Abstract
The interaction among the various Ca2+ transporters complicates the assessment of isolated systems in an intact cell. This article proposes the functionally isolated SR model (FISRM), a hybrid (experimental and mathematical) approach to study Ca2+ cycling between the cytosol and the sarcoplasmic reticulum (SR), the main source of Ca2+ for contraction in mammalian cardiomyocytes. In FISRM, the main transmembrane Ca2+ transport pathways are eliminated by using a Na+, Ca2+-free extracellular medium, and SR Ca2+ release is elicited by a train of brief caffeine pulses. Two compounds that exert opposite effects on the SR Ca2+ uptake were characterized by this approach in isolated rat ventricular cardiomyocytes. The experimental FISRM was simulated with a simple mathematical model of Ca2+ fluxes across the SR membrane, based on a previous model adapted to the present conditions. To a fair extent, the theoretical model could reproduce the experimental results, and confirm the main assumption of the experimental model: that the only relevant Ca2+ fluxes occur across the SR membrane. Thus, the FISRM seems to be a valuable framework to investigate the SR Ca2+ transport in intact cardiomyocytes under physiological and pathophysiological conditions, and to test therapeutic approaches targeting SR proteins.
1. Introduction
The Ca2+-induced Ca2+ release mechanism plays a central role in excitation–contraction coupling (ECC) in the mammalian myocardium. Briefly, sarcolemmal depolarization during the action potential allows Ca2+ influx via sarcolemmal voltage-dependent L-type Ca2+ channels, which causes the release of a much larger amount of Ca2+ from the intracellular store in the sarcoplasmic reticulum (SR). The resultant increase in the cytosolic free Ca2+ concentration ([Ca2+]i) promotes interaction of the ion with troponin C, present in the contractile apparatus, leading to the development of contraction, and ultimately to blood pumping to the circulation. SR Ca2+ release is triggered by interaction of Ca2+ with Ca2+ release channels in the SR membrane, also called ryanodine receptors (RyR) [1,2,3].
Cell relaxation, fundamental for ventricular refilling with blood to be ejected in the next contraction, is due to Ca2+ dissociation from troponin C resulting from removal of cytosolic Ca2+ by transporters stimulated by the elevated [Ca2+]i. This is accomplished mainly by the SR Ca2+-ATPase (SERCA), which pumps Ca2+ back into the SR and replenishes its stores, and, to a lesser degree, by the sarcolemmal Na+-Ca2+ exchanger (NCX), which mediates most of Ca2+ efflux from the cell. Other transporters, namely the plasma membrane Ca2+-ATPase (PMCA) and the mitochondrial Ca2+ uniporter (MCU), with low transport capacity and affinity for Ca2+, respectively, seem to play a minor role in this process (<2% [4,5]).
These mechanisms define an integrated process, in which intracellular and transsarcolemmal Ca2+ transport modulate the ECC effectiveness. The balance between Ca2+ influx and efflux determines the amount of Ca2+ available for uptake by SERCA, and, therefore, the SR Ca2+ content. The latter determines the fraction of this content to be released at the next twitch [6,7,8], which impacts not only contraction amplitude, but also the NCX-mediated Ca2+ removal. NCX function, in turn, may affect transmembrane electric potential, action potential configuration and SR Ca2+ content, evidencing an important feedback control mechanism that regulates the inotropic function in different physiological and pathophysiological scenarios [6,9]. On the other hand, Ca2+-dependent regulation of Ca2+ transporters, by, e.g., Ca2+-calmodulin-dependent enzymes, introduces additional complexity levels to an already complex interplay (for a review, see, e.g., [10,11]).
Understanding ECC and the SR function is important to identify focuses for the therapy of cardiac diseases. The SR is the main Ca2+ contributor for contraction in both healthy and failing mammalian myocardium. The SR-cytosol Ca2+ cycling is estimated to account for more than 70% of the Ca2+ fluxes during an activity cycle in mammalian cardiomyocytes, reaching 90% in the adult rat ventricle [2,4,5,12]. Accordingly, several studies have shown that defects in SR Ca2+ cycling are associated with impaired systolic/diastolic function and arrhythmias, and that SR proteins seem to be promising targets for heart failure therapy (e.g., [9,13,14,15]. However, finding an experimental model suitable for the study of the myocardial SR function is not simple. While it is difficult to reproduce physiological intracellular conditions when using isolated SR vesicles and permeabilized cardiomyocytes, the variety of Ca2+ flux pathways in intact isolated cells makes it difficult to isolate those attributable to SR Ca2+ release and uptake.
In this report, we propose an experimental model and its theoretical framework, the functionally isolated SR model (FISRM), for studying SR-cytosol Ca2+ cycling in intact isolated myocytes under electrical rest. Ca2+ transport via NCX, the main SERCA competitor, is thermodynamically suppressed by removing Na+ and Ca2+ from the extracellular medium [4,5], which also renders the cell unable to develop action potentials and abolishes Ca2+ influx. The application of brief caffeine pulses is used to trigger Ca2+ transients due solely to SR Ca2+ release, whereas [Ca2+]i decline is majorly accomplished by sequestration by SERCA. This system was paralleled by a mathematical model in which the phenomena underlying SR Ca2+ release by caffeine were introduced as an adaptation of a realistic mathematical model of rodent cardiomyocyte [16]. The results indicate that the model is suitable for investigation of the SR-cytosol Ca2+ transport in intact cells without significant interference of other transporters.
2. Materials and Methods
2.1. Cell Isolation and Perfusion Solutions
All procedures for care and use of the animals (the same as described by Penna and Bassani [17]) were in agreement with Brazilian laws and were approved by the institutional Committee for Ethics in Animal Use (CEUA/UNICAMP, N. 775-1 and 952-1).
Myocytes were isolated from the left ventricle of male adult Wistar rats by coronary perfusion with collagenase I, followed by mechanical dispersion [17]. After isolation, cell suspensions in cardioplegic solution (composition in mM: 30 KCl; 70 glutamic acid; 10 KH2PO4; 5 HEPES; 1 MgCl2; 11 glucose; 20 taurine; pH 7.4) were kept at 4 °C until use. Experiments were performed at 24–25 °C. Cells were perfused with modified Tyrode’s solution (TyN), with the following composition (mM): 140 NaCl, 6 KCl, 1.5 MgCl2, 5 HEPES, 11 glucose and 1 CaCl2; pH 7.4 at 24 °C. In the Na+-, Ca2+-free solution (Ty00), LiCl and EGTA replaced for NaCl and CaCl2 in an equimolar fashion. Caffeine (10 mM) was dissolved in Ty00 (Caf00 solution).
