Advancements in Visual Field Testing: A Systematic Review of the 24-2C Test Grid
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Collection and Risk-of-Bias Assessment
2.4. Data Synthesis and Analysis
3. Results
3.1. Summary of Included Studies
3.2. Risk-of-Bias Assessment
3.3. Summary of Findings
- (1)
- Comparison of 24-2C and 24-2 test grids
- (2)
- Comparison of 24-2C and 10-2 test grids
- (3)
- Others
4. Discussion
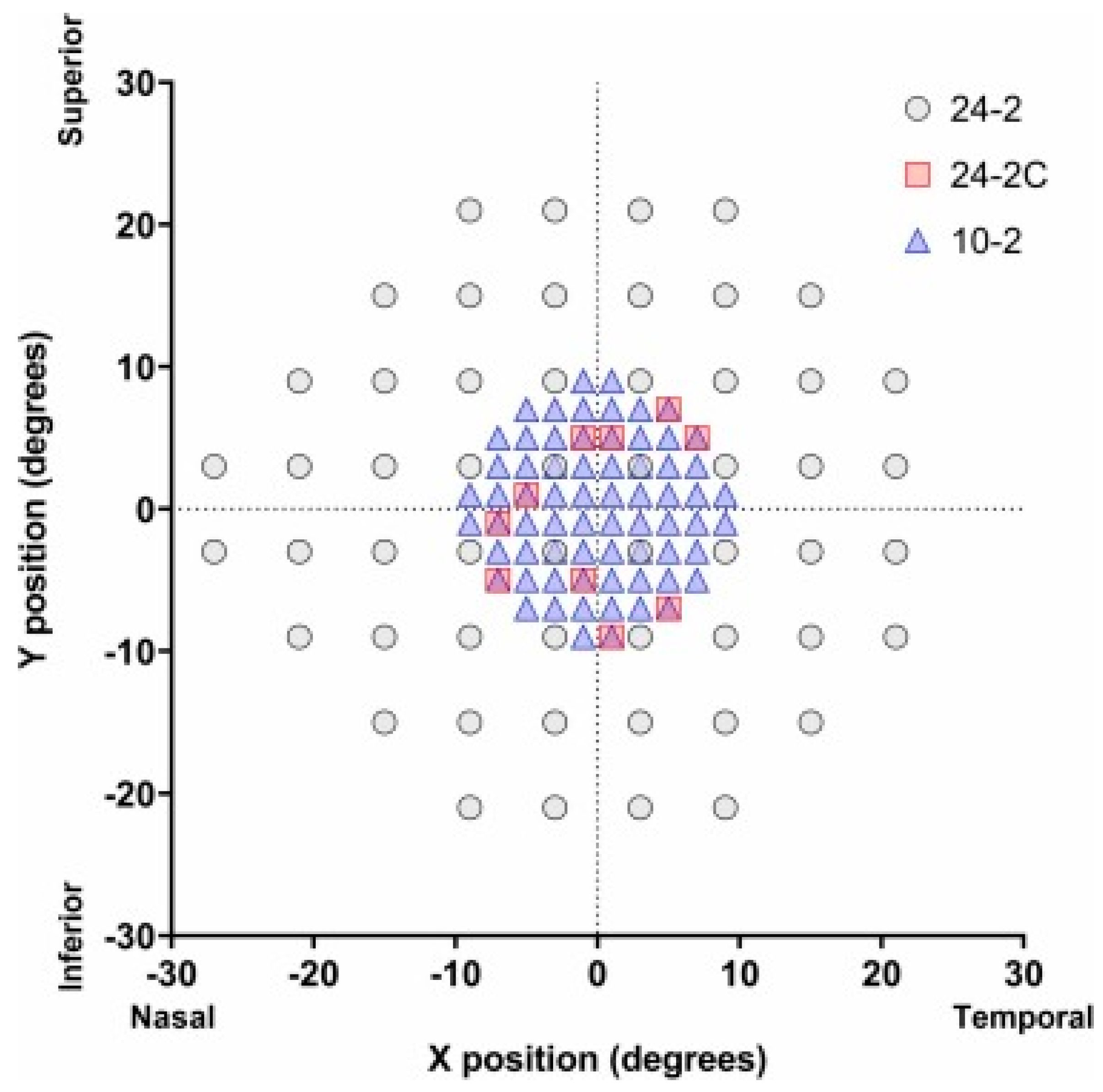
4.1. History of 24-2C
4.2. Comparing the Capabilities of the 24-2C with the 24-2 and 10-2 Grids
4.2.1. Detection of VFDs and CVFDs
4.2.2. Global Indices
4.2.3. Application in Neuro-Ophthalmology Patients
4.2.4. Macular S-F Concordance
4.2.5. Reliability Indices
4.3. Other Clinical Utilities and Implications
4.4. Limitations
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dietze, J.; Blair, K.; Zeppieri, M.; Havens, S.J. Glaucoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Morley, A.M.S. The Future of Glaucoma Clinics. Br. J. Ophthalmol. 2006, 90, 640–645. [Google Scholar] [CrossRef][Green Version]
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Broadway, D.C. Visual Field Testing for Glaucoma—A Practical Guide. Community Eye Health 2012, 25, 66–70. [Google Scholar]
- Germano, R.A.S.; Germano, C.S.; Susanna, F.N.; Susanna, R. Patterns of Visual Field Loss in Early, Moderate, and Severe Stages of Open Angle Glaucoma. J. Glaucoma 2022, 31, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.; Aspinall, P.; Papasouliotis, O.; Worton, B.; O’Brien, C. Quality of Life in Glaucoma and Its Relationship with Visual Function. J. Glaucoma 2003, 12, 139–150. [Google Scholar] [CrossRef]
- Ruia, S.; Tripathy, K. Humphrey Visual Field. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- De Moraes, C.G.; Hood, D.C.; Thenappan, A.; Girkin, C.A.; Medeiros, F.A.; Weinreb, R.N.; Zangwill, L.M.; Liebmann, J.M. 24-2 Visual Fields Miss Central Defects Shown on 10-2 Tests in Glaucoma Suspects, Ocular Hypertensives, and Early Glaucoma. Ophthalmology 2017, 124, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Medeiros, F.A.; Weinreb, R.N.; Girkin, C.A.; Zangwill, L.M. Comparing 10-2 and 24-2 Visual Fields for Detecting Progressive Central Visual Loss in Glaucoma Eyes with Early Central Abnormalities. Ophthalmol. Glaucoma 2019, 2, 95–102. [Google Scholar] [CrossRef]
- Grillo, L.M.; Wang, D.L.; Ramachandran, R.; Ehrlich, A.C.; De Moraes, C.G.; Ritch, R.; Hood, D.C. The 24-2 Visual Field Test Misses Central Macular Damage Confirmed by the 10-2 Visual Field Test and Optical Coherence Tomography. Transl. Vis. Sci. Technol. 2016, 5, 15. [Google Scholar] [CrossRef]
- Phu, J.; Kalloniatis, M. Ability of 24-2C and 24-2 Grids to Identify Central Visual Field Defects and Structure-Function Concordance in Glaucoma and Suspects. Am. J. Ophthalmol. 2020, 219, 317–331. [Google Scholar] [CrossRef]
