A Tool for the Assessment of Electromagnetic Compatibility in Active Implantable Devices: The Pacemaker Physical Twin
Abstract
1. Introduction
2. Materials and Methods
- Compatibility with Commercial Leads: The device must interface with commercially available leads, both unipolar and bipolar, through the IS-1 standard, which is widely adopted by the majority of PM and ICD manufacturers.
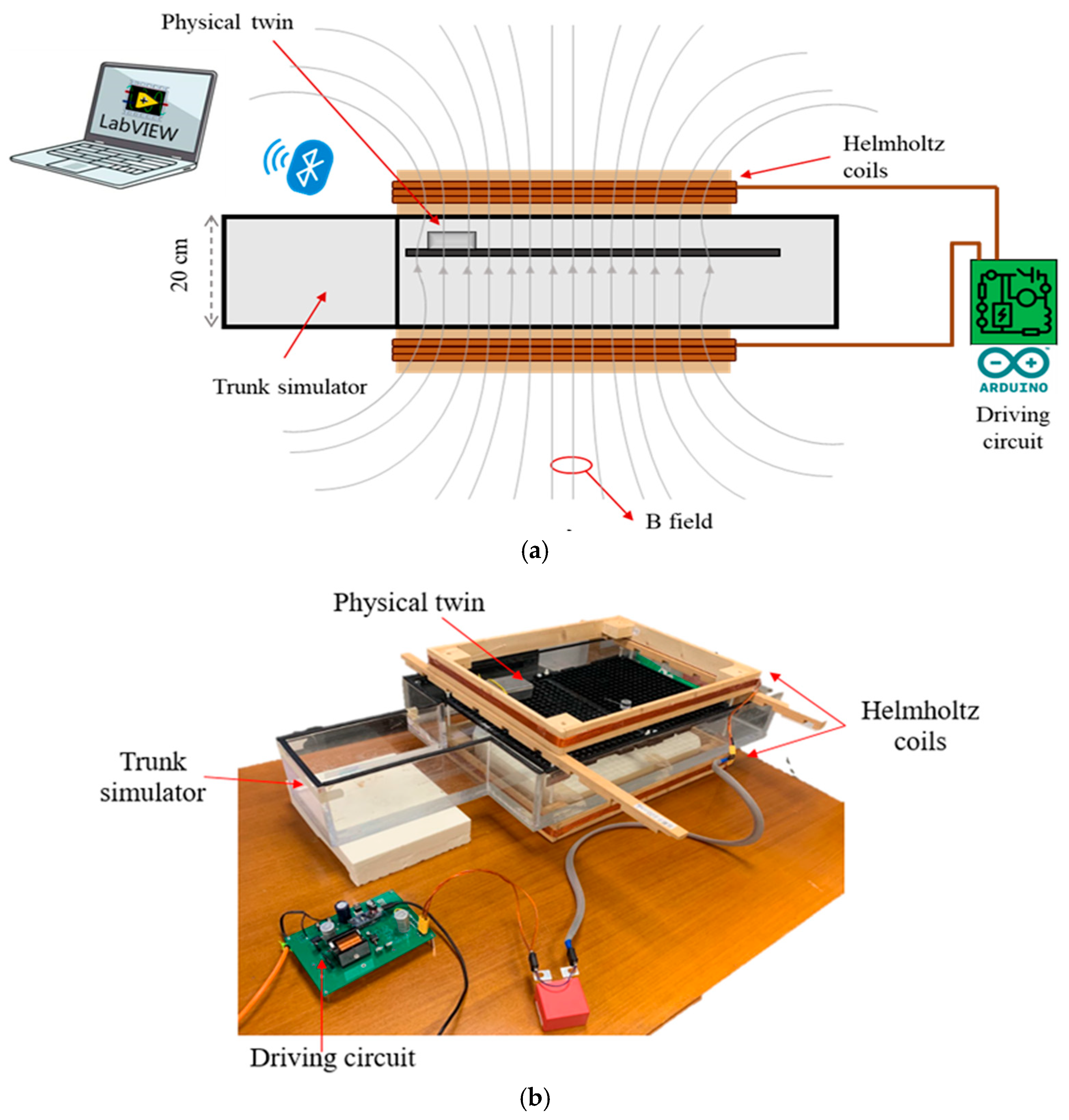
- Operation within a Human Trunk Simulator: The system must be capable of operating while immersed in a saline solution that replicates the dielectric properties of biological tissues.
