Pharmacological Intervention with 4-Phenylbutyrate Ameliorates TiAl6V4 Nanoparticles-Induced Inflammatory Osteolysis by Promoting Macrophage Apoptosis
Abstract
1. Introduction
2. Material and Methods
2.1. Ethics Statement
2.2. Nanoparticle Preparation
2.3. Murine Calvaria Resorption Model
2.4. Specimen Retrieval and Histological Processing
2.5. The Histopathologic Changes of Osteolysis
2.6. TRAP Activity and Caspase-3 Activity
2.7. Inflammatory and Osteoclastogenic Cytokine
2.8. ROS Content in Osteolytic Interface Periosteum
2.9. Western Blotting (WB)
2.10. Detection of Apoptosis Through TUNEL Staining
2.11. Immunohistochemistry
2.12. Statistical Analysis
3. Results
3.1. 4-PBA Ameliorates the Histopathologic Change of Osteolysis
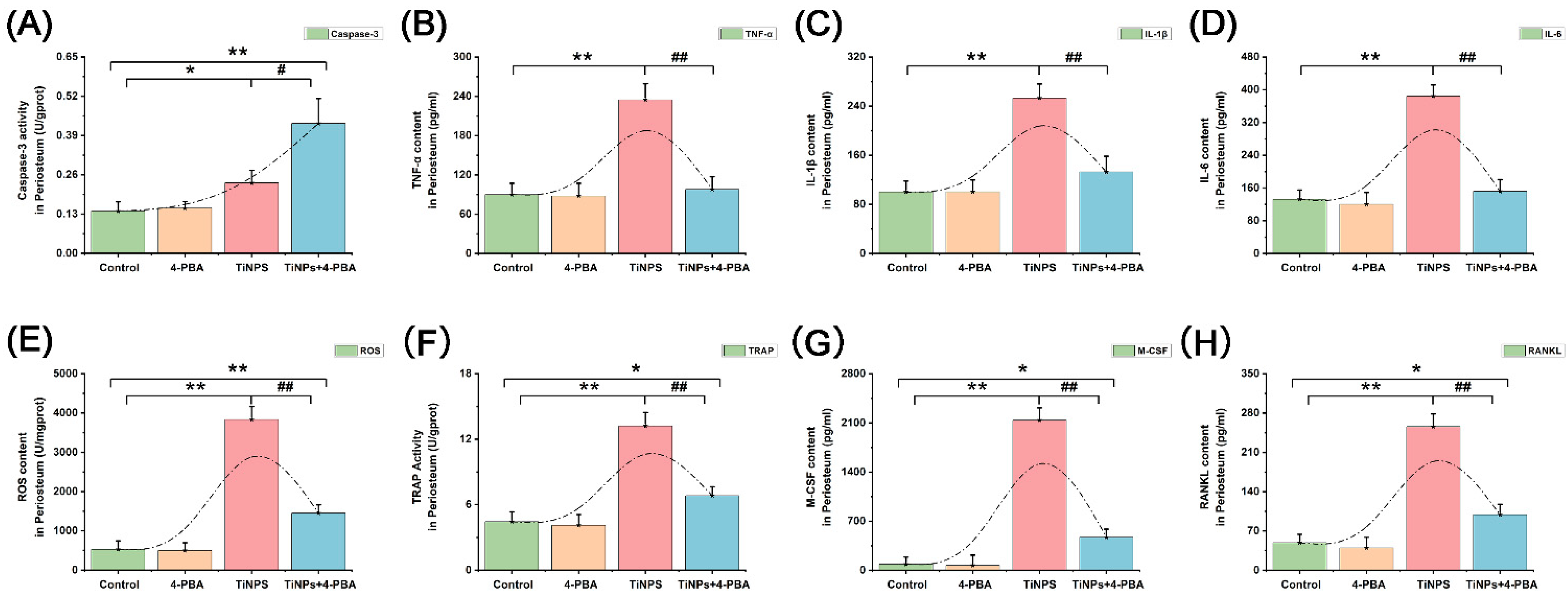
3.2. 4-PBA Promotes Particle-Induced Macrophage Apoptosis in Osteolytic Interface Periosteum
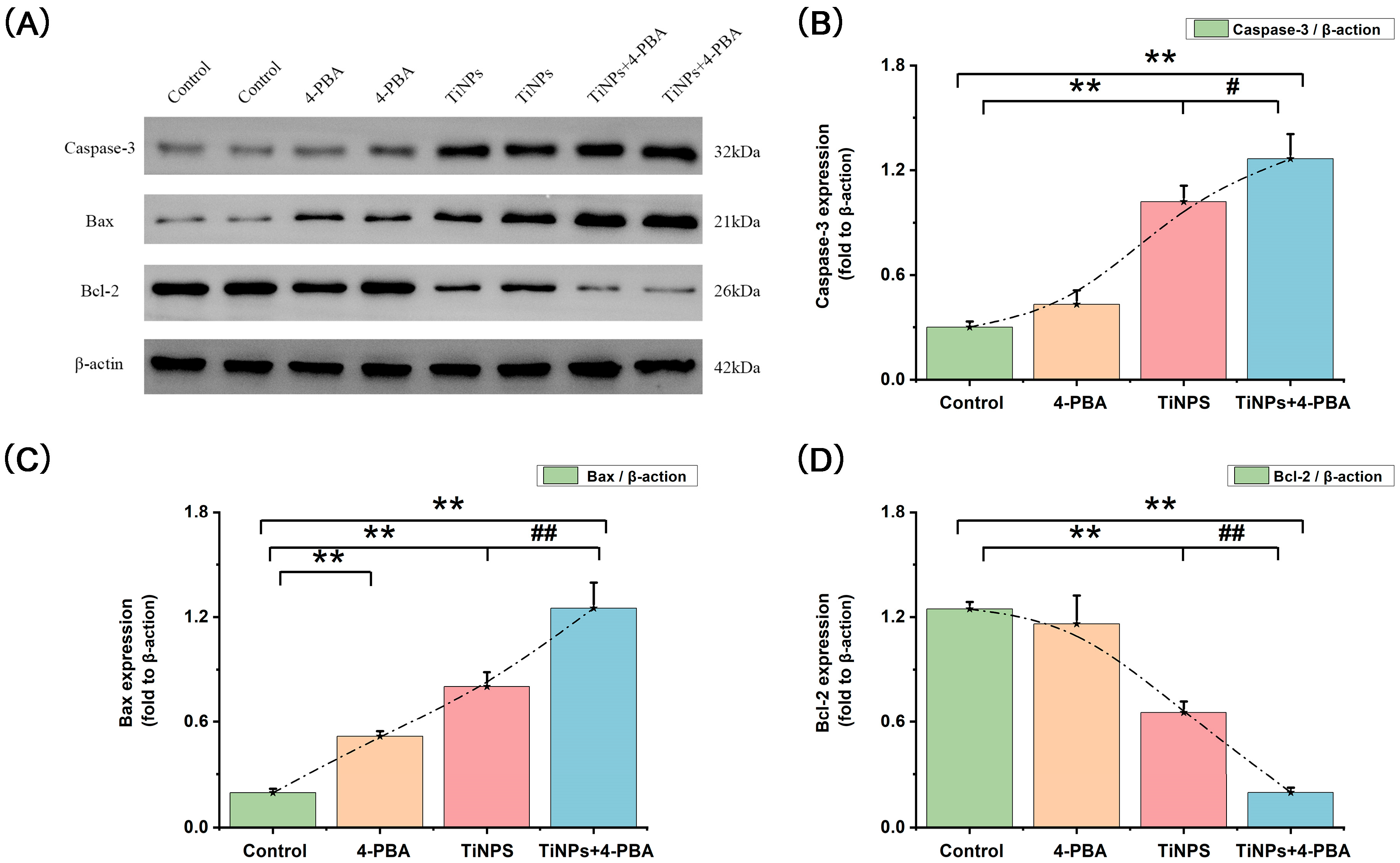
3.3. Regulators of Apoptosis (Bcl-2 and Bax) in 4-PBA-Induced Macrophage Apoptosis Within Osteolytic Interface Periosteum
3.4. Reduction of Inflammatory Factors Facilitated by 4-PBA in Osteolytic Interface Periosteum
3.5. Reduction of Osteoclastogenic Cytokines Facilitated by 4-PBA in Osteolytic Interface Periosteum
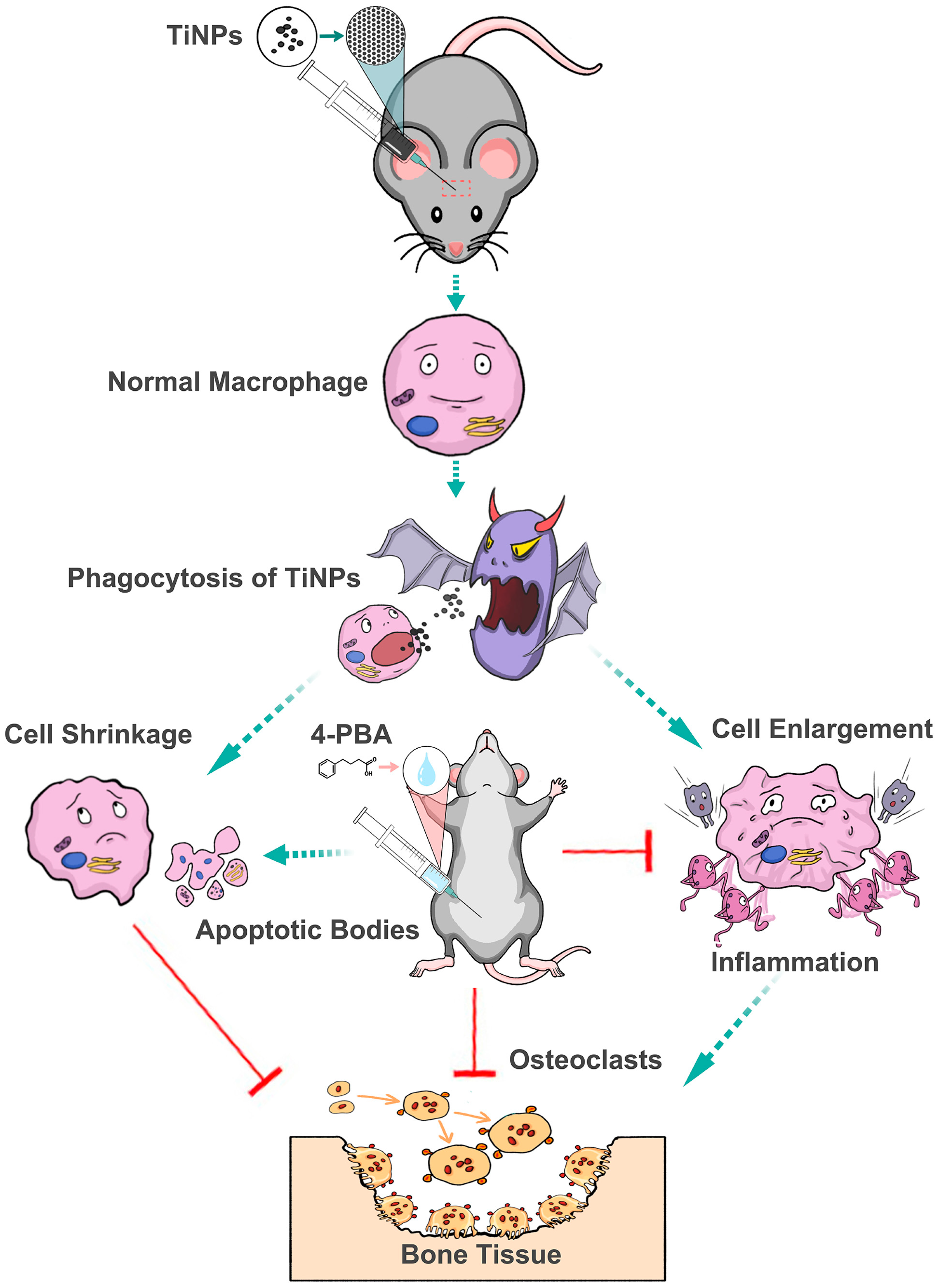
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, N.; Jacobson, M. Outcomes by Race and Ethnicity Following a Medicare Bundled Payment Program for Joint Replacement. JAMA Netw. Open 2024, 7, e2433962. [Google Scholar] [CrossRef] [PubMed]