2.2. Cell Contraction and [Ca2+]i Measurement
Myocytes were plated in a perfusion chamber of which the bottom was a collagen-treated coverslip. Cells were perfused with TyN and electrically stimulated via a pair of platinum electrodes (10 ms-long biphasic, rectangular voltage pulses delivered at 0.5 Hz). Unloaded cell shortening and Ca2+ transients were recorded simultaneously.
Systolic cell shortening (cell contraction amplitude, ΔL) was recorded with a video-edge detector and expressed as percent of resting cell length, which was measured with the aid of objective micrometer [17].
For [Ca2+]i measurement, cells were incubated with the cell-permeant Ca2+ indicator fluo-3 AM (1 µM; Life Technologies, Grand Island, NY, USA) for 20 min, followed by 20 min perfusion with TyN for fluo-3 deesterification. The dye was excited at 480 ± 20 nm, and its emission was collected at 515–560 nm using a fluorescence microscopy system previously described [18]. The background-subtracted emitted signal was converted to [Ca2+]i according to Shannon et al. [19]:
where F/F0 is the fluorescence emission normalized to that at diastole; Kd is the Ca2+-fluo-3 apparent Ca2+ dissociation constant (1.1 μM [20]), and [Ca2+]d is the diastolic [Ca2+]i taken as 0.23 μM from calibrated measurements with indo-1 [7,12].
2.3. The Experimental Protocol
The main inlets of the perfusion chamber admitted TyN or Ty00 solution, which bathed all cells in the chamber (~3 mL·min−1). A single cell was selected, and a pair of glass micropipettes was positioned aiming at it, so that solution delivered through them would perfuse the chosen cell and immediate surroundings. Each pipette delivered either Ty00 or Caf00 solution. Solutions were switched on and off with solenoid valves (LFAA mod. 12016-18H, The Lee Company, Westbrook, CT, USA) controlled by an oscillator circuit placed close to the micropipettes and chamber inlets.
Caffeine, a well-known RyR opener [21], was used to evoke SR Ca2+ release. Application of short (60 ms-long) caffeine pulses was implemented by rapid switching of the perfusion solution from the Tyr00 pipette to the Caf00 pipette (delay < 7 ms). Before and during caffeine application, the whole chamber was perfused with Ty00 via one of its main inlets.
Initially, cells were electrically stimulated for 2–5 min under perfusion with TyN for stabilization of Ca2+ transients and of free and bound [Ca2+] within the SR. Then, electrical stimulation was interrupted, and perfusion was switched to Ty00 through both one chamber inlet and one of the micropipettes. The removal of extracellular Na+ and Ca2+ ions abolished both Ca2+ efflux via NCX and Ca2+ influx, allowing isolation of intracellular SR-cytosol Ca2+ fluxes [4]. After 30 s perfusion with Ty00, caffeine pulses were applied by switching perfusion through the micropipette from Ty00 to Caf00, and then back to Ty00 60 ms later. Successive caffeine pulses (5–8) were applied at 7 s intervals to allow complete caffeine washout between pulses.
In some experiments, SR Ca2+ content was estimated to check the possibility of significant loss of SR Ca2+ during the caffeine pulses due to removal of the released Ca2+ by PMCA and MCU [4,5]. The SR Ca2+ load was estimated as in Bassani and Bers [22]. Briefly, it was defined as the total amount of Ca2+ in the SR released by caffeine ([Ca2+]SR, i.e., the sum of free and bound [Ca2+]), and expressed as µmoles Ca2+ per liter of non-mitochondrial cell water. The cytosolic total [Ca2+] ([Ca2+]T) was calculated from the recorded [Ca2+]i during sustained perfusion with Caff00 as follows:
where Bfmax is the assumed maximum concentration of Ca2+ binding sites in fluo-3 (4 μM) and Kdf is the Ca2+-fluo-3 apparent Ca2+ dissociation constant (1.1 μM [20]); Bemax and Kde are, respectively, the maximum concentration of endogenous passive Ca2+ binding sites (300 μM) and the respective apparent dissociation constant (0.52 μM) determined in permeabilized rat ventricular myocytes [23]. [Ca2+]SR was considered as the difference between the [Ca2+]T values at the Ca2+ transient peak and immediately before the caffeine application.
2.4. SERCA Stimulation and Inhibition
Two pharmacological treatments aiming at SERCA were used to test FISRM. For SERCA stimulation, cells were exposed to 10 nM isoproterenol (ISO), a β-adrenoceptor agonist that promotes intracellular accumulation of the second messenger 3′-5′-cyclic adenosine monophosphate (cAMP), which activates the cAMP-dependent protein kinase (PKA). This enzyme phosphorylates several proteins, including phospholamban (PLN), an endogenous SERCA inhibitor. Phosphorylation relieves PLN allosteric inhibition of SERCA, increasing the enzyme affinity for Ca2+, and thus the rate of SR Ca2+ uptake [15]. To cause partial inhibition of SERCA activity, 2,5-di-(tert-butyl)-1,4-benzohydroquinone (tBQ, 5 µM) was added to the perfusion solution 10 min before and during Ca2+ transient recording. This compound inhibits SERCA in muscle and non-muscle cells in a reversible fashion [24,25].
β-adrenoceptor activation with ISO has a well-know stimulating effect on sarcolemmal L-type Ca2+ channels [26,27], whereas tBQ was reported to inhibit the L-type Ca2+ current (ICaL) in cardiac myocytes [25]. Although these effects might complicate the analysis of SR-cytosol Ca2+ cycling when SR Ca2+ release depends on ICaL, they should not be a problem in the FISRM, in which this current is absent.
2.5. The Mathematical Model
A numerical analysis describing the FISRM framework can be introduced to the model proposed by Bondarenko et al. [16], which reproduces many aspects of cardiomyocyte electrophysiology and Ca2+ dynamics. Briefly, the main intracellular Ca2+ fluxes modelled are: (a) Ca2+ uptake from the cytosol into the network SR (Jup); (b) Ca2+ release from junctional SR (Jrel), in addition to SR Ca2+ leak (Jleak); and (c) Ca2+ transfer between network and junctional SR (Jtr) and between the subsarcolemmal space and the bulk myoplasm (Jxfer). Ca2+ buffering by troponin C and calmodulin (cytosol) and by calsequestrin (intra-SR) is also considered. Mathematically, Jup and Jrel, the most relevant fluxes in FISRM, are given by:
where Vmax and Km,up are, respectively, the maximum velocity and the Michaelis–Menten constant for Ca2+ uptake by SERCA; v is the maximum total RyR permeability; PO1 and PO2 are the RyR fractions in the O1 and O2 (open) states; [Ca2+]JSR and [Ca2+]SS are the [Ca2+] in the junctional SR and subsarcolemmal space, respectively; and PRyR is the RyR modulation factor for SR Ca2+ release.
