Application of a Nomogram Model in Predicting Postoperative Delirium Following Percutaneous Coronary Intervention
Abstract
1. Introduction
2. Methods
2.1. Data Source
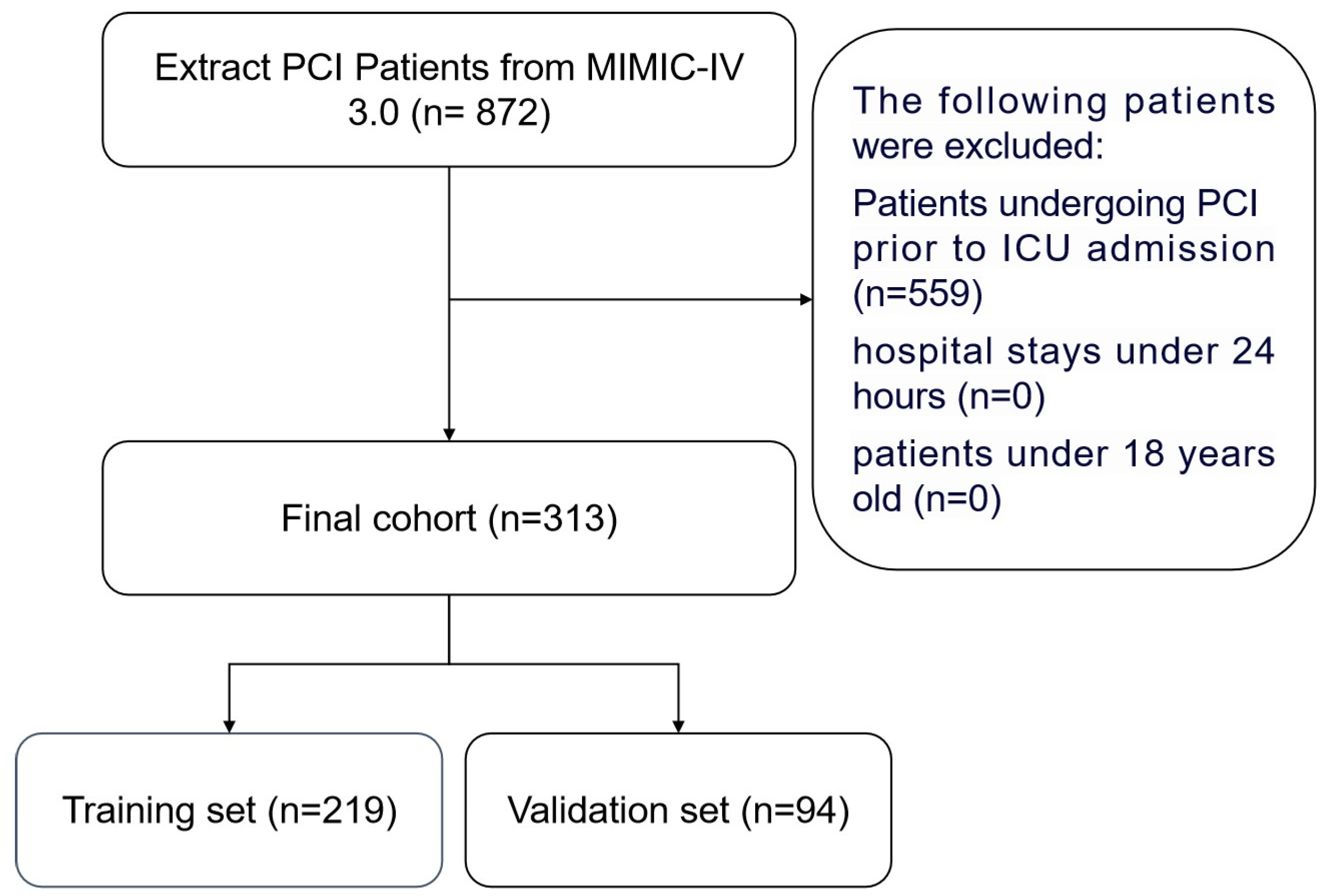
2.2. Patient Population
2.3. Data Extraction
2.4. Outcome
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients
3.2. Survival Analysis of Patients
3.3. Factors Associated with Delirium Occurrence in the Training Cohort
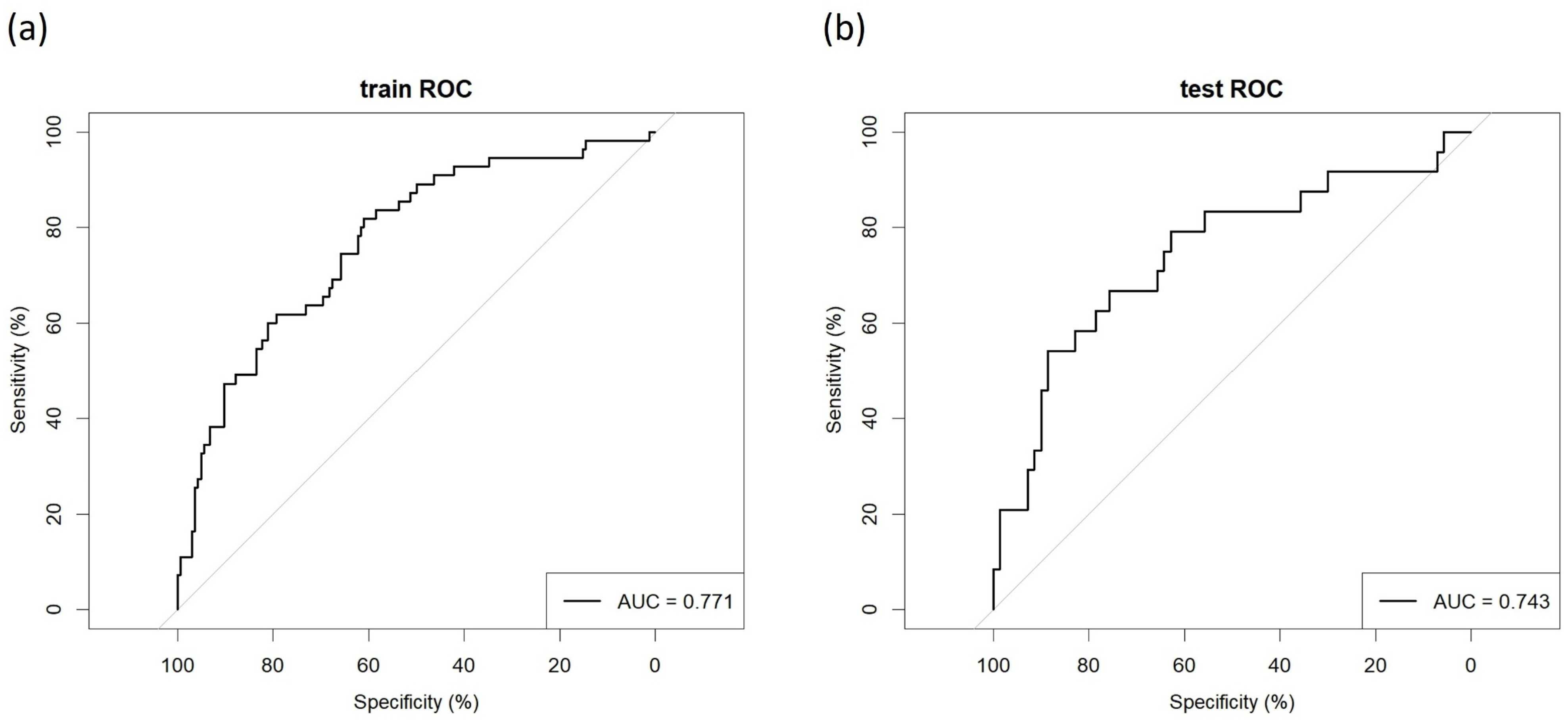
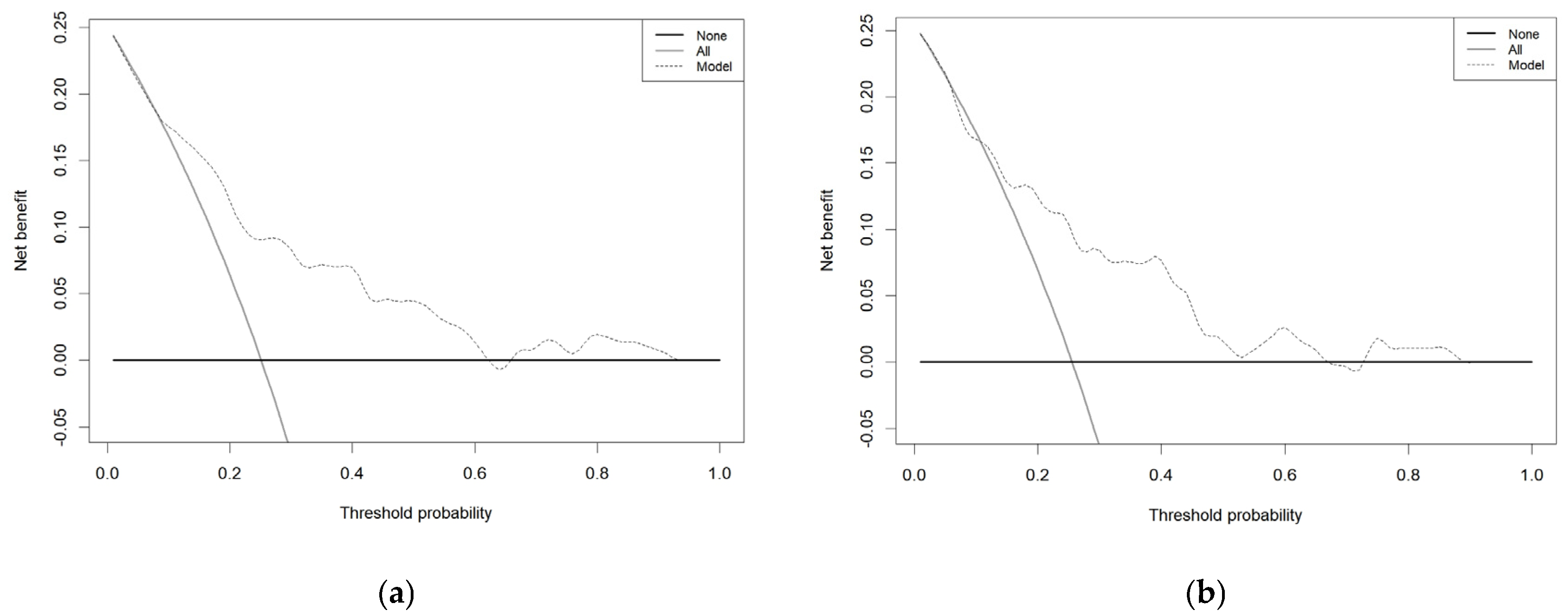
3.4. Model’s Predictive Performance and Clinical Utility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| percutaneous coronary intervention | PCI |
| intensive care unit | ICU |
| international classification of diseases | ICD |
| heart rate | HR |
| mean arterial pressure | MBP |
| blood oxygen saturation | SPO2 |
| hemoglobin | Hb |
| platelets | PLT |
| white blood cell count | WBC |
| blood urea nitrogen | BUN |
| serum creatinine | Cr |
| international normalized ratio | INR |
| activated partial thromboplastin time | APTT |
| bicarbonate, anion gap | AG |
| Kaplan–Meier | KM |
| hazard ratios | HR |
| confidence intervals | CIs |
| variance inflation factor | VIF |
| area under the receiver operating characteristic curve | AUC |
| decision curve analysis | DCA |
References
- Ibrahim, K.; McCarthy, C.P.; McCarthy, K.J.; Brown, C.H.; Needham, D.M.; Januzzi, J.L.; McEvoy, J.W. Delirium in the Cardiac Intensive Care Unit. J. Am. Heart Assoc. 2018, 7, e008568. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Beringola, A.; Vicent, L.; Martín-Asenjo, R.; Puerto, E.; Domínguez-Pérez, L.; Maruri, R.; Moreno, G.; Vidán, M.T.; Arribas, F.; Bueno, H. Diagnosis, prevention, and management of delirium in the intensive cardiac care unit. Am. Heart J. 2021, 232, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, C.; Grande, G.; Rebora, P.; Zucchelli, A.; Valsecchi, M.G.; Focà, E.; Ecarnot, F.; Marengoni, A.; Bellelli, G. Early Onset Delirium During Hospitalization Increases In-Hospital and Postdischarge Mortality in COVID-19 Patients: A Multicenter Prospective Study. J. Clin. Psychiatry 2023, 84, 22m14565. [Google Scholar] [CrossRef]
- Potter, K.M.; Prendergast, N.T.; Boyd, J.G. From Traditional Typing to Intelligent Insights: A Narrative Review of Directions Toward Targeted Therapies in Delirium. Crit. Care Med. 2024, 52, 1285–1294. [Google Scholar] [CrossRef]