- Puijk, R.; Lubbe, P.; Sierevelt, I.N.; Kristians, A.E.; Boer, J.; Nolte, P.A. The Diagnostic Characteristic and Reproducibility of Bone Scintigraphy Single-Photon Emission Computed Tomography/Computed Tomography for Diagnosing Aseptic Loosening of Uncemented Total Knee Arthroplasty. J. Arthroplast. 2024, 39, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Lubbeke, A.; Barea, C.; Zingg, M.; Lauper, N.; Hannouche, D.; Garavaglia, G. Radiographic signs and hip pain 5 years after THA with a cemented stem predict future revision for aseptic loosening: A prospective cohort study. Acta Orthop. 2024, 95, 32–38. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, Z.; Jin, J.; Wang, Y.; Bao, N.; Gao, Q.; Zhao, J. SIRT1 protects osteoblasts against particle-induced inflammatory responses and apoptosis in aseptic prosthesis loosening. Acta Biomater. 2017, 49, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, G.; Yang, X.; Dou, W.; Mao, Y.; Zhang, Y.; Shi, X.; Xia, Y.; You, Q.; Liu, M. Inhibitory effects of norcantharidin on titanium particle-induced osteolysis, osteoclast activation and bone resorption via MAPK pathways. Int. Immunopharmacol. 2024, 129, 111655. [Google Scholar] [CrossRef]
- Yu, X.; Wu, Q.; Ren, Z.; Chen, B.; Wang, D.; Yuan, T.; Ding, H.; Wang, Y.; Yuan, G.; Wang, Y.; et al. Kaempferol attenuates wear particle-induced inflammatory osteolysis via JNK and p38-MAPK signaling pathways. J. Ethnopharmacol. 2024, 318, 117019. [Google Scholar] [CrossRef]
