A Modified Bioceramic Sealer with Dual Antibacterial Mechanisms
Abstract
1. Introduction
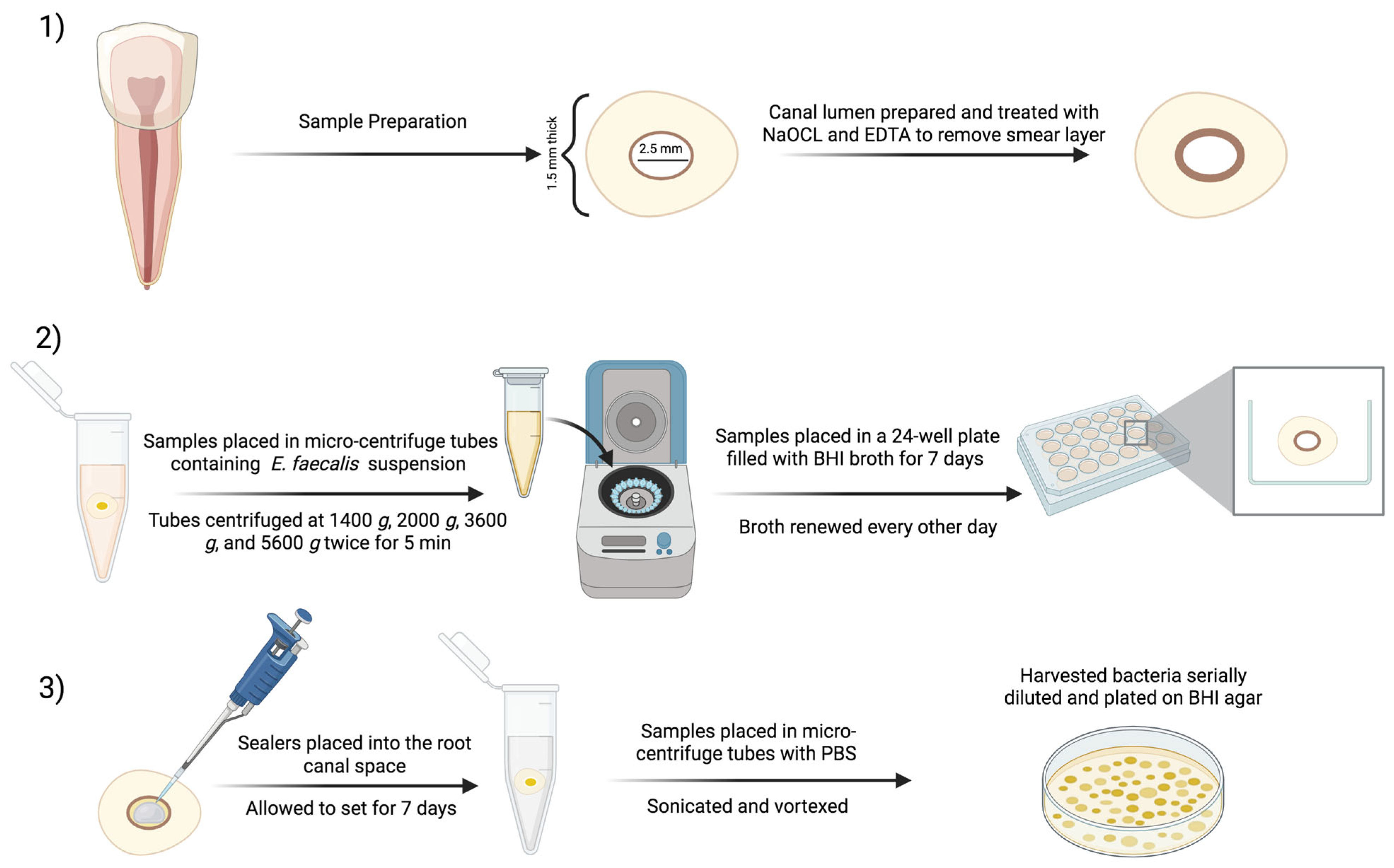
2. Materials and Methods