- Chan, P.S.; Patel, M.R.; Klein, L.W.; Krone, R.J.; Dehmer, G.J.; Kennedy, K.; Nallamothu, B.K.; Weaver, W.D.; Masoudi, F.A.; Rumsfeld, J.S.; et al. Appropriateness of percutaneous coronary intervention. JAMA 2011, 306, 53–61. [Google Scholar] [CrossRef]
- Sansanayudh, N.; Chandavimol, M.; Srimahachota, S.; Limpijankit, T.; Hutayanon, P.; Kiatchoosakun, S.; Kuanprasert, S.; Chamnarnphol, N.; Athisakul, S.; Kehasukcharoen, W.; et al. Patient Characteristics, Procedural Details, and Outcomes of Contemporary Percutaneous Coronary Intervention in Real-World Practice: Insights from Nationwide Thai PCI Registry. J. Intervent. Cardiol. 2022, 2022, 5839834. [Google Scholar] [CrossRef]
- Hashimoto, S.; Motozawa, Y.; Saito, T.; Suzuki, T. Transformation of the Cardiovascular Clinics in Japan—How to Overcome Crisis Due to Changes in the External Environment. Circ. J. 2023, 87, 240–246. [Google Scholar] [CrossRef]
- Jinnouchi, H.; Sakakura, K.; Wada, H.; Kubo, N.; Sugawara, Y.; Nakamura, T.; Funayama, H.; Ako, J.; Momomura, S. Transradial percutaneous coronary intervention for acute myocardial infarction reduces CCU stay in patients 80 or older. Int. Heart J. 2012, 53, 79–84. [Google Scholar] [CrossRef]
- Park, D.Y.; Jamil, Y.; Hu, J.-R.; Lowenstern, A.; Frampton, J.; Abdullah, A.; Damluji, A.A.; Ahmad, Y.; Soufer, R.; Nanna, M.G. Delirium in older adults after percutaneous coronary intervention: Prevalence, risks, and clinical phenotypes. Cardiovasc. Revascularization Med. 2023, 57, 60–67. [Google Scholar] [CrossRef]
- Tan, J.-F.; Duan, L.; Han, J.-C.; Cui, J.-J. Clinical characteristics of delirium in older patients with first-ever acute myocardial infarction who underwent percutaneous coronary intervention: A retrospective study. Herz 2024, 49, 456–463. [Google Scholar] [CrossRef]
- Johnson, A.E.W.; Bulgarelli, L.; Shen, L.; Gayles, A.; Shammout, A.; Horng, S.; Pollard, T.J.; Hao, S.; Moody, B.; Gow, B.; et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci. Data 2023, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Lüdtke, O.; Robitzsch, A.; Grund, S. Multiple imputation of missing data in multilevel designs: A comparison of different strategies. Psychol. Methods 2017, 22, 141–165. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.P.; Rizzo, J.D.; Zhang, M.J.; Keiding, N. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part 2: Regression modeling. Bone Marrow Transplant. 2001, 28, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y. Nomogram: An analogue tool to deliver digital knowledge. J. Thorac. Cardiovasc. Surg. 2018, 155, 1793. [Google Scholar] [CrossRef]