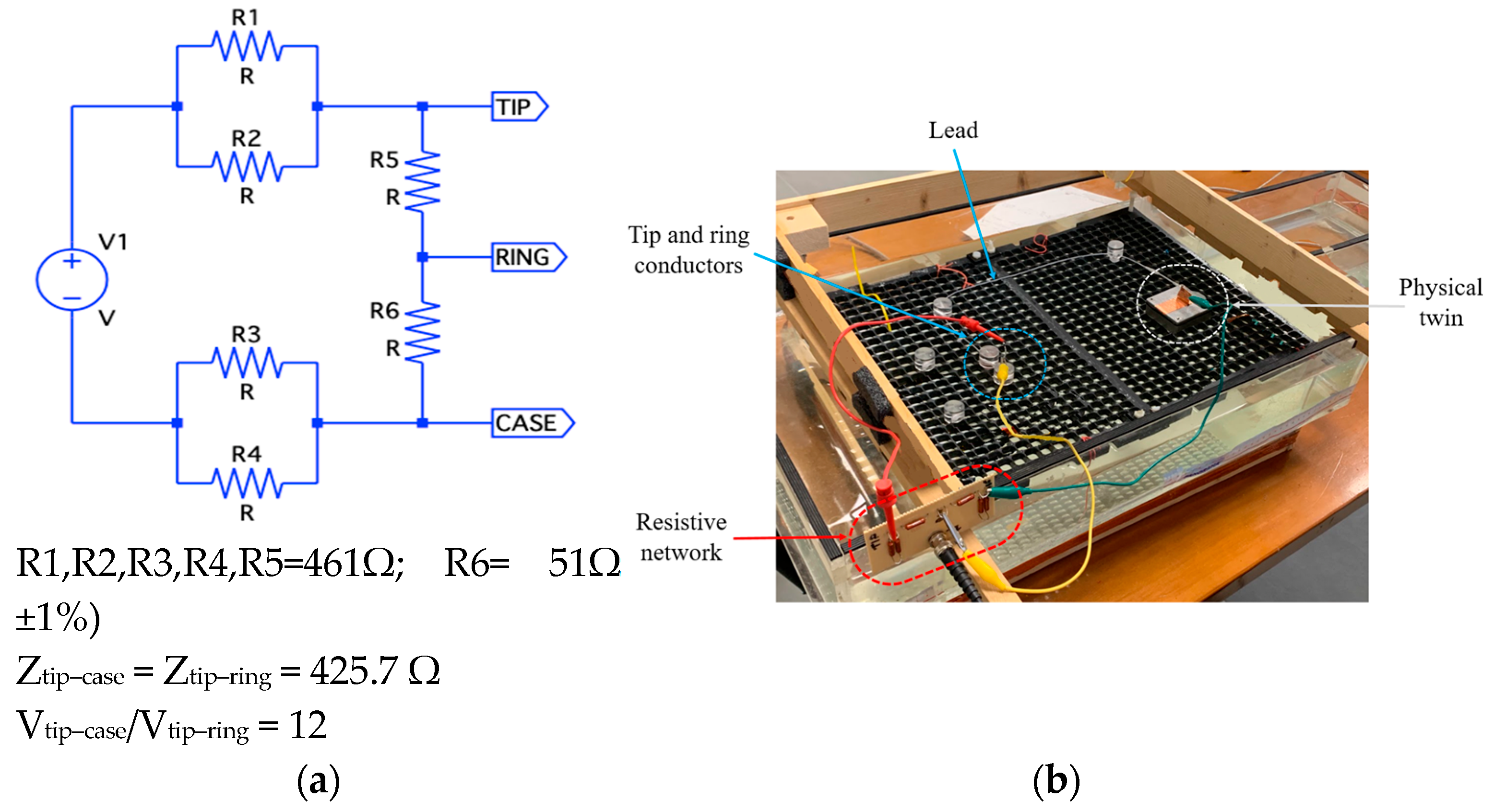
- Induced Voltage Logging: The device should continuously record the induced voltage at its input terminals in the range of 1mV-1V. The system should allow for voltage measurements between the lead tip and the metallic housing of the PM/ICD (unipolar mode) or between the two electrodes (tip and ring) of the lead (bipolar mode).