- Hong, J.W.; Baek, M.S.; Lee, J.Y.; Song, M.K.; Shin, J.W.; Kook, M.S. Comparison of the 24-2 and 24-2C Visual Field Grids in Determining the Macular Structure-Function Relationship in Glaucoma. J. Glaucoma 2021, 30, 887–894. [Google Scholar] [CrossRef]
- Chakravarti, T.; Moghadam, M.; Proudfoot, J.A.; Weinreb, R.N.; Bowd, C.; Zangwill, L.M. Agreement Between 10-2 and 24-2C Visual Field Test Protocols for Detecting Glaucomatous Central Visual Field Defects. J. Glaucoma 2021, 30, e285–e291. [Google Scholar] [CrossRef]
- WuDunn, D.; Takusagawa, H.L.; Rosdahl, J.A.; Sit, A.J.; Chopra, V.; Ou, Y.; Richter, G.M.; Knight, O.J.; Solá-Del Valle, D.; Kim, S.J. Central Visual Field Testing in Early Glaucoma. Ophthalmology 2024, 131, 240–248. [Google Scholar] [CrossRef]
- Onyekaba, N.-A.E.; Estrela, T.; Naithani, R.; McCarthy, K.M.; Jammal, A.A.; Medeiros, F.A. Comparison of 10-2 and 24-2 Perimetry to Diagnose Glaucoma Using OCT as an Independent Reference Standard. Ophthalmol. Glaucoma 2023, 6, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.C.K.; Yohannan, J.; Ramulu, P.Y.; Kalloniatis, M.; Crabb, D.P.; Crowston, J.; Phu, J. Visual Field Testing in Glaucoma Using the Swedish Interactive Thresholding Algorithm (SITA). Surv. Ophthalmol. 2025, 70, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Phu, J.; Kalloniatis, M. Comparison of 10-2 and 24-2C Test Grids for Identifying Central Visual Field Defects in Glaucoma and Suspect Patients. Ophthalmology 2021, 128, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Arab-Zozani, M.; Pezeshki, M.Z.; Khodayari-Zarnaq, R.; Janati, A. Inappropriate Rate of Admission and Hospitalization in the Iranian Hospitals: A Systematic Review and Meta-Analysis. Value Health Reg. Issues 2020, 21, 105–112. [Google Scholar] [CrossRef]
- Yamane, M.L.M.; Odel, J.G. Introducing the 24-2C Visual Field Test in Neuro-Ophthalmology. J. Neuroophthalmol. 2021, 41, e606–e611. [Google Scholar] [CrossRef]
- Meshkin, R.S.; Zhao, Y.; Elze, T.; Boland, M.V.; Friedman, D.S. Remote Video Monitoring of Simultaneous Visual Field Testing. J. Glaucoma 2022, 31, 488–493. [Google Scholar] [CrossRef]
- Behera, G.; Nath, A.; Ramasamy, A.; Kaliaperumal, S. Comparing Static Perimetry Protocols of Central Field Testing among Patients with Glaucoma. Optom. Vis. Sci. 2023, 100, 406–411. [Google Scholar] [CrossRef]
- Nishijima, E.; Fukai, K.; Sano, K.; Noro, T.; Ogawa, S.; Okude, S.; Tatemichi, M.; Lee, G.C.; Iwase, A.; Nakano, T. Comparative Analysis of 24-2C, 24–2, and 10-2 Visual Field Tests for Detecting Mild-Stage Glaucoma with Central Visual Field Defects. Am. J. Ophthalmol. 2024, 268, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Heijl, A.; Patella, V.M.; Chong, L.X.; Iwase, A.; Leung, C.K.; Tuulonen, A.; Lee, G.C.; Callan, T.; Bengtsson, B. A New SITA Perimetric Threshold Testing Algorithm: Construction and a Multicenter Clinical Study. Am. J. Ophthalmol. 2019, 198, 154–165. [Google Scholar] [CrossRef]
