The Risks Associated with Inhalation Exposure to Cosmetics and Potential for Assessment Using Lung Organoids
Abstract
1. Introduction
2. Inhalation Exposure
3. Safety Evaluation of Inhalation Exposure
3.1. Principles of Inhalation Exposure Assessments
3.2. Methods of Inhalation Exposure Evaluation
4. Safety Assessment of Lung Organoids and Their Application in Inhalable Exposed Cosmetics
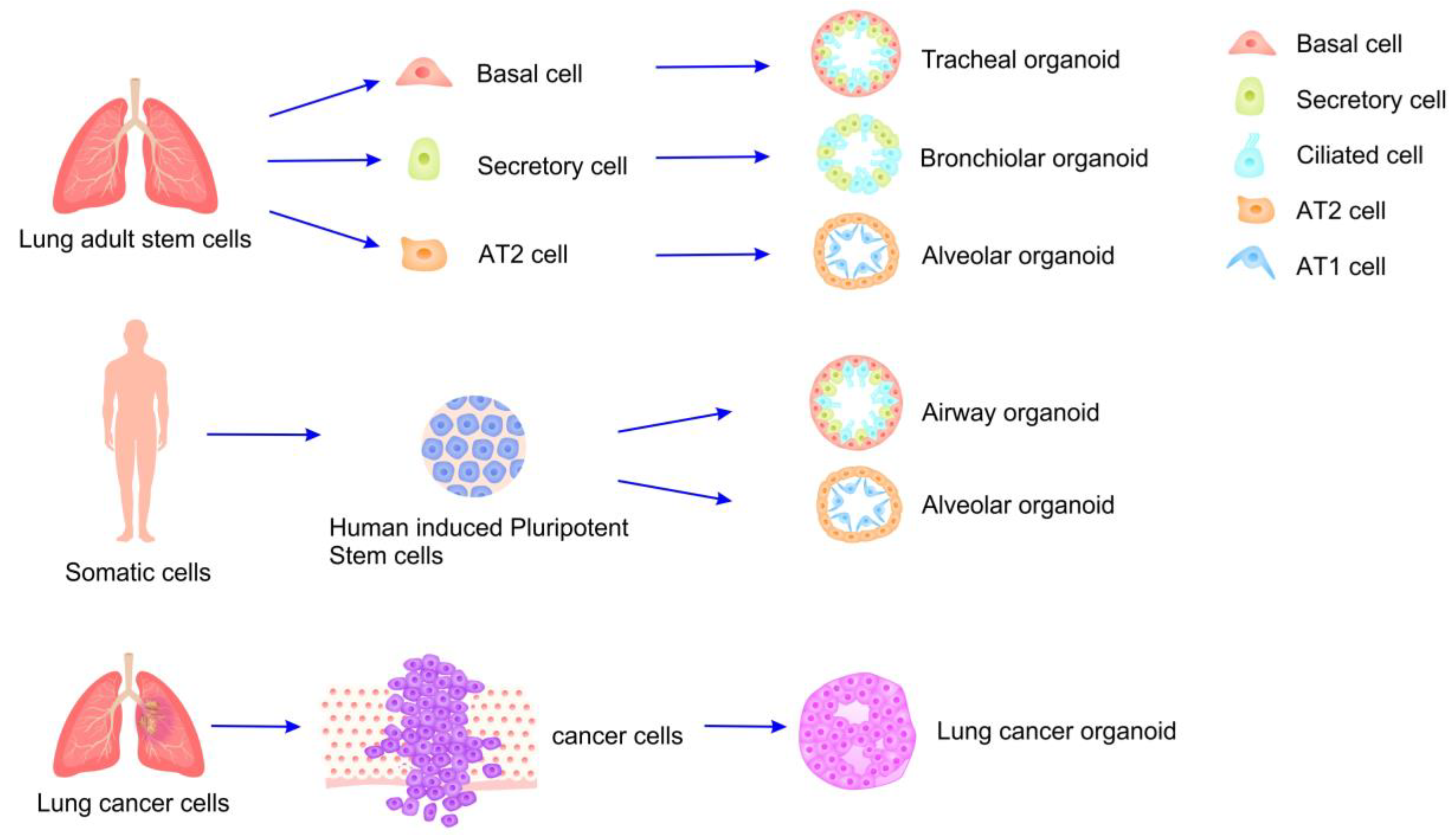
4.1. Organoids and Their Applications
4.2. Preparation of Lung Organoids and Model Evaluation
4.3. Safety Assessment of Lung Organoids Applied in Cosmetics After Inhalation Exposure
5. Future Perspectives and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grazul-Bilska, A.T.; Bilski, J.J.; Redmer, D.A.; Reynolds, L.P.; Abdullah, K.M.; Abdullah, A. Antioxidant Capacity of 3D Human Skin EpiDerm TM Model: Effects of Skin Moisturizers. Int. J. Cosmet. Sci. 2009, 31, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Jiang, Y.; Liu, J.; Wang, S.; Diao, Q. Review on the Mechanisms of Skin Adverse Reactions of Cosmetics. J. Clin. Dermatol. 2023, 52, 240–243. [Google Scholar] [CrossRef]
- Laser Cosmetology Group, Medical Aesthetics and Cosmetology Branch of Chinese Medical Association; Skin Care Product and Material Group, Committee on Skin Disease and Cosmetic Dermatology, China Association of Medical Equipment; Laser Group, Cosmetic and Plastic Surgeon Branch of Chinese Medical Doctor Association; Cosmetic Laser Group, Chinese Society of Dermatology. Guidelines for the Diagnosis and Treatment of Adverse Skin Reactions to Cosmetics (2024 Edition). Chin. J. Dermatol. 2024, 57, 485–492. [Google Scholar] [CrossRef]
- Leng, W.; He, X.; Wang, W.; Luo, Z. Clinical Characteristics of 1089 Cases with Cosmetic Dermatoses in Lanzhou Area. J. Clin. Dermatol. 2024, 53, 449–453. [Google Scholar]
- Steiling, W.; Bascompta, M.; Carthew, P.; Catalano, G.; Corea, N.; D’Haese, A.; Jackson, P.; Kromidas, L.; Meurice, P.; Rothe, H.; et al. Principle Considerations for the Risk Assessment of Sprayed Consumer Products. Toxicol. Lett. 2014, 227, 41–49. [Google Scholar] [CrossRef]
- Liu, T.; Wang, F.; Liang, Y. Review on Local Tolerance Assessment and Safety Supervision of Sprayable Cosmetic Products. Flavour Fragr. Cosmet. 2024, 2024, 50–55+158. [Google Scholar] [CrossRef]
- Thá, E.L.; Canavez, A.D.P.M.; Schuck, D.C.; Gagosian, V.S.C.; Lorencini, M.; Leme, D.M. Beyond Dermal Exposure: The Respiratory Tract as a Target Organ in Hazard Assessments of Cosmetic Ingredients. Regul. Toxicol. Pharmacol. 2021, 124, 104976. [Google Scholar] [CrossRef] [PubMed]
