Injectable and Assembled Calcium Sulfate/Magnesium Silicate 3D Scaffold Promotes Bone Repair by In Situ Osteoinduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Mg@Ca Particles
2.2. Cell Cultures and Differentiation in the Released Medium
2.3. Cell Survival Assay
2.4. RNA Extraction and Quantitative Real-Time PCR
2.5. Primers Used for Real-Time PCR
2.6. Transcriptome Sequencing
2.7. Animal Study and Surgical Procedure
2.8. Statistical Analysis
3. Results
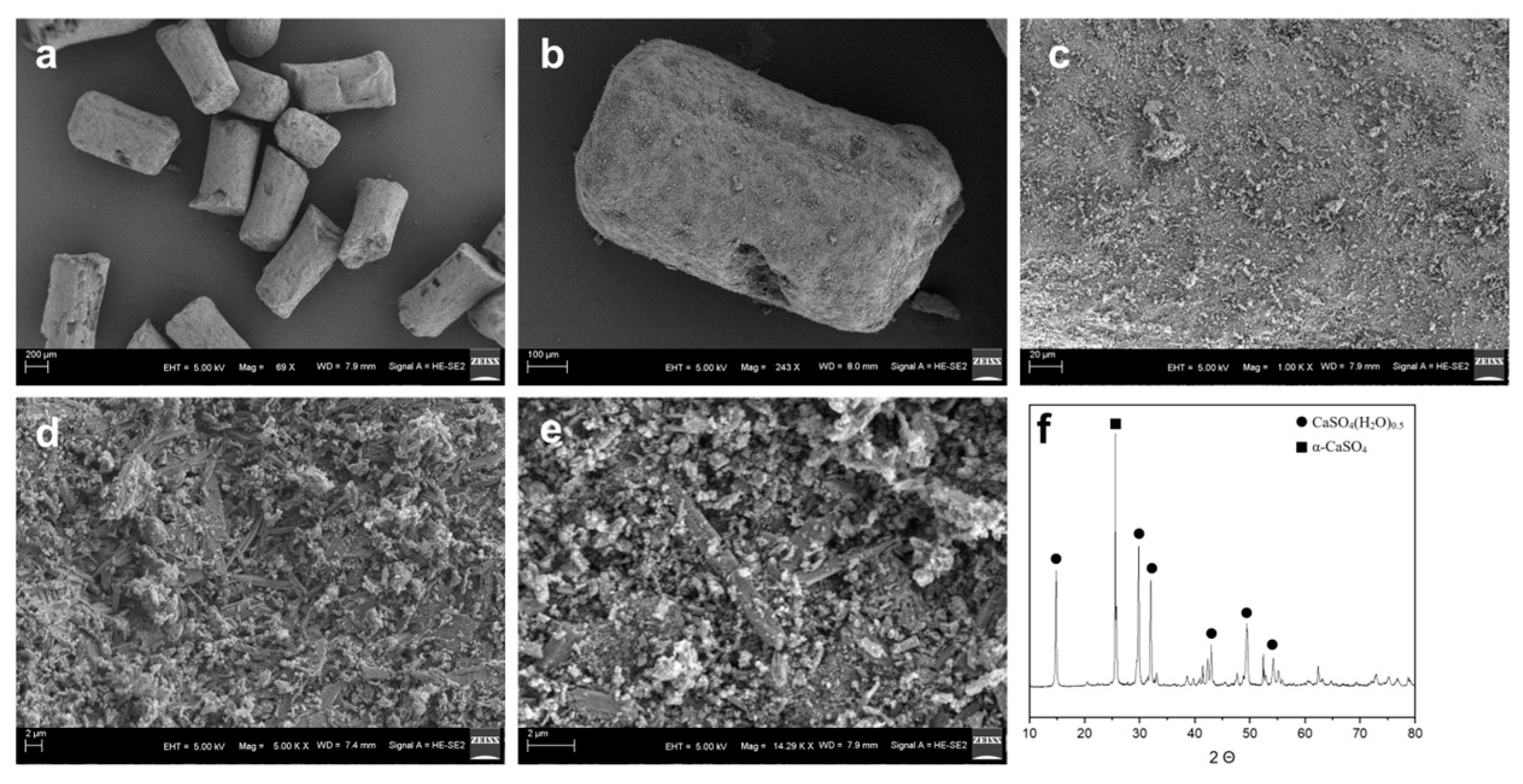
3.1. Analysis of General Physical and Chemical Properties of Mg@Ca
3.2. Assessment of Mg@Ca Scaffold Biocompatibility and Osteoinductive Properties
3.3. Gene Expression of Osteogenic Genes After Mg@Ca Treatment
3.4. Transcriptome Analysis of Mg@Ca
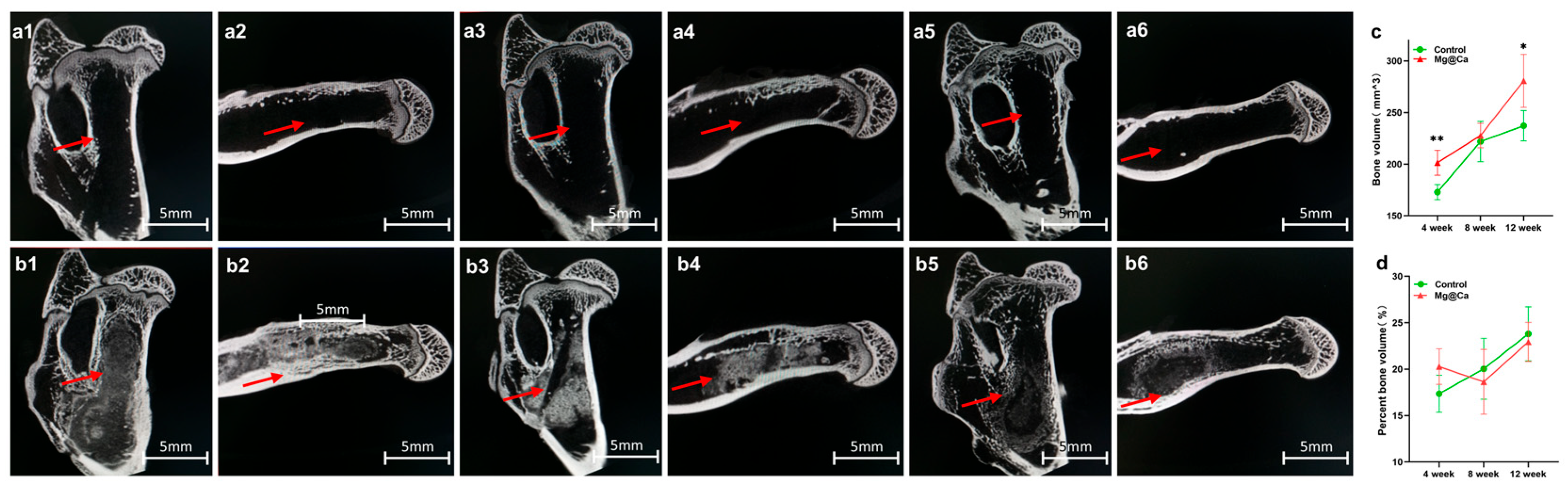
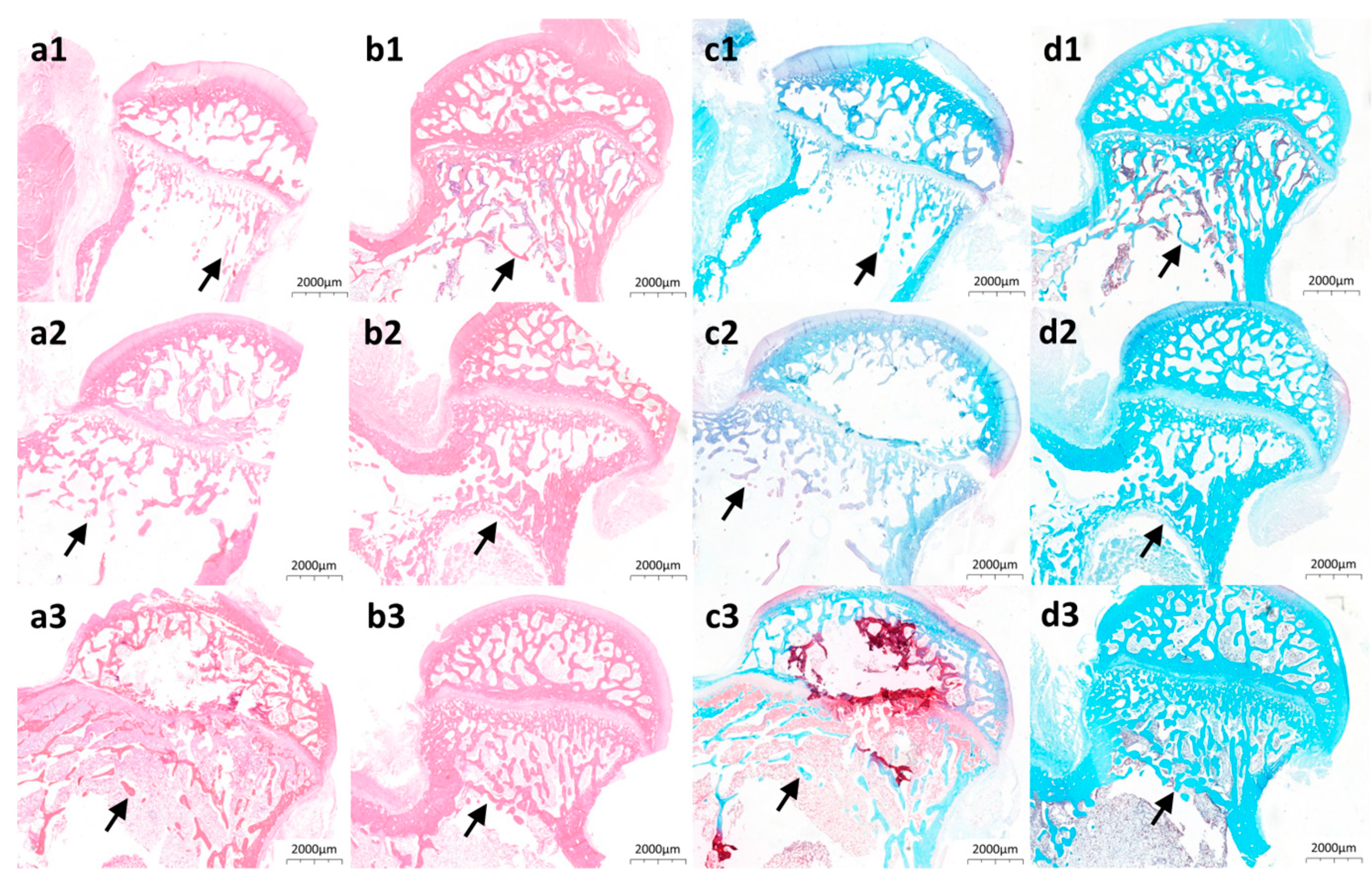
3.5. Animal Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ONFH | Osteonecrosis of the Femoral Head |
| Mg@Ca | 3D porous bioceramic scaffold combining Magnesium Silicate Hydrate with α-Hemihydrate Calcium Sulfate |
| α-CSH | α-calcium sulfate hemihydrate |
| HE | Hematoxylin-Eosin staining |
| h-BMSC | Human Bone Marrow Mesenchymal Stem Cell |
| DEGs | Differentially Expressed Genes |
| GSEA | Gene Set Enrichment Analysis |
| KEGG | Kyoto Encyclopedia of Genes and Genomes Analysis |
References
- Hungeford, D.S. Osteonecrosis—Avoiding total hip arthroplasty. J. Arthroplast. 2002, 17, 121–124. [Google Scholar] [CrossRef]
- Johnson, A.J.; Mont, M.A.; Tsao, A.K.; Jones, L.C. Treatment of Femoral Head Osteonecrosis in the United States: 16-year Analysis of the Nationwide Inpatient Sample. Clin. Orthop. Relat. Res. 2014, 472, 617–623. [Google Scholar] [CrossRef]
- Ikeuchi, K.K.; Hasegawa, Y.; Seki, T.; Takegami, Y.; Amano, T.; Ishiguro, N. Epidemiology of nontraumatic osteonecrosis of the femoral head in Japan. Mod. Rheumatol. 2015, 25, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhang, F.; Wang, B.; Liu, B.; Li, L.; Kim, S.-Y.; Goodman, S.B.; Hernigou, P.; Cui, Q.; Lineaweaver, W.C.; et al. Guidelines for clinical diagnosis and treatment of osteonecrosis of the femoral head in adults (2019 version). J. Orthop. Transl. 2020, 21, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.W.; Kim, Y.L.; Yoo, J.J.; Koo, K.-H.; Yoon, K.S.; Kim, H.J. Fate of untreated asymptomatic osteonecrosis of the femoral head. J. Bone Jt. Surg. Am. Vol. 2008, 90A, 477–484. [Google Scholar] [CrossRef]
- Ozcakir, S. Avascular Necrosis of the Hip. Turk. Fiz. Tip Ve Rehabil. Derg. Turk. J. Phys. Med. Rehabil. 2009, 55, 26–29. [Google Scholar]
- Mont, M.A.; Salem, H.S.; Piuzzi, N.S.; Goodman, S.B.; Jones, L.C. Nontraumatic Osteonecrosis of the Femoral Head: Where Do We Stand Today? A 5-Year Update. J. Bone Jt. Surg. Am. Vol. 2020, 102, 1084–1099. [Google Scholar] [CrossRef]