- Johnson, C.A.; Adams, C.W.; Lewis, R.A. Fatigue Effects in Automated Perimetry. Appl. Opt. 1988, 27, 1030. [Google Scholar] [CrossRef]
- Meier-Gibbons, F.; Töteberg-Harms, M. Influence of Cost of Care and Adherence in Glaucoma Management: An Update. J. Ophthalmol. 2020, 2020, 5901537. [Google Scholar] [CrossRef]
- Pham, A.T.; Ramulu, P.Y.; Boland, M.V.; Yohannan, J. The Effect of Transitioning from SITA Standard to SITA Faster on Visual Field Performance. Ophthalmology 2021, 128, 1417–1425. [Google Scholar] [CrossRef]
- Liu, W.W.; Shalaby, W.S.; Shiuey, E.J.; Raghu, R.; Petkovsek, D.; Myers, J.S.; Wizov, S.S.; Spaeth, G.L.; Shukla, A.G. Correlation between Central Visual Field Defects and Stereopsis in Patients with Early-to-Moderate Visual Field Loss. Ophthalmol. Glaucoma 2023, 6, 493–500. [Google Scholar] [CrossRef]
- Cloutier, M.; DeLucia, P.R. Topical Review: Impact of Central Vision Loss on Navigation and Obstacle Avoidance While Walking. Optom. Vis. Sci. 2022, 99, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.J.; Hobby, A.E.; Binns, A.M.; Crabb, D.P. How Does Age-Related Macular Degeneration Affect Real-World Visual Ability and Quality of Life? A Systematic Review. BMJ Open 2016, 6, e011504. [Google Scholar] [CrossRef] [PubMed]
- Brody, B.L.; Gamst, A.C.; Williams, R.A.; Smith, A.R.; Lau, P.W.; Dolnak, D.; Rapaport, M.H.; Kaplan, R.M.; Brown, S.I. Depression, Visual Acuity, Comorbidity, and Disability Associated with Age-Related Macular Degeneration. Ophthalmology 2001, 108, 1893–1900. [Google Scholar] [CrossRef]
- Van Landingham, S.W.; Massof, R.W.; Chan, E.; Friedman, D.S.; Ramulu, P.Y. Fear of Falling in Age-Related Macular Degeneration. BMC Ophthalmol. 2014, 14, 10. [Google Scholar] [CrossRef]
- Jackson, A.B.; Martin, K.R.; Coote, M.A.; Medeiros, F.A.; Girkin, C.A.; Fazio, M.A.; Liebmann, J.M.; De Moraes, C.G.; Weinreb, R.N.; Zangwill, L.M.; et al. Fast Progressors in Glaucoma. Ophthalmology 2023, 130, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Monhart, M.; Lee, G.; Iwase, A.; Flanagan, J. Selecting Additional Test Locations to Enhance the 24-2 Pattern Using a Scoring System. Presented at the World Glaucoma Congress, Helsinki, Finland, 28 June–1 July 2017. [Google Scholar]
- Chong, L.X.; McKendrick, A.M.; Ganeshrao, S.B.; Turpin, A. Customized, Automated Stimulus Location Choice for Assessment of Visual Field Defects. Investig. Opthalmol. Vis. Sci. 2014, 55, 3265. [Google Scholar] [CrossRef] [PubMed]