The state equations that rule RyR behavior in this model are based on the proposal of Keizer and Levine [28] modified by Jafri et al. [29]. In short, two different channel open and closed states are considered, with transition rates ( and ) that may or may not depend on [Ca2+]SS, as illustrated in the schematic transition diagram for RyR (Figure 1). RyR Ca2+ binding sites that regulate channel opening act as [Ca2+]SS sensors.
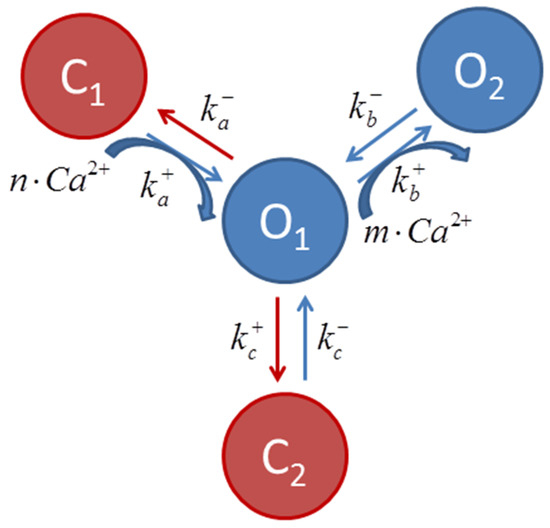
Figure 1.
Scheme describing transition among ryanodine receptor states, where and are transition rates, and m and n are the cooperativity parameters of the Ca2+ binding determined experimentally [28]. The transitions C1 ↔ O1 ↔ O2 (curved blue arrows) are Ca2+ dependent. It is considered that most channels are in the C1 state at diastolic [Ca2+]i.
In the present simulation, caffeine was assumed to diffuse passively across the sarcolemma with a first order dynamics and to modulate and rates, favoring RyR transition to the open states (Figure 1). In this case, the temporal evolution of the modulation factor Gcaff(t) can be described by:
where is defined as 1 and 150 in the absence and presence of caffeine, respectively (values chosen empirically); τcaff (time constant of increase in intracellular caffeine concentration) was considered as 50 ms, based on experimental measurements [30]. The and rates are then redefined as:
PRYR, the RyR modulation factor proposed by Bondarenko et al. [16], was adjusted for better simulation of the experimental Ca2+ transients triggered by caffeine. Here, PRYR(t) also assumed a first order dynamic behavior given by:
In this case, by setting and ms in the absence of caffeine, and and during the first 20 ms of the caffeine pulse, a bell-shaped curve for with duration similar to of the whole caffeine pulse is obtained, in analogy to that obtained by Bondarenko et al. [16] under electrical pacing. During exposure to ISO and tBQ, was adjusted to 0.15 and 0.40, respectively. For more details, see the Supplementary Materials, in which a schematic representation of the mathematical FISRM is shown (Figure S1).
2.6. Data and Statistical Analysys
The analyzed variables of twitch contractions/transients (evoked by electrical stimulation) and of caffeine evoked contractions/transients were: (a) the mean amplitude of contractions (ΔL) and of Ca2+ transients (Δ[Ca2+]i, i.e., the difference between the peak and previous diastolic [Ca2+]i values); (b) the half-time (t0.5) values of mechanical relaxation and [Ca2+]i decline, measured in 4–5 successive similar waveforms; and (c) the SR Ca2+ content. Data are presented as the means and respective standard deviations. The variables were compared with Student’s t-test for paired samples, and the statistical significance was set as p < 0.05.
3. Results
3.1. Caffeine Pulses
Figure 2 shows typical Ca2+ transients evoked by a train of brief caffeine pulses applied in the absence of extracellular Na+ and Ca2+, thus defining the FISR experimental paradigm of Ca2+ cycling. The fast rise in [Ca2+]i indicates that caffeine is able to quickly cross the sarcolemma and induce Ca2+ release from the SR. The full recovery of interpulse diastolic [Ca2+]i levels indicates that, under these experimental conditions, caffeine quickly diffuses out of the cell during its washout. A gradual decrease in the transient peak was often observed after a few pulses, and, for this reason, further measurements and comparisons were carried out only up to the 4–5th pulse.
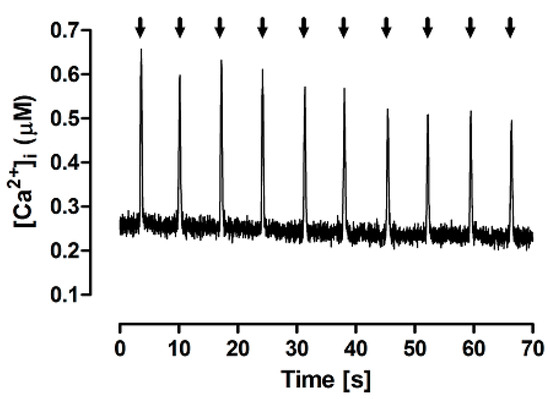
Figure 2.
Typical Ca2+ transients evoked by brief (60 ms) caffeine pulses (arrows) in the experimental FISRM.
A particularly important assumption of the FISR approach is the maintenance of stable SR Ca2+ load over successive caffeine pulses. Figure 3 illustrates the experiment performed to test this premise. Initially, myocytes were electrically stimulated until stabilization of transients. Perfusion was then switched to Ty00 and, ~45 s later, sustained perfusion with Caf00 was applied to evoke complete release of the Ca2+ stored in the SR, so that the control SR Ca2+ content could be estimated (panel A). The experiment was repeated, but five brief caffeine pulses were applied before sustained Caf00 application (panel B). In a set of seven independent experiments, the amount of Ca2+ stored in the SR after caffeine pulses (83.2 ± 17.6 µM) was not significantly different for the control value (86.6 ± 13.1 µM; p = 0.55). This confirms that application of five repeated caffeine pulses does not result in significant SR Ca2+ loss, thus allowing characterizing the model, under these conditions, as a conservative system.
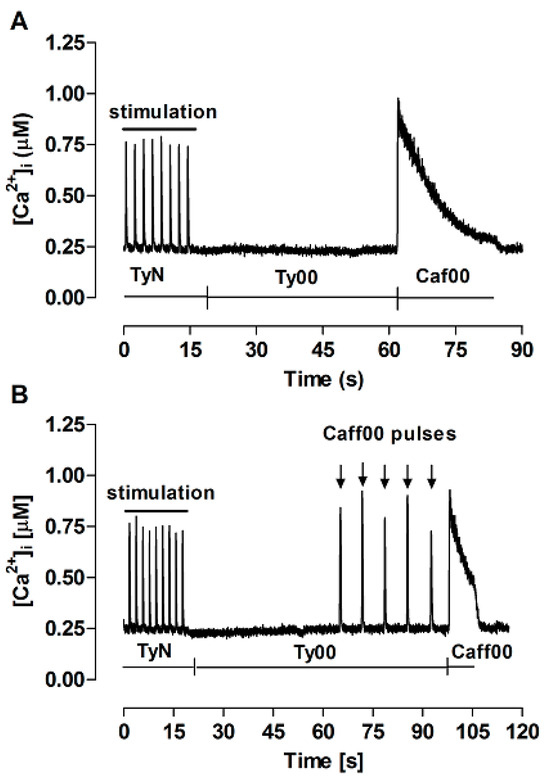
Figure 3.