- Berrada-Gomez, M.-P.; Bui, B.; Bondarenko, H.; Ferret, P.-J. Particle Size Distribution in the Evaluation of the Inhalation Toxicity of Cosmetic Spray Products. Regul. Toxicol. Pharmacol. 2023, 139, 105359. [Google Scholar] [CrossRef]
- Rothe, H.; Fautz, R.; Gerber, E.; Neumann, L.; Rettinger, K.; Schuh, W.; Gronewold, C. Special Aspects of Cosmetic Spray Safety Evaluations: Principles on Inhalation Risk Assessment. Toxicol. Lett. 2011, 205, 97–104. [Google Scholar] [CrossRef]
- Arts, J.H.E.; de Heer, C.; Woutersen, R.A. Local Effects in the Respiratory Tract: Relevance of Subjectively Measured Irritation for Setting Occupational Exposure Limits. Int. Arch. Occup. Environ. Health 2006, 79, 283–298. [Google Scholar] [CrossRef]
- Brüning, T.; Bartsch, R.; Bolt, H.M.; Desel, H.; Drexler, H.; Gundert-Remy, U.; Hartwig, A.; Jäckh, R.; Leibold, E.; Pallapies, D.; et al. Sensory Irritation as a Basis for Setting Occupational Exposure Limits. Arch. Toxicol. 2014, 88, 1855–1879. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, O.; Wiszniewska, M.; Raulf, M.; De Blay, F.; Gerth Van Wijk, R.; Moscato, G.; Nemery, B.; Pala, G.; Quirce, S.; Sastre, J.; et al. EAACI Position Paper: Irritant-Induced Asthma. Allergy 2014, 69, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H. Allergens and the Airway Epithelium Response: Gateway to Allergic Sensitization. J. Allergy Clin. Immunol. 2014, 134, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, V.D.; Vliagoftis, H. Airway Epithelium Interactions with Aeroallergens: Role of Secreted Cytokines and Chemokines in Innate Immunity. Front. Immunol. 2015, 6, 147. [Google Scholar] [CrossRef]
- Pauluhn, J. Comparative Pulmonary Response to Inhaled Nanostructures: Considerations on Test Design and Endpoints. Inhal. Toxicol. 2009, 21, 40–54. [Google Scholar] [CrossRef]
- Ciabattini, M.; Rizzello, E.; Lucaroni, F.; Palombi, L.; Boffetta, P. Systematic Review and Meta-Analysis of Recent High-Quality Studies on Exposure to Particulate Matter and Risk of Lung Cancer. Environ. Res. 2021, 196, 110440. [Google Scholar] [CrossRef]
- Fang, L.; Tang, J.; Li, X. Survey of Inhalation Exposure Assessment of Cosmetics Ingredient. Deterg. Cosmet. 2024, 47, 41–46. [Google Scholar] [CrossRef]
- Belsito, D.V.; Cohen, D.E.; Klaassen, C.D.; Liebler, D.C.; Peterson, L.A.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Zhu, J.; Toxicologist, C. Respiratory Exposure to Cosmetic Ingredients 2021. Available online: https://www.cir-safety.org/supplementaldoc/respiratory-exposure-cosmetic-ingredients-2021 (accessed on 19 May 2025).