- Labarère, J.; Renaud, B.; Fine, M.J. How to derive and validate clinical prediction models for use in intensive care medicine. Intensive Care Med. 2014, 40, 513–527. [Google Scholar] [CrossRef]
- de Haan, E.; van Rijckevorsel, V.A.J.I.M.; Bod, P.; Roukema, G.R.; de Jong, L. Dutch Hip Fracture Registry Collaboration (DHFR) Delirium After Surgery for Proximal Femoral Fractures in the Frail Elderly Patient: Risk Factors and Clinical Outcomes. Clin. Interv. Aging 2023, 18, 193–203. [Google Scholar] [CrossRef]
- Yan, E.; Veitch, M.; Saripella, A.; Alhamdah, Y.; Butris, N.; Tang-Wai, D.F.; Tartaglia, M.C.; Nagappa, M.; Englesakis, M.; He, D.; et al. Association between postoperative delirium and adverse outcomes in older surgical patients: A systematic review and meta-analysis. J. Clin. Anesth. 2023, 90, 111221. [Google Scholar] [CrossRef]
- Yokoyama, C.; Yoshitnai, K.; Ogata, S.; Fukushima, S.; Matsuda, H. Effect of postoperative delirium after cardiovascular surgery on 5-year mortality. JA Clin. Rep. 2023, 9, 66. [Google Scholar] [CrossRef]
- Hori, Y.; Mihashi, M. Relationship Between Delirium Development and Its Causative Factors in the Intensive Care Unit After Cardiac Surgery. Yonago Acta Med. 2023, 66, 214–222. [Google Scholar] [CrossRef]
- Tiwari, A.M.; Zirpe, K.G.; Khan, A.Z.; Gurav, S.K.; Deshmukh, A.M.; Suryawanshi, P.B.; Kapse, U.S.; Wankhede, P.P.; Bamne, S.N.; Bhoyar, A.P.; et al. Incidence, Subtypes, Risk factors, and Outcome of Delirium: A Prospective Observational Study from Indian Intensive Care Unit. Indian J. Crit. Care Med. 2023, 27, 111–118. [Google Scholar]
- Han, F.; Liu, X.; Huang, H.; Chu, H.; Feng, W. Effect of preoperative sleep disorders on delirium in proximal femoral surgery patients aged 60 or older. BMC Anesthesiol. 2023, 23, 376. [Google Scholar] [CrossRef] [PubMed]
- Tomite, T.; Saito, H.; Kijima, H.; Hatakeyama, Y.; Tazawa, H.; Wachi, T.; Miyakoshi, N. Delirium following total hip or knee arthroplasty: A retrospective, single-center study. J. Orthop. Sci. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.P.d.A.D.; Thirumala, P.D.; Reddy, G.; Barros, D.F.d.; Faria, V.N.R.; Shandal, V.; Kurtz, P. Risk of perioperative stroke and cerebral autoregulation monitoring: A systematic review. Arq. Neuropsiquiatr. 2022, 80, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Vu, E.L.; Brown, C.H.; Brady, K.M.; Hogue, C.W. Monitoring of cerebral blood flow autoregulation: Physiologic basis, measurement, and clinical implications. Br. J. Anaesth. 2024, 132, 1260–1273. [Google Scholar] [CrossRef]