- Hoogervorst, P.; Campbell, J.C.; Scholz, N.; Cheng, E.Y. Core Decompression and Bone Marrow Aspiration Concentrate Grafting for Osteonecrosis of the Femoral Head. J. Bone Jt. Surg. Am. Vol. 2022, 104, 54–60. [Google Scholar] [CrossRef]
- Thanh Ngoc, T.; Wolf, M.; Winter, P.; Landgraeber, S. Hip joint mechanics in patients with osteonecrosis of the femoral head following treatment by advanced core decompression. Clin. Biomech. 2022, 94, 105635. [Google Scholar]
- Yoon, T.R.; Song, E.K.; Rowe, S.M.; Park, C.H. Failure after core decompression in osteonecrosis of the femoral head. Int. Orthop. 2001, 24, 316–318. [Google Scholar] [CrossRef]
- Mihalko, W.M.; Balos, L.; Santilli, M.; Mindell, E.R. Osteonecrosis after powered core decompression. Clin. Orthop. Relat. Res. 2003, 412, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Wang, Y.; Zhu, J.; Li, C.; Wang, Z. Repair of non-traumatic femoral head necrosis by marrow core decompression with bone grafting and porous tantalum rod implantation. Pak. J. Med. Sci. 2020, 36, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhan, S.; Ling, M.; Jiang, D.; Hu, H.; Sheng, J.; Zhang, C. Biomechanical analysis of fibular graft techniques for nontraumatic osteonecrosis of the femoral head: A finite element analysis. J. Orthop. Surg. Res. 2020, 15, 335. [Google Scholar] [CrossRef]
- Korompilias, A.V.; Beris, A.E.; Lykissas, M.G.; Kostas-Agnantis, I.P.; Soucacos, P.N. Femoral head osteonecrosis: Why choose free vascularized fibula grafting. Microsurgery 2011, 31, 223–228. [Google Scholar] [CrossRef]
- Jie, K.; Feng, W.; Li, F.; Wu, K.; Chen, J.; Zhou, G.; Zeng, H.; Zeng, Y. Long-term survival and clinical outcomes of non-vascularized autologous and allogeneic fibular grafts are comparable for treating osteonecrosis of the femoral head. J. Orthop. Surg. Res. 2021, 16, 109. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, X.; Lu, X.; Sun, C.; Li, M.; Chen, G.; Long, Z.; Gao, Y.; Zhang, H.; Huang, M.; et al. Reconstructing avascular necrotic femoral head through a bioactive β-TCP system: From design to application. Bioact. Mater. 2023, 28, 495–510. [Google Scholar] [CrossRef]
- Targonska, S.; Dobrzynska-Mizera, M.; Wujczyk, M.; Rewak-Soroczynska, J.; Knitter, M.; Dopierala, K.; Andrzejewski, J.; Wiglusz, R.J. New way to obtain the poly(L-lactide-co-D,L-lactide) blend filled with nanohydroxyapatite as biomaterial for 3D-printed bone-reconstruction implants. Eur. Polym. J. 2022, 165, 110997. [Google Scholar] [CrossRef]
- Yang, J.; Fatima, K.; Zhou, X.; He, C. Meticulously engineered three-dimensional-printed scaffold with microarchitecture and controlled peptide release for enhanced bone regeneration. Biomater. Transl. 2024, 5, 69–83. [Google Scholar]
- Tonelli, M.; Faralli, A.; Ridi, F.; Bonini, M. 3D printable magnesium-based cements towards the preparation of bioceramics. J. Colloid Interface Sci. 2021, 598, 24–35. [Google Scholar] [CrossRef]
