Experiences of People with Multiple Sclerosis in Sensor-Based Jump Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures and Procedures
2.2.1. Sensor-Based Jump Assessment
2.2.2. Patient-Reported Experience Measures (PREM) Questionnaire
2.2.3. Godin Leisure Time Exercise Questionnaire (GLTEQ)
2.2.4. Expanded Disability Status Scale
2.3. Statistical Analysis
3. Results
3.1. Participants
PREM Item Outcomes for Sensor-Based Jump Assessment
3.2. Association Between PREMs and Physiotherapy Received, Fear of Falling, and Falls in the Previous Three Weeks
3.2.1. Association Between PREMs and Clinical Outcomes
3.2.2. Association Between PREMs and Age, BMI, Sex, and Physical Activity
3.3. Association Between PREM Items and CMJ Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Physiotherapy | Fear of Falling | Falls in the Previous Three Weeks | ||||
|---|---|---|---|---|---|---|
| PREM Items | PwMS with Physiotherapy (N = 42) | PwMS without Physiotherapy (N = 106) | PwMS with Fear of Falling (N = 22) | PwMS without Fear of Falling (N = 126) | PwMS with Falls in the Previous 3 Weeks (N = 9) | PwMS Without Falls in the Previous 3 Weeks (N = 139) |
| Performance difficulty total | 3 (2–5) A | 1 (0–2) A | 2 (1–6) B | 1 (0–3) B | 2 (1–4) | 2 (0–3) |
| Self-use of results | 2 (1–5) | 2 (0–5) | 3 (1–5) | 2 (0–5) | 2 (1–3) C | 5 (4–6) C |
| Usefulness of the results | 1 (0–1) A | 0 (0–1) A | 0 (0–1) | 0 (0–2) | 1 (0–1) | 0 (0–2) |
| Integration of results in therapy | 1 (0–1) A | 0 (0–1) A | 0.5 (0–2) | 0 (0–1) | 0 (0–1) | 0 (0–2) |
| Acceptance required time | 0 (0–0) | 0 (0–1) | 0 (0–1) | 0 (0–0) | 0 (0–0) | 0 (0–0.75) |
| Strain total | 4 (2–6) A | 2 (1–3) A | 5 (4–6) B | 2 (1–3) B | 3 (0–4) | 3 (1–4) |
| Safety | 3 (2–5) A | 1 (0–3) A | 5 (4–6) B | 1 (0–3) B | 3 (1–5) | 2 (0–3) |
| Comfort | 1 (0–2) A | 1 (0–2) A | 2 (1–3) B | 1 (0–2) B | 0 (0–1) | 1 (0–2) |
| Staff support performance | 0 (0–0) | 0 (0–0) | 1 (1–2) B | 0 (0–0) B | 0 (0–0) | 0 (0–0) |
| Rating of implementation frequency * | 5 (5–10) | 5 (5–5) | 5 (5–5) | 5 (5–5) | 7.5 (5–10) C | 5 (5–5) C |
| PREM | Sex | Age | EDSS | Physical Activity | BMI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female (N = 115) | Male (N = 42) | Young (18–30 Years) (N = 15) | Middle-Age (31–49 Years) (N = 113) | Old (50–65 Years) (N = 29) | EDSS 0–1.5 (N = 67) | EDSS 2–3 (N = 60) | EDSS 3.5–5.5 (N = 30) | Active (N = 111) | Moderate (N = 18) | Insufficient (N = 25) | Normal Weight (18–25) (N = 93) | Overweight (25–30) (N = 40) | Obesity (30+) (N = 24) | |
| Performance difficulty total | 2 (1–3) | 1 (1–2) | 2 (1–2) | 1 (0–2) | 2 (1–5) | 2 (1–3) | 1 (1–3) | 2 (1–5) | 1 (0–3) | 2 (1–3) | 2 (0–3) | 1 (0–3) | 2 (1–3) | 2 (0–3) |
