0.86, 3.97 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castaldi, M.A.; Villa, P.; Castaldi, A.; Castaldi, S.G. Umbilical Cord Tensile Strength Under Varying Strain Rates. Bioengineering 2025, 12, 789. https://doi.org/10.3390/bioengineering12080789
Castaldi MA, Villa P, Castaldi A, Castaldi SG. Umbilical Cord Tensile Strength Under Varying Strain Rates. Bioengineering. 2025; 12(8):789. https://doi.org/10.3390/bioengineering12080789
Chicago/Turabian StyleCastaldi, Maria Antonietta, Pietro Villa, Alfredo Castaldi, and Salvatore Giovanni Castaldi. 2025. "Umbilical Cord Tensile Strength Under Varying Strain Rates" Bioengineering 12, no. 8: 789. https://doi.org/10.3390/bioengineering12080789
APA StyleCastaldi, M. A., Villa, P., Castaldi, A., & Castaldi, S. G. (2025). Umbilical Cord Tensile Strength Under Varying Strain Rates. Bioengineering, 12(8), 789. https://doi.org/10.3390/bioengineering12080789