- Han, Y.; Wei, Q.; Chang, P.; Hu, K.; Okoro, O.V.; Shavandi, A.; Nie, L. Three-Dimensional Printing of Hydroxyapatite Composites for Biomedical Application. Crystals 2021, 11, 353. [Google Scholar] [CrossRef]
- Yue, X.; Zhao, L.; Yang, J.; Jiao, X.; Wu, F.; Zhang, Y.; Li, Y.; Qiu, J.; Ke, X.; Sun, X.; et al. Comparison of osteogenic capability of 3D-printed bioceramic scaffolds and granules with different porosities for clinical translation. Front. Bioeng. Biotechnol. 2023, 11, 1260639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, H.; Wu, L.; Ma, L.; Xing, F.; Kong, Q.; Fan, Y.; Zhou, C.; Zhang, X. 3D printing of calcium phosphate bioceramic with tailored biodegradation rate for skull bone tissue reconstruction. Bio-Des. Manuf. 2019, 2, 161–171. [Google Scholar] [CrossRef]
- Thomas, M.V.; Puleo, D.A. Calcium Sulfate: Properties and Clinical Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88B, 597–610. [Google Scholar] [CrossRef]
- Thomas, M.V.; Puleo, D.A.; Al-Sabbagh, M. Calcium sulfate: A review. J. Long-Term Eff. Med. Implant. 2005, 15, 599–607. [Google Scholar] [CrossRef]
- Baranes, D.; Kurtzman, G.M. Biphasic Calcium Sulfate as an Alternative Grafting Material in Various Dental Applications. J. Oral Implantol. 2019, 45, 247–255. [Google Scholar] [CrossRef]
- Nicoara, A.I.; Voineagu, T.G.; Alecu, A.E.; Vasile, B.S.; Maior, I.; Cojocaru, A.; Trusca, R.; Popescu, R.C. Fabrication and Characterisation of Calcium Sulphate Hemihydrate Enhanced with Zn- or B-Doped Hydroxyapatite Nanoparticles for Hard Tissue Restoration. Nanomaterials 2023, 13, 2219. [Google Scholar] [CrossRef]
- Jameel, J.; Sinha, S.; Kumar, A.; Qureshi, O.A.; Kumar, S.; Aggarwal, N.; Dua, A.; Nagori, M.J.; Khan, R. Effectiveness of Calcium Sulfate and Hydroxyapatite Composite in Collapse Prevention in Osteonecrosis of Femoral Head. Cureus J. Med. Sci. 2022, 14, e24408. [Google Scholar] [CrossRef]
- Kumar, Y.; Nalini, K.B.; Menon, J.; Patro, D.K.; Banerji, B.H. Calcium sulfate as bone graft substitute in the treatment of osseous bone defects, a prospective study. J. Clin. Diagn. Res. JCDR 2013, 7, 2926–2928. [Google Scholar] [CrossRef] [PubMed]
- Georgeanu, V.A.; Gingu, O.; Antoniac, I.V.; Manolea, H.O. Current Options and Future Perspectives on Bone Graft and Biomaterials Substitutes for Bone Repair, from Clinical Needs to Advanced Biomaterials Research. Appl. Sci. 2023, 13, 8471. [Google Scholar] [CrossRef]
- Chen, I.C.; Su, C.-Y.; Lai, C.-C.; Tsou, Y.-S.; Zheng, Y.; Fang, H.-W. Preparation and Characterization of Moldable Demineralized Bone Matrix/Calcium Sulfate Composite Bone Graft Materials. J. Funct. Biomater. 2021, 12, 56. [Google Scholar] [CrossRef]
- Xiang, H.; Wang, Y.; Chang, H.; Yang, S.; Tu, M.; Zhang, X.; Yu, B. Cerium-containing α-calcium sulfate hemihydrate bone substitute promotes osteogenesis. J. Biomater. Appl. 2019, 34, 250–260. [Google Scholar] [CrossRef]