- Khan, J.M.; Shore, A.; Lee, K.F.H.; Wood, M.D.; Maslove, D.M.; Hunt, M.; Georgescu, I.; Muscedere, J.; Boyd, J.G. Cerebral autoregulation-based mean arterial pressure targets and delirium in critically ill adults without brain injury: A retrospective cohort study. Can. J. Anaesth. 2024, 71, 107–117. [Google Scholar] [CrossRef]
- Hu, X.; Liu, L.; Da, X.; Zhu, S.; Wang, J.; Shan, M.; Liu, Y.; He, Z.; Xu, G. Anesthesia/surgery leads to blood-brain barrier disruption via the transcellular and paracellular pathways, and postoperative delirium-like behavior: A comparative study in mice of different ages. Exp. Neurol. 2025, 383, 115044. [Google Scholar] [CrossRef]
- Lu, J.; Liang, F.; Bai, P.; Liu, C.; Xu, M.; Sun, Z.; Tian, W.; Dong, Y.; Zhang, Y.; Quan, Q.; et al. Blood tau-PT217 contributes to the anesthesia/surgery-induced delirium-like behavior in aged mice. Alzheimers Dement. 2023, 19, 4110–4126. [Google Scholar] [CrossRef]
- Devinney, M.J.; Wong, M.K.; Wright, M.C.; Marcantonio, E.R.; Terrando, N.; Browndyke, J.N.; Whitson, H.E.; Cohen, H.J.; Nackley, A.G.; Klein, M.E.; et al. Role of Blood-Brain Barrier Dysfunction in Delirium following Non-cardiac Surgery in Older Adults. Ann. Neurol. 2023, 94, 1024–1035. [Google Scholar] [CrossRef]
- Coetzee, E.; Absalom, A.R. Pharmacokinetic and Pharmacodynamic Changes in the Older Adults: Impact on Anesthetics. Clin. Geriatr. Med. 2025, 41, 19–35. [Google Scholar] [CrossRef]
- Guidry, G.; Sparrow, N.A.; Marshall, H.S.; De Souza Santos, R.; Bharath, S.P.; Gezalian, M.M.; Pisarska, M.D.; Vit, J.-P.; Kelly, S.A.; Karumanchi, S.A.; et al. 17β-estradiol ameliorates delirium-like phenotypes in a murine model of urinary tract infection. Sci. Rep. 2022, 12, 19622. [Google Scholar] [CrossRef]
- Arab, H.H.; Khames, A.; Mohammad, M.K.; Alsufyani, S.E.; Ashour, A.M.; El-Sheikh, A.A.K.; Darwish, H.W.; Gad, A.M. Meloxicam Targets COX-2/NOX1/NOX4/Nrf2 Axis to Ameliorate the Depression-like Neuropathology Induced by Chronic Restraint Stress in Rats. Pharmaceuticals 2023, 16, 848. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Gunther, M. A Critical Reappraisal of Haloperidol for Delirium Management in the Intensive Care Unit: Perspective from Psychiatry. J. Clin. Med. 2025, 14, 438. [Google Scholar] [CrossRef] [PubMed]