- Ballae Ganeshrao, S.; Turpin, A.; Denniss, J.; McKendrick, A.M. Enhancing Structure–Function Correlations in Glaucoma with Customized Spatial Mapping. Ophthalmology 2015, 122, 1695–1705. [Google Scholar] [CrossRef]
- Kim, E.K.; Park, H.-Y.L.; Hong, K.E.; Shin, D.Y.; Park, C.K. Investigation of Progression Pattern and Associated Risk Factors in Glaucoma Patients with Initial Paracentral Scotomas Using Humphrey 10-2. Sci. Rep. 2021, 11, 18609. [Google Scholar] [CrossRef]
- Hu, R.; Racette, L.; Chen, K.S.; Johnson, C.A. Functional Assessment of Glaucoma: Uncovering Progression. Surv. Ophthalmol. 2020, 65, 639–661. [Google Scholar] [CrossRef]
- Åsman, P.; Heijl, A. Weighting According to Location in Computer-assisted Glaucoma Visual Field Analysis. Acta Ophthalmol. 1992, 70, 671–678. [Google Scholar] [CrossRef]
- Phu, J.; Khuu, S.K.; Nivison-Smith, L.; Zangerl, B.; Choi, A.Y.J.; Jones, B.W.; Pfeiffer, R.L.; Marc, R.E.; Kalloniatis, M. Pattern Recognition Analysis Reveals Unique Contrast Sensitivity Isocontours Using Static Perimetry Thresholds Across the Visual Field. Investig. Opthalmol. Vis. Sci. 2017, 58, 4863. [Google Scholar] [CrossRef] [PubMed]
- Banc, A.; Kedar, S. Interpretation of the Visual Field in Neuro-Ophthalmic Disorders. Curr. Neurol. Neurosci. Rep. 2024, 24, 67–81. [Google Scholar] [CrossRef]
- Drance, S.M. Diffuse Visual Field Loss in Open-Angle Glaucoma. Ophthalmology 1991, 98, 1533–1538. [Google Scholar] [CrossRef]
- Kedar, S.; Ghate, D.; Corbett, J. Visual Fields in Neuro-Ophthalmology. Indian J. Ophthalmol. 2011, 59, 103. [Google Scholar] [CrossRef]
- Hood, D.C.; Nguyen, M.; Ehrlich, A.C.; Raza, A.S.; Sliesoraityte, I.; De Moraes, C.G.; Ritch, R.; Schiefer, U. A Test of a Model of Glaucomatous Damage of the Macula With High-Density Perimetry: Implications for the Locations of Visual Field Test Points. Transl. Vis. Sci. Technol. 2014, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, J.Y.; Kim, S.-O.; Kook, M.S. Macular Structure–Function Relationship at Various Spatial Locations in Glaucoma. Br. J. Ophthalmol. 2015, 99, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Kim, K.E.; Song, W.K.; Yoon, J.; Kook, M.S. Progressive Macular Vessel Density Loss and Visual Field Progression in Open-Angle Glaucoma Eyes with Central Visual Field Damage. Ophthalmol. Glaucoma 2024, 7, 16–29. [Google Scholar] [CrossRef]
- Lee, G.A.; Kong, G.Y.X.; Liu, C. Visual Fields in Glaucoma: Where Are We Now? Clin. Experiment. Ophthalmol. 2023, 51, 162–169. [Google Scholar] [CrossRef]
- Katz, J.; Sommer, A. Screening for Glaucomatous Visual Field Loss. Ophthalmology 1990, 97, 1032–1037. [Google Scholar] [CrossRef]
- Birt, C.M.; Shin, D.H.; Samudrala, V.; Hughes, B.A.; Kim, C.; Lee, D. Analysis of Reliability Indices from Humphrey Visual Field Tests in an Urban Glaucoma Population. Ophthalmology 1997, 104, 1126–1130. [Google Scholar] [CrossRef] [PubMed]