Ca2+ transients recorded for estimation of the SR Ca2+ content. TyN: perfusion with modified Tyrode’s solution, during which twitch transients were electrically evoked at 0.5 Hz; Ty00: perfusion with Ca2+, Na+-free Tyrode’s solution; Caff00: perfusion with Ty00 containing 10 mM caffeine sustained for at least 10 s, not preceded by (A) or following brief Caf00 pulses (downward arrows) (B).
The FISRM mathematical formulation was able to reproduce the behavior of the experimental model, as shown in Figure 4. In the presence of Ty00 solution (in which [Na+]o and [Ca2+]o were decreased from 140 and 1.8 mM, respectively, to ~1 µM) and absence of electrical activity, caffeine pulses were able to evoke Ca2+ transients a little larger than those evoked by electrical stimulation in both models, possibly due to greater RyR activation by the compound than by ICaL and/or lack of NCX-mediated Ca2+ efflux. A small, but progressive decrease in the amplitude of the transients was observed, as seen in the experimental model.
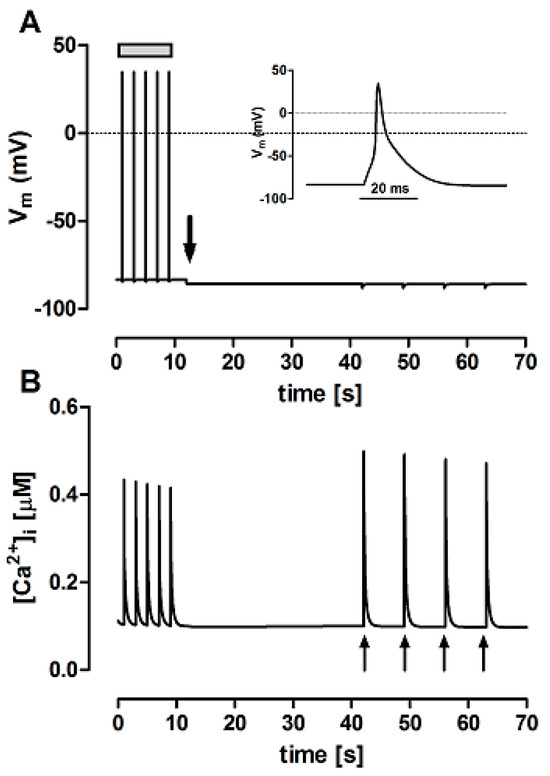
Figure 4.
Simultaneous variations in membrane potential (Vm, panel (A) and cytosolic Ca2+ concentration ([Ca2+]i, panel (B) simulated with the theoretical FISRM. Electrical stimulation (grey bar in (A) induced Ca2+ transients triggered by action potentials, one of which is shown on an expanded scale as the inset. After switching perfusion to Ty00 solution (downward arrow in (A)), electric diastole ensued, but Ca2+ transients could be evoked by application of caffeine pulses (upward arrows in (B)).
3.2. Effects of β-Adrenergic Stimulation by ISO
SERCA activity is a key target of the β-adrenoceptor-cAMP-PKA signaling cascade in the generation of positive inotropic (increase in contraction amplitude) and lusitropic (acceleration of relaxation) effects in the myocardium. As can be seen in Table 1 and Figure 5, an ISO concentration as low as 10 nM could nearly double the twitch Δ[Ca2+]i and ΔL, and decrease by ~25% the t0.5 of [Ca2+]i decline and relaxation. ISO also produced a small, but significant increase in SR Ca2+ load. These results are consistent with previous observations from this lab (e.g., [17,18] and with the well-known increase in ICaL and in SERCA activity by stimulation of myocardial β-adrenoceptors (e.g., [27,31]).

Table 1.
Effect of 10 nM isoproterenol (ISO) on the amplitude of electrically stimulated [Ca2+]i transients (Δ[Ca2+]i) and respective twitch contractions (ΔL, systolic cell shortening, expressed as percent of the resting cell length, RCL), on the decay half-time (t0.5) of both waveform types, and on the SR Ca2+ content ([Ca2+]SR, expressed as µM., i.e., µmoles Ca2+ per liter of non-mitochondrial cell water). Data are shown as the mean ± standard deviation of the mean (N = 7). p values: before (control) vs. after ISO, Student’s t-test for paired samples.
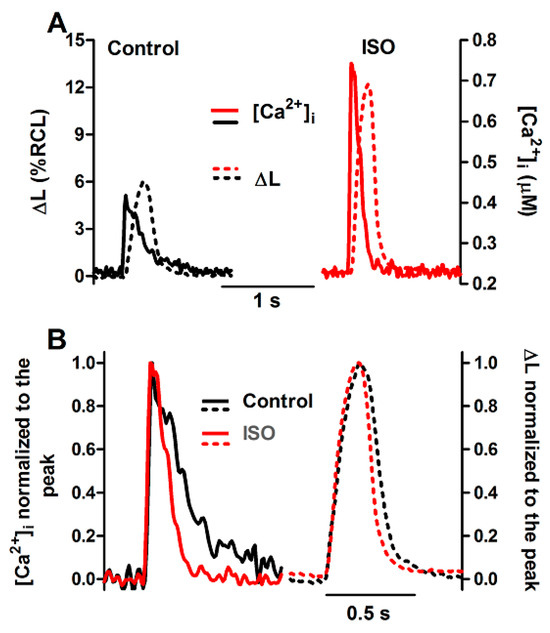
Figure 5.
Ca2+ transients and twitch contractions recorded simultaneously at 0.5 Hz from a rat ventricular cardiomyocyte before (control) and after addition of isoproterenol (ISO, 10 nM). (A): Systolic cell shortening (ΔL, expressed as percent of resting cell length, RCL) and cytosolic Ca2+ concentration ([Ca2+]i). (B): the same traces after normalization to the respective peak value.
Nevertheless, the amplitude of caffeine-triggered Ca2+ transients was not changed by ISO, although this agent could produce a significant positive lusitropic effect, reducing the t0.5 of [Ca2+]i decline by a similar extent as in the twitch (~25%; Table 2 and Figure 6).

Table 2.
Effects of isoproterenol (ISO, 10 nM) and 2,5-di-(tert-butyl)-1,4-benzohydroquinone (tBQ, 5 µM) on the amplitude (Δ[Ca2+]i) and half-time of decline (t0.5 [Ca2+]i) of Ca2+ transients elicited by brief caffeine pulses. Data are shown as in Table 1.
