Do Preparation Techniques Transform the Metabolite Profile of Platelet-Rich Plasma?
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. PRP Preparation Procedure
- Stage 1: Blood Collection
- Stage 2: Centrifugation
- Stage 3: Fractionation and PRP Collection
2.3. Q-TOF LC–MS Analysis
2.4. Data Analysis
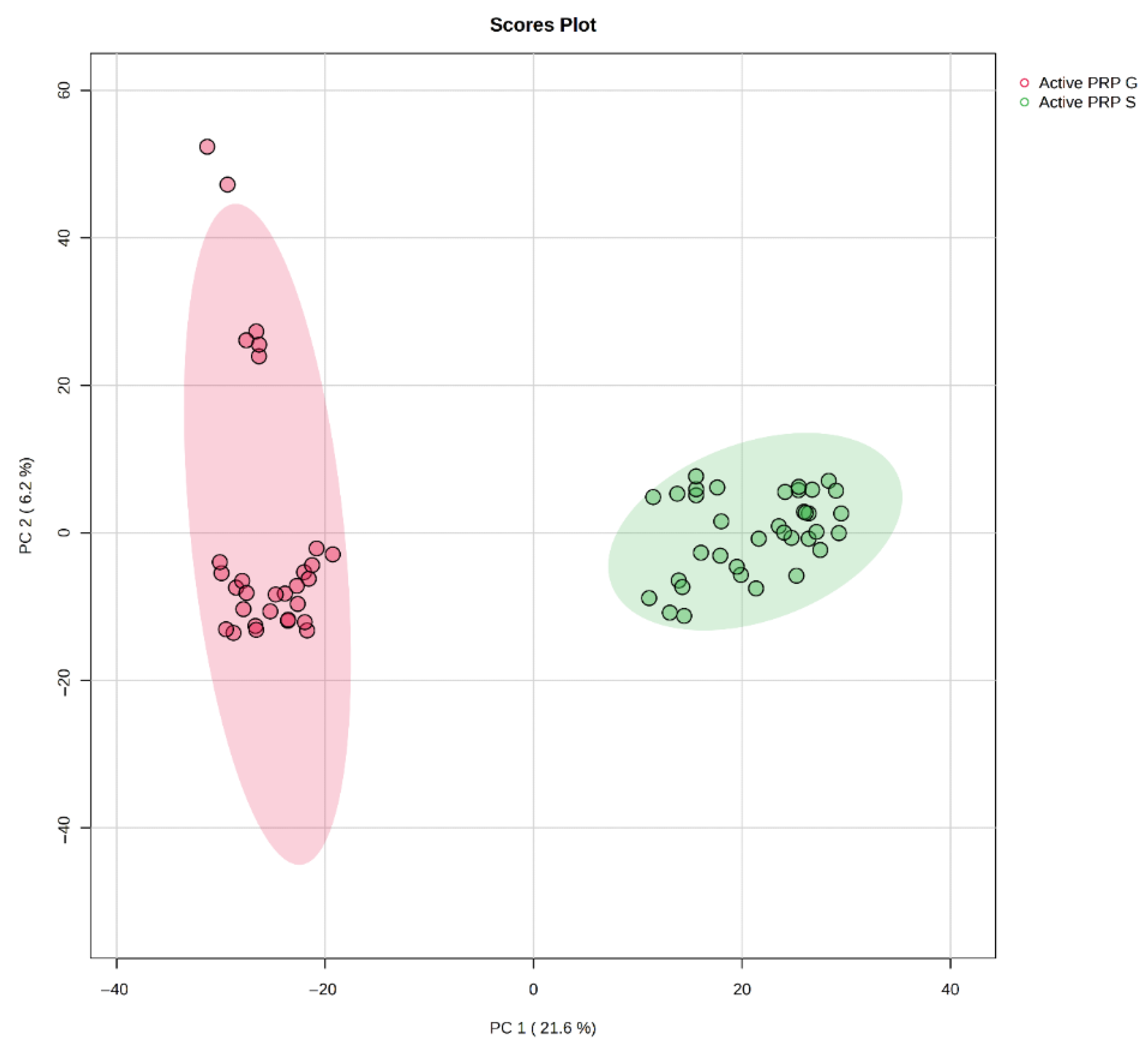
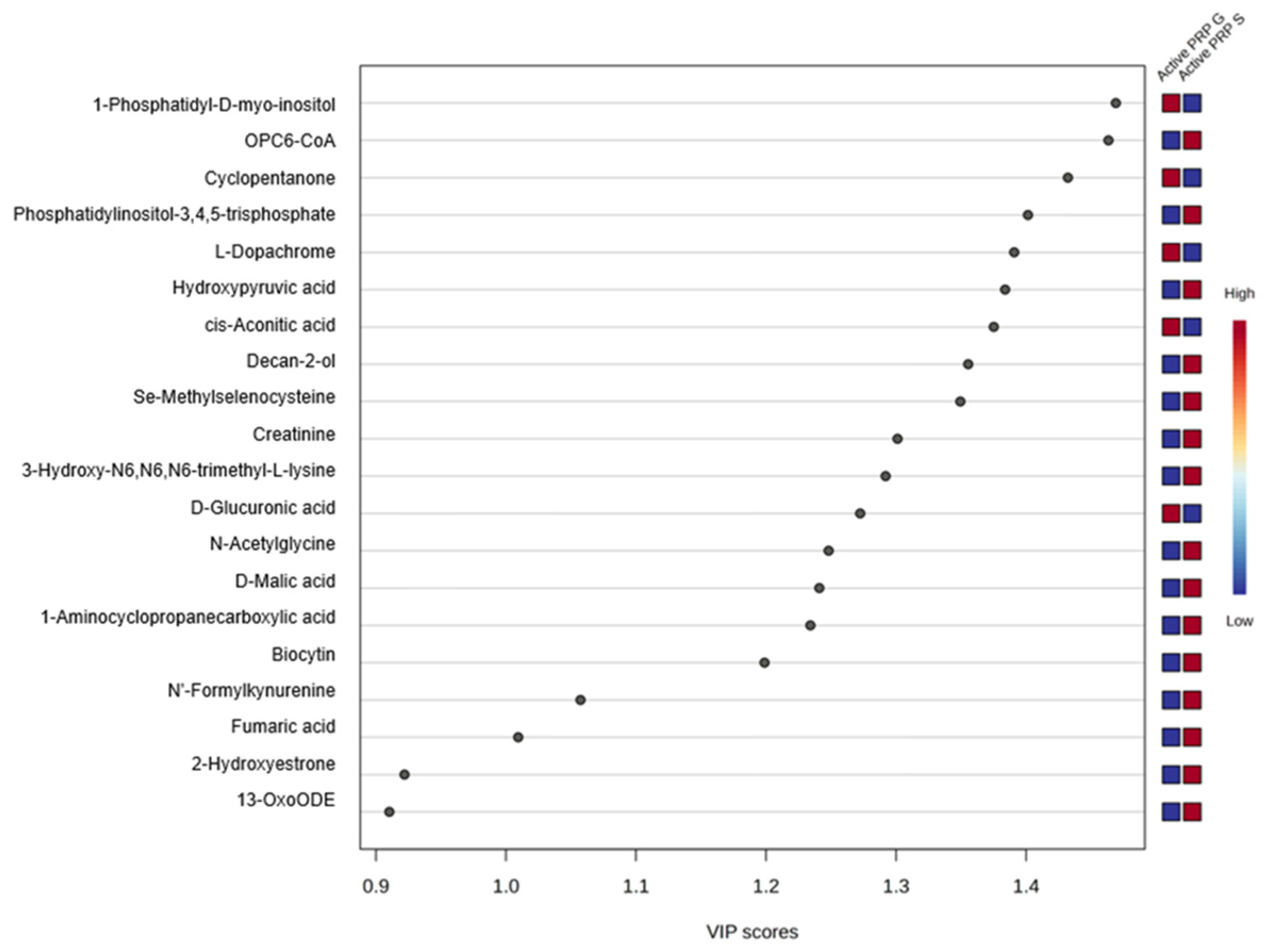
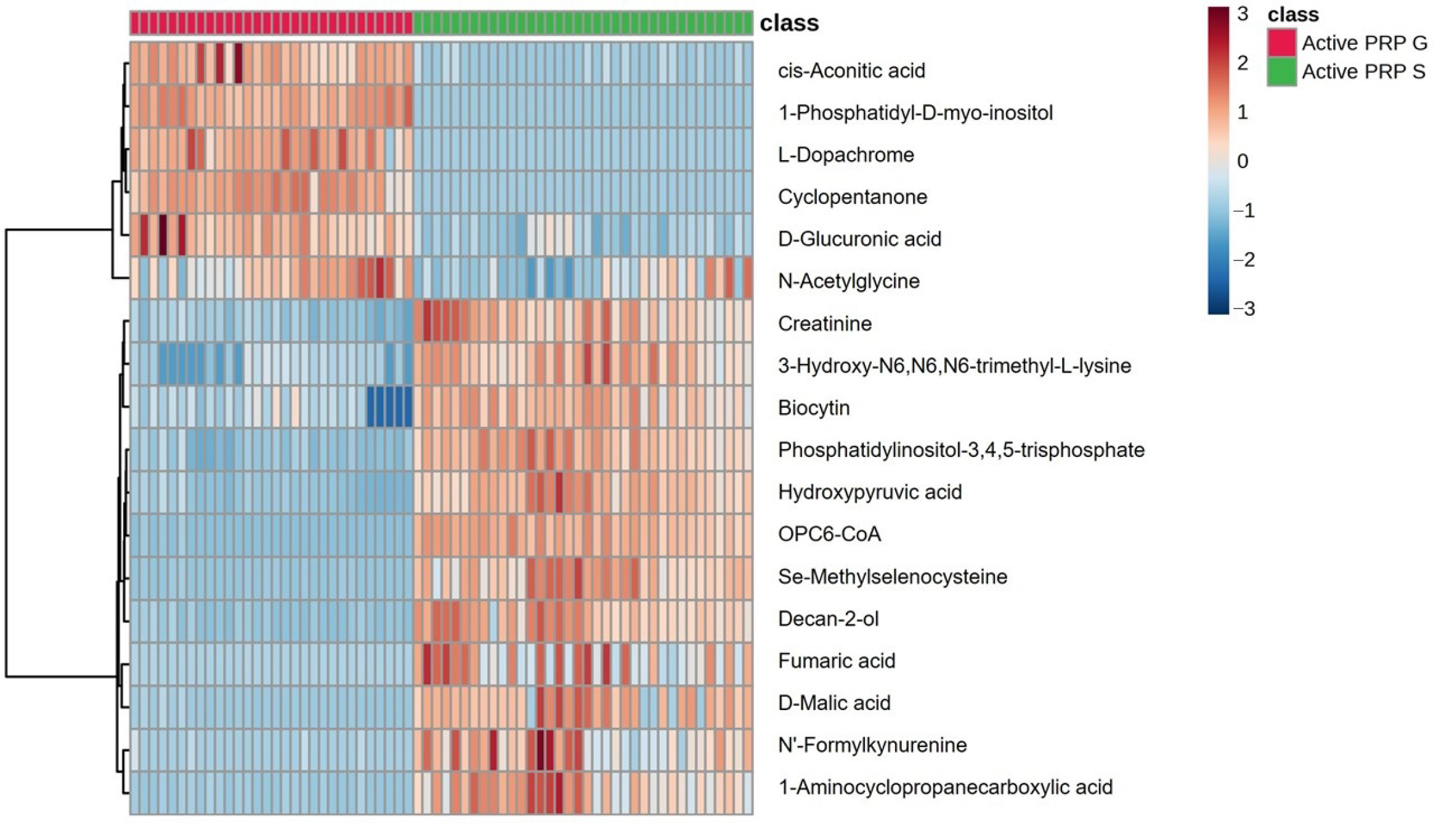
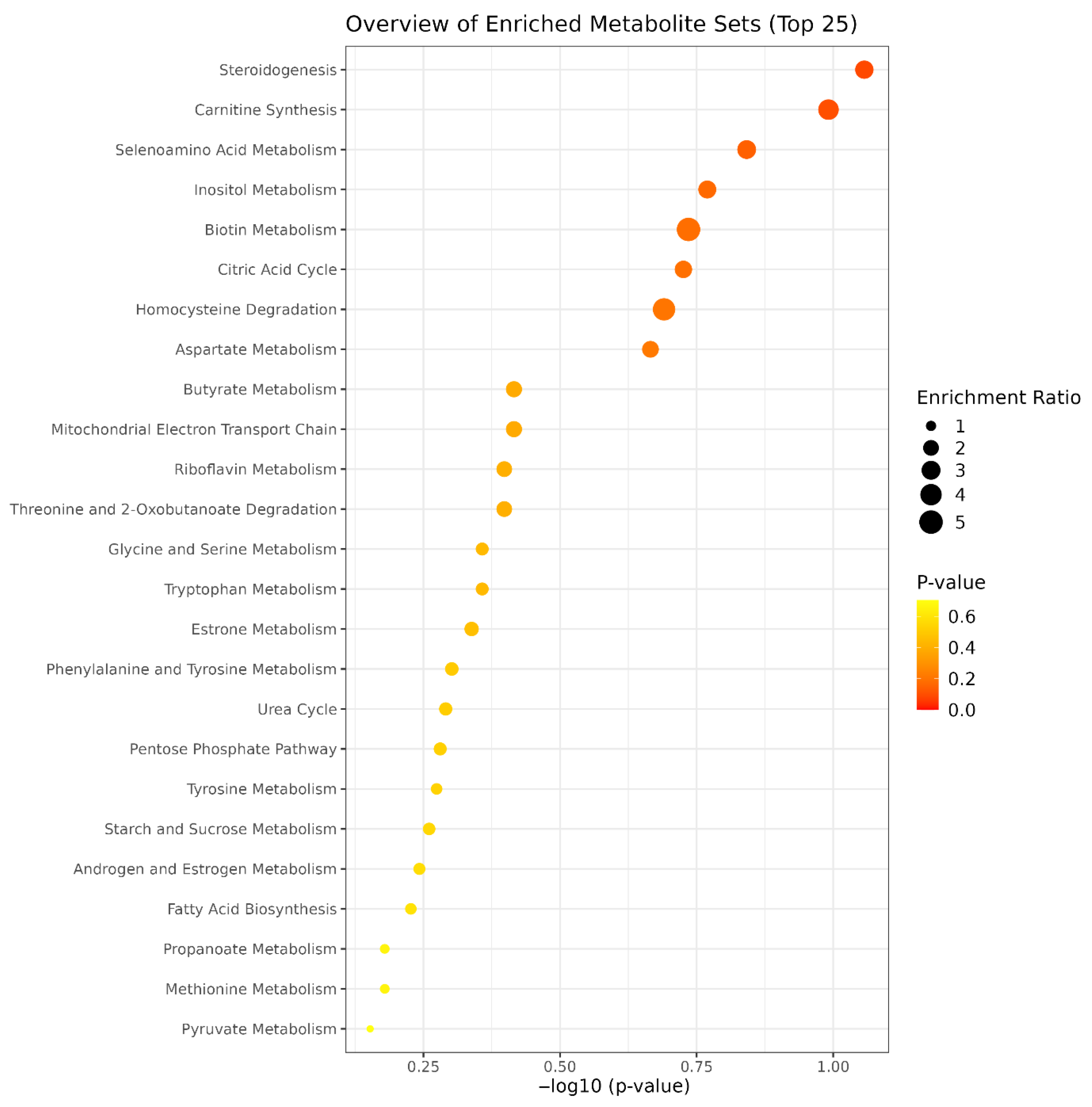
3. Results
4. Discussion
4.1. Reproducibility of the Kits
4.2. Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PRP | platelet-rich plasma |
| P-PRP | leukocyte-poor platelet-rich plasma |
| L-PRP | leukocyte-rich platelet-rich plasma |
| PRF | platelet-rich fibrin |
| PRP-G | activated PRP using Quantix IDRIA G kit |
| PRP-S | activated PRP using Quantix IDRIA S+ kit |
| A-PRP | activated platelet-rich plasma |
| TFF-PRP | tangential flow filtration PRP |
| C_L-PRP | CellPhi leukocyte-rich PRP |
| C_P-PRP | CellPhi platelet-rich PRP |
| PRP-P | premixed PRP |
| B7-PRP | B7 system PRP kit |
| PRM-PRP | premade PRP (based on MSK kits) |
| LC–MS | liquid chromatography–mass spectrometry |
| Q-TOF LC–MS | quadrupole time-of-flight liquid chromatography–mass spectrometry |
| PCA | principal component analysis |
| PLS-DA | partial least squares discriminant analysis |
| VIP | variable importance in projection |
| HMDB | Human Metabolome Database |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MS | mass spectrometry |
| SD | standard deviation |
| ICMJE | International Committee of Medical Journal Editors |
References
- Santos, R.G.D.; Santos, G.S.; Alkass, N.; Chiesa, T.L.; Azzini, G.O.; da Fonseca, L.F.; Santos, A.F.D.; Rodrigues, B.L.; Mosaner, T.; Lana, J.F. The regenerative mechanisms of platelet-rich plasma: A review. Cytokine 2021, 144, 155560. [Google Scholar] [CrossRef] [PubMed]
- Arnoczky, S.P.; Shebani-Rad, S. The basic science of platelet-rich plasma (PRP): What clinicians need to know. Sports Med. Arthrosc. Rev. 2013, 21, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Calabrese, C.; De Angelis, B.; Dionisi, L.; Pizzicannella, J.; Kothari, A.; De Fazio, D.; Garcovich, S. Impact of the different preparation methods to obtain autologous non-activated platelet-rich plasma (A-PRP) and activated platelet-rich plasma (AA-PRP) in plastic surgery: Wound healing and hair regrowth evaluation. Int. J. Mol. Sci. 2020, 21, 431. [Google Scholar] [CrossRef] [PubMed]