| 10SHT | 2 (1–3) | 2 (0–3) | 2 (1–2) | 2 (1–3) | 3 (1–5) | 1 (0.75–2) | 2 (0–3) | 3 (1–6) | 2 (0–4) | 2 (1–5) | 2 (1–3) | 2 (0–3) | 2 (1–4) | 1 (0–3) |
| CMJ | 2 (1–3) | 1 (0–2) | 2 (1–2) | 2 (1–3) | 2 (0–5) | 1 (0–2) | 1 (0–3) | 3 (1–5) | 2 (0–3) | 1 (1–2) | 1 (0–3) | 1 (0–3) | 2 (0.5–3) | 1 (0–3) |
| SLCMJ | 4 (2–6) | 3 (1–4.5) | 4 (2–6) | 2 (1–5) | 4 (1–8) | 3 (1–5) | 3 (2–5) | 5 (1.5–8) | 5 (3–6) | 3 (1.5–5.5) | 3 (2–5) | 3 (1.5–5) | 3 (2–6) | 5 (2–7) |
| Self-use of results | 2 (0–5) | 2 (0–4) | 3 (1–3) | 2 (1–3) | 2 (1–3) | 2 (0.75–5) | 2 (0–4) | 4 (0–2) | 2 (0–4.5) | 2.5 (0–5) | 2 (0–5) | 2 (0–4) | 2 (0–5) | 3 (1–7) |
| Usefulness of the results | 0 (0–2) | 0 (0–1) | 1 (0–4) | 0 (0–1) | 3 (1–5) | 0 (0–2) | 0 (0–1) | 0 (0–1) | 0 (0–2) | 0.5 (0–2) | 0 (0–1) | 0 (0–1) | 0 (0–2) | 0 (0–1.5) |
| Integration of results in therapy | 0 (0–2) | 0 (0–1) | 1 (0–4) | 0 (0–2) | 1 (1–3) | 0 (0–2) | 0 (0–1) | 0 (0–2) | 0 (0–1) | 0 (0–2) | 0 (0–2) | 0 (0–1) | 0 (0–1) | 0 (0–2) |
| Acceptance required time | 0 (0–1) | 0 (0–0) | 3 (1–4) | 2 (1–3) | 3 (1–5) | 0 (0–0) | 0 (0–1) | 0 (0–1) | 0 (0–0) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–0.5) | 0 (0–0) |
| Strain total | 3 (1–5) | 2 (0–3) | 2 (1–3) | 2 (1–2) | 3 (1–5) | 2 (1–3) | 2 (1–4) | 3 (2–6) | 3 (1–5) | 2 (1–5) | 3 (1–4) | 2 (1–3) | 3 (1–4) | 3 (2–5) |
| 10SHT | 2 (1–4) | 1 (0–3) | 1 (0–2) | 1 (0–2) | 4 (3–5.75) | 1 (1–3) | 2 (1–4) | 4 (2–6) | 3 (1–4) | 2.5 (1–6) | 2 (1–4) | 2 (1–4) | 2 (1–5) | 2 (1–5) |
| CMJ | 2 (1–4) | 1 (0–2) | 2 (1–2) | 2 (1–3) | 5 (3–6.75) | 2 (1–3) | 2 (1–3) | 4 (2–6) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 3 (1–5) |
| SLCMJ | 4 (2–6) | 3 (1–4) | 3 (2–5) | 3 (2–4) | 5 (3–7) | 3 (1–4) | 4 (2–6) | 6 (3–8) | 5 (3–6) | 3 (2–4) | 3 (2–5) | 3 (2–5) | 3 (2–5) | 4 (2–6) |
| Safety jump assessment | 2 (1–4) | 1 (0–2) | 2 (1–3) | 1 (0.25–3) | 3 (0.5–5) | 1 (0–3) | 1 (0–3) | 4 (2–6) | 3 (1–4) | 2 (1–4) | 1 (0–3) | 1 (0–3) | 2 (1–3) | 2 (1–5) |
| Comfort jump assessment | 1 (0–2) | 0 (0–1) | 1 (0–1) | 1 (0.25–3) | 0.5 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0 (0–2) | 0 (0–2) | 1 (0–2) | 1 (0–2) |
| Staff support performance | 0 (0–0) | 0 (0–0) | 0 (0–0) | 2 (0–3) | 0 (0–3) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Rating of implementation frequency * | 5 (5–5) | 5 (5–5) | 5 (0–5) | 5 (0–5) | 5 (0–5) | 5 (5–5) | 5 (5–5) | 5 (5–5) | 5 (5–5) | 5 (5–5) | 5 (5–5) | 5 (5–5) | 5 (5–5) | 5 (5–5) |
References
- Goldenberg, M.M. Multiple Sclerosis Review. Pharm. Ther. 2012, 37, 175–184. [Google Scholar]
- Jørgensen, M.L.K.; Dalgas, U.; Wens, I.; Hvid, L.G. Muscle Strength and Power in Persons with Multiple Sclerosis–A Systematic Review and Meta-Analysis. J. Neurol. Sci. 2017, 376, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Stagsted, R.A.W.; Ramari, C.; Skjerbaek, A.G.; Thrue, C.; Dalgas, U.; Hvid, L.G. Lower Extremity Muscle Power–A Critical Determinant of Physical Function in Aging and Multiple Sclerosis. Exp. Gerontol. 2021, 150, 111347. [Google Scholar] [CrossRef] [PubMed]