- Matiș, L.; Alexandru, B.A.; Ghitea, T.C. Catecholamine Variations in Pediatric Gastrointestinal Disorders and Their Neuropsychiatric Expression. Biomedicines 2023, 11, 2600. [Google Scholar] [CrossRef] [PubMed]
- Sadhu, A.; Valencia, C.; Fatima, H.; Nwankwo, I.; Anam, M.; Maharjan, S.; Amjad, Z.; Abaza, A.; Vasavada, A.M.; Khan, S. Haloperidol Versus Atypical Antipsychotics for Treating Delirium in Intensive Care Unit Patients: A Systematic Review. Cureus 2022, 14, e30641. [Google Scholar] [CrossRef]
- Yabo, W.; Dongxu, L.; Xiao, L.; Qi, A. Cardiac surgery outcomes: The efficacy of dexmedetomidine in reducing postoperative delirium—A bibliometric study. Curr. Probl. Cardiol. 2025, 50, 102984. [Google Scholar] [CrossRef]
- Ma, H.; Ahrens, E.; Wachtendorf, L.J.; Suleiman, A.; Shay, D.; Munoz-Acuna, R.; Tartler, T.M.; Teja, B.; Wagner, S.; Subramaniam, B.; et al. Intraoperative Use of Phenylephrine versus Ephedrine and Postoperative Delirium: A Multicenter Retrospective Cohort Study. Anesthesiology 2024, 140, 657–667. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, B.; Fu, J.; Peng, H.; Chen, Y.; Hu, X. Effect of phenylephrine versus ephedrine on the incidence of postoperative delirium in olderly adults undergoing knee arthroplasty under general anesthesia: A single-center trial. Sci. Rep. 2024, 14, 17333. [Google Scholar] [CrossRef]
- Creagh-Brown, B.; Wunsch, H.; Martin, P.; Harlet, P.; Forni, L.; Moonesinghe, S.R.; Jammer, I. The incidence of postoperative vasopressor usage: Protocol for a prospective international observational cohort study (SQUEEZE). Perioper. Med. 2023, 12, 8. [Google Scholar] [CrossRef]
- Douglas, N.; Leslie, K.; Darvall, J.N. Vasopressors to treat postoperative hypotension after adult noncardiac, non-obstetric surgery: A systematic review. Br. J. Anaesth. 2023, 131, 813–822. [Google Scholar] [CrossRef]
- Li, W.; Li, S.; Shang, Y.; Zhuang, W.; Yan, G.; Chen, Z.; Lyu, J. Associations between dietary and blood inflammatory indices and their effects on cognitive function in elderly Americans. Front. Neurosci. 2023, 17, 1117056. [Google Scholar] [CrossRef]
- Ma, Y.; Li, C.; Peng, W.; Wan, Q. The influence of delirium on mortality and length of ICU stay and analysis of risk factors for delirium after liver transplantation. Front. Neurol. 2023, 14, 1229990. [Google Scholar] [CrossRef] [PubMed]