- AboElenein, M.; Alashhab, M.; Kandil, W.; Ahmed, A. Intraarticular Injection of Platelet Rich Plasma in Knee Osteoarthritis. Benha J. Appl. Sci. 2021, 5, 109–119. [Google Scholar] [CrossRef]
- Laver, L.; Filardo, G.; Sanchez, M.; Magalon, J.; Tischer, T.; Abat, F.; Bastos, R.; Cugat, R.; Iosifidis, M.; Kocaoglu, B. The use of injectable orthobiologics for knee osteoarthritis: A European ESSKA-ORBIT consensus. Part 1—Blood-derived products (platelet-rich plasma), Knee Surgery, Sports Traumatology. Arthroscopy 2024, 32, 783–797. [Google Scholar]
- Dai, P.; Wu, Y.; Gao, Y.; Li, M.; Zhu, M.; Xu, H.; Feng, X.; Jin, Y.; Zhang, X. Multiomics analysis of platelet-rich plasma promoting biological performance of mesenchymal stem cells. BMC Genom. 2024, 25, 564. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.R.; Pires, L.; Martins, R.A.; Santos, M.; Santos, G.S.; Lana, J.V.; Costa, B.R.; Santos, N.; de Macedo, A.P.; Kruel, A. Orthobiologics Revisited: A Concise Perspective on Regenerative Orthopedics. Curr. Issues Mol. Biol. 2025, 47, 247. [Google Scholar] [CrossRef]
- Ali, Z.; Sharma, S.; Ain, S.; Ain, Q. Recent Advances in Osteoarthritis: Pathophysiology, Diagnosis, and Therapeutic Strategies. Cuest. Fisioter. 2025, 54, 787–811. [Google Scholar]
- Miroshnychenko, O.; Chalkley, R.J.; Leib, R.D.; Everts, P.A.; Dragoo, J.L. Proteomic analysis of platelet-rich and platelet-poor plasma. Regen. Ther. 2020, 15, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Sağlam, N.; Korkusuz, F.; Şam, M. Nano-Biomaterials in Tissue Repair and Regeneration: Clinical Aspect—Soft Tissues; Springer Nature: New York, NY, USA, 2024. [Google Scholar]
- Stiller, H.L.; Perumal, N.; Manicam, C.; Trzeciak, E.R.; Todt, J.; Jurk, K.; Tuettenberg, A.; Schumann, S.; Schiegnitz, E.; Blatt, S. First-vs. Second-Generation Autologous Platelet Concentrates and Their Implications for Wound Healing: Differences in Proteome and Secretome. Bioengineering 2024, 11, 1171. [Google Scholar] [CrossRef] [PubMed]
- LaCroix, I.S.; Cohen, M.; Moore, E.E.; Dzieciatkowska, M.; Silliman, C.C.; Hansen, K.C.; D’Alessandro, A. Omics markers of platelet transfusion in trauma patients. Transfusion 2023, 63, 1447–1462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Fabricant, P.D.; Ishmael, C.R.; Wang, J.C.; Petrigliano, F.A.; Jones, K.J. Utilization of platelet-rich plasma for musculoskeletal injuries: An analysis of current treatment trends in the United States. Orthop. J. Sports Med. 2016, 4, 2325967116676241. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, D.; Seghatchian, J.; Acker, J.P. Platelet rich plasma in treatment of musculoskeletal pathologies. Transfus. Apher. Sci. 2019, 58, 102675. [Google Scholar] [CrossRef] [PubMed]