- Geßner, A.; Stölzer-Hutsch, H.; Trentzsch, K.; Schriefer, D.; Ziemssen, T. Countermovement Jumps Detect Subtle Motor Deficits in People with Multiple Sclerosis below the Clinical Threshold. Biomedicines 2023, 11, 774. [Google Scholar] [CrossRef]
- Jette, A.M. Toward a Common Language for Function, Disability, and Health. Phys. Ther. 2006, 86, 726–734. [Google Scholar] [CrossRef]
- Ramari, C.; Hvid, L.G.; de David, A.C.; Dalgas, U. The Importance of Lower-Extremity Muscle Strength for Lower-Limb Functional Capacity in Multiple Sclerosis: Systematic Review. Ann. Phys. Rehabil. Med. 2020, 63, 123–137. [Google Scholar] [CrossRef]
- Dillenseger, A.; Weidemann, M.L.; Trentzsch, K.; Inojosa, H.; Haase, R.; Schriefer, D.; Voigt, I.; Scholz, M.; Akgün, K.; Ziemssen, T. Digital Biomarkers in Multiple Sclerosis. Brain Sci. 2021, 11, 1519. [Google Scholar] [CrossRef]
- Voigt, I.; Inojosa, H.; Wenk, J.; Akgün, K.; Ziemssen, T. Building a Monitoring Matrix for the Management of Multiple Sclerosis. Autoimmun. Rev. 2023, 22, 103358. [Google Scholar] [CrossRef]
- Voigt, I.; Inojosa, H.; Dillenseger, A.; Haase, R.; Akgün, K.; Ziemssen, T. Digital Twins for Multiple Sclerosis. Front. Immunol. 2021, 12, 669811. [Google Scholar] [CrossRef]
- Inojosa, H.; Schriefer, D.; Ziemssen, T. Clinical Outcome Measures in Multiple Sclerosis: A Review. Autoimmun. Rev. 2020, 19, 102512. [Google Scholar] [CrossRef]
- Ziemssen, T.; Kern, R.; Thomas, K. Multiple Sclerosis: Clinical Profiling and Data Collection as Prerequisite for Personalized Medicine Approach. BMC Neurol. 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, M.C.; Downer, M.B.; Holloway, B.J.; Wallack, E.M.; Lockyer, E.J.; Buckle, N.C.M.; Abbott, C.L.; Ploughman, M. Bipedal Hopping Reveals Evidence of Advanced Neuromuscular Aging Among People With Mild Multiple Sclerosis. J. Mot. Behav. 2017, 49, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, M.C.; Wadden, K.P.; Ploughman, M. Bipedal Hopping as a New Measure to Detect Subtle Sensorimotor Impairment in People with Multiple Sclerosis. Disabil. Rehabil. 2020, 44, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Geßner, A.; Hartmann, M.; Trentzsch, K.; Stölzer-Hutsch, H.; Schriefer, D.; Ziemssen, T. The Association of Age, Sex, and BMI on Lower Limb Neuromuscular and Muscle Mechanical Function in People with Multiple Sclerosis. Biomedicines 2024, 12, 971. [Google Scholar] [CrossRef]
- Geßner, A.; Hartmann, M.; Vágó, A.; Trentzsch, K.; Schriefer, D.; Mehrholz, J.; Ziemssen, T. Sensitive Identification of Asymmetries and Neuromuscular Deficits in Lower Limb Function in Early Multiple Sclerosis. Neurorehabil. Neural Repair 2024, 38, 1–12. [Google Scholar] [CrossRef]
