Far-Red Component Enhances Paramylon Production in Photoautotrophic Euglena gracilis
Abstract
1. Introduction
2. Culture System Design
3. Material and Methods
3.1. Culture Conditions and Growth Curves
3.2. Pigment Quantification
3.3. Oxygen Evolution and Oxygen Consumption
3.4. Paramylon Quantification
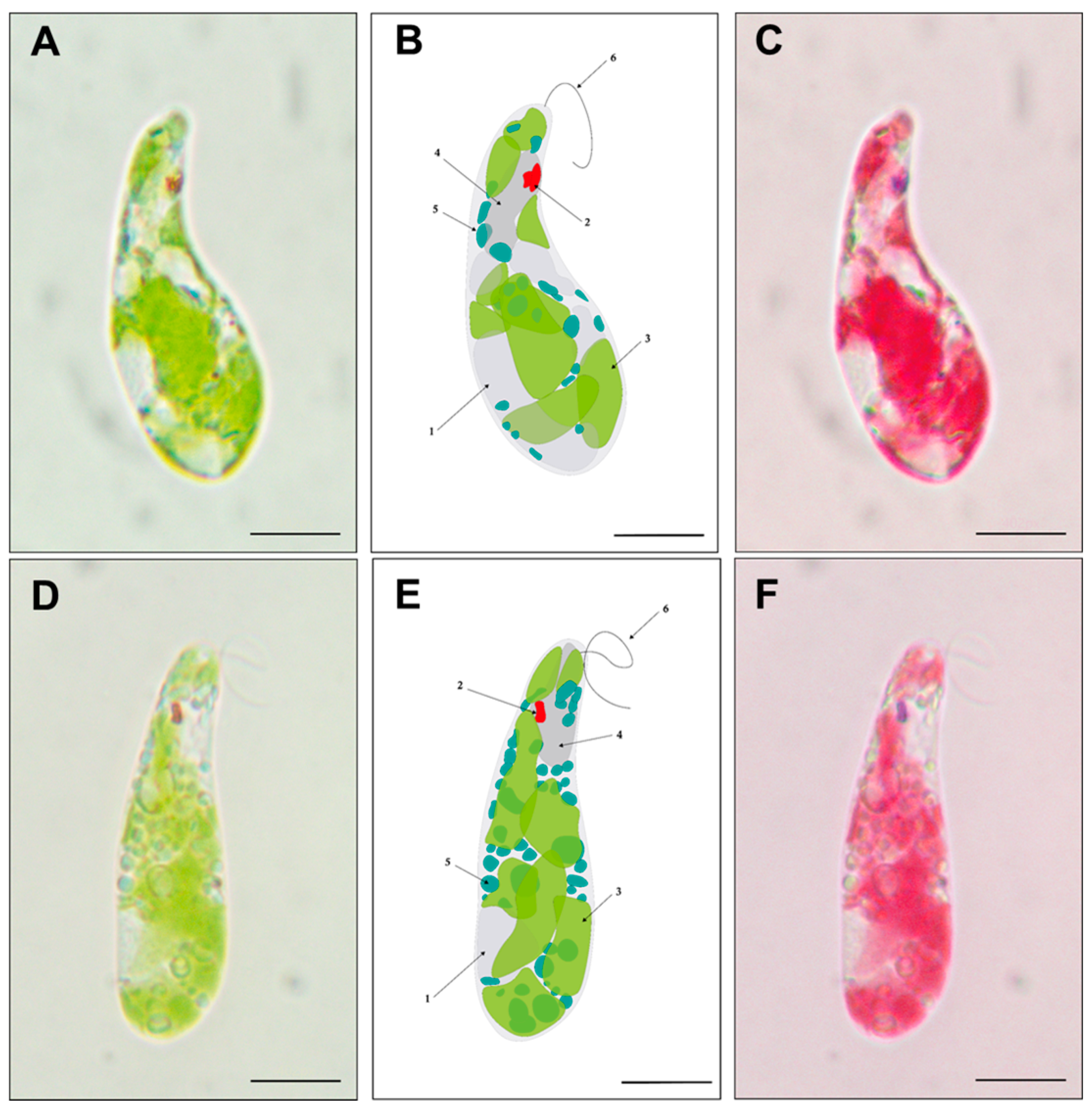
3.5. Brightfield Microscopy
3.6. Statistical Analysis
4. Results
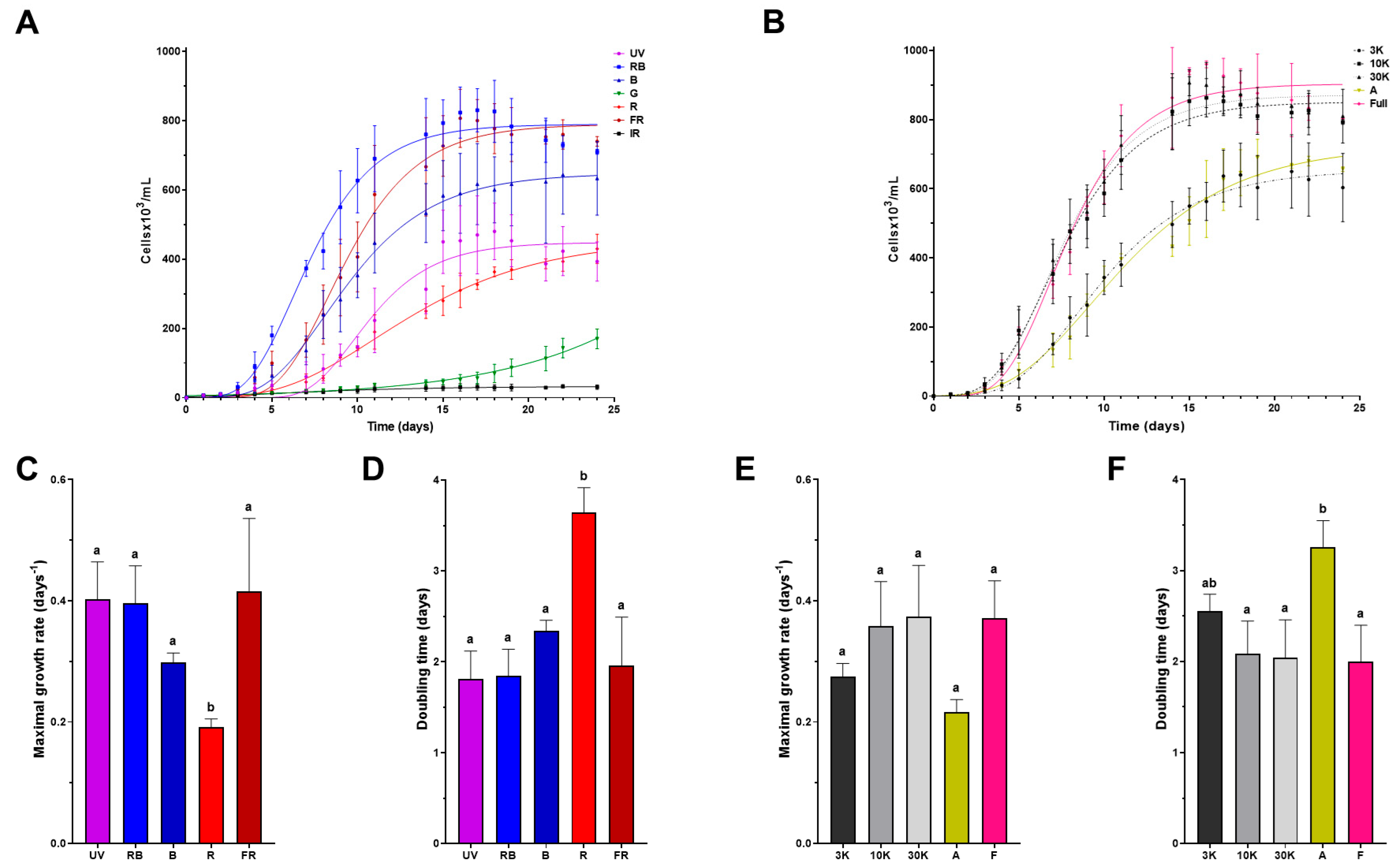
4.1. Light Wavelength as an Environmental Factor Affecting Euglena gracilis Growth
4.2. E. gracilis Adapts Its Pigment Composition as an Acclimatization Strategy to Specific Light Regimes
4.3. E. gracilis Photosystem II Can Use Different Wavelengths for Oxygen Evolution
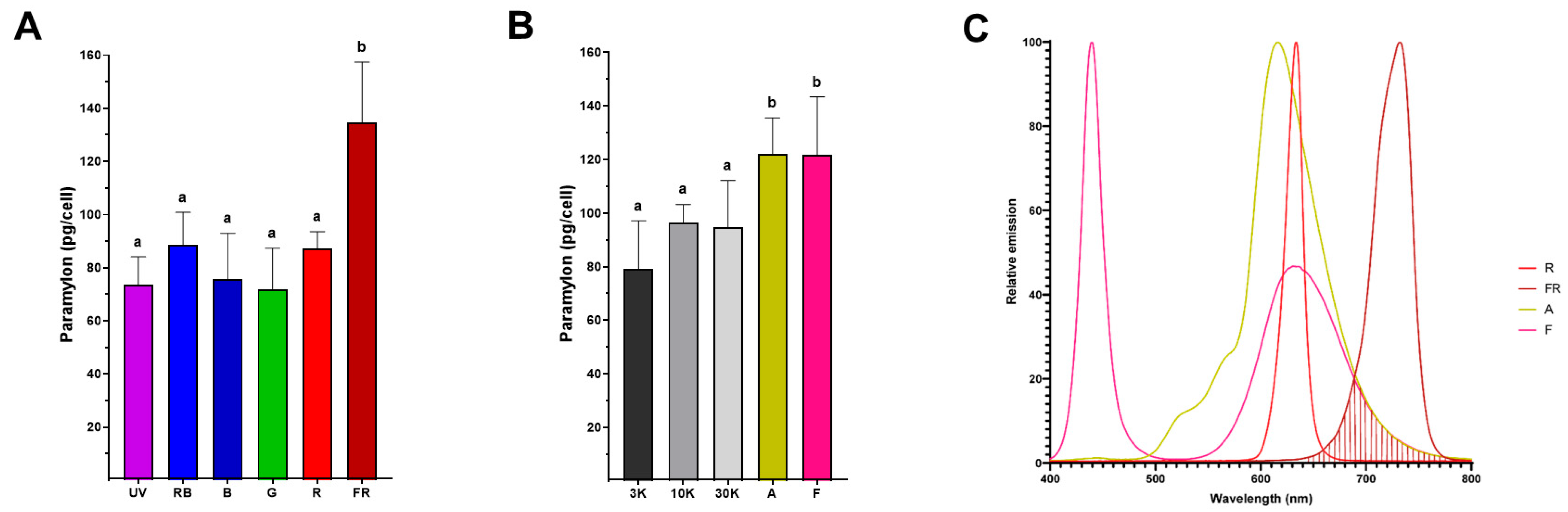
4.4. Far-Red Light Improves Paramylon Production in Photoautotrophically Grown E. gracilis
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UV | Ultraviolet light |
| RB | Royal blue light |
| B | Blue light |
| G | Green light |
| R | Red light |
| FR | Far-red light |
| IR | Infrared light |
| 3K | White light, 3000 K temperature |
| 10K | White light, 10,000 K temperature |
| 30K | White light, 30,000 K temperature |
| A | Amber light |
| F | Full-spectrum light |
| Chl a | Chlorophyll a |
| Chl b | Chlorophyll b |
| TMP | Tris-minimum-phosphate medium |
References
- Yen, H.W.; Hu, I.C.; Chen, C.Y.; Ho, S.H.; Lee, D.J.; Chang, J.S. Microalgae-based biorefinery—From biofuels to natural products. Bioresour. Technol. 2013, 135, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Rial, R.C. Biofuels versus climate change: Exploring potentials and challenges in the energy transition. Renew. Sustain. Energy Rev. 2024, 196, 114369. [Google Scholar] [CrossRef]
- Nelson, N.; Ben-Shem, A. The complex architecture of oxygenic photosynthesis. Nat. Rev. Mol. Cell Biol. 2004, 5, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Ulhassan, Z.; Brestic, M.; Zivcak, M.; Zhou, W.; Allakhverdiev, S.I.; Yang, X.; Safdar, M.E.; Yang, W.; Liu, W. Photosynthesis research under climate change. Photosynth. Res. 2021, 150, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Li, J.; Qian, J.; Wang, B.; Liu, J.; Xu, R.; Chen, P.; Zhou, W. Recent advances in CO. Chemosphere 2023, 319, 137987. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.L.; McCauley, J.; Labeeuw, L.; Ray, P.; Kuzhiumparambil, U.; Hall, C.; Doblin, M.; Nguyen, L.N.; Ralph, P.J. How microalgal biotechnology can assist with the UN Sustainable Development Goals for natural resource management. Curr. Res. Environ. Sustain. 2021, 3, 100050. [Google Scholar] [CrossRef]
- Sousa, V.; Pereira, R.N.; Vicente, A.A.; Dias, O.; Geada, P. Microalgae biomass as an alternative source of biocompounds: New insights and future perspectives of extraction methodologies. Food Res. Int. 2023, 173, 113282. [Google Scholar] [CrossRef] [PubMed]
- Leander, B.S.; Lax, G.; Karnkowska, A.; Simpson, A.G.B. Euglenida. In Handbook of the Protists; Archibald, J.M., Simpson, A.G.B., Slamovits, C.H., Margulis, L., Melkonian, M., Chapman, D.J., Corliss, J.O., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–42. [Google Scholar]
- Monfils, A.K.; Triemer, R.E.; Bellairs, E.F. Characterization of paramylon morphological diversity in photosynthetic euglenoids (Euglenales, Euglenophyta). Phycologia 2011, 50, 156–169. [Google Scholar] [CrossRef]
- Ebenezer, T.E.; Low, R.S.; O’Neill, E.C.; Huang, I.; DeSimone, A.; Farrow, S.C.; Field, R.A.; Ginger, M.L.; Guerrero, S.A.; Hammond, M.; et al. Euglena International Network (EIN): Driving euglenoid biotechnology for the benefit of a challenged world. Biol. Open 2022, 11, bio059561. [Google Scholar] [CrossRef] [PubMed]
- Gissibl, A.; Sun, A.; Care, A.; Nevalainen, H.; Sunna, A. Bioproducts From Euglena gracilis: Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Bedard, S.; Roxborough, E.; O’Neill, E.; Mangal, V. The biomolecules of Euglena gracilis: Harnessing biology for natural solutions to future problems. Protist 2024, 175, 126044. [Google Scholar] [CrossRef] [PubMed]