- Chiang, C.-C.; Hsieh, M.-K.; Wang, C.-Y.; Tuan, W.-H.; Lai, P.-L. Cytotoxicity and cell response of preosteoblast in calcium sulfate-augmented PMMA bone cement. Biomed. Mater. 2021, 16, 055014. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Qiu, L.; Zhang, Y.; Gao, B. Assessment of α-calcium sulfate hemihydrate/nanocellulose composite bone graft material for bone healing in a rabbit femoral condyle model. Mater. Express 2021, 11, 1497–1504. [Google Scholar] [CrossRef]
- Kok, J.; Sirka, A.; Grassi, L.; Raina, D.B.; Tarasevicius, S.; Tagil, M.; Lidgren, L.; Isaksson, H. Fracture strength of the proximal femur injected with a calcium sulfate/hydroxyapatite bone substitute. Clin. Biomech. 2019, 63, 172–178. [Google Scholar] [CrossRef]
- Hasandoost, L.; Alhalawani, A.; Rodriguez, O.; Yazdi, A.R.; Zalzal, P.; Schemitsch, E.H.; Waldman, S.D.; Papini, M.; Towler, M.R. Calcium sulfate-containing glass polyalkenoate cement for revision total knee arthroplasty fixation. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2020, 108, 3356–3369. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rai, S.; Ze, R.; Tang, X.; Liu, R.; Hong, P. Injectable calcium sulfate vs mixed bone graft of autologous iliac bone and allogeneic bone. Which is the better bone graft material for unicameral bone cyst in humerus? Medicine 2020, 99, e20563. [Google Scholar] [CrossRef]
- Wang, J.; Xie, L.; Wang, X.; Zheng, W.; Chen, H.; Cai, L.; Chen, L. The effects of oyster shell/alpha-calcium sulfate hemihydrate/platelet-rich plasma/bone mesenchymal stem cells bioengineering scaffold on rat critical-sized calvarial defects. J. Mater. Sci. Mater. Med. 2020, 31, 96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zou, J.; Wang, B.; Wu, Z.; Jia, Y.; Cheeseman, C.R. Characterization of Magnesium Silicate Hydrate (MSH) Gel Formed by Reacting MgO and Silica Fume. Materials 2018, 11, 909. [Google Scholar] [CrossRef]
- Yoo, K.-H.; Kim, Y.-I.; Yoon, S.-Y. Physicochemical and Biological Properties of Mg-Doped Calcium Silicate Endodontic Cement. Materials 2021, 14, 1843. [Google Scholar] [CrossRef]
- Zhai, Z.; Qu, X.; Li, H.; Yang, K.; Wan, P.; Tan, L.; Ouyang, Z.; Liu, X.; Tian, B.; Xiao, F.; et al. The effect of metallic magnesium degradation products on osteoclast-induced osteolysis and attenuation of NF-κB and NFATc1 signaling. Biomaterials 2014, 35, 6299–6310. [Google Scholar] [CrossRef]
- Weng, Y.; Jian, Y.; Huang, W.; Xie, Z.; Zhou, Y.; Pei, X. Alkaline earth metals for osteogenic scaffolds: From mechanisms to applications. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2023, 111, 1447–1474. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Ni, Y.; Wei, H.; Duan, Y.; Chen, J.; Xiao, Q.; Gao, J.; Yu, Y.; Cui, Y.; Ouyang, S.; et al. Ion incorporation into bone grafting materials. Periodontology 2000 2024, 94, 213–230. [Google Scholar] [CrossRef]