- Wireless Data Transmission: The system must support wireless transfer of recorded data to an external computer for real-time monitoring and storage.
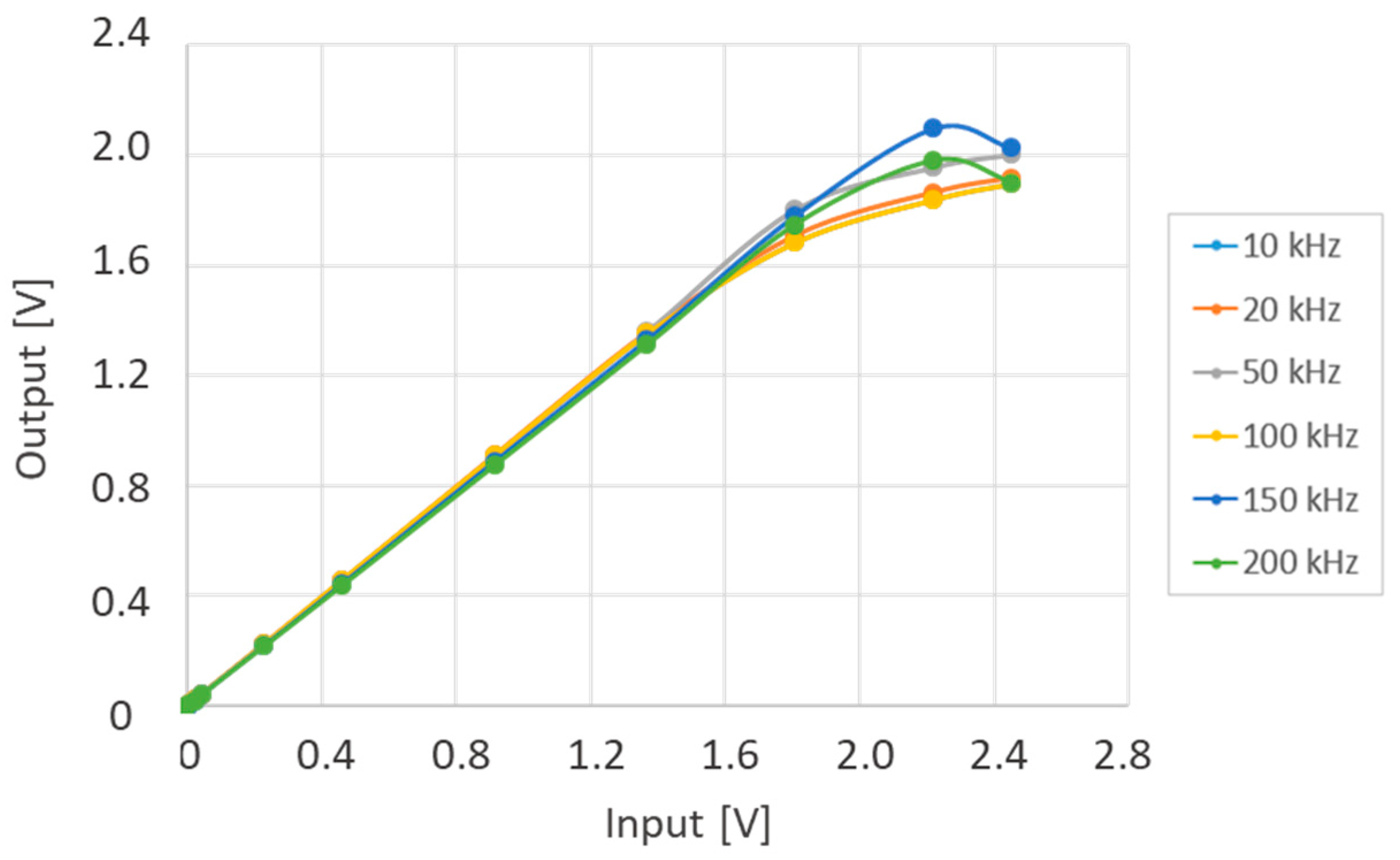
- Frequency and Amplitude Range: The device should reliably measure sinusoidal signals across a frequency range of 10 kHz to 200 kHz, with amplitudes up to the reference levels specified by the ICNIRP guidelines (50–100 µT).