- Pasqui, E.; de Donato, G.; Brancaccio, B.; Casilli, G.; Ferrante, G.; Cappelli, A.; Palasciano, G. The Predictive Role of Inflammatory Biochemical Markers in Post-Operative Delirium After Vascular Surgery Procedures. Vasc. Health Risk Manag. 2022, 18, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Zheng, K.; Dai, W.; Wang, Y.; Hao, P. Comparative analysis of inflammatory markers as predictive markers for postoperative delirium in cardiac surgery patients: An observational study. Front. Med. 2025, 12, 1515940. [Google Scholar] [CrossRef]
- Wang, P.; Huang, J.; Xu, L.; Hu, R. The association between the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio and delirium in ischemic stroke patients. Front. Med. 2024, 11, 1456742. [Google Scholar] [CrossRef]
- Liu, J.; Qian, J.; Wang, X.; Lin, J.; Yang, S.; Hu, R.; Xian, J.; Feng, H.; Chen, Y.; Tan, B. Identifying hormones and other perioperative risk factors for postoperative delirium after endoscope-assisted transsphenoidal pituitary adenoma resection: A retrospective, matched cohort study. Brain Behav. 2023, 13, e3041. [Google Scholar] [CrossRef]
- Ketema, B.; Mengistu, G.; Melka, D.; Zenebe, Y.; Zebenigus, M.; Leul, F. A multicenter prospective study on the prevalence of Post Stroke Delirium and associated risk factors in Addis Ababa, Ethiopia. BMC Neurol. 2025, 25, 114. [Google Scholar] [CrossRef]
- Baron, M.; Devor, M. From molecule to oblivion: Dedicated brain circuitry underlies anesthetic loss of consciousness permitting pain-free surgery. Front. Mol. Neurosci. 2023, 16, 1197304. [Google Scholar] [CrossRef]
- Barra, M.E.; Solt, K.; Yu, X.; Edlow, B.L. Restoring consciousness with pharmacologic therapy: Mechanisms, targets, and future directions. Neurotherapeutics 2024, 21, e00374. [Google Scholar] [CrossRef]
- Yang, C.-C.; Wang, X.-Y.; Chou, P.-H.; Lin, C.-H. Valproate-related neutropenia and lithium-related leukocytosis in patients treated with clozapine: A retrospective cohort study. BMC Psychiatry 2023, 23, 170. [Google Scholar] [CrossRef]
- Kanyo, R.; Lamothe, S.M.; Urrutia, A.; Goodchild, S.J.; Allison, W.T.; Dean, R.; Kurata, H.T. Site and Mechanism of ML252 Inhibition of Kv7 Voltage-Gated Potassium Channels. Function 2023, 4, zqad021. [Google Scholar] [CrossRef]