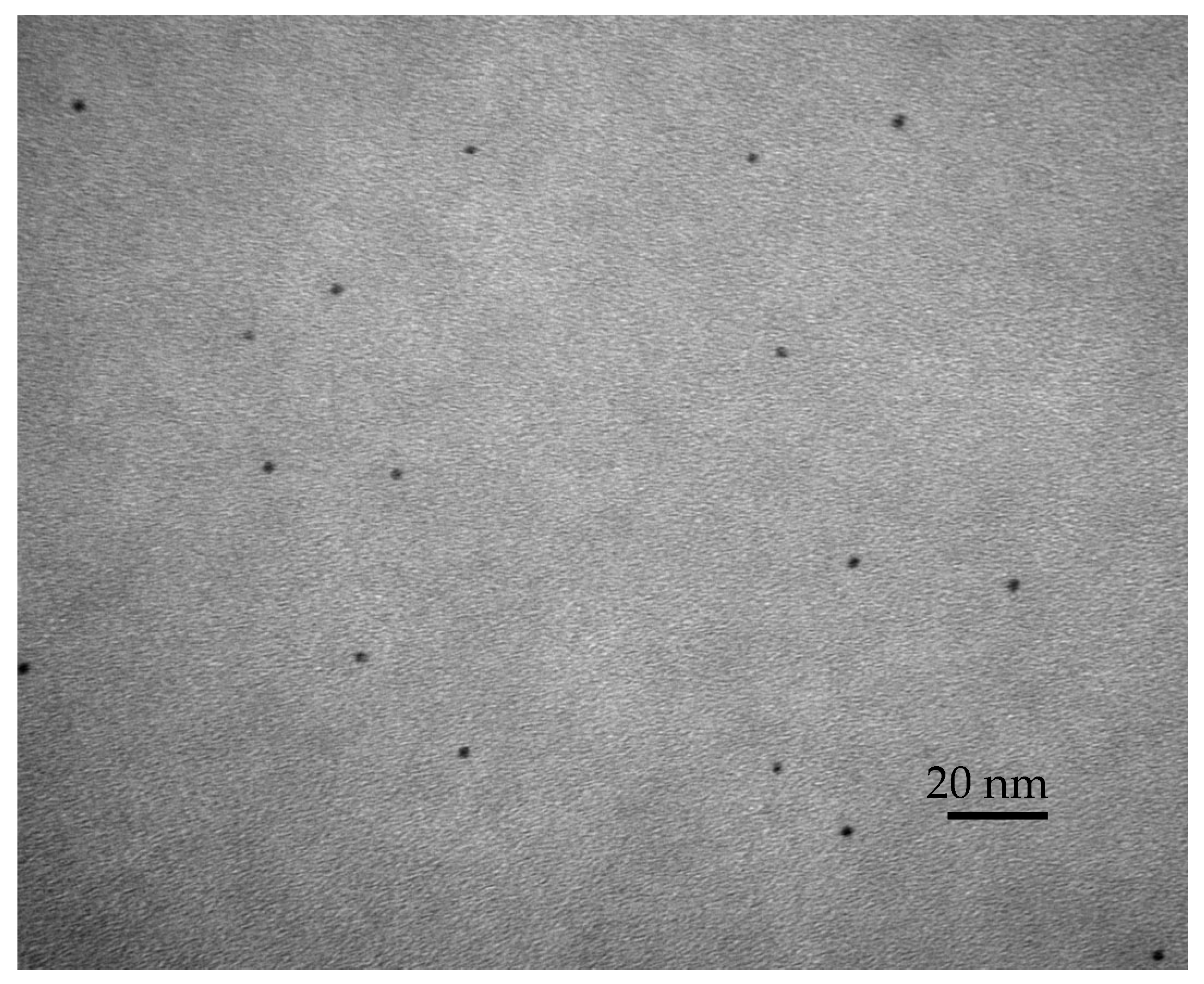
2.1. Preparation of the Modified Root Canal Sealer
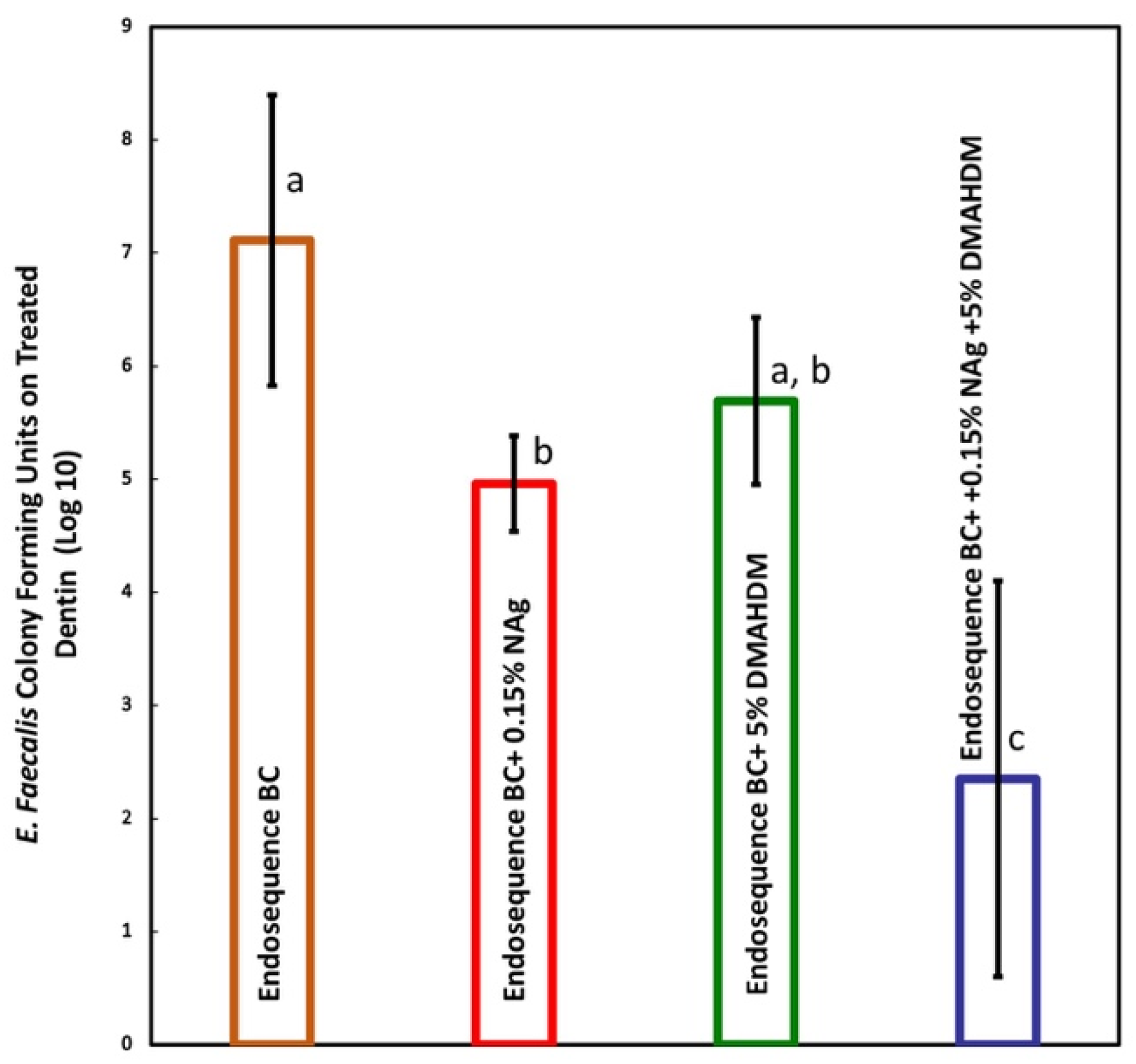
- Group 1: EndoSequence BC sealer only;
- Group 2: EndoSequence BC sealer + 0.15% NAg;
- Group 3: EndoSequence BC sealer + 5% DMAHDM;
- Group 4: EndoSequence BC sealer + 0.15% NAg + 5% DMAHDM.