- Haase, R.; Kullmann, J.S.; Ziemssen, T. Therapy Satisfaction and Adherence in Patients with Relapsing-Remitting Multiple Sclerosis: The THEPA-MS Survey. Ther. Adv. Neurol. Disord. 2016, 9, 250–263. [Google Scholar] [CrossRef]
- Becker, V.; Heeschen, V.; Schuh, K.; Schieb, H.; Ziemssen, T. Patient Satisfaction and Healthcare Services in Specialized Multiple Sclerosis Centres in Germany. Ther. Adv. Neurol. Disord. 2018, 11, 1756285617748845. [Google Scholar] [CrossRef]
- Bull, C.; Byrnes, J.; Hettiarachchi, R.; Downes, M. A Systematic Review of the Validity and Reliability of Patient-Reported Experience Measures. Health Serv. Res. 2019, 54, 1023–1035. [Google Scholar] [CrossRef]
- Sutliff, M.H.; Naft, J.M.; Stough, D.K.; Lee, J.C.; Arrigain, S.S.; Bethoux, F.A. Efficacy and Safety of a Hip Flexion Assist Orthosis in Ambulatory Multiple Sclerosis Patients. Arch. Phys. Med. Rehabil. 2008, 89, 1611–1617. [Google Scholar] [CrossRef]
- Midaglia, L.; Mulero, P.; Montalban, X.; Graves, J.; Hauser, S.L.; Julian, L.; Baker, M.; Schadrack, J.; Gossens, C.; Scotland, A.; et al. Adherence and Satisfaction of Smartphone- and Smartwatch-Based Remote Active Testing and Passive Monitoring in People With Multiple Sclerosis: Nonrandomized Interventional Feasibility Study. J. Med. Internet Res. 2019, 21, e14863. [Google Scholar] [CrossRef]
- Thotam, S.M.; Buhse, M. Patient Satisfaction with Physicians and Nurse Practitioners in Multiple Sclerosis Centers. Int. J. MS Care 2020, 22, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Sikes, E.M.; Richardson, E.V.; Cederberg, K.J.; Sasaki, J.E.; Sandroff, B.M.; Motl, R.W. Use of the Godin Leisure-Time Exercise Questionnaire in Multiple Sclerosis Research: A Comprehensive Narrative Review. Disabil. Rehabil. 2019, 41, 1243–1267. [Google Scholar] [CrossRef] [PubMed]
- Motl, R.W.; Bollaert, R.E.; Sandroff, B.M. Validation of the Godin Leisure-Time Exercise Questionnaire Classification Coding System Using Accelerometry in Multiple Sclerosis. Rehabil. Psychol. 2018, 63, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Godin, G. The Godin-Shephard Leisure-Time Physical Activity Questionnaire. Heal. Fit. J. Can. 2011, 4, 18–22. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating Neurologic Impairment in Multiple Sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- van Munster, C.E.P.; Uitdehaag, B.M.J. Outcome Measures in Clinical Trials for Multiple Sclerosis. CNS Drugs 2017, 31, 217–236. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Science; Routledge: New York, NY, USA, 1988; p. 567. [Google Scholar] [CrossRef]