- Alldritt, I.; Greenhaff, P.L.; Wilkinson, D.J. Metabolomics as an important tool for determining the mechanisms of human skeletal muscle deconditioning. Int. J. Mol. Sci. 2021, 22, 13575. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Pramanik, J.; Mahata, B. Revisiting steroidogenesis and its role in immune regulation with the advanced tools and technologies. Genes Immun. 2021, 22, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Mahata, B.; Raman, C.; Bereshchenko, O. Steroids and Secosteroids in the Modulation of Inflammation and Immunity; Frontiers Media SA: Lausanne, Switzerland, 2021; p. 825577. [Google Scholar]
- Daniel, J.L.; Dangelmaier, C.; Jin, J.; Ashby, B.; Smith, J.B.; Kunapuli, S.P. Molecular basis for ADP-induced platelet activation: I. Evidence for three distinct ADP receptors on human platelets. J. Biol. Chem. 1998, 273, 2024–2029. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Inositol trisphosphate and calcium signalling mechanisms. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2009, 1793, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Varga-Szabo, D.; Braun, A.; Nieswandt, B. Calcium signaling in platelets. J. Thromb. Haemost. 2009, 7, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.W. The role of the carnitine system in human metabolism. Ann. N. Y. Acad. Sci. 2004, 1033, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.V.; Costa, A.M.; Grzeskowiak, L. Oxidative stress and tissue repair: Mechanism, biomarkers, and therapeutics. Oxidative Med. Cell. Longev. 2021, 2021, 6204096. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lou, L. The Central Role of the Citric Acid Cycle in Energy Metabolism: From Metabolic Intermediates to Regulatory Mechanisms. Biol. Evid. 2024, 14, 110–121. [Google Scholar] [CrossRef]
- Dupont, G. Modeling the intracellular organization of calcium signaling. Wiley Interdiscip. Rev. Syst. Biol. Med. 2014, 6, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Tsui, M.M.; York, J.D. Roles of inositol phosphates and inositol pyrophosphates in development, cell signaling and nuclear processes. Adv. Enzym. Regul. 2010, 50, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Estevez, B.; Du, X. New concepts and mechanisms of platelet activation signaling. Physiology 2017, 32, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Townsend, D.; Tew, K. The antioxidant role of selenium and seleno-compounds. Biomed. Pharmacother. 