- Qi, L.; Zhao, T.; Yan, J.; Ge, W.; Jiang, W.; Wang, J.; Gholipourmalekabadi, M.; Lin, K.; Wang, X.; Zhang, L. Advances in magnesium-containing bioceramics for bone repair. Biomater. Transl. 2024, 5, 3–20. [Google Scholar] [PubMed]
- Liu, G.; Mei, Y.; Ma, H.; Lu, Q.; Meng, H.; Quan, Q.; Zhang, Y.; Zhao, J.; Li, H.; Wang, A.; et al. Effects of bone-resorptive lesion on stress distribution of the femoral head and on progression in patients with osteonecrosis of the femoral head. Chin. J. Orthop. 2020, 40, 408–416. [Google Scholar]
- Ding, M.; Overgaard, S. 3-D microarchitectural properties and rod- and plate-like trabecular morphometric properties of femur head cancellous bones in patients with rheumatoid arthritis, osteoarthritis, and osteoporosis. J. Orthop. Transl. 2021, 28, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Li, W.; Chen, K.; Feng, B.; Zhou, L.; Weng, X.; Cui, S.; Engqvist, H.; Xia, W. Injectable and assembled 3D solid structure for free-to-fixed shape in bone reconstruction. Appl. Mater. Today 2020, 21, 100823. [Google Scholar] [CrossRef]
- Boontanapibul, K.; Steere, J.T.; Amanatullah, D.F.; Huddleston, J.I.; Maloney, W.J.; Goodman, S.B. Diagnosis of Osteonecrosis of the Femoral Head: Too Little, Too Late, and Independent of Etiology. J. Arthroplast. 2020, 35, 2342–2349. [Google Scholar] [CrossRef]
- Kraeutler, M.J.; Hernandez, E.; Samuelsson, K.; Mei-Dan, O. The Principles of Hip Joint Preservation. Arthrosc. J. Arthrosc. Relat. Surg. 2024, 40, 1958–1960. [Google Scholar] [CrossRef]
- Piuzzi, N.S.; Chahla, J.; Schrock, J.B.; LaPrade, R.F.; Pascual-Garrido, C.; Mont, M.A.; Muschler, G.F. Evidence for the Use of Cell-Based Therapy for the Treatment of Osteonecrosis of the Femoral Head: A Systematic Review of the Literature. J. Arthroplast. 2017, 32, 1698–1708. [Google Scholar] [CrossRef]
- Zhao, J.; Shen, F.; Sheng, S.; Wang, J.; Wang, M.; Wang, F.; Jiang, Y.; Jing, Y.; Xu, K.; Su, J. Cartilage-on-chip for osteoarthritis drug screening. Orig. Res. 2025, 1, 846. [Google Scholar]
- Wu, D.Y.; Wang, S.S.; Wu, C.S. A new composite fabricated from hydroxyapatite, gelatin-MgO microparticles, and compatibilized poly(butylene succinate) with osteogenic functionality. Biomater. Adv. 2023, 154, 213586. [Google Scholar] [CrossRef]
- Tao, Z.; Yuan, Z.; Zhou, D.; Qin, L.; Xiao, L.; Zhang, S.; Liu, C.; Zhao, J.; Li, Y. Fabrication of magnesium-doped porous polylactic acid microsphere for bone regeneration. Biomater. Transl. 2023, 4, 280–290. [Google Scholar]
- Lin, W.C.; Chen, P.W.; Chen, T.Y.; Liu, B.Y.; Su, Y.P.; Hwang, D.F. Effects of magnesium ion supplement on physicochemical properties and bioactivities of medical bone materials converted from oyster shell. J. Ceram. Soc. Jpn. 2023, 131, 368–375. [Google Scholar] [CrossRef]