- Kramer, B.C.; Musch, D.C.; Niziol, L.M.; Weizer, J.S. Reliability of Simultaneous Visual Field Testing. Ophthalmology 2012, 119, 304–307. [Google Scholar] [CrossRef]
- Le, C.T.; Fiksel, J.; Ramulu, P.; Yohannan, J. Differences in Visual Field Loss Pattern When Transitioning from SITA Standard to SITA Faster. Sci. Rep. 2022, 12, 7001. [Google Scholar] [CrossRef]
- Bengtsson, B.; Heijl, A. SITA Fast, a New Rapid Perimetric Threshold Test. Description of Methods and Evaluation in Patients with Manifest and Suspect Glaucoma. Acta Ophthalmol. Scand. 1998, 76, 431–437. [Google Scholar] [CrossRef]
- Kelly, S.R.; Bryan, S.R.; Crabb, D.P. Does Eye Examination Order for Standard Automated Perimetry Matter? Acta Ophthalmol. 2019, 97, e833–e838. [Google Scholar] [CrossRef]

| S/N | Author | Year | Country (City) | Number of Participants (M/F) | Age, Mean (SD) | Type of Participants | Other Visual Field Tests Included |
|---|---|---|---|---|---|---|---|
| 1 | Hong et al. [12] | 2021 | Korea (Seoul) | 150(58/92) | 62.6(NA) | OAG | 24-2 Faster |
| 2 | Chakravarti et al. [13] | 2021 | USA | 92(46/46) | 66.9 (63.9, 69.9) | OAG, glaucoma suspects | 10-2 Standard |
| 3 | Phu et al. [18] | 2021 | Australia (New South Wales) | 188 (103/85) | Glaucoma: 65.0 (59.5, 71.0) Glaucoma suspect: 64.0 (57.0, 70.0) | Glaucoma (unspecified), glaucoma suspects | 10-2 Fast |
| 4 | Phu et al. [11] | 2020 | Australia (New South Wales) | 104 (59/45) | SITA Faster: 62.0 (IQR 52.75, 69.0) SITA Standard: 59.0 (IQR 52.0, 67.8) | Glaucoma (unspecified), glaucoma suspects | 24-2 Standard, 24-2 Faster |
| 5 | Meshkin et al. [21] | 2022 | USA | 474 (224/250) | 64.4 (15.7) | Patients that require visual field testing | Not applicable |
| 6 | Behera et al. [22] | 2023 | India (Puducherry) | 60 (32/28) | 55.18 (12.19) | Glaucoma (Unspecified) | 24-2 Standard, 10-2 Standard |
| 7 | Yamane et al. [20] | 2021 | USA (Columbia) | 25 (NA) | 54.8 (19.4) | Neuro-ophthalmology patients | 10-2 Fast |
| 8 | Nishijima et al. [23] | 2024 | Japan | 146 (74/72) | With CVFD (59.5 (57.3–61.6)) Without CVFD (63.2 (55.6–70.8)) | POAG, NTG | 24-2 Standard, 10-2 Standard |
| Test Grids | 24-2C Faster | 24-2 Standard | 24-2 Faster | 10-2 Standard | 10-2 Fast | |
|---|---|---|---|---|---|---|
| Outcomes | ||||||
| Overall VFD detection | ↔ [22,23] | Not compared | Not compared | ↔ [20] | ||
| CVFD detection | − [11,22,23] | − [11] | + [13,22] | + [18] | ||
| Visual field indices (MD, PSD, and CMD) | ↔ [11,22] | ↔ [11] | ↔ [22] | ↔ [18] | ||
| Structure–function concordance | Not compared | − [11,12] | Not compared | + [18] | ||
| Test duration (speed) | − [11,22,23] | + [11] | − [22,23] | − [20] | ||
| Reliability (false +/− rates) | + [22] | Not compared | + [13] | + [20] | ||