2003, 57, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Kipp, A.P.; Frombach, J.; Deubel, S.; Brigelius-Flohe, R. Selenoprotein W as biomarker for the efficacy of selenium compounds to act as source for selenoprotein biosynthesis. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 87–112. [Google Scholar]
- Karachaliou, C.-E.; Livaniou, E. Biotin homeostasis and human disorders: Recent findings and perspectives. Int. J. Mol. Sci. 2024, 25, 6578. [Google Scholar] [CrossRef] [PubMed]
- Wal, A.; Sasmal, A.; Singh, R.; Yadav, P.; Singh, Y.; Garg, V.; Wal, P. Regulatory role, mechanism, and metabolic profile of Biotin in gene expression. Curr. Pharmacogenomics Pers. Med. 2023, 20, 73–86. [Google Scholar] [CrossRef]
- van Wonderen, S.F.; van Baarle, F.L.; de Bruin, S.; Peters, A.L.; de Korte, D.; van Bruggen, R.; Vlaar, A.P. Biotinylated platelets: A promising labeling technique? Transfus. Med. Rev. 2023, 37, 150719. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Singh, M.; Sharma, R. A comparative analysis of serological parameters and oxidative stress in osteoarthritis and rheumatoid arthritis. Rheumatol. Int. 2012, 32, 2377–2382. [Google Scholar] [CrossRef] [PubMed]
- Karaçoban, L.; Korkmaz, M.F.; Uğur, R.; Fidan, S.B. Donor tissue type alters the effects of mesenchymal stem cells on human osteoarthritic chondrocytes and their metabolomic profiles. Res. Sports Med. 2025, 33, 412–426. [Google Scholar] [CrossRef] [PubMed]
- Neves, M., Jr.; Gualano, M.S.; Roschel, R.; Lima, H.; Pereira, C.L.; Otaduy, B. Beneficial effect of creatine supplementation in knee osteoarthritis. Med. Sci. Sports Exerc. 2011, 43, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.W.; Ryu, D.J.; Suh, Y.J. Association between low serum creatinine levels and knee osteoarthritis in Koreans without renal insufficiency. Yonsei Med. J. 2024, 65, 519. [Google Scholar] [CrossRef] [PubMed]
- Inoguchi, T.; Sonoda, N.; Maeda, Y. Bilirubin as an important physiological modulator of oxidative stress and chronic inflammation in metabolic syndrome and diabetes: A new aspect on old molecule. Diabetol. Int. 2016, 7, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Higgins, A.; Lees, P. The acute inflammatory process, arachidonic acid metabolism and the mode of action of anti-inflammatory drugs. Equine Vet. J. 1984, 16, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Brash, A.R. Arachidonic acid as a bioactive molecule. J. Clin. Investig. 