- Liu, X.S.; Pei, J.; Zhao, D.C.; Yan, Y.G. A novel strategy for calcium magnesium phosphate/carboxymethyl chitosan composite bone cements with enhanced physicochemical properties, excellent cytocompatibility and osteogenic differentiation. Biomed. Mater. 2024, 19, 055014. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, S.; Matsuzaki, H. Short-term dietary magnesium deficiency downregulates the expression of bone formation-related genes in rats. Magnes. Res. 2023, 36, 23–29. [Google Scholar] [CrossRef]
- Duan, H.; Cao, C.; Wang, X.; Tao, J.; Li, C.; Xin, H.; Yang, J.; Song, Y.; Ai, F. Magnesium-alloy rods reinforced bioglass bone cement composite scaffolds with cortical bone-matching mechanical properties and excellent osteoconductivity for load-bearing bone in vivo regeneration. Sci. Rep. 2020, 10, 18193. [Google Scholar] [CrossRef]
- Niknam, Z.; Zali, H.; Mansouri, V.; Rezaei Tavirani, M.; Omidi, M. Morphological and Molecular Analysis of Osteoblasts Differentiated from Mesenchymal Stem Cells in Polycaprolactone/Magnesium Oxide/Graphene Oxide Scaffold. Int. J. Organ Transplant. Med. 2019, 10, 171–182. [Google Scholar]
- Wu, Q.; Xu, S.; Wang, F.; He, B.; Wang, X.; Sun, Y.; Ning, C.; Dai, K. Double-edged effects caused by magnesium ions and alkaline environment regulate bioactivities of magnesium-incorporated silicocarnotite in vitro. Regen. Biomater. 2021, 8, rbab016. [Google Scholar]
- Zofkova, I.; Nemcikova, P.; Kuklik, M. Polymorphisms Associated With Low Bone Mass and High Risk of Atraumatic Fracture. Physiol. Res. 2015, 64, 621–631. [Google Scholar] [CrossRef]
- Selvaraj, V.; Sekaran, S.; Dhanasekaran, A.; Warrier, S. Type 1 collagen: Synthesis, structure and key functions in bone mineralization. Differentiation 2024, 136, 100757. [Google Scholar] [CrossRef]
- Mohammadi, S.M.; Saniee, N.; Mousaviasl, S.; Radmanesh, E.; Doustimotlagh, A.H. The Role of Osteocalcin in Patients with Osteoporosis: A Systematic Review. Iran. J. Public Health 2024, 53, 2432–2439. [Google Scholar] [CrossRef]
- Smith, C.; Lin, X.Z.; Parker, L.; Yeap, B.B.; Hayes, A.; Levinger, I. The role of bone in energy metabolism: A focus on osteocalcin. Bone 2024, 188, 117238. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, J.K.; Jakubowska-Pietkiewicz, E. Osteocalcin: Beyond Bones. Endocrinol. Metab. 2024, 39, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, L.; Xu, R.N.; Ti, Y.F.; Zhao, Y.L.; Zhou, L.W.; Zhao, J.N. BDKRB2+9/−9 bp polymorphisms influence BDKRB2 expression levels and NO production in knee osteoarthritis. Exp. Biol. Med. 2017, 242, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Li, J.; Shan, L.Q.; Fan, Q.Y. The Effect of Bradykinin B2 Receptor Polymorphisms on the Susceptibility and Severity of Osteoarthritis in a Chinese Cohort. J. Biomed. Biotechnol. 2012, 2012, 597637. [Google Scholar] [CrossRef]