- Loretz, L.; Api, A.M.; Barraj, L.; Burdick, J.; Davis, D.A.; Dressler, W.; Gilberti, E.; Jarrett, G.; Mann, S.; Laurie Pan, Y.H.; et al. Exposure Data for Personal Care Products: Hairspray, Spray Perfume, Liquid Foundation, Shampoo, Body Wash, and Solid Antiperspirant. Food Chem. Toxicol. 2006, 44, 2008–2018. [Google Scholar] [CrossRef]
- SCCS Members. The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation, 11th Revision, 30–31 March 2021, SCCS/1628/21. Regul. Toxicol. Pharmacol. 2021, 127, 105052. [Google Scholar] [CrossRef]
- Biesterbos, J.W.H.; Dudzina, T.; Delmaar, C.J.E.; Bakker, M.I.; Russel, F.G.M.; Von Götz, N.; Scheepers, P.T.J.; Roeleveld, N. Usage Patterns of Personal Care Products: Important Factors for Exposure Assessment. Food Chem. Toxicol. 2013, 55, 8–17. [Google Scholar] [CrossRef]
- Dearman, R.J.; Basketter, D.A.; Kimber, I. Inter-Relationships between Different Classes of Chemical Allergens. J. Appl. Toxicol. 2013, 33, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Périz, M.; Pérez-Cano, F.J.; Rodríguez-Lagunas, M.J.; Cambras, T.; Pastor-Soplin, S.; Best, I.; Castell, M.; Massot-Cladera, M. Development and Characterization of an Allergic Asthma Rat Model for Interventional Studies. Int. J. Mol. Sci. 2020, 21, 3841. [Google Scholar] [CrossRef] [PubMed]
- Pauluhn, J.; Mohr, U. Experimental Approaches to Evaluate Respiratory Allergy in Animal Models. Exp. Toxicol. Pathol. 2005, 56, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Lalko, J.F.; Kimber, I.; Frank Gerberick, G.; Foertsch, L.M.; Api, A.M.; Dearman, R.J. The Direct Peptide Reactivity Assay: Selectivity of Chemical Respiratory Allergens. Toxicol. Sci. 2012, 129, 421–431. [Google Scholar] [CrossRef]
- Dik, S.; Rorije, E.; Schwillens, P.; Van Loveren, H.; Ezendam, J. Can the Direct Peptide Reactivity Assay Be Used for the Identification of Respiratory Sensitization Potential of Chemicals? Toxicol. Sci. 2016, 153, 361–371. [Google Scholar] [CrossRef]
- Teubner, W.; Mehling, A.; Schuster, P.X.; Guth, K.; Worth, A.; Burton, J.; van Ravenzwaay, B.; Landsiedel, R. Computer Models versus Reality: How Well Do in Silico Models Currently Predict the Sensitization Potential of a Substance. Regul. Toxicol. Pharmacol. 2013, 67, 468–485. [Google Scholar] [CrossRef]
- Seed, M.; Agius, R. Further Validation of Computer-Based Prediction of Chemical Asthma Hazard. Occup. Med. (Chic. Ill) 2009, 60, 115–120. [Google Scholar] [CrossRef]
- Seed, M.J.; Cullinan, P.; Agius, R.M. Methods for the Prediction of Low-Molecular-Weight Occupational Respiratory Sensitizers. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 103–109. [Google Scholar] [CrossRef]