- Feuzing, F.; Mbakidi, J.P.; Marchal, L.; Bouquillon, S.; Leroy, E. A review of paramylon processing routes from microalga biomass to non-derivatized and chemically modified products. Carbohydr. Polym. 2022, 288, 119181. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Hata, S.; Suzuki, K.; Yoshida, E.; Nakano, R.; Mitra, S.; Arashida, R.; Asayama, Y.; Yabuta, Y.; Takeuchi, T. Oral administration of paramylon, a beta-1,3-D-glucan isolated from Euglena gracilis Z inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. J. Vet. Med. Sci. 2010, 72, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lim, D.; Lee, D.; Yu, J.; Lee, T. Valorization of corn steep liquor for efficient paramylon production using Euglena gracilis: The impact of precultivation and light-dark cycle. Algal Res. 2022, 61, 102587. [Google Scholar] [CrossRef]
- Rodríguez-Bolaños, M.; Vargas-Romero, G.; Jaguer-García, G.; Aguilar-Gonzalez, Z.I.; Lagos-Romero, V.; Miranda-Astudillo, H.V. Antares I: A Modular Photobioreactor Suitable for Photosynthesis and Bioenergetics Research. Appl. Biochem. Biotechnol. 2024, 196, 2176–2195. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, J.; Qin, H.; Wang, Q.; Chen, Z.; Liu, C.; Zheng, L.; Wang, J. Paramylon from Euglena gracilis Prevents Lipopolysaccharide-Induced Acute Liver Injury. Front. Immunol. 2021, 12, 797096. [Google Scholar] [CrossRef] [PubMed]
- Rubiyatno; Matsui, T.; Mori, K.; Toyama, T. Paramylon production by Euglena gracilis via mixotrophic cultivation using sewage effluent and waste organic compounds. Bioresour. Technol. Rep. 2021, 15, 100735. [Google Scholar] [CrossRef]
- Rodríguez-Zavala, J.S.; Ortiz-Cruz, M.A.; Mendoza-Hernández, G.; Moreno-Sánchez, R. Increased synthesis of α-tocopherol, paramylon and tyrosine by Euglena gracilis under conditions of high biomass production. J. Appl. Microbiol. 2010, 109, 2160–2172. [Google Scholar] [CrossRef] [PubMed]
- Aoe, S.; Yamanaka, C.; Koketsu, K.; Nishioka, M.; Onaka, N.; Nishida, N.; Takahashi, M. Effects of Paramylon Extracted from Euglena gracilis EOD-1 on Parameters Related to Metabolic Syndrome in Diet-Induced Obese Mice. Nutrients 2019, 11, 1674. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Sugimoto, R.; Suzuki, K.; Shirakata, Y.; Hashiguchi, T.; Yoshida, C.; Nakano, Y. Anti-fibrotic activity of Euglena gracilis and paramylon in a mouse model of non-alcoholic steatohepatitis. Food Sci. Nutr. 2019, 7, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Ogushi, M.; Nakashima, A.; Nakano, Y.; Suzuki, K. Accelerated Wound Healing on the Skin Using a Film Dressing with β-Glucan Paramylon. In Vivo 2018, 32, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Zhao, J.; Li, H.; Fan, D. Paramylon hydrogel: A bioactive polysaccharides hydrogel that scavenges ROS and promotes angiogenesis for wound repair. Carbohydr. Polym. 2022, 289, 119467. [Google Scholar] [CrossRef] [PubMed]
- Vadiveloo, A.; Moheimani, N.R.; Cosgrove, J.J.; Parlevliet, D.; Bahri, P.A. Effects of different light spectra on the growth, productivity and photosynthesis of two acclimated strains of Nannochloropsis sp. J. Appl. Phycol. 2017, 29, 1765–1774. [Google Scholar] [CrossRef]
- Xin, K.; Guo, R.; Wang, X.; Yu, Z.; Mao, W.; Cheng, C.; Che, G.; Qian, L.; Cheng, J.; Yang, W.; et al. Photoautotrophic Growth and Cell Division of Euglena gracilis with Mixed Red and Blue Wavelengths. Ind. Eng. Chem. Res. 2024, 63, 4746–4755. [Google Scholar] [CrossRef]
- Ritchie, R.J. Consistent Sets of Spectrophotometric Chlorophyll Equations for Acetone, Methanol and Ethanol Solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Caspers, H.J.D.H. Strickland and T. R. Parsons: A Practical Handbook of Seawater Analysis. Ottawa: Fisheries Research Board of Canada, Bulletin 167, 1968. 293 pp. $ 7.50. Int. Rev. Gesamten Hydrobiol. Hydrogr. 1970, 55, 167. [Google Scholar] [CrossRef]
- Gain, G.; Vega de Luna, F.; Cordoba, J.; Perez, E.; Degand, H.; Morsomme, P.; Thiry, M.; Baurain, D.; Pierangelini, M.; Cardol, P. Trophic state alters the mechanism whereby energetic coupling between photosynthesis and respiration occurs in Euglena gracilis. New Phytol. 2021, 232, 1603–1617. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; van Iersel, M.W.; Bugbee, B. Photosynthesis in sun and shade: The surprising importance of far-red photons. New Phytol. 