| Outcome | Studies (n = Number of Participants) | Effect Estimate |
|---|---|---|
| Ability to detect CVFD: TD/TP | 3 studies (n = 326) | 5.5 more defective points in 24-2C Faster (17.5 vs. 12, p < 0.001) (n = 60) More central upper CVFDs detected in 24-2C Faster [AUC = 0.85, 95% CI = (0.78, 0.91) vs. AUC = 0.75 95% CI = (0.67, 0.83)], no difference in central lower CVFDs (p > 0.05) (n = 162) Tendency to identify more CVFD clusters but not statistically significant across all criteria (p > 0.05) (n = 104) |
| Ability to detect CVFD: PD | 3 studies (n = 326) | 2 more defective points in 24-2C Faster (6 vs. 4, p < 0.001) (n = 60) More central lower CVFDs detected in 24-2C Faster [AUC = 0.81, 95% CI = (0.72, 0.90) vs. AUC = 0.64, 95% CI = (0.53, 0.75), p < 0.05], no difference in central upper CVFDs (p > 0.05) (n = 162) |
| Ability to detect peripheral VFD | 1 study (n = 60) | No significant difference (p > 0.05) (n = 60) |
| Conventional visual field indices: MD | 2 studies (n = 164) | 0.891 dB [95% LOA = (−5.125, 3.344)] lower in 24-2 Standard, no systemic difference (ICC = 0.95, 95% CI 0.92–0.97, p < 0.001) (n = 60) 0.73 dB, [96.2% CI =(−1.01, −0.06), p = 0.0038] lower in 24-2C Faster (n = 104) |
| Conventional visual field indices: PSD | 2 studies (n = 164) | 0.694 dB [95% LOA = (−2.409, 3.797)] higher in 24-2 Standard, no systemic difference (ICC = 0.93, 95% CI 0.89–0.96, p < 0.001) (n = 60) No significant difference (p > 0.05) (n = 104) |
| Conventional visual field indices: CMD | 1 study (n = 104) | 0.35 dB [96.2% CI = (−0.70, −0.03), p = 0.0226] lower in 24-2C Faster (n = 104) |
| Macular S-F concordance | 1 study (n = 104) | Higher ratio of identified functional defects compared to structural defects in 24-2C Faster (p < 0.0001) (n = 104) |
| Time taken | 3 studies (n = 326) | Faster in 24-2C Faster: 155.0 s, IQR = (140.0–174.0 s) vs. 314.0 s, IQR = (283.8–338.0 s), p < 0.001, (n = 104) 210.5 s, IQR = (176.25–247.25 s) vs. 408 s, IQR = (338.75–471.5 s), p < 0.001, (n = 60) 165.0 s, SD = (161–170 s) vs. 308.0 s, SD = (302–315 s), p < 0.001, (n = 162) |
| Sensitivity/false negatives | 1 study (n = 60) | Lower in 24-2 Standard [4.5%, IQR = (0–7%) vs. 7.5%, IQR = (0.0–12.75%), p < 0.01] (n = 60) |
| Specificity/false positives | 1 study (n = 60) | Lower in 24-2C Faster but not significant [0%, IQR = (0–4%) vs. 1%, IQR = (0.0–2.8%), p = 0.34] (n = 60) |
| Outcome | Studies (n = Number of Participants) | Effect Estimate |
|---|---|---|
| Ability to detect CVFD | 1 study (n = 104) | 2 more clusters of CVFD out of 64 (3.1%) identified by 24-2C Faster, tendency to identify more CVFD clusters but not statistically significant across all criteria (p > 0.05) (n = 104) |
| Ability to detect overall VFD | - | Not reported |