- Cunningham, A.R.; Cunningham, S.L.; Consoer, D.M.; Moss, S.T.; Karol, M.H. Development of an Information-Intensive Structure-Activity Relationship Model and Its Application to Human Respiratory Chemical Sensitizers. SAR QSAR Environ. Res. 2005, 16, 273–285. [Google Scholar] [CrossRef]
- Dik, S.; Ezendam, J.; Cunningham, A.R.; Carrasquer, C.A.; Van Loveren, H.; Rorije, E. Evaluation of In Silico Models for the Identification of Respiratory Sensitizers. Toxicol. Sci. 2014, 142, 385–394. [Google Scholar] [CrossRef]
- Marsee, A.; Roos, F.J.M.; Verstegen, M.M.A.; Roos, F.; Verstegen, M.; Clevers, H.; Vallier, L.; Takebe, T.; Huch, M.; Peng, W.C.; et al. Building Consensus on Definition and Nomenclature of Hepatic, Pancreatic, and Biliary Organoids. Cell Stem Cell 2021, 28, 816–832. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.A.; Zhang, Y.; Rathnam, C.; Pongkulapa, T.; Lee, K.B. Bioengineering Approaches for the Advanced Organoid Research. Adv. Mater. 2021, 33, 2007949. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhang, W.; Wu, X.; Zhang, Y.; Xu, K.; Su, J. Organoid Assessment Technologies. Clin. Transl. Med. 2023, 13, e1499. [Google Scholar] [CrossRef] [PubMed]
- Strobel, H.A.; Moss, S.M.; Hoying, J.B. Vascularized Tissue Organoids. Bioengineering 2023, 10, 124. [Google Scholar] [CrossRef]
- Huang, K.C.; Wang, M.L.; Chen, S.J.; Kuo, J.C.; Wang, W.J.; Nhi Nguyen, P.N.; Wahlin, K.J.; Lu, J.F.; Tran, A.A.; Shi, M.; et al. Morphological and Molecular Defects in Human Three-Dimensional Retinal Organoid Model of X-Linked Juvenile Retinoschisis. Stem Cell Rep. 2019, 13, 906–923. [Google Scholar] [CrossRef]
- Taylor, J.; Sellin, J.; Kuerschner, L.; Krähl, L.; Majlesain, Y.; Förster, I.; Thiele, C.; Weighardt, H.; Weber, E. Generation of Immune Cell Containing Adipose Organoids for in Vitro Analysis of Immune Metabolism. Sci. Sci. Rep. 2020, 10, 21104. [Google Scholar] [CrossRef]
- Pasch, C.A.; Favreau, P.F.; Yueh, A.E.; Babiarz, C.P.; Gillette, A.A.; Sharick, J.T.; Karim, M.R.; Nickel, K.P.; DeZeeuw, A.K.; Sprackling, C.M.; et al. Patient-Derived Cancer Organoid Cultures to Predict Sensitivity to Chemotherapy and Radiation. Clin. Cancer Res. 2019, 25, 5376–5387. [Google Scholar] [CrossRef]
- Sun, C.P.; Lan, H.R.; Fang, X.L.; Yang, X.Y.; Jin, K.T. Organoid Models for Precision Cancer Immunotherapy. Front. Immunol. 2022, 13, 770465. [Google Scholar] [CrossRef]
- Kong, J.C.H.; Guerra, G.R.; Millen, R.M.; Roth, S.; Xu, H.; Neeson, P.J.; Darcy, P.K.; Kershaw, M.H.; Sampurno, S.; Malaterre, J.; et al. Tumor-Infiltrating Lymphocyte Function Predicts Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. JCO Precis Oncol. 2018, 2, 1–15. [Google Scholar] [CrossRef]
- Yao, Q.; Cheng, S.; Pan, Q.; Yu, J.; Cao, G.; Li, L.; Cao, H. Organoids: Development and Applications in Disease Models, Drug Discovery, Precision Medicine, and Regenerative Medicine. MedComm 2024, 5, e735. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Q.; Li, Y.; Feng, Y.; Wang, Y.; Cheng, W. Low-Dose of Polystyrene Microplastics Induce Cardiotoxicity in Mice and Human-Originated Cardiac Organoids. Environ. Int. 2023, 179, 108171. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Li, X.; Zhou, Y.; Yu, H.; Xie, Y.; Guo, H.; Wang, H.; Li, Y.; Feng, Y.; Wang, Y. Polystyrene Microplastics Induce Hepatotoxicity and Disrupt Lipid Metabolism in the Liver Organoids. Sci. Total Environ. 2022, 806, 150328. [Google Scholar] [CrossRef] [PubMed]
- Astashkina, A.I.; Jones, C.F.; Thiagarajan, G.; Kurtzeborn, K.; Ghandehari, H.; Brooks, B.D.; Grainger, D.W. Nanoparticle Toxicity Assessment Using an in Vitro 3-D Kidney Organoid Culture Model. Biomaterials 2014, 35, 6323–6331. [Google Scholar] [CrossRef]
- Xu, H.; Kang, J.; Gao, X.; Lan, Y.; Li, M. Towards a Better Understanding of the Human Health Risk of Per-and Polyfluoroalkyl Substances Using Organoid Models. Bioengineering 2025, 12, 393. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.; Xia, C.; Chandra, A.; Hamon, M.; Lee, G.; Yang, C.; Guo, Z.; Sun, B. Full-Thickness Perfused Skin-on-a-Chip with In Vivo-Like Drug Response for Drug and Cosmetics Testing. Bioengineering 2024, 11, 1055. [Google Scholar] [CrossRef]
- Van Gele, M.; Geusens, B.; Speeckaert, R.; Dynoodt, P.; Vanhoecke, B.; Van Den Bossche, K.; Lambert, J. Development of a 3D Pigmented Skin Model to Evaluate RNAi-Induced Depigmentation. Exp. Dermatol. 2011, 20, 773–775. [Google Scholar] [CrossRef]
- Duval, C.; Schmidt, R.; Regnier, M.; Facy, R.; Asselineau, D.; Bernerd, O. The Use of Reconstructed Human Skin to Evaluate UV-Induced Modifications and Sunscreen Efficacy. Exp. Dermatol. 2003, 12 (Suppl. S2), 64–70. [Google Scholar] [CrossRef]
- Tao, T.P.; Brandmair, K.; Gerlach, S.; Przibilla, J.; Géniès, C.; Jacques-Jamin, C.; Schepky, A.; Marx, U.; Hewitt, N.J.; Maschmeyer, I.; et al. Demonstration of the First-Pass Metabolism in the Skin of the Hair Dye, 4-Amino-2-Hydroxytoluene, Using the Chip2 Skin–Liver Microphysiological Model. J. Appl. Toxicol. 2021, 41, 1553–1567. [Google Scholar] [CrossRef]
- Li, T.; Yang, J.; Yang, W. Advances of in Vitro Culture Models Derived from Lung Adult Stem Cells. Chin. J. Biotechnol 2022, 38, 3255–3266. [Google Scholar] [CrossRef]
- Kastlmeier, M.T.; Guenther, E.M.; Stoeger, T.; Voss, C. Lung Organoids for Hazard Assessment of Nanomaterials. Int. J. Mol. Sci. 2022, 23, 15666. [Google Scholar] [CrossRef]
- Nikolić, M.Z.; Rawlins, E.L. Lung Organoids and Their Use To Study Cell-Cell Interaction. Curr. Pathobiol. Rep. 2017, 5, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Kumar, R.; Buragohain, L.; Kumari, A.; Ghosh, M. Organoid Technology: A Reliable Developmental Biology Tool for Organ-Specific Nanotoxicity Evaluation. Front. Cell Dev. Biol. 2021, 9, 696668. [Google Scholar] [CrossRef] [PubMed]
- Sachs, N.; Papaspyropoulos, A.; Zomer-van Ommen, D.D.; Heo, I.; Böttinger, L.; Klay, D.; Weeber, F.; Huelsz-Prince, G.; Iakobachvili, N.; Amatngalim, G.D.; et al. Long-term Expanding Human Airway Organoids for Disease Modeling. EMBO J. 2019, 38, e100300. [Google Scholar] [CrossRef]
- Kong, J.; Wen, S.; Cao, W.; Yue, P.; Xu, X.; Zhang, Y.; Luo, L.; Chen, T.; Li, L.; Wang, F.; et al. Lung Organoids, Useful Tools for Investigating Epithelial Repair after Lung Injury. Stem Cell Res. Ther. 2021, 12, 95. [Google Scholar] [CrossRef]
- Choi, S.Y.; Cho, Y.H.; Kim, D.S.; Ji, W.; Choi, C.M.; Lee, J.C.; Rho, J.K.; Jeong, G.S. Establishment and Long-Term Expansion of Small Cell Lung Cancer Patient-Derived Tumor Organoids. Int. J. Mol. Sci. 2021, 22, 1349. [Google Scholar] [CrossRef]
- Kim, M.; Mun, H.; Sung, C.O.; Cho, E.J.; Jeon, H.J.; Chun, S.M.; Jung, D.J.; Shin, T.H.; Jeong, G.S.; Kim, D.K.; et al. Patient-Derived Lung Cancer Organoids as in Vitro Cancer Models for Therapeutic Screening. Nat. Commun. 2019, 10, 3991. [Google Scholar] [CrossRef]
- Thacker, V.V.; Dhar, N.; Sharma, K.; Barrile, R.; Karalis, K.; McKinney, J.D. A Lung-on-Chip Model of Early m. Tuberculosis Infection Reveals an Essential Role for Alveolar Epithelial Cells in Controlling Bacterial Growth. Elife 2020, 9, e59961. [Google Scholar] [CrossRef]
- Fonseca, K.L.; Rodrigues, P.N.S.; Olsson, I.A.S.; Saraiva, M. Experimental Study of Tuberculosis: From Animal Models to Complex Cell Systems and Organoids. PLoS Pathog. 2017, 13, e1006421. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Fang, H.; Liu, X.; Fu, Z.; Zhu, F. Progress in Research and Application of Lung Organoids. Chin. J. Pathophysiol. 2024, 40, 1146. [Google Scholar] [CrossRef]
- Hild, M.; Jaffe, A.B. Production of 3-D Airway Organoids from Primary Human Airway Basal Cells and Their Use in High-Throughput Screening. Curr. Protoc. Stem Cell Biol. 2016, 37, IE.9.1–IE.9.15. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Rana, T.M. Generation of 3D Lung Organoids from Human Induced Pluripotent Stem Cells for Modeling of Lung Development and Viral Infection. Heliyon 2023, 9, e19601. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J.; Dye, B.R.; Ferrer-Torres, D.; Hill, D.R.; Overeem, A.W.; Shea, L.D.; Spence, J.R. Generation of Lung Organoids from Human Pluripotent Stem Cells in Vitro. Nat. Protoc. 2019, 14, 518–540. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Yuan Hsin, H.; Ingber, D.E. Reconstituting Organ-Level Lung Functions on a Chip. Science (1979) 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A.S.; Cherubini, A.; Rusconi, F.; Santo, N.; Madaschi, L.; Pistoni, C.; Moschetti, G.; Sarnicola, M.L.; Crosti, M.; Rosso, L.; et al. Human Airway Organoids and Microplastic Fibers: A New Exposure Model for Emerging Contaminants. Environ. Int. 2022, 163, 107200. [Google Scholar] [CrossRef]
- Huang, S.; Wiszniewski, L.; Constant, S.; Roggen, E. Potential of in Vitro Reconstituted 3D Human Airway Epithelia (MucilAirTM) to Assess Respiratory Sensitizers. Toxicol. Vitr. 2013, 27, 1151–1156. [Google Scholar] [CrossRef]
- Chary, A.; Serchi, T.; Moschini, E.; Hennen, J.; Cambier, S.; Ezendam, J.; Blömeke, B.; Gutleb, A.C. An in Vitro Coculture System for the Detection of Sensitization Following Aerosol Exposure. ALTEX 2019, 36, 403–418. [Google Scholar] [CrossRef]
- Mizoguchi, I.; Ohashi, M.; Chiba, Y.; Hasegawa, H.; Xu, M.; Owaki, T.; Yoshimoto, T. Prediction of Chemical Respiratory and Contact Sensitizers by OX40L Expression in Dendritic Cells Using a Novel 3D Coculture System. Front. Immunol. 2017, 8, 929. [Google Scholar] [CrossRef] [PubMed]
- Czekala, L.; Simms, L.; Stevenson, M.; Tschierske, N.; Maione, A.G.; Walele, T. Toxicological Comparison of Cigarette Smoke and E-Cigarette Aerosol Using a 3D in Vitro Human Respiratory Model. Regul. Toxicol. Pharmacol. 2019, 103, 314–324. [Google Scholar] [CrossRef]
- Giralt, A.; Iskandar, A.R.; Martin, F.; Moschini, E.; Serchi, T.; Kondylis, A.; Marescotti, D.; Leroy, P.; Ortega-Torres, L.; Majeed, S.; et al. Comparison of the Biological Impact of Aerosol of E-Vapor Device with MESH® Technology and Cigarette Smoke on Human Bronchial and Alveolar Cultures. Toxicol. Lett. 2021, 337, 98–110. [Google Scholar] [CrossRef]
- Iskandar, A.R.; Martin, F.; Leroy, P.; Schlage, W.K.; Mathis, C.; Titz, B.; Kondylis, A.; Schneider, T.; Vuillaume, G.; Sewer, A.; et al. Comparative Biological Impacts of an Aerosol from Carbon-Heated Tobacco and Smoke from Cigarettes on Human Respiratory Epithelial Cultures: A Systems Toxicology Assessment. Food Chem. Toxicol. 2018, 115, 109–126. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, L.; Du, C.; Li, Y.; Cheng, W.; Bi, H.; Li, G.; Zhuang, M.; Ren, D.; Wang, H.; et al. Human Airway Organoids as 3D in Vitro Models for a Toxicity Assessment of Emerging Inhaled Pollutants: Tire Wear Particles. Front. Bioeng. Biotechnol. 2023, 10, 1105710. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, R.; Chen, Y.; Zhao, X.; Chen, S.; Yang, X.; Cheng, Z.; Hu, B.; Liang, X.; Yin, N.; et al. Development of Human Lung Induction Models for Air Pollutants’ Toxicity Assessment. Environ. Sci. Sci. Technol. 2021, 55, 2440–2451. [Google Scholar] [CrossRef] [PubMed]