2001, 107, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Lash, T.D.; Mani, U.N.; Drinan, M.A.; Zhen, C.; Hall, T.; Jones, M.A. Normal and abnormal heme biosynthesis. 1. Synthesis and metabolism of di-and monocarboxylic porphyrinogens related to coproporphyrinogen-III and harderoporphyrinogen: A model for the active site of coproporphyrinogen oxidase. J. Org. Chem. 1999, 64, 464–477. [Google Scholar] [CrossRef]
- Dailey, H.A.; Medlock, A.E. A primer on heme biosynthesis. Biol. Chem. 2022, 403, 985–1003. [Google Scholar] [CrossRef] [PubMed]
- NaveenKumar, S.K.; SharathBabu, B.N.; Hemshekhar, M.; Kemparaju, K.; Girish, K.S.; Mugesh, G. The role of reactive oxygen species and ferroptosis in heme-mediated activation of human platelets. ACS Chem. Biol. 2018, 13, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Campelo, A.E.; Cutini, P.H.; Massheimer, V.L. Testosterone modulates platelet aggregation and endothelial cell growth through nitric oxide pathway. J. Endocrinol. 2012, 213, 77. [Google Scholar] [CrossRef]
- Bos-Mikich, A.; de Oliveira, R.; Frantz, N. Platelet-rich plasma therapy and reproductive medicine. J. Assist. Reprod. Genet. 2018, 35, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Paglia, G.; Sigurjónsson, Ó.E.; Rolfsson, Ó.; Hansen, M.B.; Brynjólfsson, S.; Gudmundsson, S.; Palsson, B.O. Metabolomic analysis of platelets during storage: A comparison between apheresis-and buffy coat–derived platelet concentrates. Transfusion 2015, 55, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.W.; Serrano, K.; Stefanoni, D.; D’Alessandro, A.; Devine, D.V. In vitro characterization and metabolomic analysis of cold-stored platelets. J. Proteome Res. 2021, 20, 2251–2265. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Lee, K.; Ju, J.H. Recent updates of diagnosis, pathophysiology, and treatment on osteoarthritis of the knee. Int. J. Mol. Sci. 2021, 22, 2619. [Google Scholar] [CrossRef] [PubMed]
- Young, S.N. L-tyrosine to alleviate the effects of stress? J. Psychiatry Neurosci. 2007, 32, 224. [Google Scholar] [PubMed]
- Sutton, E.E.; Coll, M.R.; Deuster, P.A. Ingestion of tyrosine: Effects on endurance; muscle strength; anaerobic performance. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Li, J.; Jiang, W.; Jiang, Y.; Wan, Y.; Wang, Y.; Xin, F.; Zhang, W. Strategies for the biological synthesis of D-glucuronic acid and its derivatives. World J. Microbiol. Biotechnol. 2024, 40, 94. [Google Scholar] [CrossRef] [PubMed]