- Bolte, J.; Dilger, E.-M.; Brütt, A.L. Guideline-recommended standardized instruments in multiple sclerosis: A comparative content analysis based on the International Classification of Functioning, Disability and Health (ICF). Nervenarzt 2024, 95, 1116–1123. [Google Scholar] [CrossRef]
- Van Hooren, B.; Zolotarjova, J. The Difference Between Countermovement and Squat Jump Performances: A Review of Underlying Mechanisms with Practical Applications. J. Strength Cond. Res. 2017, 31, 2011–2020. [Google Scholar] [CrossRef]
- Seiberl, W.; Hahn, D.; Power, G.A.; Fletcher, J.R.; Siebert, T. Editorial: The Stretch-Shortening Cycle of Active Muscle and Muscle-Tendon Complex: What, Why and How It Increases Muscle Performance? Front. Physiol. 2021, 12, 693141. [Google Scholar] [CrossRef]
- Kragstrup, T.W.; Kjaer, M.; Mackey, A.L. Structural, Biochemical, Cellular, and Functional Changes in Skeletal Muscle Extracellular Matrix with Aging. Scand. J. Med. Sci. Sport. 2011, 21, 749–757. [Google Scholar] [CrossRef]
- Sanai, S.A.; Saini, V.; Benedict, R.H.B.; Zivadinov, R.; Teter, B.E.; Ramanathan, M.; Weinstock-Guttman, B. Aging and Multiple Sclerosis. Mult. Scler. 2016, 22, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Magyari, M. Gender Differences in Multiple Sclerosis Epidemiology and Treatment Response. Dan. Med. J. 2016, 63, 1–18. [Google Scholar]
- Haizlip, K.M.; Harrison, B.C.; Leinwand, L.A. Sex-Based Differences in Skeletal Muscle Kinetics and Fiber-Type Composition. Physiol. (Bethesda) 2015, 30, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska-Skrendo, A.; Leznicka, K.; Leońska-Duniec, A.; Wilk, M.; Filip, A.; Ciȩszczyk, P.; Sawczuk, M. Genetics of Muscle Stiffness, Muscle Elasticity and Explosive Strength. J. Hum. Kinet. 2020, 74, 143–159. [Google Scholar] [CrossRef]
- Arpan, I.; Shah, V.V.; McNames, J.; Harker, G.; Carlson-Kuhta, P.; Spain, R.; El-Gohary, M.; Mancini, M.; Horak, F.B. Fall Prediction Based on Instrumented Measures of Gait and Turning in Daily Life in People with Multiple Sclerosis. Sensors 2022, 22, 5940. [Google Scholar] [CrossRef]
- Van Liew, C.; Huisinga, J.M.; Peterson, D.S. Evaluating the Contribution of Reactive Balance to Prediction of Fall Rates Cross-Sectionally and Longitudinally in Persons with Multiple Sclerosis. Gait Posture 2022, 92, 30–35. [Google Scholar] [CrossRef]
- Focke, A.; Strutzenberger, G.; Jekauc, D.; Worth, A.; Woll, A.; Schwameder, H. Effects of Age, Sex and Activity Level on Counter-Movement Jump Performance in Children and Adolescents. Eur. J. Sport Sci. 2013, 13, 518–526. [Google Scholar] [CrossRef]
- Ntai, A.; Zahou, F.; Paradisis, G.; Smirniotou, A.; Tsolakis, C. Anthropometric Parameters and Leg Power Performance in Fencing. Age, Sex and Discipline Related Differences. Sci. Sport. 2017, 32, 135–143. [Google Scholar] [CrossRef]