- Yu, X.; Ding, H.; Wang, D.; Ren, Z.; Chen, B.; Wu, Q.; Yuan, T.; Liu, Y.; Zhang, L.; Zhao, J.; et al. Particle-induced osteolysis is mediated by endoplasmic reticulum stress-associated osteoblast apoptosis. Chem. Biol. Interact. 2023, 383, 110686. [Google Scholar] [CrossRef]
- Yu, X.; Hu, J.; Yang, X.; Xu, Q.; Chen, H.; Zhan, P.; Zhang, B. Sesamin inhibits RANKL-induced osteoclastogenesis and attenuates LPS-induced osteolysis via suppression of ERK and NF-kappaB signalling pathways. J. Cell. Mol. Med. 2024, 28, e18056. [Google Scholar] [CrossRef]
- Xie, X.; Chen, W.; Xu, M.; Chen, J.; Yang, T.; Wang, C.; Su, Y.; Zhao, J.; Xu, J.; Liu, Q. IKK/NF-kappaB and ROS signal axes are involved in Tenacissoside H mediated inhibitory effects on LPS-induced inflammatory osteolysis. Cell Prolif. 2024, 57, e13535. [Google Scholar] [CrossRef]
- Niu, J.; Bi, F.; Tian, Q.; Tian, K. Melittin Treats Periprosthetic Osteolysis in a Rat Model by Inhibiting the NF-kB Pathway and Regulating the Ratio of Receptor Activator of Nuclear Factor Kappa B Ligand/Osteoprotegerin. J. Arthroplast. 2024, 39, 1845–1855. [Google Scholar] [CrossRef]
- Ma, T.; Chen, S.; Wang, J.; Liang, S.; Chen, M.; Liu, Q.; Zhang, Z.; Liu, G.; Yang, Y.; Hu, Y.; et al. Enhanced Osteolysis Targeted Therapy through Fusion of Exosomes Derived from M2 Macrophages and Bone Marrow Mesenchymal Stem Cells: Modulating Macrophage Polarization. Small 2024, 20, e2303506. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, K.; Bai, M.; Deng, Z.; Gan, J.; Zhou, G.; Qian, H.; Bao, N.; Zhao, J. Probiotics protect mice from CoCrMo particles-induced osteolysis. Int. J. Nanomed. 2017, 12, 5387–5397. [Google Scholar] [CrossRef]