- Chęciński, M.; Chlubek, D.; Sikora, M. Effects of Hyaluronic Acid (HA) and Platelet-Rich Plasma (PRP) on Mandibular Mobility in Temporomandibular Joint Disorders: A Controlled Clinical Trial. Biomolecules 2024, 14, 1216. [Google Scholar] [CrossRef] [PubMed]
- Elhassan, Y.S.; Kluckova, K.; Fletcher, R.S.; Schmidt, M.S.; Garten, A.; Doig, C.L.; Cartwright, D.M.; Oakey, L.A.; Sauchanka, O.; Laginha, I.; et al. Nicotinamide riboside augments the aged human skeletal muscle NAD+ metabolome and induces transcriptomic and anti-inflammatory signatures. Cell Rep. 2019, 28, 1717–1728. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Boffa, A.; Andriolo, L.; Romandini, I.; Altamura, S.A.; Cenacchi, A.; Roverini, V.; Zaffagnini, S.; Filardo, G. Leukocyte-Rich versus Leukocyte-Poor Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis: A Double-Blind Randomized Trial. Am. J. Sports Med. 2022, 50, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Assirelli, E.; Filardo, G.; Mariani, E.; Kon, E.; Roffi, A.; Vaccaro, F.; Marcacci, M.; Facchini, A.; Pulsatelli, L. Effect of two different preparations of platelet-rich plasma on synoviocytes, Knee Surgery, Sports Traumatology. Arthroscopy 2015, 23, 2690–2703. [Google Scholar]
- Saadati, S.; O’Connell, J.; de Courten, M.A.; Ekinci, E.I.; de Courten, B. The Effect of Carnosine Supplementation on Musculoskeletal Health in Adults with Prediabetes and Type 2 Diabetes: A Secondary Analysis of a Randomized Controlled Trial. Nutrients 2024, 16, 4328. [Google Scholar] [CrossRef] [PubMed]
- West, D.W.D.; Phillips, S.M. Anabolic Processes in Human Skeletal Muscle: Restoring the Identities of Growth Hormone and Testosterone. Physician Sportsmed. 2010, 38, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.M.; Phillips, S.M. Branched-chain amino acids (leucine, isoleucine, and valine) and skeletal muscle. In Nutrition and Skeletal Muscle; Academic Press: Cambridge, MA, USA, 2019; pp. 283–298. [Google Scholar]
- Candreva, T.; de Oliveira, F.A.; Rosado-de-Castro, P.H.; Mendez-Otero, R.; Santiago, M.F.; Da Poian, A.T.; Torres, A.G. Docosahexaenoic acid slows inflammation resolution and impairs the quality of healed skin tissue. Clin. Sci. 2019, 133, 2345–2360. [Google Scholar] [CrossRef] [PubMed]
- El-barbary, A.M.; Hussein, M.R.; Abu-Zaid, A.; Mansour, H.H. Assessment of lipid peroxidation and antioxidant status in rheumatoid arthritis and osteoarthritis patients. Egypt. Rheumatol. 2011, 33, 179–185. [Google Scholar] [CrossRef][Green Version]