2.2. Flow Properties of the Sealers
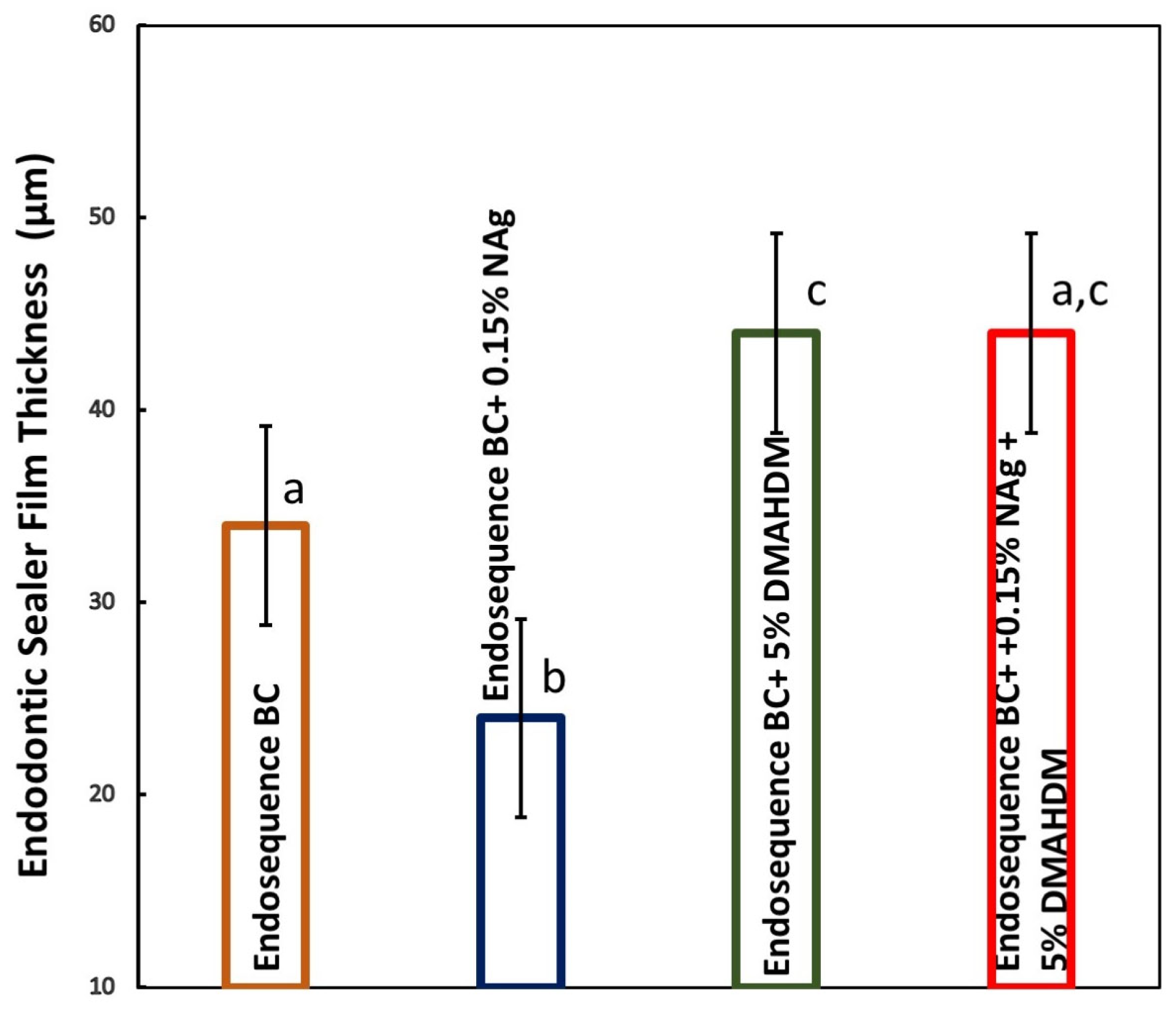
2.3. Film Thickness Properties of the Sealers
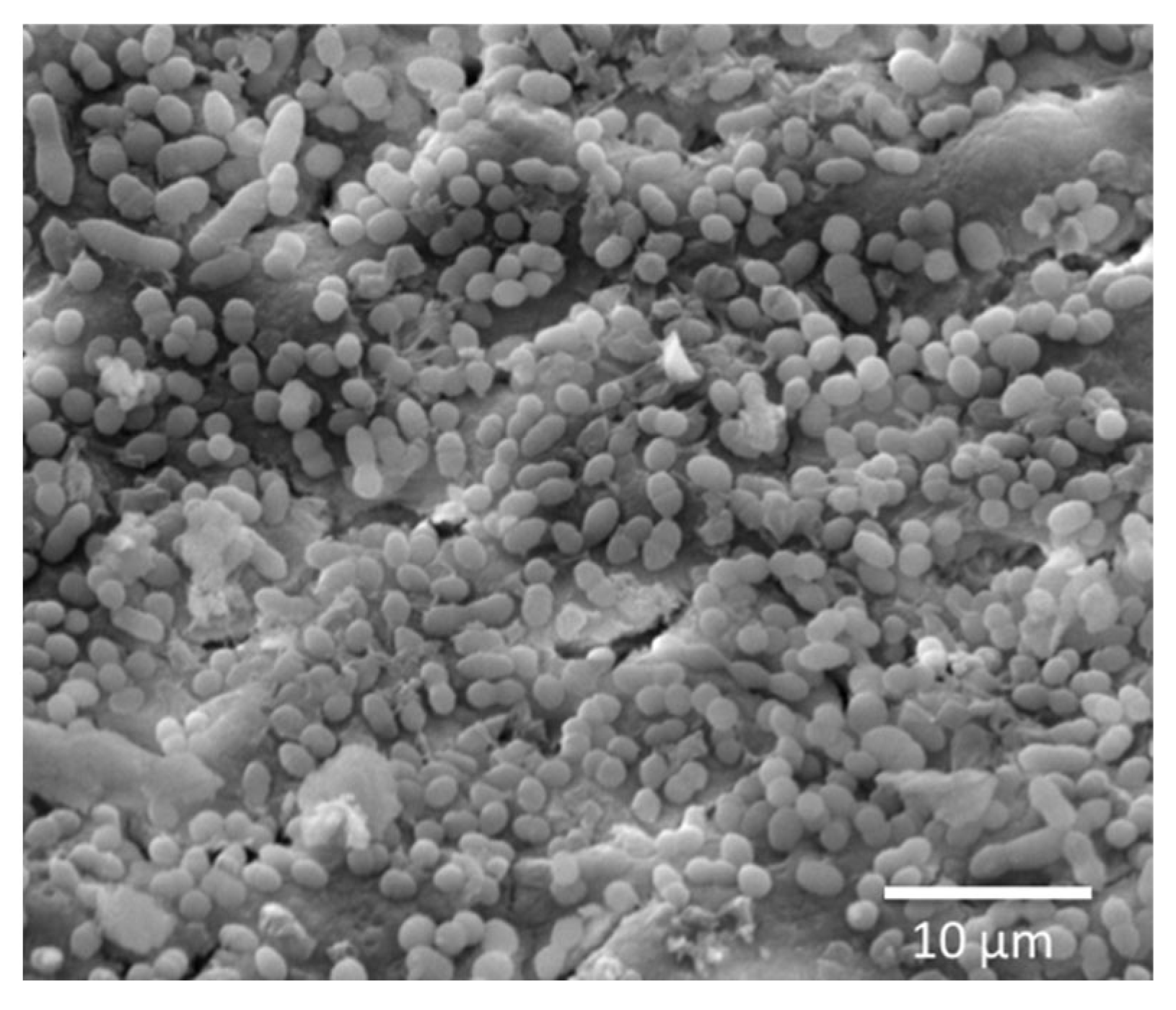
2.4. Antibiofilm Testing of E. faecalis-Impregnated Dentin Discs
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kakehashi, S.; Stanley, H.R.; Fitzgerald, R.J. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg. Oral Med. Oral Pathol. 1965, 20, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Möller, A.J.; Fabricius, L.; Dahlen, G.; Ohman, A.E.; Heyden, G. Influence on periapical tissue of indigenous oral bacteria and necrotic pulp in monkeys. Eur. J. Oral Sci. 1981, 89, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.S.; Jacobsen, E.L.; BeGole, E.A.; Remeikis, N.A. A comparison of five irrigating solutions: A scanning electron microscopic study. J. Endod. 1986, 12, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Schilder, H. Filling Root canals in three dimensions. J. Endod. 2006, 32, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Chávez de Paz, L.E.; Bergenholtz, G.; Svensäter, G. The Effects of Antimicrobials on Endodontic Biofilm Bacteria. J. Endod. 2010, 36, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, U.; Figdor, D.; Persson, S.; Sundqvist, G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int. Endod. J. 1997, 30, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.N.R.; Sjögren, U.; Krey, G.; Kahnberg, K.E.; Sundqvist, G. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: A long-term light and electron microscopic follow-up study. J. Endod. 1990, 16, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Love, R.M. Invasion of Dentinal Tubules by Root Canal Bacteria. Endod. Top. 2004, 9, 52–65. [Google Scholar] [CrossRef]