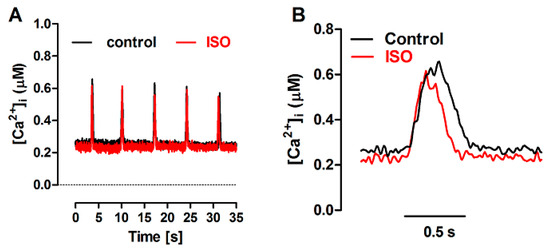
Figure 6.
(A): Ca2+ transients evoked by caffeine pulses in the experimental FISRM before (control) and during exposure to 10 nM isoproterenol (ISO). (B): Single caffeine transients in an expanded timescale.
The action of ISO was simulated in the mathematical model as a 40% increase in the permeability of the sarcolemmal Ca2+ L-type channels (PCaL), associated with a 35% decrease in the SERCA Km [8], leaving unaltered the RyR parameters. The latter change simulated the relief of SERCA inhibition due to phopholamban phosphorylation. As shown in Figure 7, the mathematical FISRM reproduced the marked increase observed experimentally in Δ[Ca2+]i of twitches, but not of caffeine-evoked transients. [Ca2+]i decline in twitches and caffeine transients was also faster, although in the latter it was accelerated in the later phase of the decay, rather than at the t0.5 level, as in the experiments. Nevertheless, in both experiment and simulation, a 25–30% decrease in the total duration of [Ca2+]i decline was observed.
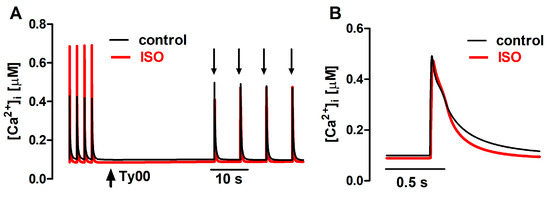
Figure 7.
(A): Ca2+ transients generated with the mathematical FISRM, evoked by electrical stimulation and by caffeine pulses (downward arrows) in the absence and presence of isoproterenol (ISO). (B): Single caffeine-induced transients in an expanded timescale.
3.3. Effects of SERCA Inhibition by tBQ
The effects of partial SERCA inhibition with 5 µM tBQ were also examined in paced cells and in the FISRM. The compound exerted effects opposite to those of ISO: a negative inotropic effect, which was rather variable in magnitude among cells, resulting marginally or not statistically significant. But a stronger negative lusitropic effect was observed for both contraction and Ca2+ transients, in addition to a significant loss of the SR Ca2+ load (Table 3). Electrically evoked twitch Ca2+ transients and contractions recorded before and during exposure to tBQ are shown in Figure 8. In the experimental FISRM, tBQ depressed the amplitude of caffeine-triggered Ca2+ transients, and, as observed for twitches, prolonged [Ca2+]i decline (Table 2; Figure 9).

Table 3.
Effect of 5 μM 2,5-di-(tert-butyl)-1,4-benzohydroquinone (tBQ) on the amplitude of electrically stimulated [Ca2+]i transients and respective twitch contractions. Data are presented as in Table 1.
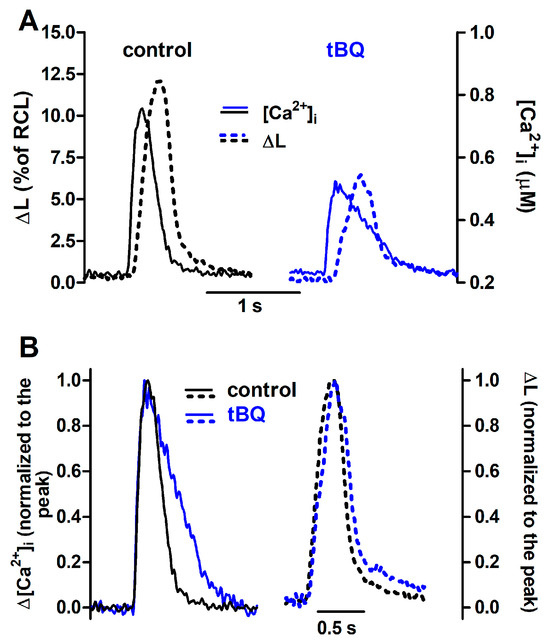
Figure 8.
Ca2+ transients and twitch contractions recorded simultaneously from a rat ventricular cardiomyocyte before (control) and after addition of 2,5-di-(tert-butyl)-1,4-benzohydroquinone (tBQ, 5 μM). (A): Systolic cell shortening (ΔL, expressed as percent of resting cell length, RCL) and cytosolic Ca2+ concentration ([Ca2+]i). (B): the same traces after normalization to the respective peak value.
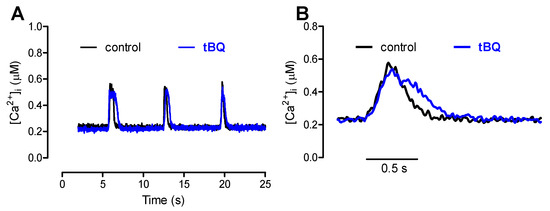
Figure 9.
(A): Ca2+ transients evoked by brief caffeine pulses in the experimental FISRM before (control) and during exposure to 5 μM 2,5-di-(tert-butyl)-1,4-benzohydroquinone (tBQ). (B): Single caffeine-induced transients in an expanded timescale.
The action of tBQ was simulated in the mathematical model as a 40% decrease in the maximal velocity of Ca2+ uptake by SERCA (Vmax). This change decreased Δ[Ca2+]i by ~40% and doubled the t0.5 for [Ca2+]i decay of twitches, effects that were qualitatively similar to those seen experimentally, although of greater magnitude. Amplitude decrease and lengthening were also observed for simulated caffeine-evoked transients, but changes were less pronounced than those in twitches (Figure 10). Additionally, prolongation of [Ca2+]i decay at 50% decline was smaller than that seen experimentally, but comparable at 90% decay.
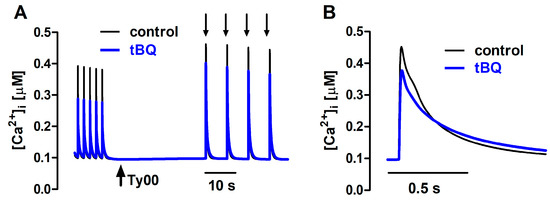
Figure 10.
(A) Ca2+ transients generated with the mathematical FISRM before (control) and after exposure to 5 μM 2,5-di-(tert-butyl)-1,4-benzohydroquinone (tBQ), presented as described in Figure 7. (B) Single caffeine-induced transients in an expanded timescale.