- Kohara, Y.; Kitazawa, R.; Haraguchi, R.; Imai, Y.; Kitazawa, S. Macrophages are requisite for angiogenesis of type H vessels during bone regeneration in mice. Bone 2022, 154, 116200. [Google Scholar] [CrossRef]
- Toor, S.M.; Wani, S.; Albagha, O.M.E. Comprehensive Transcriptomic Profiling of Murine Osteoclast Differentiation Reveals Novel Differentially Expressed Genes and LncRNAs. Front. Genet. 2021, 12, 781272. [Google Scholar] [CrossRef]
- Yi, X.; Wu, P.; Fan, Y.Y.; Gong, Y.; Liu, J.Y.; Xiong, J.J.; Xu, X.Y. Identification of candidate genes simultaneously shared by adipogenesis and osteoblastogenesis from human mesenchymal stem cells. Folia Histochem. Cytobiol. 2022, 60, 179–190. [Google Scholar] [CrossRef]
- Yang, C.Y.; Liu, Y.; Wang, Z.Y.; Lin, M.M.; Liu, C. Controlled mechanical loading improves bone regeneration by regulating type H vessels in a S1Pr1-dependent manner. Faseb J. 2022, 36, e22530. [Google Scholar] [CrossRef]
- Chen, H.Y.; Wu, X.Y.; Lan, Y.N.; Zhou, X.J.; Zhang, Y.; Long, L.; Zhong, Y.L.; Hao, Z.G.; Zhang, W.J.; Xue, D.T. SCUBE3 promotes osteogenic differentiation and mitophagy in human bone marrow mesenchymal stem cells through the BMP2/TGF-β signaling pathway. Faseb J. 2024, 38, e70011. [Google Scholar] [CrossRef]
- Yang, H.Y.; Xiong, C.W.; Yu, Z.T.; Yang, Z.C.; Zhang, Y.; Zhang, J.J.; Huang, Y.; Xu, N.W.; Zhou, X.D.; Jiang, M.Q.; et al. A functional polymorphism at the miR-25-3p binding site in the 30-untranslated region of the S1PR1 gene decreases the risk of osteoporosis in Chinese postmenopausal women. Arab. J. Chem. 2023, 16, 104888. [Google Scholar] [CrossRef]
- Ma, W.J.; Li, C. Enhancing postmenopausal osteoporosis: A study of KLF2 transcription factor secretion and PI3K-Akt signaling pathway activation by PIK3CA in bone marrow mesenchymal stem cells. Arch. Med. Sci. 2024, 20, 918–937. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.H.; Deng, C.N.; He, L.; Wu, Q.; Xu, L.; Yu, Y.N. Fluoride induces osteoblast autophagy by inhibiting the PI3K/AKT/mTOR signaling pathway in vivo and in vitro. Exp. Biol. Med. 2023, 248, 1159–1172. [Google Scholar]
- Long, G.N.; Liu, F.; Cheng, H.M.; Guo, J.; Wang, P.; Luo, Y.F.; Li, Z.H.; Tong, F. miR-374-5p inhibits osteogenesis by targeting PTEN/PI3K/AKT signaling pathway. J. Orthop. Surg. Res. 2025, 20, 283. [Google Scholar] [CrossRef]
- Jing, Y.; Zhou, J.; Guo, F.; Yu, L.; Ren, X.; Yin, X. Betaine regulates adipogenic and osteogenic differentiation of hAD-MSCs. Mol. Biol. Rep. 2023, 50, 5081–5089. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J. Time series clustering analysis of genes during osteogenic differentiation of human mesenchymal stem cells. Genes Genet. Syst. 2022, 97, 209–218. [Google Scholar] [CrossRef]
- Feng, K.; Yu, M.; Lou, X.; Wang, D.; Wang, L.; Ren, W. Multi-omics analysis of bone marrow mesenchymal stem cell differentiation differences in osteoporosis. Genomics 2023, 115, 110668. [Google Scholar] [CrossRef]