- Wyle, Y.; Lu, N.; Hepfer, J.; Sayal, R.; Martinez, T.; Wang, A. The Role of Biophysical Factors in Organ Development: Insights from Current Organoid Models. Bioengineering 2024, 11, 619. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Wu, Y.; Li, G.; Zhang, W.; Zhang, H.; Su, J. AI-Enabled Organoids: Construction, Analysis, and Application. Bioact. Mater. 2024, 31, 525–548. [Google Scholar] [CrossRef]
- Mo, Y.; Wang, J.; Yang, H.; Bai, L.; Xu, K.; Su, J. Artificial Intelligence for Organoids Multidimensional Assessment. SmartMat 2025, 6, e70016. [Google Scholar] [CrossRef]


| Particle Diameter (μm) | Sedimentation Site |
|---|---|
| >100 | hard to enter the respiratory tract |
| 10~100 | upper respiratory tract |
| 5~10 | bronchiole, pulmonary acinus |
| <5 | pulmonary acinus |
| Ingredient | Examples |
|---|---|
| Solvent | Water, oil, ethanol, isopropanol, etc. |
| Propellant | Propane, butane, etc. |
| Humectant | Glycerin, hyaluronic acid, propylene glycol, etc. |
| Active ingredients | Vitamins, plant extracts, peptides, etc. |
| Preservative | Phenoxyethanol, parabens, etc. |
| Fragrance and pigment | Natural fragrance, synthetic fragrance, natural pigment, synthetic pigment, etc. |
| Other additives | Thickener, antioxidant, etc. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Luo, X.; Hu, R.; Tang, L.; Xiang, Q. The Risks Associated with Inhalation Exposure to Cosmetics and Potential for Assessment Using Lung Organoids. Bioengineering 2025, 12, 652. https://doi.org/10.3390/bioengineering12060652
Li Y, Luo X, Hu R, Tang L, Xiang Q. The Risks Associated with Inhalation Exposure to Cosmetics and Potential for Assessment Using Lung Organoids. Bioengineering. 2025; 12(6):652. https://doi.org/10.3390/bioengineering12060652
Chicago/Turabian StyleLi, Yiguang, Xin Luo, Rong Hu, Lifeng Tang, and Qi Xiang. 2025. "The Risks Associated with Inhalation Exposure to Cosmetics and Potential for Assessment Using Lung Organoids" Bioengineering 12, no. 6: 652. https://doi.org/10.3390/bioengineering12060652
APA StyleLi, Y., Luo, X., Hu, R., Tang, L., & Xiang, Q. (2025). The Risks Associated with Inhalation Exposure to Cosmetics and Potential for Assessment Using Lung Organoids. Bioengineering, 12(6), 652. https://doi.org/10.3390/bioengineering12060652