- Desomer, A.; Van den Heede, K.; Triemstra, M.; Paget, J.; De Boer, D.; Kohn, L.; Cleemput, I. Use of Patient-Reported Outcome and Experience Measures in Patient Care and Policy. In KCE Report 303; KCE: Brussels, Belgium, 2018. [Google Scholar] [CrossRef]
- Minvielle, E.; Fierobe, A.; Fourcade, A.; Ferrua, M.; di Palma, M.; Scotté, F.; Mir, O. The Use of Patient-Reported Outcome and Experience Measures for Health Policy Purposes: A Scoping Review in Oncology. Health Policy 2023, 129, 104702. [Google Scholar] [CrossRef]
- Knapp, A.; Harst, L.; Hager, S.; Schmitt, J.; Scheibe, M. Use of Patient-Reported Outcome Measures and Patient-Reported Experience Measures Within Evaluation Studies of Telemedicine Applications: Systematic Review. J. Med. Internet Res. 2021, 23, e30042. [Google Scholar] [CrossRef]
- Trentzsch, K.; Weidemann, M.L.; Torp, C.; Inojosa, H.; Scholz, M.; Haase, R.; Schriefer, D.; Akgün, K.; Ziemssen, T. The Dresden Protocol for Multidimensional Walking Assessment (DMWA) in Clinical Practice. Front. Neurosci. 2020, 14, 582046. [Google Scholar] [CrossRef]
- Scholz, M.; Haase, R.; Trentzsch, K.; Stölzer-Hutsch, H.; Ziemssen, T. Improving Digital Patient Care: Lessons Learned from Patient-Reported and Expert-Reported Experience Measures for the Clinical Practice of Multidimensional Walking Assessment. Brain Sci. 2021, 11, 786. [Google Scholar] [CrossRef]

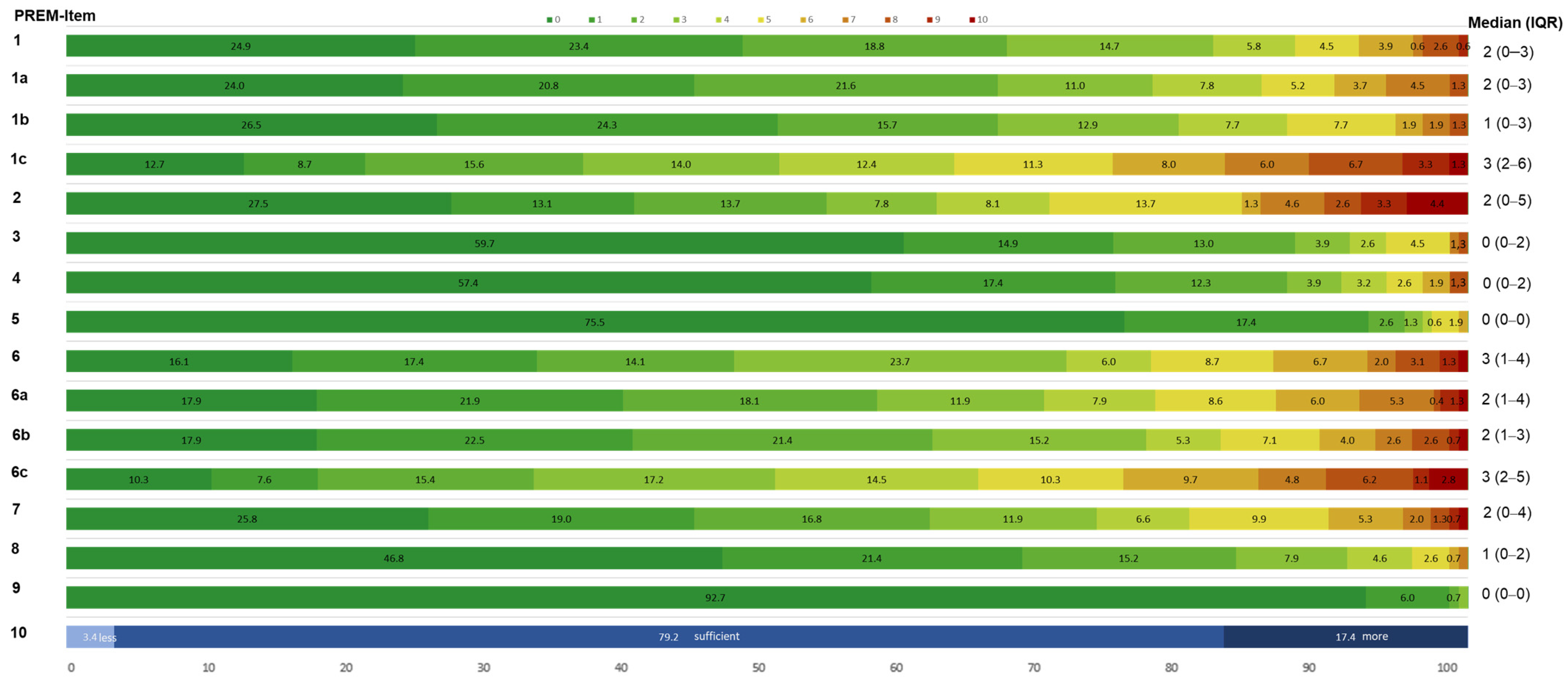



| PREM Items | PREM Questions | Interpretation Scale 0–10 | |
|---|---|---|---|
| 1 | Difficulty total (and for each jump test: 10SHT, CMJ, SLCMJ) | How easy or difficult would you rate the jump assessment? (and each jump tests) | 0 = easy; 10 = difficult |
| 2 | Self-use of results | To which extent do you use the results for your own review? | 0 = always; 10 = not at all |
| 3 | Usefulness of the results | How useful do you think is incorporating the results into your progress monitoring? | 0 = useful; 10 = not useful |
| 4 | Integration of results in MS therapy | How convinced are you that the results will be used for your disease progression? | 0 = convinced; 10 = not at all convinced |
| 5 | Acceptance required time | How do you rate the time required to perform the jump assessment? | 0 = appropriate; 10 = too time-consuming |
| 6 | Strain total (and for each jump test: 10SHT, CMJ, SLCMJ) | How exhausting do you experience the jump assessment? | 0 = not exhausting; 10 = exhausting |
| 7 | Safety | How do you rate the safety of the jump assessment? | 0 = safe; 10 = unsafe |
| 8 | Comfort | How do you rate the comfort of the jump assessment? | 0 = comfortable; 10 = very uncomfortable |
| 9 | Staff support performance | How do you rate the support and explanations provided by the staff? | 0 = very good; 10 = bad |