4. Discussion and Conclusions
The present study proposes the FISRM, devised to study the bidirectional Ca2+ transport between the cytosol and the SR in intact, contracting, isolated myocytes. The condition of SR functional isolation can be easily reversed by simply switching perfusion to a physiological solution containing Na+ and Ca2+, which restores the cell ability to develop electrical activity and the transsarcolemmal Ca2+ transport. In this model, SR Ca2+ release is achieved by brief, local application of caffeine that evokes concerted RyR opening, resulting in a robust, phasic Ca2+ transient and a subsequent contraction not dissimilar in shape to those evoked by electric stimulation.
Short caffeine pulses were also used by Su et al. [30] to compare the SR ability to take up Ca2+ in cardiomyocytes from different species. However, cells were internally perfused, which may affect cytosolic composition and cell volume, in contrast with the present experimental model, in which cell membrane is intact and the cytoplasmic environment is left undisturbed. Additionally, caffeine pulses were briefer (60 vs. 100 ms in [30]), which allows faster caffeine washout and less interference of prolonged Ca2+ release on the transient decay.
It was possible to confirm experimentally the important assumption that Ca2+ cycling was largely conservative in the FISRM, as the SR Ca2+ content was not significantly changed by a short series of successive caffeine-induced Ca2+ release events. This supports the notion that cytosolic Ca2+ removal can be attributable majorly to SERCA, with negligible contribution of the transporters not inhibited in this model (PMCA and MCU). These transporters seem to play a minor role in the Ca2+ transient decay in cardiomyocytes [5]. Also, changes in twitch Ca2+ transients and in SR Ca2+ content in mature cardiomyocytes were not observed after pharmacological PMCA inhibition [32]. Additionally, twitch Ca2+ transients under basal conditions are not affected by PMCA [33] or MCU knockout [34]. Simulation of Ca2+ transients with the mathematical FISRM closely reproduced the experimental results, which supports the assumption that the activity of only two Ca2+ transport paths (i.e., RyR and SERCA) is sufficient to account for the Ca2+ fluxes involved during the first caffeine-induced transients.
Nevertheless, one of the limitations of the experimental FISRM is that the amplitude of Ca2+ transients decays over repeated caffeine applications. Partial SR Ca2+ depletion due to cumulative removal of cytosolic Ca2+ by PMCA and MCU might be involved in this decay. The observation that a smaller progressive decline in the transient amplitude also in the mathematical FISRM, which included the Ca2+ flux via PMCA but not via MCU, lends support to this possibility, making it unlikely that changes in RyR sensitivity are involved. Nevertheless, results showed that the transient features and the SR Ca2+ load are largely preserved during the first five caffeine pulses, thus delimiting the reliable range of pulses.
SERCA stimulation and inhibition by ISO and tBQ, respectively, showed to result in opposite changes in twitch amplitude and in decline timecourse, as expected. ISO mimicks the β-adrenergic stimulation seen during sympathetic activation seen during exercise and the fight-or-flight (acute stress) response. In cardiomyocytes, β-adrenoceptor activation accelerates [Ca2+]i decay via SERCA stimulation and increases transient amplitude by augmenting L-type Ca2+ channel activity, increasing the SR Ca2+ content and, depending on the stimulation level, sensitizing RyR to Ca2+ (e.g., [27,31]. Enhancing both Ca2+ influx and the SR Ca2+ store contributes to increase the fraction of the SR Ca2+ load that is released at a twitch [6]. Simulation of ISO action was achieved by increasing both L-type Ca2+ channel permeability and SERCA affinity for Ca2+, resulting in accelerated [Ca2+]i decline in both types of contraction, but enhanced Δ[Ca2+]i only in electrically evoked twitches, in which greater Ca2+ current (absent in the FISR model) can trigger greater release of SR Ca2+ [6,35]. However, one might expect that increase in the fractional SR Ca2+ release (because of higher Ca2+ content) and/or RyR phosphorylation would lead to greater Ca2+ release and transient amplitude [6,8,33,36]. It is possible that the magnitude of these changes was insufficient to increase caffeine-induced Δ[Ca2+]i content release at this low, more physiological level of adrenergic stimulation.
As in the FISRM, Ca2+ influx via L-type channels is absent due to the removal of extracellular Ca2+, it is possible to isolate the effects of β-adrenergic stimulation on the SR function to better characterize, for instance, the role of RyR phosphorylation by different protein kinases at diverse sites in the modulation of systolic and diastolic SR Ca2+ release in intact cells from hearts under healthy and pathophysiological conditions [36,37,38], as well as in the search for therapies [26,39].
SERCA inhibition by tBQ, on the other hand, was simulated by diminishing the maximal Ca2+ uptake capacity, equivalent to a decrease in the number of active enzyme molecules, based on the observation that tBQ binds to the ATPase, forming a dead-end complex with interruption of the Ca2+ transport cycling [24,40]. In the experimental model, tBQ effects were on average stronger on amplitude of electrically stimulated twitches than of caffeine-induced transients, which was not reproduced by the simulations, in which a milder and comparable depression of Δ[Ca2+]i was observed in both kinds of transient. It is likely that these discrepancies are related to the inhibition of L-type Ca2+ current by this agent at concentrations required for SERCA inhibition [25], which was eliminated by extracellular Ca2+ removal and not included in the computational model as one of the mechanisms affected by tBQ.
Regarding the [Ca2+]i decline before and after treatment with ISO and tBQ, although able to simulate the qualitative effects of ISO and tBQ observed experimentally (i.e., acceleration and prolongation, respectively), the computational FISRM could not replicate the decay timecourse, showing a stronger effect in the late phases of the transient decay. This might be explained by the formulation of passive cytosolic Ca2+ buffers solely at equilibrium in the model of Bondarenko et al. [16], when the existence of fast buffers has been reported in cardiomyocytes [41]. Another possibility is the appearance of biphasic [Ca2+]i decline in the presence of agents that promote SR Ca2+ leak, such as ISO [42]. Curiously, tBQ has been reported to also transiently stimulate SR Ca2+ leak in skeletal muscle [43]. Thus, more studies should be performed to improve the models, and to explore the regulation of mechanisms involved in Ca2+ release.
In conclusion, simulations closely reproduced the experimental results, in agreement with the assumptions of the model. Thus, the proposed hybrid FISRM seems to represent a valuable tool for accessing and investigating the SR function in intact cardiac myocytes in pathophysiological conditions, and in the development and test of pharmacological therapies targeting the SR. Nevertheless, the use of this experimental model might also be extended to other cell types (e.g., skeletal and smooth muscle) to investigate Ca2+ mobilization from the sarco/endoplasmic reticulum by RyR, without the interference of transsarcolemmal Ca2+ fluxes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/bioengineering12060627/s1.