2022, 236, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Schulze, P.S.; Barreira, L.A.; Pereira, H.G.; Perales, J.A.; Varela, J.C. Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol. 2014, 32, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Slavens, S.; Crunkleton, D.W.; Johannes, T.W. Interactive effect of light quality and temperature on Chlamydomonas reinhardtii growth kinetics and lipid synthesis. Algal Res. 2021, 53, 102127. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, Y.; Han, S.H.; Hwang, S.J. The effects of wavelength and wavelength mixing ratios on microalgae growth and nitrogen, phosphorus removal using Scenedesmus sp. for wastewater treatment. Bioresour. Technol. 2013, 130, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Smayda, T.J.; Mitchell-Innes, B. Dark survival of autotrophic, planktonic marine diatoms. Mar. Biol. 1974, 25, 195–202. [Google Scholar] [CrossRef]
- Mattos, E.R.; Singh, M.; Cabrera, M.L.; Das, K.C. Enhancement of biomass production in Scenedesmus bijuga high-density culture using weakly absorbed green light. Biomass Bioenergy 2015, 81, 473–478. [Google Scholar] [CrossRef]
- Kubín, Š.; Borns, E.; Doucha, J.; Seiss, U. Light Absorption and Production Rate of Chlorella vulgaris in Light of Different Spectral Composition. Biochem. Und Physiol. Der Pflanz. 1983, 178, 193–205. [Google Scholar] [CrossRef]
- Ievina, B.; Romagnoli, F. Unveiling underlying factors for optimizing light spectrum to enhance microalgae growth. Bioresour. Technol. 2025, 418, 131980. [Google Scholar] [CrossRef] [PubMed]
- Paper, M.; Glemser, M.; Haack, M.; Lorenzen, J.; Mehlmer, N.; Fuchs, T.; Schenk, G.; Garbe, D.; Weuster-Botz, D.; Eisenreich, W.; et al. Efficient Green Light Acclimation of the Green Algae. Front. Bioeng. Biotechnol. 2022, 10, 885977. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.X.; Schiff, J.A. Chlorophyll-Protein Complexes from Euglena gracilis and Mutants Deficient in Chlorophyll b: II. Polypeptide Composition. Plant Physiol. 1986, 80, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Astudillo, H.; Arshad, R.; Vega de Luna, F.; Aguilar-Gonzalez, Z.; Forêt, H.; Feller, T.; Gervasi, A.; Nawrocki, W.; Counson, C.; Morsomme, P.; et al. A Unique LHCE Light-Harvesting protein Family is involved in Photosystem I and II Far-Red Absorption in Euglena gracilis. bioRxiv 2025. [Google Scholar] [CrossRef]