- Zhang, D.; Xiang, W.; Liu, J.; Li, W.; Qiao, Z.; Wang, K.; Shao, L. Design, synthesis, and structure-activity relationship of 5,7- dimethylbenzo[d]thiazoles as novel Kv7.2/7.3 activators with antiepileptic effects. Eur. J. Med. Chem. 2025, 292, 117660. [Google Scholar] [CrossRef]



| Characteristic | No Delirium, N = 234 | Delirium Present, N = 79 | p-Value |
|---|---|---|---|
| Gender | 0.883 | ||
| Male | 138 (59%) | 48 (61%) | |
| Female | 96 (41%) | 31 (39%) | |
| Age | 71.00 (62.00, 80.00) | 73.00 (62.00, 82.00) | 0.452 |
| Admission Type | 0.799 | ||
| Emergency | 109 (47%) | 40 (51%) | |
| Urgent | 97 (41%) | 31 (39%) | |
| Other | 28 (12%) | 8 (10%) | |
| Hb | 11.80 (10.20, 13.30) | 11.30 (9.35, 13.15) | 0.226 |
| PLTs | 203.00 (153.25, 264.75) | 215.00 (158.00, 291.00) | 0.302 |
| WBC | 9.85 (7.30, 13.50) | 12.20 (8.30, 16.75) | <0.001 |
| BUN | 23.00 (16.00, 30.00) | 27.00 (18.50, 44.50) | 0.007 |
| Cr | 1.10 (0.80, 1.50) | 1.40 (0.90, 1.95) | 0.019 |
| INR | 1.20 (1.10, 1.40) | 1.20 (1.10, 1.45) | 0.128 |
| APTT | 35.45 (29.33, 59.93) | 36.40 (29.55, 61.25) | 0.718 |
| Bicarbonate | 24.00 (21.00, 26.00) | 23.00 (19.00, 26.00) | 0.124 |
| Serum Potassium | 4.10 (3.80, 4.70) | 4.30 (3.65, 4.70) | 0.590 |
| Serum Sodium | 138.00 (135.25, 140.00) | 138.00 (135.00, 141.00) | 0.785 |
| Blood Glucose | 135.00 (106.00, 179.75) | 154.00 (122.00, 201.50) | 0.032 |
| AG | 15.00 (13.00, 18.00) | 16.00 (14.00, 19.00) | 0.104 |
| MBP | 80.00 (70.00, 93.00) | 81.00 (71.00, 100.00) | 0.208 |
| HR | 85.50 (71.00, 102.00) | 94.00 (75.00, 111.00) | 0.037 |
| SP02 | 97.00 (94.00, 99.00) | 98.00 (95.00, 100.00) | 0.045 |
| Benzodiazepine Use | 75 (32%) | 44 (56%) | <0.001 |
| Vasoactive Drug Therapy | 44 (19%) | 45 (57%) | <0.001 |
| Hospital LOS | 8.00 (4.00, 13.00) | 15.00 (11.00, 24.00) | <0.001 |
| Hospital Mortality | 10 (4.3%) | 15 (19%) | <0.001 |
| 28-day mortality | 18 (7.7%) | 15 (19%) | 0.009 |
| 180-day mortality | 38 (16%) | 24 (30%) | 0.010 |
| 360-day mortality | 51 (22%) | 28 (35%) | 0.024 |
| No Delirium (n = 234) | Delirium Present (n = 79) | ||
|---|---|---|---|
| HR (95%CI) | p-Value | ||
| 28-day mortality | |||
| Unadjusted | Reference | 2.684 (1.353, 5.327) | 0.005 |
| Adjusted | Reference | 2.833 (1.193, 6.728) | 0.018 |
| 180-day mortality | |||
| Unadjusted | Reference | 2.103 (1.261, 3.506) | 0.004 |
| Adjusted | Reference | 1.599 (0.841, 3.040) | 0.153 |
| 360-day mortality | |||
| Unadjusted | Reference | 1.854 (1.169, 2.940) | 0.009 |
| Adjusted | Reference | 1.640 (0.931, 2.888) | 0.087 |