- Campo, G.M.; Avenoso, A.; Campo, S.; Ferlazzo, A.M.; Altavilla, D.; Calatroni, A. Antioxidant activity of chondroitin sulfate. In Advances in Pharmacology; Academic Press: Cambridge, MA, USA, 2006; pp. 417–431. [Google Scholar]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Dudaric, L.; Gnjidic, Z.; Bralic, M.; Vukojevic, K.; Petrovic, O.; Glavina-Durdov, M. Bone remodeling in osteoarthritis—biological and radiological aspects. Medicina 2023, 59, 1613. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.B.; Tuan, R.S. Anabolic/catabolic balance in pathogenesis of osteoarthritis: Identifying molecular targets. PMR 2011, 3, S3–S11. [Google Scholar] [CrossRef] [PubMed]

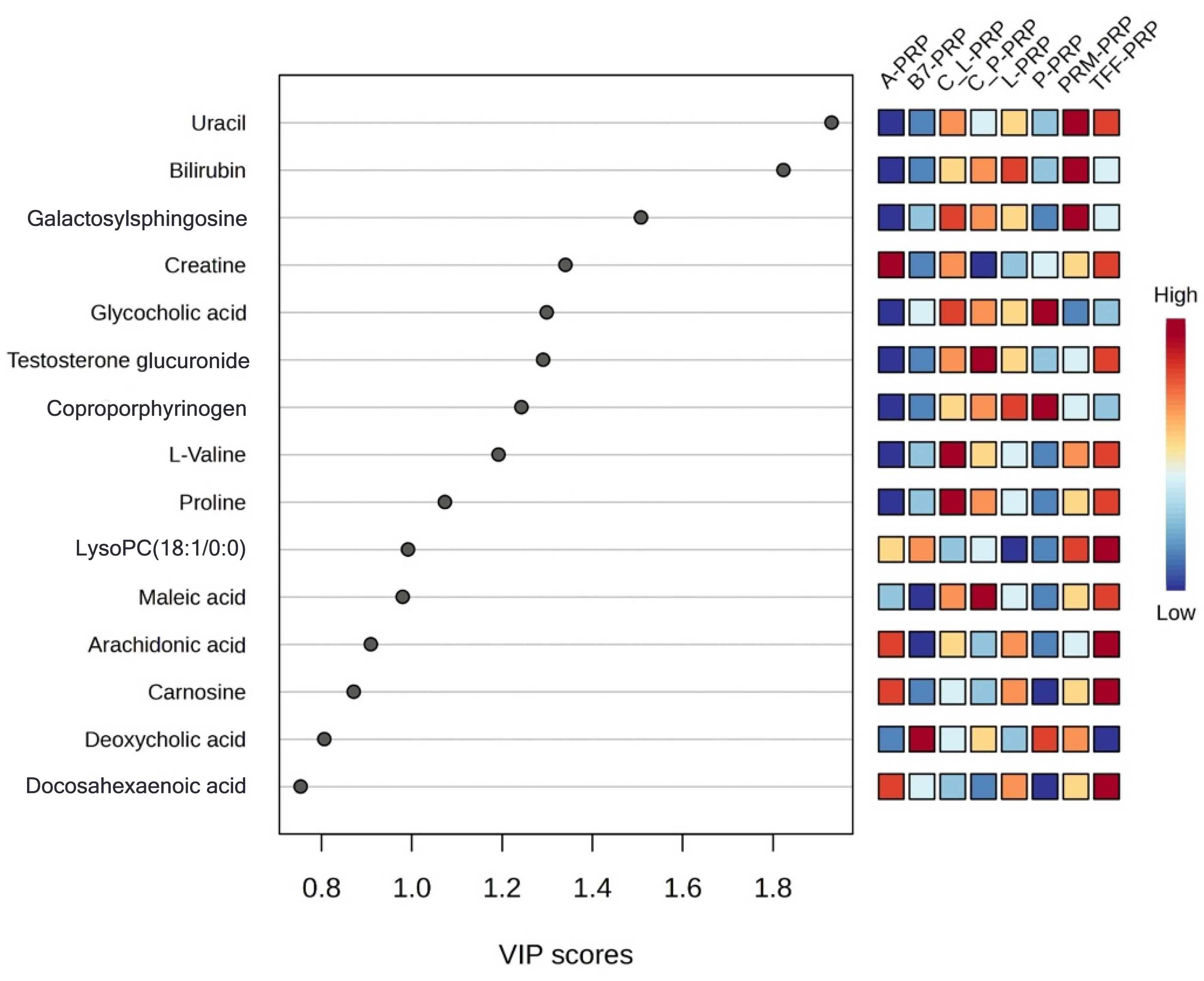

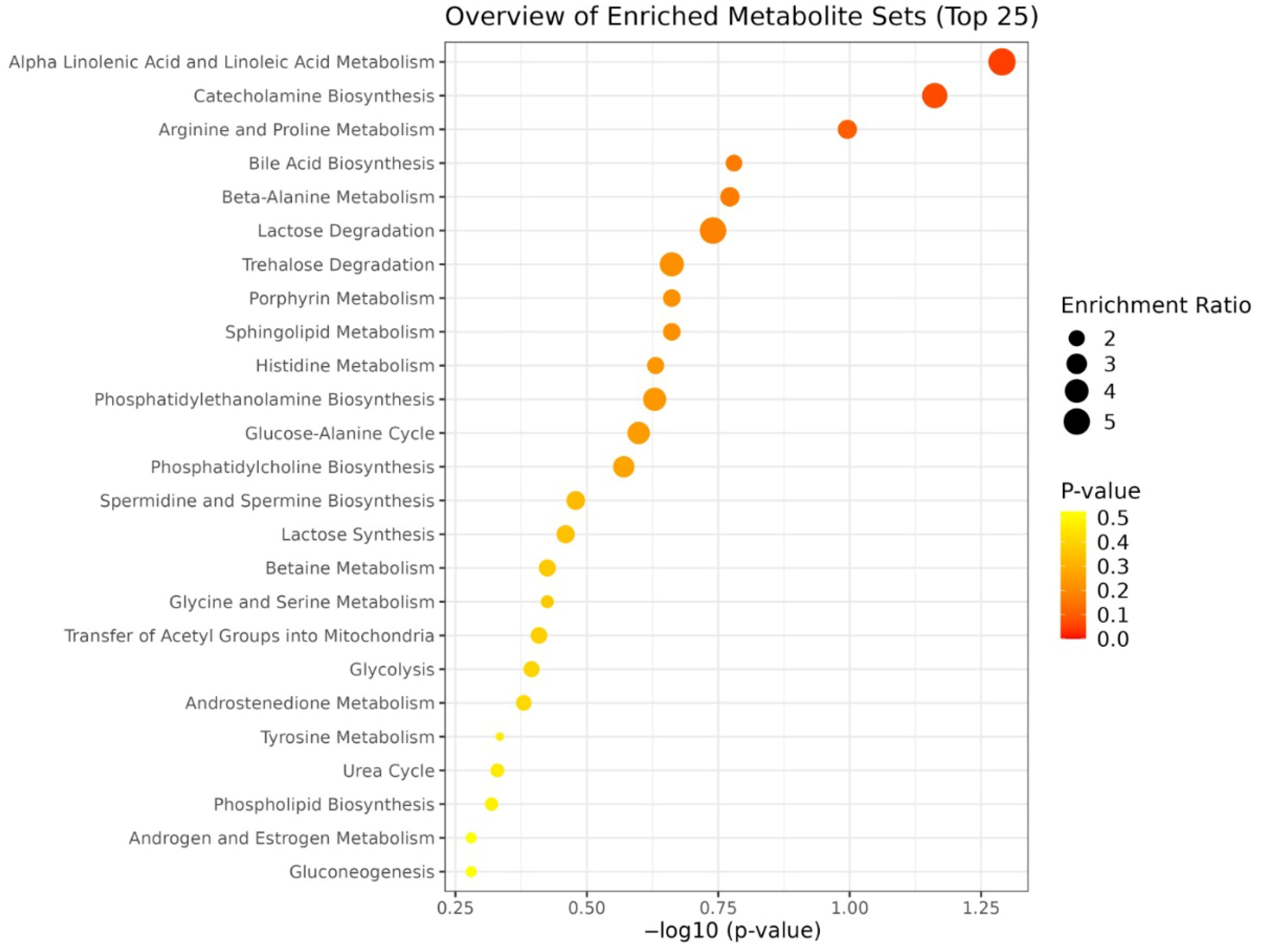
| Variables | Mean ± SD or (%) |
|---|---|
| Gender | Male, 6 (100%) |
| Age (years) | 22.0 ± 1.7 |
| Height (cm) | 178.8 ± 6.2 |
| Weight (kg) | 81.2 ± 13.2 |
| Body mass index (kg/m2) | 26.0 ± 5.0 |
| Comorbidities | Present: 1 (16.6%) Absent: 5 (83.4%) |
| Smoking status | Smoker: 1 (16.6%) Non-smoker: 5 (83.4%) |
| PRP Type | Kit Name | Centrifugation (Single/Double Spin) | RPM (Revolutions per Minute) | Duration (min) | Yield (mL) | Tube Material | Activated | Leukocyte Count | Erythrocyte Count | Platelet Count |
|---|---|---|---|---|---|---|---|---|---|---|
| PRP-P | MSK Premium PRP | Single | 3500 | 8 | 2–3 | Gel-based plastic | No | 0.04 × 103/μL | ||
| A-PRP | MSK Activated PRP | Single | 3000 | 6 | 2–3 | Gel-based plastic | Yes | 0.03 × 103/μL | ||
| B7-PRP | MSK Biotin PRP | Single | 3000 | 6 | 4–5 | Gel-based plastic | Yes | 0.01 × 103/μL | ||
| P-PRP | Neogenesis | Single | 3100 | 10 | ~3 | Plastic | No | 0 | ||
| L-PRP | Neogenesis | Single | 3100 | 10 | ~4 | Plastic | No | 6.49 × 103/μL | 0.04 × 103/μL | 447 × 103/μL |
| C P-PRP | CellPhi | Single | 3400 | 10 | 5.5 | Plastic | No | 0 | ||
| C L-PRP | CellPhi | Single | 3400 | 10 | 5.5 | Plastic | No | 1.67 × 103/μL | ||
| TFF-PRP | Manson HA-PRP | Single | 3500 | 8 | 2–3 | Gel-based plastic | Yes | 0.56 × 103/μL | ||
| PRP-G | Quantix IDRIA G | Double | 4150/3100 | 3/8 | ~13 | Gel-based plastic | Yes | NA | NA | NA |
| PRP-S | Quantix IDRIA S | Single | 4000 | 10 | ~13 | Glass beads based plastic | Yes | NA | NA | NA |
| PRP Type | Key Metabolites (Heatmap/VIP) | Main Effect/Discussion | Suggested Clinical Application |
|---|---|---|---|
| A-PRP | Bilirubin, arachidonic acid, coproporphyrinogen, creatine | High redox buffering, early-phase inflammatory activation, enhanced energy support | Acute regeneration, rapid cellular mobilization, controlled inflammation |
| TFF-PRP | Carnosine, testosterone glucuronide, L-valine, DHA | Antioxidant effect, tissue remodeling, anabolic profile | Early osteoarthritis or conditions with oxidative/inflammatory damage |
| L-PRP | L-tyrosine, D-glucuronic acid, nicotinamide riboside | High immune activation, tissue remodeling, anti-inflammatory potential | Acute musculoskeletal injuries, tissue remodeling scenarios |
| P-PRP | Low L-tyrosine & D-glucuronic acid, balanced profile | Low inflammatory, matrix-preserving profile | Chronic degenerative conditions, early osteoarthritis, intra-articular uses where minimal inflammation is desired |
| C_L-PRP | Creatine, maleic acid (slightly elevated) | Sustained energy support, redox balance | Chronic tendon repair, late-stage osteoarthritis |
| C_P-PRP | Testosterone glucuronide (slightly elevated), creatine | Androgenic/anabolic stimulus, energy support | Early cartilage regeneration, subacute joint injuries |
| B7-PRP | Moderate levels of various metabolites | Balanced, non-polarized biochemical profile | General-purpose or maintenance-phase applications |
| PRM-PRP | Moderate levels of various metabolites | Balanced, non-polarized biochemical profile | Supportive, maintenance, or non-targeted application |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fidan, B.B.; Koç, E.; Özotuk, E.Ç.; Kaplan, O.; Çelebier, M.; Korkusuz, F. Do Preparation Techniques Transform the Metabolite Profile of Platelet-Rich Plasma? Bioengineering 2025, 12, 774. https://doi.org/10.3390/bioengineering12070774
Fidan BB, Koç E, Özotuk EÇ, Kaplan O, Çelebier M, Korkusuz F. Do Preparation Techniques Transform the Metabolite Profile of Platelet-Rich Plasma? Bioengineering. 2025; 12(7):774. https://doi.org/10.3390/bioengineering12070774
Chicago/Turabian StyleFidan, Bilge Başak, Emine Koç, Emine Çiftçi Özotuk, Ozan Kaplan, Mustafa Çelebier, and Feza Korkusuz. 2025. "Do Preparation Techniques Transform the Metabolite Profile of Platelet-Rich Plasma?" Bioengineering 12, no. 7: 774. https://doi.org/10.3390/bioengineering12070774
APA StyleFidan, B. B., Koç, E., Özotuk, E. Ç., Kaplan, O., Çelebier, M., & Korkusuz, F. (2025). Do Preparation Techniques Transform the Metabolite Profile of Platelet-Rich Plasma? Bioengineering, 12(7), 774. https://doi.org/10.3390/bioengineering12070774