- Battery Operation: The system should be battery-operated to avoid any possible ground path which could affect the induced voltage.
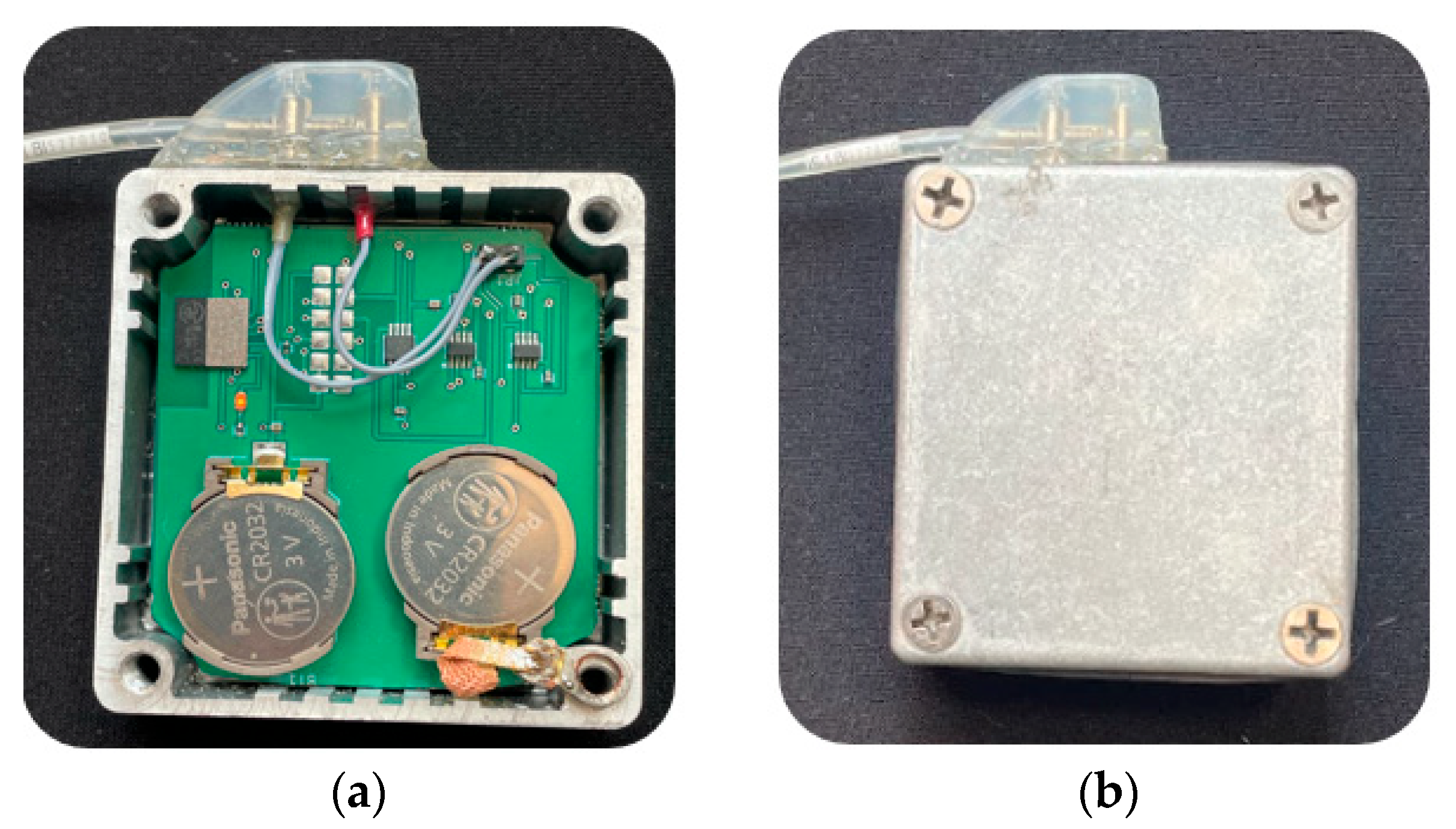
2.1. The Physical Twin: Hardware Components
- RMS-to-DC Converter (LTC1968, Analog Devices, Wilmington, NC, USA): This component outputs a DC voltage proportional to the RMS value of the input signal. It supports both single-ended inputs (referenced to ground) and differential inputs, maintaining a stable gain across the 50 Hz to 500 kHz frequency range.