- Landgraeber, S.; von Knoch, M.; Loer, F.; Wegner, A.; Tsokos, M.; Hussmann, B.; Totsch, M. Extrinsic and intrinsic pathways of apoptosis in aseptic loosening after total hip replacement. Biomaterials 2008, 29, 3444–3450. [Google Scholar] [CrossRef]
- Liu, G.; Guo, T.; Zhang, Y.; Liu, N.; Chen, J.; Chen, J.; Zhang, J.; Zhao, J. Apoptotic pathways of macrophages within osteolytic interface membrane in periprosthestic osteolysis after total hip replacement. Apmis 2017, 125, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Li, Y.; Lu, Z.; Zhang, L.; Gao, Z.; Jin, Q. Suppression of titanium particle-induced TNF-alpha expression and apoptosis in human U937 macrophages by siRNA silencing. Int. J. Artif. Organs 2013, 36, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wu, W.; Cao, L.; Huang, Y.; Zhu, Z.; Tang, T.; Dai, K. Pathways of macrophage apoptosis within the interface membrane in aseptic loosening of prostheses. Biomaterials 2011, 32, 9159–9167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tian, Z.; Li, W.; Wang, X.; Man, Z.; Sun, S. Inhibitory effect of quercetin on titanium particle-induced endoplasmic reticulum stress (ERS)-related apoptosis and in vivoosteolysis. Biosci. Rep. 2017, 37, BSR20170961. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, Y.; Wang, Q.; Sun, Y.; Xiong, C.; Cao, L.; Wang, B.; Bao, N.; Zhao, J. Tumor necrosis factor-like weak inducer of apoptosis regulates particle-induced inflammatory osteolysis via the p38 mitogen-activated protein kinase signaling pathway. Mol. Med. Rep. 2015, 12, 1499–1505. [Google Scholar] [CrossRef]