- Rôças, I.; Siqueira, J.; Santos, K. Association of Enterococcus faecalis with different forms of periradicular diseases. J. Endod. 2004, 30, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, B.; Spångberg, L.; Barry, J.; Fouad, A.F. Enterococcus spp. in endodontically treated teeth with and without periradicular lesions. J. Endod. 2005, 31, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Molander, A.; Reit, C.; Dahlén, G.; Kvist, T. Microbiological status of root-filled teeth with apical periodontitis. Int. Endod. J. 1998, 31, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hussein, F.E.; Liew, A.K.; Ramlee, R.A.; Abdullah, D.; Chong, B.S. Factors associated with apical periodontitis: A multilevel analysis. J. Endod. 2016, 42, 1441–1445. [Google Scholar] [CrossRef] [PubMed]
- Grossman, L.I. Endodontic Practice, 10th ed.; Henry Kimpton Publishers: Philadelphia, PA, USA, 1981; p. 297. [Google Scholar]
- Šimundić Munitić, M.; Poklepović Peričić, T.; Utrobičić, A.; Bago, I.; Puljak, L. Antimicrobial efficacy of commercially available endodontic bioceramic root canal sealers: A systematic review. PLoS ONE 2019, 17, e0223575. [Google Scholar] [CrossRef] [PubMed]
- Ørstavik, D. Antibacterial properties of root canal sealers, cements and pastes. Int. Endod. J. 1981, 14, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Gonçalves, R.B. Antibacterial activities of root canal sealers against selected anaerobic bacteria. J. Endod. 1996, 22, 79–80. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Favieri, A.; Gahyva, S.M.; Moraes, S.R.; Lima, K.C.; Lopes, H.P. Antimicrobial activity and flow rate of newer and established root canal sealers. J. Endod. 2000, 26, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Baras, B.H.; Melo, M.A.S.; Sun, J.; Oates, T.W.; Weir, M.D.; Xie, X.; Bai, Y.; Xu, H.H.K. Novel endodontic sealer with dual strategies of dimethylaminohexadecyl methacrylate and nanoparticles of silver to inhibit root canal biofilms. Dent. Mater. 