| Conventional visual field indices: MD | 1 study (n = 104) | 0.02 dB [96.7% CI = (−0.33, −0.30 dB), p = 0.9715] lower in 24-2C Faster (n = 104) |
| Conventional visual field indices: PSD | - | Not reported |
| Conventional visual field indices: CMD | 1 study (n = 104) | 0.13 dB [96.7% CI = (0.27, 0.04), p = 0.2769] lower in 24-2 Faster (n = 104) |
| Macular S-F concordance | 2 studies (n = 336) | Greater in average, hemimacular, superotemporal, inferotemporal parafoveal sectors in 24-2 Faster (p < 0.05), similar in superocentral, superonasal, inferocentral, inferonasal parafoveal sectors (p > 0.10) (n = 232) More significant defects at regions with structural loss in 24-2C Faster at p < 0.05 and p < 0.01, higher ratio of identified functional defects compared to structural defects in 24-2C Faster (p < 0.0001) (n = 104) |
| Time taken | 1 study (n = 162) | Faster in 24-2 Faster: 125.5 s, IQR = (110.5–148.8 s) vs. 154.5 s, IQR = (136.3–181.8 s); median difference = 26.0 s, p< 0.0001 (n = 162) |
| Sensitivity/false negatives | - | Not reported |
| Specificity/false positives | - | Not reported |
| Outcome | Studies (n = Number of Participants) | Effect Estimate |
|---|---|---|
| Ability to detect CVFD: TD | 1 study (n = 165) | Moderate-to-substantial agreement between 10-2 Standard and 24-2C Faster (ĸ = 0.477–0.708) (n = 165) |
| Ability to detect CVFD: PD | 1 study (n = 165) | Moderate-to-substantial agreement between 10-2 Standard and 24-2C Faster (ĸ = 0.397–0.689) (n = 165) |
| Ability to detect overall VFD | - | Not reported |
| Conventional visual field indices: MD | 1 study (n = 60) | 1.635 dB [95% LOA = (−9.124 to 5.854)] lower in 24-2C Faster, no systematic difference [ICC = 0.80, 95% CI = (0.687, 0.876), p < 0.001] (n = 60) |
| Conventional visual field indices: PSD | 1 study (n = 60) | 0.776 dB [95% LOA = (−4.678 to 6.230)] higher in 24-2C Faster, no systematic difference [ICC = 0.80, 95% CI = (0.690–0.877), p < 0.001] (n = 60) |
| Conventional visual field indices: CMD | - | Not reported |
| Macular S-F concordance | - | Not reported |
| Time taken | 2 studies (n = 222) | Faster in 24-2C Faster: 165.0 s, SD = (161–170 s) vs. 343 s, SD = (334–353 s), p < 0.001 (n = 162) Median = 210.5 s vs. 244.5 s (n = 60) |
| Sensitivity/false negatives | 1 study (n = 165) | Higher sensitivity in 10-2 Standard (1.0 vs. 0.97) (n = 165) |
| Specificity/false positives | 1 study (n = 165) | Higher specificity in 10-2 Standard (1.0 vs. 0.94) (n = 165) |
| Outcome | Studies (n = Number of Participants) | Effect Estimate |
|---|---|---|