- Di Martino, A.; Valtetsiotis, K.; Rossomando, V.; Brunello, M.; Bordini, B.; D’Agostino, C.; Ruta, F.; Traina, F.; Faldini, C. Efficacy of Bisphosphonates in Total Hip Arthroplasty Patients: Systematic Review and Meta-Analysis. Biomedicines 2024, 12, 1778. [Google Scholar] [CrossRef]
- Lee, A.; Durst, C.R.; Rajaee, S.S. Initiation of Bisphosphonates Prior to Total Joint Arthroplasty Does Not Lower Periprosthetic Fracture Risk. J. Arthroplast. 2024, 39, 1459–1462. [Google Scholar] [CrossRef]
- Mcdonald, C.L.; Lemme, N.J.; Testa, E.J.; Aaron, R.; Hartnett, D.A.; Cohen, E.M. Bisphosphonates in Total Joint Arthroplasty: A Review of Their Use and Complications. Arthroplast. Today 2022, 14, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Pichler, K.; Karall, D.; Kotzot, D.; Steichen-Gersdorf, E.; Rummele-Waibel, A.; Mittaz-Crettol, L.; Wanschitz, J.; Bonafe, L.; Maurer, K.; Superti-Furga, A.; et al. Bisphosphonates in multicentric osteolysis, nodulosis and arthropathy (MONA) spectrum disorder—An alternative therapeutic approach. Sci. Rep. 2016, 6, 34017. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, G.; Ha, D.P.; Wang, J.; Xiong, M.; Lee, A.S. ER chaperone GRP78/BiP translocates to the nucleus under stress and acts as a transcriptional regulator. Proc. Natl. Acad. Sci. USA 2023, 120, e1991519176. [Google Scholar] [CrossRef]
- Liu, G.; Wu, J.; Wang, Y.; Xu, Y.; Xu, C.; Fang, G.; