Bone Mineral Density and Intermuscular Fat Derived from Computed Tomography Images Using Artificial Intelligence Are Associated with Fracture Healing
Abstract
1. Introduction
2. Methods
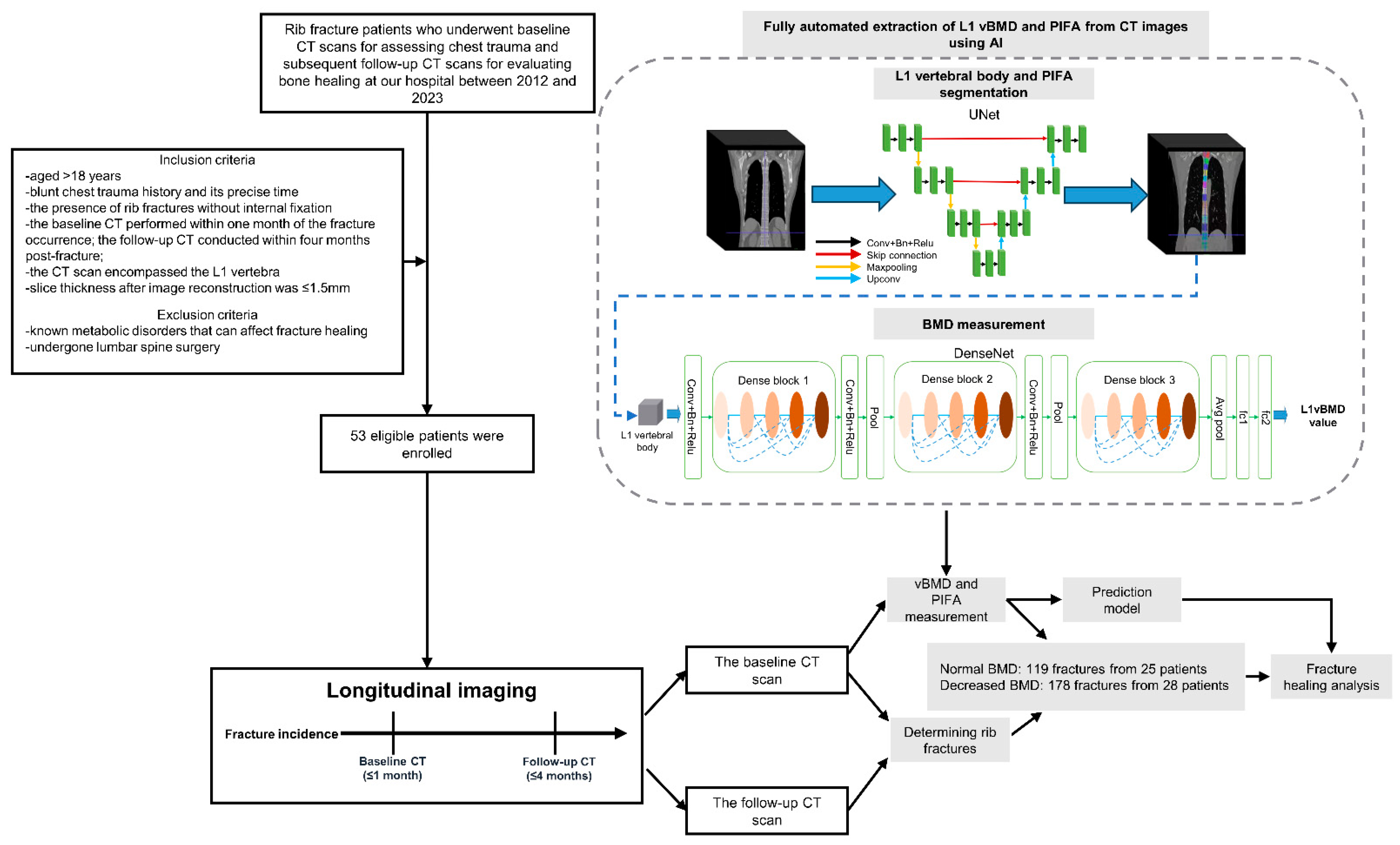
2.1. Study Design and Data Selection
2.2. CT Acquisition Protocols
2.3. BMD and PIFA Evaluated Using AI
2.4. Image Analysis
2.5. Statistical Analyses
3. Results
3.1. Patient and Fracture Characteristics
3.2. Associations of BMD and PIFA with Callus Formation
3.3. Associations of BMD and PIFA with Callus Volume Increase
3.4. Associations of BMD and PIFA with Poor Fracture Healing
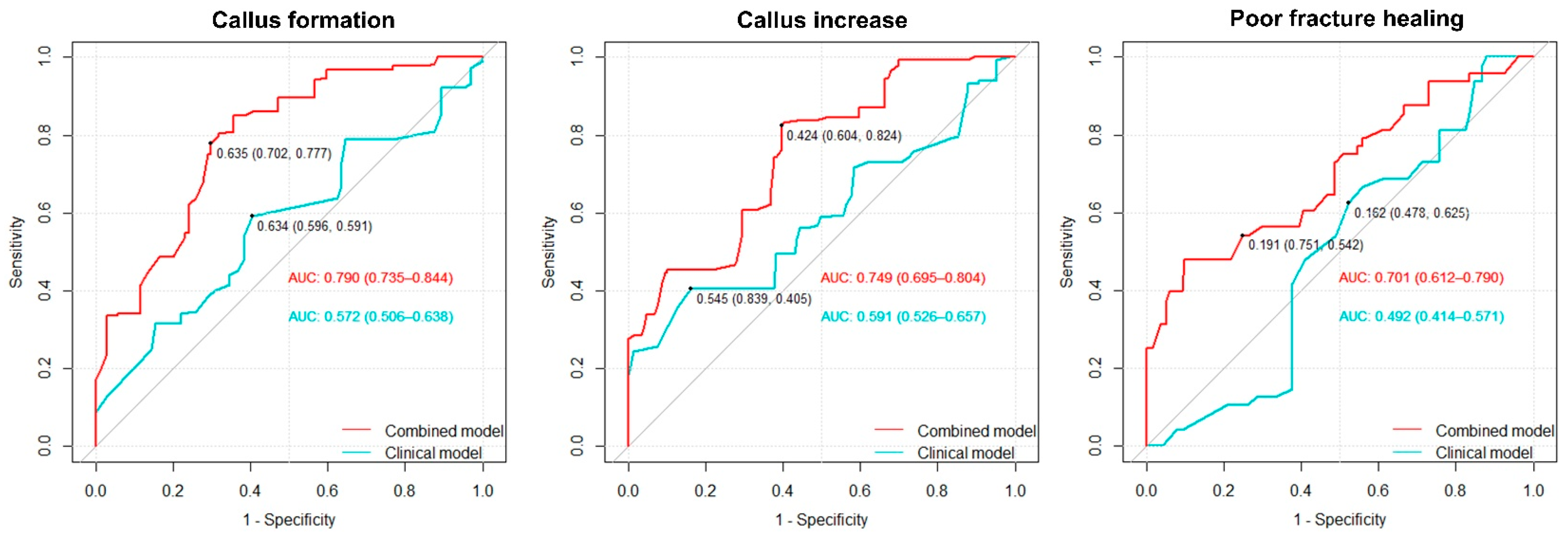
3.5. Predictive Value of BMD and PIFA for Fracture Healing Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rizzoli, R.; Bianchi, M.L.; Garabédian, M.; McKay, H.A.; Moreno, L.A. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone 2010, 46, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Shoback, D. Mechanisms Underlying Normal Fracture Healing and Risk Factors for Delayed Healing. Curr. Osteoporos. Rep. 2019, 17, 36–47. [Google Scholar] [CrossRef] [PubMed]
- American College of Radiology. ACR–SPR–SSR Practice Parameter for the Performance of Musculoskeletal Quantitative Computed Tomography (QCT); American College of Radiology: Reston, VA, USA, 2018. [Google Scholar]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Nie, T.; Wu, F.; Heng, Y.; Cai, W.; Liu, Z.; Qin, L.; Cao, Y.; Zheng, C. Influence of skeletal muscle and intermuscular fat on postoperative complications and long-term survival in rectal cancer patients. J. Cachexia Sarcopenia Muscle 2024, 15, 702–717. [Google Scholar] [CrossRef] [PubMed]
- Correa-de-Araujo, R.; Addison, O.; Miljkovic, I.; Goodpaster, B.H.; Bergman, B.C.; Clark, R.V.; Elena, J.W.; Esser, K.A.; Ferrucci, L.; Harris-Love, M.O.; et al. Myosteatosis in the Context of Skeletal Muscle Function Deficit: An Interdisciplinary Workshop at the National Institute on Aging. Front. Physiol. 2020, 11, 963. [Google Scholar] [CrossRef] [PubMed]
- Marasco, S.; Liew, S.; Edwards, E.; Varma, D.; Summerhayes, R. Analysis of bone healing in flail chest injury: Do we need to fix both fractures per rib? J. Trauma Acute Care Surg. 2014, 77, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.A.; Makaram, N.; Simpson, A.; Keating, J.F. Fracture nonunion in long bones: A literature review of risk factors and surgical management. Injury 2021, 52 (Suppl. S2), S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Habibi, H.; Takahashi, S.; Hoshino, M.; Takayama, K.; Sasaoka, R.; Tsujio, T.; Yasuda, H.; Kanematsu, F.; Kono, H.; Toyoda, H.; et al. Impact of paravertebral muscle in thoracolumbar and lower lumbar regions on outcomes following osteoporotic vertebral fracture: A multicenter cohort study. Arch. Osteoporos. 2021, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Katsu, M.; Ohba, T.; Ebata, S.; Haro, H. Comparative study of the paraspinal muscles after OVF between the insufficient union and sufficient union using MRI. BMC Musculoskelet. Disord. 2018, 19, 143. [Google Scholar] [CrossRef] [PubMed]
- Therkildsen, J.; Nissen, L.; Jørgensen, H.S.; Thygesen, J.; Ivarsen, P.; Frost, L.; Isaksen, C.; Langdahl, B.L.; Hauge, E.M.; Boettcher, M.; et al. Thoracic Bone Mineral Density Derived from Cardiac CT Is Associated with Greater Fracture Rate. Radiology 2020, 296, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, T.; Dockery, F. Osteoporosis and fracture risk in older people. Clin. Med. 2014, 14, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.; Zagar, C.A.; Lawrence, K.V. Osteoporosis: Common Questions and Answers. Am. Fam. Physician 2023, 107, 238–246. [Google Scholar] [PubMed]
- Gruenewald, L.D.; Koch, V.; Martin, S.S.; Yel, I.; Eichler, K.; Gruber-Rouh, T.; Lenga, L.; Wichmann, J.L.; Alizadeh, L.S.; Albrecht, M.H.; et al. Diagnostic accuracy of quantitative dual-energy CT-based volumetric bone mineral density assessment for the prediction of osteoporosis-associated fractures. Eur. Radiol. 2022, 32, 3076–3084. [Google Scholar] [CrossRef] [PubMed]
- Kajiki, Y.; Tsuji, H.; Misawa, H.; Nakahara, R.; Tetsunaga, T.; Yamane, K.; Oda, Y.; Takao, S.; Ozaki, T. Psoas muscle index predicts osteoporosis and fracture risk in individuals with degenerative spinal disease. Nutrition 2022, 93, 111428. [Google Scholar] [CrossRef] [PubMed]
- Tokashiki, T.; Igarashi, T.; Shiraishi, M.; Kano, R.; Ojiri, H. Evaluation of the association between osteoporotic vertebral compression fractures and psoas major/paraspinal muscle mass and ADC measured on MRI. Skelet. Radiol. 2024, 53, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Vitale, J.; Sconfienza, L.M.; Galbusera, F. Cross-sectional area and fat infiltration of the lumbar spine muscles in patients with back disorders: A deep learning-based big data analysis. Eur. Spine J. 2024, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.I.; Zheng, K.; Lin, C.; Mei, L.; Lu, L.; Li, W.; Chen, F.P.; Wang, Y.; Zhou, X.; Wang, F.; et al. Automated bone mineral density prediction and fracture risk assessment using plain radiographs via deep learning. Nat. Commun. 2021, 12, 5472. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yuan, H.; Cheng, J.; Weng, X.; Xu, H.; Gao, J.; Huang, M.; Wáng, Y.X.J.; Wu, Y.; Xu, W.; et al. Chinese expert consensus on the diagnosis of osteoporosis by imaging and bone mineral density. Quant. Imaging Med. Surg. 2020, 10, 2066–2077. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.S.; Kazam, J.J.; Fufa, D.; Bartolotta, R.J. Radiologic evaluation of fracture healing. Skelet. Radiol. 2019, 48, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Ji, G.; Bao, T.; Fu, H.; Yang, L.; Yang, M. Diagnosing sarcopenia and myosteatosis based on chest computed tomography images in healthy Chinese adults. Insights Imaging 2021, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Stoppino, L.P.; Piscone, S.; Saccone, S.; Ciccarelli, S.A.; Marinelli, L.; Milillo, P.; Gallo, C.; Macarini, L.; Vinci, R. Vertebral and Femoral Bone Mineral Density (BMD) Assessment with Dual-Energy CT versus DXA Scan in Postmenopausal Females. J. Imaging 2024, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Kang, W.Y.; Park, H.; Yang, Z.; Lee, J.; Kim, C.; Woo, O.H.; Hong, S.J. Evaluation of deep learning-based quantitative computed tomography for opportunistic osteoporosis screening. Sci. Rep. 2024, 14, 363. [Google Scholar] [CrossRef] [PubMed]
- Linder, A.; Eggebrecht, T.; Linder, N.; Stange, R.; Schaudinn, A.; Blüher, M.; Denecke, T.; Busse, H. Stand-alone MRI tool for semiautomatic volumetry of abdominal adipose compartments in patients with obesity. Sci. Rep. 2025, 15, 9354. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.; Eggebrecht, T.; Linder, A.; Linder, N.; Schaudinn, A.; Blüher, M.; Denecke, T.; Busse, H. Abdominal fat quantification using convolutional networks. Eur. Radiol. 2023, 33, 8957–8964. [Google Scholar] [CrossRef] [PubMed]
- Weston, A.D.; Korfiatis, P.; Kline, T.L.; Philbrick, K.A.; Kostandy, P.; Sakinis, T.; Sugimoto, M.; Takahashi, N.; Erickson, B.J. Automated Abdominal Segmentation of CT Scans for Body Composition Analysis Using Deep Learning. Radiology 2019, 290, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Magudia, K.; Bridge, C.P.; Bay, C.P.; Babic, A.; Fintelmann, F.J.; Troschel, F.M.; Miskin, N.; Wrobel, W.C.; Brais, L.K.; Andriole, K.P.; et al. Population-Scale CT-based Body Composition Analysis of a Large Outpatient Population Using Deep Learning to Derive Age-, Sex-, and Race-specific Reference Curves. Radiology 2021, 298, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Shin, Y.; Park, J.; Kim, H.; Lee, I.S.; Seo, D.W.; Huh, J.; Lee, T.Y.; Park, T.; Lee, J.; et al. Development and Validation of a Deep Learning System for Segmentation of Abdominal Muscle and Fat on Computed Tomography. Korean J. Radiol. 2020, 21, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.H.; Yi, J.; Shanbhag, A.; Marcinkiewicz, A.; Patel, K.K.; Lemley, M.; Ramirez, G.; Geers, J.; Chareonthaitawee, P.; Wopperer, S.; et al. Deep learning-quantified body composition from positron emission tomography/computed tomography and cardiovascular outcomes: A multicentre study. Eur. Heart J. 2025, 46, 2336–2347. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Marcinkiewicz, A.M.; Shanbhag, A.; Miller, R.J.H.; Geers, J.; Zhang, W.; Killekar, A.; Manral, N.; Lemley, M.; Buchwald, M.; et al. AI-based volumetric six-tissue body composition quantification from CT cardiac attenuation scans for mortality prediction: A multicentre study. Lancet Digit. Health 2025, 7, 100862. [Google Scholar] [CrossRef] [PubMed]
- Ziegelmayer, S.; Häntze, H.; Mertens, C.; Busch, F.; Lemke, T.; Kather, J.N.; Truhn, D.; Kim, S.H.; Wiestler, B.; Graf, M.; et al. Intermuscular adipose tissue and lean muscle mass assessed with MRI in people with chronic back pain in Germany: A retrospective observational study. Lancet Reg. Health Eur. 2025, 54, 101323. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Widmer, A.; Swartz, A.Z.; Koethe, J.R.; Silver, H.J. Density of ectopic fat depots predict distinct biomarkers of glycemic and insulinemic status in persons with HIV. Nutr. Diabetes 2025, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Roll, W.; Plagwitz, L.; Ventura, D.; Masthoff, M.; Backhaus, C.; Varghese, J.; Rahbar, K.; Schindler, P. Prognostic value of body composition out of PSMA-PET/CT in prostate cancer patients undergoing PSMA-therapy. Eur. J. Nucl. Med. Mol. Imaging, 2025; ahead of print. [Google Scholar] [CrossRef]
- Zura, R.; Xu, Z.J.; Della Rocca, G.J.; Mehta, S.; Steen, R.G. When Is a Fracture Not “Fresh”? Aligning Reimbursement With Patient Outcome After Treatment With Low-Intensity Pulsed Ultrasound. J. Orthop. Trauma 2017, 31, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Lafuente-Gracia, L.; Borgiani, E.; Nasello, G.; Geris, L. Towards in silico Models of the Inflammatory Response in Bone Fracture Healing. Front. Bioeng. Biotechnol. 2021, 9, 703725. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, L.; Geisler, C.; Pourhassan, M.; Braun, W.; Glüer, C.C.; Bosy-Westphal, A.; Müller, M.J. What is the best reference site for a single MRI slice to assess whole-body skeletal muscle and adipose tissue volumes in healthy adults? Am. J. Clin. Nutr. 2015, 102, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, P.J. Value-added Opportunistic CT Screening: State of the Art. Radiology 2022, 303, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.P.; Yu, Z. Pan, Y.; Wang, H.; Lu, Y. The Consistency of Artificial Intelligence Bone Mineral Density Measurement System and QCT in Measuring Bone Mineral Density. Chin. Comput. Med. Imaging 2023, 29, 178–183. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, X.; Wang, M.; Lian, Y.; Hou, P.; Chai, X.; Dai, Q.; Qian, B.; Jiang, Y.; Gao, J. Artificial intelligence assisted automatic screening of opportunistic osteoporosis in computed tomography images from different scanners. Eur. Radiol. 2025, 35, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.A.; Palanca, A.A.; Bellino, M.J.; Lowenberg, D.W. Assessment of compromised fracture healing. J. Am. Acad. Orthop. Surg. 2012, 20, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.R.; Taitsman, L.A.; Barei, D.P.; Nork, S.E. Femoral nonunion: Risk factors and treatment options. J. Am. Acad. Orthop. Surg. 2008, 16, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and its associated cutoff point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Unal, I. Defining an Optimal Cut-Point Value in ROC Analysis: An Alternative Approach. Comput. Math. Methods Med. 2017, 2017, 3762651. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Fujikawa, K.; Matsuki-Fukushima, M.; Nakamura, M. Effect of ovariectomy induced osteoporosis on metaphysis and diaphysis repair process. Injury 2021, 52, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Fischer, V.; Haffner-Luntzer, M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin. Cell Dev. Biol. 2022, 123, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cheng, S.; Sun, K.; Wang, J.; Liu, X.; Zhao, Y.; Yang, J.; Zhao, D.; Xue, C.; Tao, Y.; et al. Changes in macrophage and inflammatory cytokine expressions during fracture healing in an ovariectomized mice model. BMC Musculoskelet. Disord. 2021, 22, 494. [Google Scholar] [CrossRef] [PubMed]
- Kawai, R.; Kawashima, I.; Maeda, A.; Tsukada, M.; Aoshiba, H.; Kusaka, Y.; Tsukahara, T. The factors affecting the timing of bone union after closing-wedge high tibial osteotomy. J. Clin. Orthop. Trauma 2020, 11, S526–S529. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Qi, J.; Gao, J.; Sun, J.; Wang, G. The Impact of Bone Mineral Density on Bone Metabolism and the Fracture Healing Process in Elderly Chinese Patients With Osteoporotic Vertebral Compression Fractures. J. Clin. Densitom. 2021, 24, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Gorter, E.A.; Gerretsen, B.M.; Krijnen, P.; Appelman-Dijkstra, N.M.; Schipper, I.B. Does osteoporosis affect the healing of subcapital humerus and distal radius fractures? J. Orthop. 2020, 22, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Sheng, R.; Cao, M.; Song, M.; Wang, M.; Zhang, Y.; Shi, L.; Xie, T.; Li, Y.; Wang, J.; Rui, Y. Muscle-bone crosstalk via endocrine signals and potential targets for osteosarcopenia-related fracture. J. Orthop. Transl. 2023, 43, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chim, Y.N.; Wang, J.; Wong, R.M.Y.; Chow, S.K.H.; Cheung, W.H. Impaired Fracture Healing in Sarco-Osteoporotic Mice Can Be Rescued by Vibration Treatment Through Myostatin Suppression. J. Orthop. Res. 2020, 38, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tian, Y.; Zhang, L.; Zhang, T.; Wang, P.; Wang, J. Type II collagen from squid cartilage mediated myogenic IGF-I and irisin to activate the Ihh/PThrp and Wnt/β-catenin pathways to promote fracture healing in mice. Food Funct. 2021, 12, 6502–6512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chow, S.K.H.; Leung, K.S.; Lee, H.H.; Cheung, W.H. An animal model of co-existing sarcopenia and osteoporotic fracture in senescence accelerated mouse prone 8 (SAMP8). Exp. Gerontol. 2017, 97, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.H.; Cho, W.; Park, S.B.; Choo, J.; Kim, J.H.; Kim, S.W.; Shin, C.S. A Computed Tomography-Based Fracture Prediction Model With Images of Vertebral Bones and Muscles by Employing Deep Learning: Development and Validation Study. J. Med. Internet Res. 2024, 26, e48535. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, P.J.; Perez, A.A.; Garrett, J.W.; Graffy, P.M.; Zea, R.; Summers, R.M. Fully Automated Deep Learning Tool for Sarcopenia Assessment on CT: L1 Versus L3 Vertebral Level Muscle Measurements for Opportunistic Prediction of Adverse Clinical Outcomes. Am. J. Roentgenol. 2022, 218, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Lenchik, L.; Barnard, R.; Boutin, R.D.; Kritchevsky, S.B.; Chen, H.; Tan, J.; Cawthon, P.M.; Weaver, A.A.; Hsu, F.C. Automated Muscle Measurement on Chest CT Predicts All-Cause Mortality in Older Adults From the National Lung Screening Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Storlino, G.; Sanesi, L.; Colucci, S.; Grano, M. Myokines and Osteokines in the Pathogenesis of Muscle and Bone Diseases. Curr. Osteoporos. Rep. 2020, 18, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Gielen, E.; Dupont, J.; Dejaeger, M.; Laurent, M.R. Sarcopenia, osteoporosis and frailty. Metabolism 2023, 145, 155638. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Zanker, J.; Duque, G. Osteosarcopenia: Epidemiology, diagnosis, and treatment-facts and numbers. J. Cachexia Sarcopenia Muscle 2020, 11, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Seuvic, F.M.; Gillet, R.; Louis, M.; Germain, É.; Lombard, C.; Blum, A.; Gondim Teixeira, P.A. Association between opportunistic vertebral bone density measurements and new vertebral fractures after percutaneous vertebral cementoplasty: A case-control study. Eur. Radiol. 2023, 33, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liao, M.; Wang, Y.; Chen, L.; He, L.; Ji, Y.; Xiao, Y.; Lu, Y.; Fan, W.; Nie, Z.; et al. Opportunistic osteoporosis screening using chest CT with artificial intelligence. Osteoporos. Int. 2022, 33, 2547–2561. [Google Scholar] [CrossRef] [PubMed]
- Poullain, F.; Champsaur, P.; Pauly, V.; Knoepflin, P.; Le Corroller, T.; Creze, M.; Pithioux, M.; Bendahan, D.; Guenoun, D. Vertebral trabecular bone texture analysis in opportunistic MRI and CT scan can distinguish patients with and without osteoporotic vertebral fracture: A preliminary study. Eur. J. Radiol. 2023, 158, 110642. [Google Scholar] [CrossRef] [PubMed]
- Page, J.H.; Moser, F.G.; Maya, M.M.; Prasad, R.; Pressman, B.D. Opportunistic CT Screening-Machine Learning Algorithm Identifies Majority of Vertebral Compression Fractures: A Cohort Study. J. Bone Miner. Res. Plus 2023, 7, e10778. [Google Scholar] [CrossRef] [PubMed]
- Bodden, J.; Dieckmeyer, M.; Sollmann, N.; Burian, E.; Rühling, S.; Löffler, M.T.; Sekuboyina, A.; El Husseini, M.; Zimmer, C.; Kirschke, J.S.; et al. Incidental vertebral fracture prediction using neuronal network-based automatic spine segmentation and volumetric bone mineral density extraction from routine clinical CT scans. Front. Endocrinol. 2023, 14, 1207949. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, V.S.; Efstathopoulos, N.; Kontakis, G.; Kanakaris, N.K.; Giannoudis, P.V. The influence of osteoporosis in femoral fracture healing time. Injury 2009, 40, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Mastmeyer, A.; Engelke, K.; Fuchs, C.; Kalender, W.A. A hierarchical 3D segmentation method and the definition of vertebral body coordinate systems for QCT of the lumbar spine. Med. Image Anal. 2006, 10, 560–577. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, D.; Zhang, Y.; Li, W.; Jiang, C.; Ni, M.; Liao, N.; Yuan, H. Utilizing artificial intelligence to determine bone mineral density using spectral CT. Bone 2025, 192, 117321. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total | Normal BMD | Decreased BMD | p-Value |
|---|---|---|---|---|
| Number of patients | 53 | 25 | 28 | |
| Age (years) | 53.64 ± 10.64 | 48.72 ± 8.18 | 54.95 ± 10.11 | <0.001 |
| Sex (% male) | 29 (54.7%) | 15 (60%) | 14 (50%) | 0.650 |
| Number of fractures on the ribs | 5 (3–8) | 5 (3–6.5) | 5.5 (2.25–10) | 0.299 |
| Rib fractures | ||||
| Number of fractures | 297 | 119 | 178 | |
| Age (years) | 56.35 ± 10.96 | 49.45 ± 8.66 | 60.96 ± 9.88 | <0.001 |
| Sex (% male) | 162 (54.5%) | 63 (52.9%) | 99 (59.9%) | 0.650 |
| Fractured site | ||||
| 1–3 | 38 (12.8%) | 10 (8.4%) | 28 (15.7%) | 0.064 |
| 4–7 | 175 (58.9%) | 71 (59.7%) | 104 (58.4%) | 0.832 |
| 8–12 | 84 (28.3%) | 38 (31.9%) | 46 (21.4%) | 0.253 |
| Fracture type | ||||
| Displaced | 104 (39.2%) | 44 (37.0%) | 60 (33.7%) | 0.563 |
| Non-displaced | 193 (60.8%) | 75 (63.0%) | 118 (66.3%) | |
| Callus | ||||
| Baseline CT | 123 (41.4%) | 55 (46.2%) | 68 (38.2%) | 0.169 |
| Follow-up CT | 193 (65.0%) | 85 (71.4%) | 108 (60.7%) | 0.057 |
| Parameters | Callus in Baseline CT | Callus in Follow-Up CT | Callus Increase | Poor Fracture Healing | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Decreased BMD | ||||||||||||
| Unadjusted | 0.72 | 0.45–1.15 | 0.719 | 0.62 | 0.38–1.02 | 0.58 | 0.65 | 0.41–1.03 | 0.069 | 2.96 | 1.41–6.20 | 0.004 |
| Age- and sex-adjusted | 1.47 | 0.81–2.65 | 0.207 | 0.41 | 0.22–0.75 | 0.004 | 0.36 | 0.13–0.49 | <0.001 | 4.78 | 2.07–11.06 | <0.001 |
| BMD, per 1 SD decrease | ||||||||||||
| Unadjusted | 0.73 | 0.58–0.93 | 0.009 | 1.22 | 0.96–1.56 | 0.11 | 0.91 | 0.73–1.15 | 0.439 | 1.82 | 1.27–2.62 | 0.001 |
| Age- and sex-adjusted | 0.95 | 0.72–1.26 | 0.74 | 0.66 | 0.49–0.89 | 0.007 | 0.64 | 0.47–0.86 | 0.003 | 2.28 | 1.52–3.42 | <0.001 |
| Age-, sex-, and PIFA-adjusted | 0.94 | 0.70–1.25 | 0.647 | 0.69 | 0.50–0.97 | 0.032 | 0.70 | 0.51–0.96 | 0.026 | 2.08 | 1.38–3.13 | <0.001 |
| PIFA, per 1 SD increase | ||||||||||||
| Unadjusted | 0.96 | 0.76–1.22 | 0.761 | 0.32 | 0.22–0.45 | <0.001 | 0.45 | 0.33–0.62 | <0.001 | 2.00 | 1.49–2.69 | <0.001 |
| Age- and sex-adjusted | 1.09 | 0.86–1.39 | 0.479 | 0.25 | 0.16–0.38 | <0.001 | 0.33 | 0.22–0.48 | <0.001 | 2.16 | 1.59–2.94 | <0.001 |
| Age-, sex-, and BMD-adjusted | 1.10 | 0.86–1.41 | 0.438 | 0.24 | 0.16–0.37 | <0.001 | 0.33 | 0.23–0.49 | <0.001 | 2.09 | 1.50–2.93 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Wang, X.; Li, M.; Jin, L. Bone Mineral Density and Intermuscular Fat Derived from Computed Tomography Images Using Artificial Intelligence Are Associated with Fracture Healing. Bioengineering 2025, 12, 785. https://doi.org/10.3390/bioengineering12070785
Tang Y, Wang X, Li M, Jin L. Bone Mineral Density and Intermuscular Fat Derived from Computed Tomography Images Using Artificial Intelligence Are Associated with Fracture Healing. Bioengineering. 2025; 12(7):785. https://doi.org/10.3390/bioengineering12070785
Chicago/Turabian StyleTang, Yilin, Xiaodong Wang, Ming Li, and Liang Jin. 2025. "Bone Mineral Density and Intermuscular Fat Derived from Computed Tomography Images Using Artificial Intelligence Are Associated with Fracture Healing" Bioengineering 12, no. 7: 785. https://doi.org/10.3390/bioengineering12070785
APA StyleTang, Y., Wang, X., Li, M., & Jin, L. (2025). Bone Mineral Density and Intermuscular Fat Derived from Computed Tomography Images Using Artificial Intelligence Are Associated with Fracture Healing. Bioengineering, 12(7), 785. https://doi.org/10.3390/bioengineering12070785