2019, 35, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Zubizarreta-Macho, Á.; Rico-Romano, C.; María Jesús Fernández-Aceñero, M.; Mena-Álvarez, J.; Cabal, B.; Díaz, L.A.; Torrecillas, R.; Moya, J.S.; López-Píriz, R. Adding Two Antimicrobial Glasses to an Endodontic Sealer to Prevent Bacterial Root Canal Reinfection: An In Vivo Pilot Study in Dogs. Antibiotics 2021, 28, 1183. [Google Scholar] [CrossRef] [PubMed]
- Baras, B.H.; Melo, M.A.S.; Thumbigere-Math, V.; Tay, F.R.; Fouad, A.F.; Oates, T.W.; Weir, M.D.; Cheng, L.; Xu, H.H.K. Novel bioactive and therapeutic root canal sealers with antibacterial and remineralization properties. Materials 2020, 13, 1096. [Google Scholar] [CrossRef] [PubMed]
- Talapko, J.; Matijević, T.; Juzbašić, M.; Antolović-Požgain, A.; Škrlec, I. Antibacterial Activity of Silver and Its Application in Dentistry, Cardiology and Dermatology. Microorganisms 2020, 8, 1400. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Zeiger, D.N.; Howarter, J.A.; Zhang, X.; Lin, N.J.; Antonucci, J.M.; Lin-Gibson, S. In situ formation of silver nanoparticles in photocrosslinking polymers. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Yudovin-Farber, I.; Bahir, R.; Domb, A.J.; Weiss, E.I. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials 2006, 27, 3995–4002. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, F.; Weir, M.D.; Xu, H.H.K. Dental plaque microcosm response to bonding agents containing quaternary ammonium methacrylates with different chain lengths and charge densities. J. Dent. 2013, 41, 1122–1123. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Söderling, E.; Liu, F.; He, J.; Lassila, L.V.J.; Vallittu, P.K. Optimizing the concentration of quaternary ammonium dimethacrylate monomer in bis-GMA/TEGDMA dental resin system for antibacterial activity and mechanical properties. J. Mater. Sci. Mater. Med. 2014, 25, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Cheng, L.; Weir, M.D.; Bai, Y.X.; Xu, H.H.K. Effects of quaternary ammonium chain length on the antibacterial and remineralizing effects of a calcium phosphate nanocomposite. Int. J. Oral Sci. 2016, 8, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Weir, M.D.; Xu, H.H.K. Effects of quaternary ammonium chain length on antibacterial bonding agents. J. Dent. Res. 2013, 92, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Loushine, B.A.; Bryan, T.E.; Looney, S.W.; Gillen, B.M.; Loushine, R.J.; Weller, R.N.; Pashley, D.H.; Tay, F.R. Setting properties and cytotoxicity evaluation of a premixed bioceramic root canal sealer. J. Endod. 2011, 37, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shen, Y.; Ruse, N.D.; Haapasalo, M. Antibacterial Activity of Endodontic Sealers by Modified Direct Contact Test Against Enterococcus faecalis. J. Endod. 2009, 35, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Abduljabbar, S.; Abumostafa, A. Antimicrobial effect of different calcium silicate – Based bioceramic endodontic sealers against Enterococcus faecalis. An in vitro study. Saudi J. Oral. Sci. 2021, 8, 48–52. [Google Scholar] [CrossRef]
- Seung, J.; Weir, M.D.; Melo, M.A.S.; Romberg, E.; Nosrat, A.; Xu, H.H.K.; Tordik, P.A. A modified Resin Sealer: Physical and Antibacterial Properties. J. Endod. 2018, 44, 1553–1557. [Google Scholar] [CrossRef] [PubMed]
- Baras, B.H.; Sun, J.; Melo, M.A.S.; Tay, F.R.; Oates, T.W.; Zhang, K.; Weir, M.D.; Xu, H.H.K. Novel root canal sealer with dimethylaminohexadecyl methacrylate, nano-silver, and nano-calcium phosphate to kill bacteria inside root dentin and increase dentin hardness. Dent. Mater. 2019, 35, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- ISO 6876; Dentistry: Root Canal Sealing Materials. International Organization for Standardization: Geneva, Switzerland, 2012.