- Tan, B.; Zhao, N.; Guo, W.; Huang, F.; Hu, H.; Chen, Y.; Li, J.; Ling, Z.; Zou, Z.; Hu, R.; et al. Biomimetic hydroxyapatite coating on the 3D-printed bioactive porous composite ceramic scaffolds promoted osteogenic differentiation via PI3K/AKT/mTOR signaling pathways and facilitated bone regeneration in vivo. J. Mater. Sci. Technol. 2023, 136, 54–64. [Google Scholar] [CrossRef]
- Gong, C.; Yang, J.; Zhang, X.; Wang, X.; Wei, Z.; Huang, X.; Guo, W. Surface functionalization of calcium magnesium phosphate cements with alginate sodium for enhanced bone regeneration via TRPM7/PI3K/Akt signaling pathway. Int. J. Biol. Macromol. 2024, 266, 130998. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, F.; Sun, S.; Li, H.; Li, L.; Xu, H.; Wang, J.; Shao, M.; Li, C.; Wang, H.; et al. Brushite-coated Mg-Nd-Zn-Zr alloy promotes the osteogenesis of vertebral laminae through IGF2/PI3K/AKT signaling pathway. Biomater. Adv. 2023, 152, 213505. [Google Scholar] [CrossRef]


| Genes | Sequences (5′-3′) Sense | Sequences (5′-3′) Antisense |
|---|---|---|
| GAPDH | ACCCACTCCTCCACCTTTGA | CATACCAGGAAATGAGCTTGACAA |
| RUNX2 | ATGGCGGGTAACGATGAAA | TTGTGAAGACGGTTATGGTCAAG |
| ALP | GACCTCCTCGGAAGACACTCTG | CGCCTGGTAGTTGTTGTGAGC |
| BGLAP | GAGGGCAGCGAGGTAGTGA | TGTGGTCAGCCAACTCGTCA |
| OPN | TGGGAGGGCTTGGTTGTCA | CAGAATCAGCCTGTTTAACTGGTAT |
| COL1A1 | GTGCGATGACGTGATCTGTGA | GTTTCTTGGTCGGTGGGTGA |
| COL2A1 | CGCTGTCCTTCGGTGTCAG | CCTTGATGTCTCCAGGTTCTCC |
| OGN | TGAGGATAAATACCTGGAT | TGCGTAAAGATAGGCTGA |
| VEGFA | AGGGCAGAATCATCACGAAGT | AGGGTCTCGATTGGATGGCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Zhao, T.; Wang, H.; Liu, G.; Bian, Y.; Wang, Q.; Xia, W.; Cai, S.; Weng, X. Injectable and Assembled Calcium Sulfate/Magnesium Silicate 3D Scaffold Promotes Bone Repair by In Situ Osteoinduction. Bioengineering 2025, 12, 599. https://doi.org/10.3390/bioengineering12060599
Zhu W, Zhao T, Wang H, Liu G, Bian Y, Wang Q, Xia W, Cai S, Weng X. Injectable and Assembled Calcium Sulfate/Magnesium Silicate 3D Scaffold Promotes Bone Repair by In Situ Osteoinduction. Bioengineering. 2025; 12(6):599. https://doi.org/10.3390/bioengineering12060599
Chicago/Turabian StyleZhu, Wei, Tianhao Zhao, Han Wang, Guangli Liu, Yixin Bian, Qi Wang, Wei Xia, Siyi Cai, and Xisheng Weng. 2025. "Injectable and Assembled Calcium Sulfate/Magnesium Silicate 3D Scaffold Promotes Bone Repair by In Situ Osteoinduction" Bioengineering 12, no. 6: 599. https://doi.org/10.3390/bioengineering12060599
APA StyleZhu, W., Zhao, T., Wang, H., Liu, G., Bian, Y., Wang, Q., Xia, W., Cai, S., & Weng, X. (2025). Injectable and Assembled Calcium Sulfate/Magnesium Silicate 3D Scaffold Promotes Bone Repair by In Situ Osteoinduction. Bioengineering, 12(6), 599. https://doi.org/10.3390/bioengineering12060599