| Ability to detect CVFD: TD | 3 studies (n = 267) | 2.6 times more defective points identified by 24-2C Faster (46 vs. 17.5) (n = 60) More CVFD identified by 24-2C Faster (58 (43.9%) vs. 42 (31.8%)) (n = 165) Similar between 24-2C Faster and 10-2 Fast at p < 0.05 (24-2C vs. 10-2; 4.16 (0.71) vs. 4.28(0.73), p = 0.767), p < 0.02 (24-2 vs. 10-2; 3.15 (0.69) vs. 3.28 (0.69), p = 0.791) and p < 0.01 significance (24-2C vs. 10-2; 2.45 (0.63) vs. 2.58 (0.63), p = 0.651) (n = 42) |
| Ability to detect CVFD: PD | 3 studies (n = 267) | 2.8 times more defective points identified by 24-2C Faster (17 vs. 6) (n = 60) More CVFD identified by 24-2C Faster (56 (42.4%) vs. 42 (31.8%)) (n = 165) Similar between 24-2C Faster and 10-2 Fast at p < 0.05, p < 0.02, p < 0.01 levels (p > 0.70) (n = 42) |
| Ability to detect CVFD: Others | 1 study (n = 188) | More central locations with a visual field defect detected by 10-2 Fast (p = 0.02), more CVFDs for all contiguity conditions identified by 10-2 Fast (2+ to 6+; p < 0.0001) (n = 188) |
| Ability to detect overall VFD | - | Not reported |
| Conventional visual field indices: MD | 1 study (n = 188) | Similar between 24-2C Faster and 10-2 Fast: mean bias = 0.70 dB, 95% LOA = (−3.8 to 5.2 dB) (n = 188) |
| Conventional visual field indices: PSD | 1 study (n = 188) | Similar between 24-2C Faster and 10-2 Fast: mean bias = 0.92 dB, 95% LOA = (−5.0 to 3.2 dB) (n = 188) |
| Conventional visual field indices: CMD | - | Not reported |
| Macular S-F concordance | 1 study (n = 188) | Higher S-F concordance in 10-2 Fast (p < 0.0001) (n = 188) |
| Time taken | 1 study (n = 42) | Faster in 24-2C Faster: 3:09 vs. 3:58 min, p < 0.001 (n = 42) |
| Sensitivity/false negatives | 1 study (n = 42) | Lower false-negative rate in 10-2 Fast 1.15% vs. 3.89% p = 0.002 (n = 42) |
| Specificity/false positives | 1 study (n = 42) | Lower false-positive rate in 10-2 Fast (1.09% vs. 2.57%, p = 0.043) (n = 42) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, E.; Wong, N.S.Q.; Goh, C.X.Y.; Stewart, M.W.; Dorairaj, S.; Ang, B.C.H. Advancements in Visual Field Testing: A Systematic Review of the 24-2C Test Grid. Bioengineering 2025, 12, 711. https://doi.org/10.3390/bioengineering12070711
Jin E, Wong NSQ, Goh CXY, Stewart MW, Dorairaj S, Ang BCH. Advancements in Visual Field Testing: A Systematic Review of the 24-2C Test Grid. Bioengineering. 2025; 12(7):711. https://doi.org/10.3390/bioengineering12070711
Chicago/Turabian StyleJin, Eric, Natalie Shi Qi Wong, Claire Xin Yi Goh, Michael W. Stewart, Syril Dorairaj, and Bryan Chin Hou Ang. 2025. "Advancements in Visual Field Testing: A Systematic Review of the 24-2C Test Grid" Bioengineering 12, no. 7: 711. https://doi.org/10.3390/bioengineering12070711
APA StyleJin, E., Wong, N. S. Q., Goh, C. X. Y., Stewart, M. W., Dorairaj, S., & Ang, B. C. H. (2025). Advancements in Visual Field Testing: A Systematic Review of the 24-2C Test Grid. Bioengineering, 12(7), 711. https://doi.org/10.3390/bioengineering12070711