- Digitally Controlled Switches (ADG619, CMOS SPDT, Analog Devices, Wilmington, NC, USA): Two switches allow dynamic selection of the input to the RMS-to-DC converter. They enable switching between unipolar mode (single-ended measurement referenced to ground) and bipolar mode (differential measurement between two input signals).
- Integrated Microcontroller with Bluetooth Module (ISP1507-AX, Insight SIP, Sophia-Antipolis, France): This module manages the switch states, acquires data from the RMS converter via its ADC, and transmits the measured signals wirelessly to an external receiver.
- Power Supply (Two CR2032 Lithium Coin Cells): These batteries provide a nominal supply voltage of 3 V, ensuring full portability and compliance with the design requirements for battery-powered operation.
2.2. The Physical Twin: Firmware
- Unipolar tip measurement: The induced voltage is measured between the lead tip and the metal enclosure simulating the PM/ICD chassis.
- Unipolar ring measurement: The induced voltage is measured between the lead ring electrode and the metal enclosure.
- Bipolar measurement: The induced voltage is measured between the tip and ring electrodes of the lead.
- Remote activation and pairing with the measurement board.
- Battery monitoring during continuous operation.
- Real-time data acquisition and visualization of induced voltage.
- On-the-fly switching between measurement modes.
- Monitoring of the measurement modality and power-down to conserve battery life.
2.3. The Physical Twin: Performace Characterization and Validation
- C-shaped configuration: The loop, formed by the lead, the metal case, and the connection through the saline solution between the lead tip and the case, resulted in a geometrical induction area of approximately 450 cm2.
- S-shaped configuration: The loop formed two opposite areas, yielding opposing contributions to the induced voltage, which resulted in a geometrical induction area of almost zero.
3. Results
3.1. Physical Twin Chatacterization
3.2. Measurement of the Induced Voltage
4. Discussion
- Initial measurement of the voltage induced by the EMF source using the physical twin. This step assesses whether the measured value, at the frequency of interest, exceeds limits set by international standards.
- If the induced voltage is below regulatory thresholds, the risk of EMI can be considered low.
- If the induced voltage exceeds these thresholds, a potential risk of EMI events cannot be excluded. In such cases, additional measurements using actual devices should be carried out for further risk assessment.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PM | Pacemaker |
| ICD | Implantable Cardioverter/Defibrillator |
| CIED | Cardiac Implantable Electronic Device |
| RFID | Radiofrequency Identification |
| WPT | Wireless Power Transfer |
| EAS | Electronic Article Surveillance |
| EMF | Electromagnetic Field |
| EMC | Electromagnetic Compatibility |
| EMI | Electromagnetic Interference |
References
- Seidman, S.J.; Brockman, R.; Lewis, B.M.; Guag, J.; Shein, M.J.; Clement, W.J.; Kippola, J.; Digby, D.; Barber, C.; Huntwork, D. In vitro tests reveal sample radiofrequency identification readers inducing clinically significant electromagnetic interference to implantable pacemakers and implantable cardioverter-defibrillators. Heart Rhythm. 2010, 7, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Mattei, E.; Lucano, E.; Censi, F.; Triventi, M.; Calcagnini, G. Provocative Testing for the Assessment of the Electromagnetic Interference of RFID and NFC Readers on Implantable Pacemaker. IEEE Trans. Electromagn. Compat. 2016, 58, 314–322. [Google Scholar] [CrossRef]
- Campi, T.; Cruciani, S.; Maradei, F.; Feliziani, M. Pacemaker Lead Coupling with an Automotive Wireless Power Transfer System. IEEE Trans. Electromagn. Compat. 2019, 61, 1935–1943. [Google Scholar] [CrossRef]
- Campi, T.; Cruciani, S.; Maradei, F.; Feliziani, M. Electromagnetic Interference in Cardiac Implantable Electronic Devices Due to Dynamic Wireless Power Systems for Electric Vehicles. Energies 2023, 16, 3822. [Google Scholar] [CrossRef]
- Vivarelli, C.; Censi, F.; Calcagnini, G.; Freschi, F.; Giaccone, L.; Canova, A.; Mattei, E. Electromagnetic Immunity of Pacemakers and Implantable Defibrillators to Wireless Power Transfer Systems for Automotive: A Provocative Study. IEEE Trans. Electromagn. Compat. 2023, 66, 97–107. [Google Scholar] [CrossRef]
- ISO 14117:2019; Active Implantable Medical Devices—Electromagnetic Compatibility—EMC Test Protocols for Implantable Cardiac Pacemakers, Implantable Cardioverter Defibrillators and Cardiac Resynchronization Devices. International Standard Organization: Geneva, Switzerland, 2019.
- Driessen, S.; Napp, A.; Schmiedchen, K.; Kraus, T.; Stunder, D. Electromagnetic interference in cardiac electronic implants caused by novel electrical appliances emitting electromagnetic fields in the intermediate frequency range: A systematic review. Eurospace 2019, 21, 219–229. [Google Scholar] [CrossRef]
- Misiri, J.; Kusumoto, F.; Goldschlager, N. Electromagnetic interference and implanted cardiac devices: The nonmedical environment (part I). Clin. Cardiol. 2012, 35, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Wilkoff, B.L.; Albert, T.; Lazebnik, M.; Park, S.M.; Edmonson, J.; Herberg, B.; Golnitz, J.; Wixon, S.; Peltier, J.; Yoon, H.; et al. Safe magnetic resonance imaging scanning of patients with cardiac rhythm devices: A role for computer modeling. Heart Rhythm. 2013, 10, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- ISO/TS 10974:2018; Assessment of the Safety of Magnetic Resonance Imaging for Patients with an Active Implantable Medical Device. International Standard Organization: Geneva, Switzerland, 2018.
- IEC/IEEE Standard 62704-1:2017; Determining the Peak Spatial-Average SAR in the Human Head from Wireless Communications Devices: General Requirements for Using the Finite-Difference Time-Domain (FDTD) Method. International Electrotechnical Commission/Institute of Electrical and Electronics Engineers: Geneva, Switzerland, 2017.