| Variables | OR | 95% CI | p-Value |
|---|---|---|---|
| Gender | |||
| Male | Reference | — | — |
| Female | 0.784 | (0.331–1.808) | 0.573 |
| Age | 1.048 | (1.013–1.086) | 0.008 |
| Admission Type | |||
| Emergency | Reference | — | — |
| Urgent | 1.450 | (0.632–3.388) | 0.382 |
| Other | 1.640 | (0.461–5.460) | 0.427 |
| Hb | 1.153 | (0.941–1.423) | 0.175 |
| PLTs | 1.003 | (0.999–1.007) | 0.166 |
| WBC | 1.071 | (1.019–1.136) | 0.011 |
| BUN | 1.021 | (0.997–1.046) | 0.090 |
| Cr | 1.116 | (0.739–1.612) | 0.576 |
| INR | 0.923 | (0.557–1.444) | 0.738 |
| APTT | 0.993 | (0.979–1.006) | 0.302 |
| Bicarbonate | 0.947 | (0.830–1.074) | 0.407 |
| Serum Potassium | 0.462 | (0.231–0.881) | 0.023 |
| Serum Sodium | 1.047 | (0.960–1.155) | 0.330 |
| Blood Glucose | 1.003 | (0.997–1.008) | 0.364 |
| AG | 0.939 | (0.804–1.093) | 0.423 |
| MBP | 1.023 | (1.000–1.048) | 0.053 |
| HR | 1.012 | (0.993–1.032) | 0.211 |
| SP02 | 1.063 | (0.963–1.190) | 0.253 |
| Benzodiazepine Use | |||
| NO | Reference | — | — |
| YES | 2.486 | (1.055–5.990) | 0.038 |
| Vasoactive Drug Therapy | |||
| NO | Reference | — | — |
| YES | 3.815 | (1.575–9.479) | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Y.; Meng, Z.; Sun, J.; Qi, Y.; Wang, K.; Huang, P.; Yang, Q.; Fan, R.; Guan, J.; Zhao, M.; et al. Application of a Nomogram Model in Predicting Postoperative Delirium Following Percutaneous Coronary Intervention. Bioengineering 2025, 12, 637. https://doi.org/10.3390/bioengineering12060637
Xiong Y, Meng Z, Sun J, Qi Y, Wang K, Huang P, Yang Q, Fan R, Guan J, Zhao M, et al. Application of a Nomogram Model in Predicting Postoperative Delirium Following Percutaneous Coronary Intervention. Bioengineering. 2025; 12(6):637. https://doi.org/10.3390/bioengineering12060637
Chicago/Turabian StyleXiong, Yaxin, Ze Meng, Jiuyue Sun, Yucheng Qi, Kuo Wang, Ping Huang, Qiuyue Yang, Renliang Fan, Jiaman Guan, Mingyan Zhao, and et al. 2025. "Application of a Nomogram Model in Predicting Postoperative Delirium Following Percutaneous Coronary Intervention" Bioengineering 12, no. 6: 637. https://doi.org/10.3390/bioengineering12060637
APA StyleXiong, Y., Meng, Z., Sun, J., Qi, Y., Wang, K., Huang, P., Yang, Q., Fan, R., Guan, J., Zhao, M., & Meng, X. (2025). Application of a Nomogram Model in Predicting Postoperative Delirium Following Percutaneous Coronary Intervention. Bioengineering, 12(6), 637. https://doi.org/10.3390/bioengineering12060637