- Tanno, Y.; Kato, S.; Takahashi, S.; Tamaki, S.; Takaichi, S.; Kodama, Y.; Sonoike, K.; Shinomura, T. Light dependent accumulation of β-carotene enhances photo-acclimation of Euglena gracilis. J. Photochem. Photobiol. B 2020, 209, 111950. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.X.; Gantt, E. One ring or two? Determination of ring number in carotenoids by lycopene epsilon-cyclases. Proc. Natl. Acad. Sci. USA 2001, 98, 2905–2910. [Google Scholar] [CrossRef] [PubMed]
- Mutschlechner, M.; Walter, A.; Colleselli, L.; Griesbeck, C.; Schöbel, H. Enhancing carotenogenesis in terrestrial microalgae by UV-A light stress. J. Appl. Phycol. 2022, 34, 1943–1955. [Google Scholar] [CrossRef]
- Xu, Y.; Harvey, P.J. Carotenoid Production by Dunaliella salina under Red Light. Antioxidants 2019, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liu, Q.; Cheng, Y.; Feng, L.; Wu, X.; Fan, Y.; Raza, M.A.; Wang, X.; Yong, T.; Liu, W.; et al. Low red/far-red ratio as a signal promotes carbon assimilation of soybean seedlings by increasing the photosynthetic capacity. BMC Plant Biol. 2020, 20, 148. [Google Scholar] [CrossRef] [PubMed]
- Piiparinen, J.; Barth, D.; Eriksen, N.T.; Teir, S.; Spilling, K.; Wiebe, M.G. Microalgal CO2 capture at extreme pH values. Algal Res. 2018, 32, 321–328. [Google Scholar] [CrossRef]
- Krajčovič, J.; Matej, V.; Schwartzbach, S.D. Euglenoid flagellates: A multifaceted biotechnology platform. J. Biotechnol. 2015, 202, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Grimm, P.; Risse, J.M.; Cholewa, D.; Müller, J.M.; Beshay, U.; Friehs, K.; Flaschel, E. Applicability of Euglena gracilis for biorefineries demonstrated by the production of α-tocopherol and paramylon followed by anaerobic digestion. J. Biotechnol. 2015, 215, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Inui, H.; Miyatake, K.; Nakano, Y.; Kitaoka, S. Production and Composition of Wax Esters by Fermentation of Euglena gracilis. Agric. Biol. Chem. 1983, 47, 2669–2671. [Google Scholar] [CrossRef]