| 10 | Rating of implementation frequency | Do you think the jump assessment once a year is sufficient (if no relapses occur)? | a. jump assessment should be less frequent b. frequency is sufficient c. jump assessment should be more frequent |
| pwMS (N = 157) | ||
|---|---|---|
| Age (years) | 39.66 (± 9.75) | |
| Females N (%) | 115 (73.2 %) | |
| BMI | 25.21 (± 5.01) | |
| GLTEQ Score (N = 154) | 41.22 (± 28.17) | |
| Insufficiently active N (%) | 25 (16.2%) | |
| Moderate active N (%) | 18 (11.7%) | |
| Active N (%) | 111 (72.1%) | |
| Disease duration (years) | 8.9 (±6.57) | |
| MS Type N (%) | RRMS | 147 (93.6%) |
| PPMS | 8 (5.1%) | |
| SPMS | 2 (1.3%) | |
| EDSS Median (IQR) | Score | 2.0 (1.5–3.0) |
| Pyramidal FSS | 1.0 (1.0–2.0) | |
| Cerebellar FSS | 0 (0–1.0) | |
| Sensory FSS | 1.0 (0–2.0) | |
| Ambulation | 0 (0–1.0) | |
| PREM Items | Sex | Age | BMI | Physical Activity | Disease Duration | EDSS Score | FSS Pyramidal | FSS Cerebellar | FSS Sensory | Ambulation Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Difficulty total | 0.167 * | 0.093 * | 0.110 * | 0.093 * | −0.077 | 0.099 * | 0.207 * | 0.167 * | 0.095 | 0.185 * |
| 10SHT | 0.071 | 0.213 * | 0.085 | −0.057 | 0.028 | 0.235 * | 0.312 * | 0.246 * | 0.072 | 0.302 * |
| CMJ | 0.187 * | 0.242 * | 0.064 | −0.081 | −0.002 | 0.228 * | 0.283 * | 0.271 * | 0.156 * | 0.257 * |
| SLCMJ | 0.168 * | 0.156 * | 0.200 * | −0.143 * | 0.031 | 0.136 * | 0.226 * | 0.120 * | 0.077 | 0.126 * |
| Self-use of results | 0.103 * | −0.03 | 0.103 * | −0.013 | 0.141 * | −0.024 | −0.035 | −0.086 | 0.014 | 0.060 |
| Usefulness of the results | 0.141 * | −0.144 * | 0.059 | −0.060 | 0.066 | −0.062 | −0.009 | −0.165 * | −0.034 | 0.052 |
| Integration of results in therapy | 0.250 * | −0.081 | 0.000 | 0.088 | 0.055 | −0.034 | 0.04 | −0.056 | −0.007 | −0.035 |
| Acceptance required time | 0.080 | 0.013 | −0.010 | 0.084 | −0.010 | 0.043 | 0.124 * | 0.042 | 0.031 | 0.027 |
| Strain total | 0.215 * | 0.118 * | 0.237 * | −0.093 | 0.048 | 0.200 * | 0.247 * | 0.170 * | 0.068 | 0.200 * |
| 10SHT | 0.152 * | 0.190 * | 0.081 | −0.155 * | −0.038 | 0.266 * | 0.299 * | 0.217 * | 0.062 | 0.283 * |
| CMJ | 0.258 * | 0.255 * | 0.162 * | −0.123 * | 0.004 | 0.291 * | 0.362 * | 0.334 * | 0.194 * | 0.386 * |
| SLCMJ | 0.258 * | 0.186 * | 0.197 * | −0.121 * | 0.065 | 0.252 * | 0.323 * | 0.218 * | 0.087 | 0.292 * |
| Safety jump assessment | 0.282 * | 0.100 * | 0.169 * | −0.187 * | 0.036 | 0.272 * | 0.326 * | 0.250 * | 0.130 * | 0.340 * |
| Comfort jump assessment | 0.209 * | −0.067 | 0.095 * | −0.161 * | 0.031 | 0.023 | −0.026 | −0.073 | 0.031 | 0.041 |
| Staff support performance | 0.117 * | 0.017 | −0.035 | −0.233 * | 0.083 | 0.091 | 0.139 * | 0.045 | 0.029 | 0.114 * |