Author Contributions
Conceptualization, D.C.S., J.W.M.B. and R.A.B.; methodology, D.C.S., J.W.M.B. and R.A.B.; computational modeling, D.C.S.; experiment conduction, D.C.S.; data analysis and interpretation, D.C.S. and R.A.B.; writing, R.A.B. and D.C.S.; supervision, J.W.M.B. and R.A.B.; funding acquisition, J.W.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by CNPq (Brasil) No. 304087/2022-0 and No. 313970/2023-8; Brazilian Health Ministry/FINEP (Conv. 0113021400, ref. 0441/12); FAPESP (Proc. N. 05/52601-1).
Institutional Review Board Statement
The animal study protocol was approved by the institutional Committee for Ethics in Animal Research (protocol No. 775-1 and 952-1).
Informed Consent Statement
Not applicable.
Data Availability Statement
A link for the source-code of the mathematical FISRM is available in the Supplementary Materials. The original contributions presented in this study are included in this article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
Authors are indebted to Elizângela S. Oliveira, Sérgio Moura and Mauro Martinazo for the invaluable technical support.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study, in the collection, analyses, or interpretation of data; in the writing of this manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| [Ca2+]i | Cytosolic free Ca2+ concentration |
| cAMP | 3’,5’-cyclyc adenosine monophosphate |
| Caf00 | Na+-Ca2+-free Tyrode’s solution containing caffeine |
| ECC | Excitation–contraction coupling |
| FISRM | Functionally isolated sarcoplasmic reticulum model |
| ISO | Isoproterenol |
| MCU | Mitochondrial Ca2+ uniporter |
| NCX | Na+/Ca2+ exchanger |
| PKA | cAMP-dependent protein kinase |
| PLN | Phospholamban |
| PMCA | Plasma membrane Ca2+-ATPase |
| RyR | Ryanodine receptors |
| SERCA | Sarco/endoplasmic Ca2+-ATPase |
| SR | Sarcoplasmic reticulum |
| tBQ | 2,5-di-(tert-butyl)-1,4-benzohydroquinone |
| TyN | Modified Tyrode’s solution |
| Ty00 | Na+-Ca2+-free Tyrode’s solution |
| ΔL | Contraction amplitude (cell shortening) |
| Δ[Ca2+]i | Ca2+ transient amplitude |
References
- Fabiato, A. Calcium-induced release of calcium from cardiac sarcoplasmic reticulum. J. Physiol. 1983, 245, C1–C14. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Eisner, D.A.; Caldwell, J.L.; Kistamás, K.; Trafford, A.W. Calcium and excitation-contraction coupling in the heart. Circ. Res. 2017, 121, 181–195. [Google Scholar] [CrossRef]
- Bassani, R.A.; Bassani, J.W.M.; Bers, D.M. Mitochondrial and sarcolemmal Ca transport reduce [Ca2+]i during caffeine contractures in rabbit cardiac myocytes. J. Physiol. 1992, 453, 591–608. [Google Scholar] [CrossRef]
- Bassani, J.W.M.; Bassani, R.A.; Bers, D.M. Relaxation in rabbit and rat cardiac cells: Species-dependent differences in cellular mechanism. J. Physiol. 1994, 476, 279–293. [Google Scholar] [CrossRef]
- Bassani, J.W.M.; Yuan, W.; Bers, D.M. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am. J. Physiol.-Cell Physiol. 1995, 268, C1313–C1319. [Google Scholar] [CrossRef]
- Bassani, R.A.; Altamirano, J.; Puglisi, J.L.; Bers, D.M. Action potential duration determines sarcoplasmic reticulum Ca2+ reloading in mammalian ventricular myocytes. J. Physiol. 2004, 559, 591–607. [Google Scholar] [CrossRef]
- Shannon, T.R.; Wang, F.; Bers, D.M. Regulation of cardiac sarcoplasmic reticulum Ca release by luminal [Ca] and altered gating assessed with mathematical model. Biophys. J. 2005, 89, 4096–4110. [Google Scholar] [CrossRef]
- Kho, C.; Lee, A.; Hajjar, R.J. Altered sarcoplasmic reticulum calcium cycling: Targets for heart failure therapy. Nat. Rev. Cardiol. 2012, 9, 717–733. [Google Scholar] [CrossRef]
- Mattiazzi, A.; Bassani, R.A.; Escobar, A.L.; Palomeque, J.; Valverde, C.A.; Vila Petroff, M.; Bers, D.M. Chasing cardiac physiology and pathology down the CaMKII cascade. Am. J. Physiol. Heart Circul. Physiol. 2015, 308, H1177–H1191. [Google Scholar] [CrossRef][Green Version]
- Gaido, O.E.R.; Nkashama, L.J.; Schole, K.L.; Wang, Q.; Umapathi, P.; Mesubi, O.O.; Konstantinidis, K.; Luczak, E.D.; Anderson, M.E. CaMKII as a therapeutic target in cardiovascular disease. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 249–272. [Google Scholar] [CrossRef] [PubMed]
- Bassani, R.A.; Ricardo, R.A.; Bassani, J.W.M. Estimation of the fractional sarcoplasmic reticulum Ca2+ release in intact cardiomyocytes using integrated Ca2+ fluxes. Gen. Physiol. Biophys. 2012, 31, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Zhihao, L.; Jungyu, N.; Lan, L.; Michael, S.; Rui, G.; Xiyun, B.; Xiaozhi, L.; Guanwei, F. SERCA2a: A key protein in the Ca2+ cycle of the heart failure. Heart Fail. Rev. 2020, 25, 523–535. [Google Scholar] [CrossRef]
- Kansakar, U.; Varzideh, F.; Jankauskas, S.S.; Gambardella, J.; Trimarco, B.; Santulli, G. Advances in the understanding of excitation-contraction coupling: The pulsing quest for drugs against heart failure and arrhythmias. Eur. Heart J. 2021, 7, e91–e93. [Google Scholar] [CrossRef]
- Mattiazzi, A.; Kranias, E.G. Unleashing the power of genetics: PLN ablation, phospholambanopathies and evolving challenges. Circ. Res. 2024, 134, 138–142. [Google Scholar] [CrossRef]
- Bondarenko, V.E.; Szigeti, G.P.; Bett, G.C.L.; Kim, S.-J.; Rasmusson, R.L. Computer model of action potential of mouse ventricular myocytes. Am. J. Physiol. Heart Circul. Physiol. 2004, 287, H1378–H1403. [Google Scholar] [CrossRef]
- Penna, L.B.; Bassani, R.A. Increased spontaneous activity and reduced inotropic response to catecholamines in ventricular myocytes from footshock-stressed rats. Stress. 2010, 13, 73–82. [Google Scholar] [CrossRef]