- Silny, A.; Scholten, J. The Interference Threshold of Unipolar Cardiac Pacemakers in Extremely Low Frequency Magnetic Fields. J. Med. Eng. Technol. 2011, 25, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Seidman, S.; Bassen, H. Determining EMC Test Levels for Implantable Devices in Bipolar Lead Configuration. Biomed. Instrum. Technol. 2021, 55, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Kourtiche, D.; Claudel, J.; Deschamps, F.; Magne, I.; Roth, P.; Schmitt, P.; Nadi, M. Interference voltage measurement and analysis of cardiac implants exposed to electric fields at extremely low frequency. Biomed. Phys. Eng. Express 2024, 10, 045060. [Google Scholar] [CrossRef] [PubMed]
- Irnich, W. Electronic security systems and active implantable medical devices. Pacing Clin. Electrophysiol. 2002, 25, 1235–1258. [Google Scholar] [CrossRef] [PubMed]
- Hille, S.; Eichhorn, K.; Gonschorek, K. Interference voltage and interference threshold in pacemakers with unipolar and bipolar electrodes. In Proceedings of the IEEE International Symposium on Electromagnetic Compatibility, Austin, TX, USA, 17–21 August 2009; pp. 147–152. [Google Scholar]
- Mattei, E.; Censi, F.; Delogu, A.; Ferrara, A.; Calcagnini, G. Setups for in vitro assessment of RFID interference on pacemakers. Phys. Med. Biol. 2013, 58, 5301–5316. [Google Scholar] [CrossRef]
- Stunder, D. Electromagnetic Inference of Cardiac Implants in the Intermediate Frequency Range; Federal Institute for Occupational Safety and Health: Dortmund, Germany; Berlin, Germany; Dresden, Germany, 2020; ISBN 978-3-88261-086-4. [Google Scholar]
- Andreuccetti, D.; Fossi, R.; Petrucci, C. An Internet Resource for the Calculation of the Dielectric Properties of Body Tissues in the Frequency Range 10 Hz–100 GHz. Italian National Research Council, Institute for Applied Physics. 1997. Available online: http://niremf.ifac.cnr.it/tissprop/ (accessed on 23 June 2025).
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). ICNIRP guidelines for limiting exposure to time-varying electric, magnetic and electromagnetic fields (up to 300 GHZ). Health Phys. 1998, 74, 494–522. [Google Scholar]
- EN 50527-2-1:2016; Procedure for the Assessment of the Exposure of Workers with Implanted Medical Devices to Electromagnetic Fields. Part 2-1: Specific Assessment for Workers with Cardiac Pacemakers. European Committee for Electrotechnical Standardization (CENELEC): Brussels, Belgium, 2016.




| GAIN | Measurement Range (mV) | Resolution (mV) | Accuracy @ 8 Samp. Averaging (mV) 1 | Accuracy @ 16 Samp. AVERAGING (mV) 1 |
|---|---|---|---|---|
| 1/4 | 2400 | 0.59 | ±1.6 | ±1.2 |
| 1/3 | 1800 | 0.44 | ±1.4 | ±1.0 |
| 1/2 | 1200 | 0.29 | ±0.8 | ±0.6 |
| 1 | 600 | 0.15 | ±0.6 | ±0.4 |
| 2 | 300 | 0.07 | ±0.4 | ±0.2 |
| 4 | 150 | 0.04 | ±0.4 | ±0.2 |
| 16 μT | 30 μT | 50 μT | 100 μT | 150 μT | 200 μT | |
|---|---|---|---|---|---|---|
| 10 kHz | ||||||
| 20 kHz | ||||||
| 50 kHz | ||||||
| 100 kHz | ||||||
| 150 kHz | ||||||
| 200 kHz |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vivarelli, C.; Mattei, E.; Ricci, F.; D'Eramo, S.; Calcagnini, G. A Tool for the Assessment of Electromagnetic Compatibility in Active Implantable Devices: The Pacemaker Physical Twin. Bioengineering 2025, 12, 689. https://doi.org/10.3390/bioengineering12070689
Vivarelli C, Mattei E, Ricci F, D'Eramo S, Calcagnini G. A Tool for the Assessment of Electromagnetic Compatibility in Active Implantable Devices: The Pacemaker Physical Twin. Bioengineering. 2025; 12(7):689. https://doi.org/10.3390/bioengineering12070689
Chicago/Turabian StyleVivarelli, Cecilia, Eugenio Mattei, Federica Ricci, Sara D'Eramo, and Giovanni Calcagnini. 2025. "A Tool for the Assessment of Electromagnetic Compatibility in Active Implantable Devices: The Pacemaker Physical Twin" Bioengineering 12, no. 7: 689. https://doi.org/10.3390/bioengineering12070689
APA StyleVivarelli, C., Mattei, E., Ricci, F., D'Eramo, S., & Calcagnini, G. (2025). A Tool for the Assessment of Electromagnetic Compatibility in Active Implantable Devices: The Pacemaker Physical Twin. Bioengineering, 12(7), 689. https://doi.org/10.3390/bioengineering12070689