- Yagi, K.; Hamada, K.; Hlrata, K.; Miyamoto, K.; Miura, Y.; Akano, T.; Fukatu, K.; Ikuta, Y.; Nakamura, H.K. Stimulatory effect of red light on starch accumulation in a marine green alga,chlamydomonas sp. strain MGA161. Appl. Biochem. Biotechnol. 1994, 45, 225–232. [Google Scholar] [CrossRef]
- Six, A.; Dimitriades-Lemaire, A.; Lancelon-Pin, C.; Putaux, J.L.; Dauvillée, D.; Petroutsos, D.; Alvarez Diaz, P.; Sassi, J.F.; Li-Beisson, Y.; Fleury, G. Red light induces starch accumulation in Chlorella vulgaris without affecting photosynthesis efficiency, unlike abiotic stress. Algal Res. 2024, 80, 103515. [Google Scholar] [CrossRef]
- Meeuse, B.J.D. Breakdown of paramylon and laminarin by digestive enzymes of Lamellibranchs—An important ecological feature. Basteria 1964, 28, 67–79. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Gonzalez, Z.I.; Rico-Luna, A.; Takahashi-Íñiguez, T.; Miranda-Astudillo, H.V. Far-Red Component Enhances Paramylon Production in Photoautotrophic Euglena gracilis. Bioengineering 2025, 12, 763. https://doi.org/10.3390/bioengineering12070763
Aguilar-Gonzalez ZI, Rico-Luna A, Takahashi-Íñiguez T, Miranda-Astudillo HV. Far-Red Component Enhances Paramylon Production in Photoautotrophic Euglena gracilis. Bioengineering. 2025; 12(7):763. https://doi.org/10.3390/bioengineering12070763
Chicago/Turabian StyleAguilar-Gonzalez, Zhaida I., Anaiza Rico-Luna, Tóshiko Takahashi-Íñiguez, and Héctor V. Miranda-Astudillo. 2025. "Far-Red Component Enhances Paramylon Production in Photoautotrophic Euglena gracilis" Bioengineering 12, no. 7: 763. https://doi.org/10.3390/bioengineering12070763
APA StyleAguilar-Gonzalez, Z. I., Rico-Luna, A., Takahashi-Íñiguez, T., & Miranda-Astudillo, H. V. (2025). Far-Red Component Enhances Paramylon Production in Photoautotrophic Euglena gracilis. Bioengineering, 12(7), 763. https://doi.org/10.3390/bioengineering12070763