- Soriano, D.C.; Bassani, R.A.; Bassani, J.W.M. Instrumentation for measurement of intracellular Ca2+ concentration: Effects of beta-adrenergic stimulation on isolated cardiac myocytes. Braz. J. Biomed. Eng. 2007, 23, 123–130. [Google Scholar]
- Shannon, T.R.; Ginsburg, K.S.; Bers, D.M. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ. Res. 2002, 91, 594–600. [Google Scholar] [CrossRef]
- Harkins, A.B.; Kurebayashi, N.; Baylor, S.M. Resting myoplasmic calcium in frog skeletal muscle fibers estimated with fluo-3. Biophys. J. 1993, 65, 865–881. [Google Scholar] [CrossRef]
- Sitsapesan, R.; Williams, A.J. Mechanisms of caffeine activation of single calcium-release channels of sheep cardiac sarcoplasmic reticulum. J. Physiol. 1990, 423, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Bassani, R.A.; Bers, D.M. Rate of diastolic Ca release from sarcoplasmic reticulum of intact rabbit and rat ventricular myocytes. Biophys. J. 1995, 68, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Bassani, R.A.; Shannon, T.R.; Bers, D.M. Passive Ca2+ binding in ventricular myocardium of neonatal and adult rats. Cell Calcium 1998, 23, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Nakasaki, Y.; Matsuda, N.; Shigekawa, M. Inhibition of sarcoplasmic reticulum Ca2+-ATPase by 2,5-di(tert-butyl)-1,4-benzohydroquinone. J. Biochem. 1992, 112, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.; Greensmith, D.J.; Sankaranaraynan, R.; O’Neill, S.C.; Eisner, D.A. The effect of 2,5-di-(tert-butyl)-1,4-benzohydroquinone (TBQ) on intracellular Ca2+ handling in rat ventricular myocytes. Cell Calcium 2015, 58, 208–214. [Google Scholar] [CrossRef]
- Parichatikanond, W.; Duangrat, R.; Kurose, H.; Mangmool, S. Regulation of β-adrenergic receptors in the heart: A review on emerging therapeutic strategies for heart failure. Cells 2024, 13, 1674. [Google Scholar] [CrossRef]
- Yang, B.; Wang, S.; Yang, H. β-adrenergic regulation of Ca2+ signaling in heart cells. Biophys. Rep. 2024, 10, 274–282. [Google Scholar] [CrossRef]
- Keizer, J.; Levine, L. Ryanodine receptor adaptation and Ca2+-induced Ca2+ release–dependent Ca2+ oscillations. Biophys. J. 1996, 71, 3477–3487. [Google Scholar] [CrossRef]
- Jafri, M.S.; Rice, J.J.; Winslow, R.L. Cardiac Ca2+ dynamics: The roles of ryanodine receptor adaption and sarcoplasmic reticulum load. Biophys. J. 1998, 74, 1149–1169. [Google Scholar] [CrossRef]
- Su, Z.; Li, F.; Spitzer, K.W.; Yao, A.; Ritter, M.; Barry, W.H. Comparison of sarcoplasmic reticulum Ca2+-ATPase function in human, dog, rabbit, and mouse ventricular myocytes. J. Mol. Cell. Cardiol. 2003, 35, 761–767. [Google Scholar] [CrossRef]
- Ginsburg, K.S.; Weber, C.R.; Bers, D.M. Control of maximum sarcoplasmic reticulum Ca load in intact ferret ventricular myocytes: Effects of thapsigargin and isoproterenol. J. Gen. Physiol. 1998, 111, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Bassani, R.A.; Bassani, J.W.M. Higher sarcolemmal Ca-ATPase activity in neonatal rat ventricle. Biophys. J. 2002, 82, 596a. [Google Scholar]
- Wilson, C.; Stafford, N.; Zi, M.; Chelu, A.; Niort, B.C.; Li, Y.; Baudoin, F.; Prehar, S.; Trafford, A.W.; Cartwright, E.J. Cardiomyocyte-specific loss of plasma membrane calcium ATPase 1 impacts cardiac rhythm and is associated with ventricular repolarisation dysfunction. J. Mol. Cell. Cardiol. 2022, 172, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Luongo, T.S.; Lambert, J.P.; Yuan, A.; Zhang, X.; Gross, P.; Song, J.; Shanmughapriya, S.; Gao, E.; Jain, M.; Houser, S.R.; et al. The mitochondrial calcium uniporter matches energetic supply with cardiac workload during stress and modulates permeability transition. Cell Rep. 2015, 12, 23–34. [Google Scholar] [CrossRef]
- Ginsburg, K.S.; Bers, D.M. Modulation of excitation-contraction coupling by isoproterenol in cardiomyocytes with controlled SR Ca2+ load and Ca2+ current trigger. J. Physiol. 2004, 556, 463–480. [Google Scholar] [CrossRef]
- Ullrich, N.D.; Valdivia, H.H.; Niggli, E. PKA phosphorylation of cardiac ryanodine receptor modulates SR luminal Ca2+ sensitivity. J. Mol. Cell. Cardiol. 2012, 53, 33–42. [Google Scholar] [CrossRef][Green Version]
- Bovo, E.; Huke, S.; Blatter, L.A.; Zima, A.V. The effect of PKA-mediated phosphorylation of ryanodine receptor on SR Ca2+ leak in ventricular myocytes. J. Mol. Cell Cardiol. 2017, 104, 9–16. [Google Scholar] [CrossRef]
- Benitah, J.P.; Perrier, R.; Mercadier, J.J.; Pereira, L.; Gómez, A.M. RyR2 and calcium release in heart failure. Front. Physiol. 2021, 12, 734210. [Google Scholar] [CrossRef]
- Arici, M.; Hsu, S.C.; Ferrandi, M.; Barassi, P.; Ronchi, C.; Torre, E.; Luraghi, A.; Chang, G.J.; Ferrari, P.; Bianchi, G.; et al. Selective SERCA2a activator as a candidate for chronic heart failure therapy. J. Transl. Med. 2024, 22, 77. [Google Scholar] [CrossRef]
- Tadini-Buoninsegni, F.; Smeazzetto, S.; Gualdani, R.; Moncelli, M.R. Drug interactions with the Ca2+-ATPase from sarco(endo)plasmic reticulum (SERCA). Front. Mol. Biosci. 2018, 5, 36. [Google Scholar] [CrossRef]
- Berlin, J.R.; Bassani, J.W.; Bers, D.M. Intrinsic cytosolic calcium buffering properties of single rat cardiac myocytes. Biophys. J. 1994, 67, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.; Li, Y.; Greensmith, D.J.; Eisner, D.A.; Venetucci, L. Biphasic decay of the Ca transient results from increased sarcoplasmic reticulum Ca leak. J. Physiol. 2016, 594, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Dettbarn, C.; Palade, P. Effects of three sarcoplamic/endoplasmic reticulum Ca++ pump inhibitors on relase channels of intracellular stores. J. Pharmacol. Exp. Ther. 1998, 285, 739–745. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).