| Rating of implementation frequency | 0.220 * | 0.041 | −0.054 | 0.129 * | −0.016 | 0.104 * | 0.07 | 0.063 | 0.103 * | 0.110 * |
| Peak Force (N/kg) | Peak Power (W/kg) | Force at Zero Velocity (N/kg) | Flight Time (s) | Flight Time-Contraction Time Ratio | Jump Height (cm) | Propulsive Time (s) | Braking Time (s) | Negative Work (J) | Positive Work (J) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Difficulty CMJ | −0.308 * | −0.461 * | −0.148 * | −0.430 * | −0.287 * | −0.448 * | 0.053 | 0.029 | 0.064 | −0.264 * |
| Self-use of results | −0.216 * | −0.247 * | −0.085 | −0.224 * | −0.194 * | −0.222 * | 0.106 * | 0.018 | 0.005 | −0.007 |
| Usefulness of the results | −0.104 * | −0.187 * | −0.033 | −0.207 * | −0.128 * | −0.190 * | 0.031 | −0.077 | 0.093 * | −0.054 |
| Integration of results in therapy | −0.123 * | −0.216 * | −0.026 | −0.202 * | −0.121 * | −0.231 * | 0.005 | −0.019 | 0.063 | −0.139 * |
| Acceptance required time | 0.046 | −0.076 | 0.067 | −0.072 | 0.031 | −0.099 * | −0.113 * | −0.119 * | 0.074 | −0.113 * |
| Strain CMJ | −0.353 * | −0.472 * | −0.201 * | −0.465 * | −0.319 * | −0.465 * | 0.087 | 0.012 | 0.089 | −0.203 * |
| Safety | −0.329 * | −0.411 * | −0.0232 * | −0.433 * | −0.359 * | −0.417 * | 0.147 * | 0.050 | 0.087 | −0.160 * |
| Comfort | −0.105 * | −0.221 * | −0.049 | −0.210 * | −0.137 * | −0.232 * | −0.009 | −0.021 | 0.054 | −0.127 * |
| Staff support performance | 0.069 | −0.112 * | 0.098 * | −0.139 * | −0.020 | −0.133 * | −0.143 * | −0.165 * | 0.134 * | −0.176 * |
| Implementation frequency | 0.045 | 0.139 * | −0.037 | 0.132 * | 0.016 | 0.125 * | 0.014 | 0.054 | −0.018 | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geßner, A.; Vágó, A.; Stölzer-Hutsch, H.; Schriefer, D.; Hartmann, M.; Trentzsch, K.; Ziemssen, T. Experiences of People with Multiple Sclerosis in Sensor-Based Jump Assessment. Bioengineering 2025, 12, 610. https://doi.org/10.3390/bioengineering12060610
Geßner A, Vágó A, Stölzer-Hutsch H, Schriefer D, Hartmann M, Trentzsch K, Ziemssen T. Experiences of People with Multiple Sclerosis in Sensor-Based Jump Assessment. Bioengineering. 2025; 12(6):610. https://doi.org/10.3390/bioengineering12060610
Chicago/Turabian StyleGeßner, Anne, Anikó Vágó, Heidi Stölzer-Hutsch, Dirk Schriefer, Maximilian Hartmann, Katrin Trentzsch, and Tjalf Ziemssen. 2025. "Experiences of People with Multiple Sclerosis in Sensor-Based Jump Assessment" Bioengineering 12, no. 6: 610. https://doi.org/10.3390/bioengineering12060610
APA StyleGeßner, A., Vágó, A., Stölzer-Hutsch, H., Schriefer, D., Hartmann, M., Trentzsch, K., & Ziemssen, T. (2025). Experiences of People with Multiple Sclerosis in Sensor-Based Jump Assessment. Bioengineering, 12(6), 610. https://doi.org/10.3390/bioengineering12060610