- Ma, J.; Wang, Z.; Shen, Y.; Haapasalo, M. A New noninvasive model to study the effectiveness of dentin disinfection by using confocal laser scanning microscopy. J. Endod. 2011, 37, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.T.P.; Nagata, J.; Bottino, M.C. Antimicrobial efficacy of triple antibiotic–eluting polymer nanofibers against multispecies biofilm. J. Endod. 2017, 43, S51–S56. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shen, Y.; Haapasalo, M. Dentin extends the antibacterial effect of endodontic sealers against Enterococcus faecalis biofilms. J. Endod. 2014, 40, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Mitwalli, H.; Alsahafi, R.; Balhaddad, A.A.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Emerging Contact-Killing Antibacterial Strategies for Developing Anti-Biofilm Dental Polymeric Restorative Materials. Bioengineering 2020, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Balhaddad, A.A.; Ibrahim, M.S.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Concentration dependence of quaternary ammonium monomer on the design of high-performance bioactive composite for root caries restorations. Dent. Mater. 2020, 36, e266–e278. [Google Scholar] [CrossRef] [PubMed]
- Alharamlah, F.; AlTuwaijri, F.; AlQuorain, H.; Khan, A.S.; Alonaizan, F.; Alsahafi, R.; Weir, M.D.; Xu, H.H.K.; Balhaddad, A.A. The impact of dimethylaminohexadecyl methacrylates on the physical and antibacterial properties of endodontic sealers. Front. Oral Health 2025, 31, 1524541. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Weir, M.D.; Xu, H.H.K.; Antonucci, J.M.; Kraigsley, A.M.; Lin, N.J.; Lin-Gibson, S.; Zhou, X. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent. Mater. 2012, 28, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Ge, S. Application of Antimicrobial Nanoparticles in Dentistry. Molecules 2019, 24, 1033. [Google Scholar] [CrossRef] [PubMed]
- Chaloupka, K.; Malam, Y.; Seifalian, A. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef] [PubMed]
- El-Tayeb, M.M.N.; Nabeel, M. Antimicrobial efficacy of EndoSequence bioceramic selaer incorporated with silver and chitosan nanoparticles: An in-vitro study. J. Endod. 2023, 49, 512–518. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baras, B.; Almohaimede, A.; Alshibani, Y.; Alzahrani, F.; Alageel, R.; Weir, M.D.; Xu, H.H.K. A Modified Bioceramic Sealer with Dual Antibacterial Mechanisms. Bioengineering 2025, 12, 768. https://doi.org/10.3390/bioengineering12070768
Baras B, Almohaimede A, Alshibani Y, Alzahrani F, Alageel R, Weir MD, Xu HHK. A Modified Bioceramic Sealer with Dual Antibacterial Mechanisms. Bioengineering. 2025; 12(7):768. https://doi.org/10.3390/bioengineering12070768
Chicago/Turabian StyleBaras, Bashayer, Amal Almohaimede, Yara Alshibani, Farah Alzahrani, Raseel Alageel, Michael D. Weir, and Hockin H. K. Xu. 2025. "A Modified Bioceramic Sealer with Dual Antibacterial Mechanisms" Bioengineering 12, no. 7: 768. https://doi.org/10.3390/bioengineering12070768
APA StyleBaras, B., Almohaimede, A., Alshibani, Y., Alzahrani, F., Alageel, R., Weir, M. D., & Xu, H. H. K. (2025). A Modified Bioceramic Sealer with Dual Antibacterial Mechanisms. Bioengineering, 12(7), 768. https://doi.org/10.3390/bioengineering12070768







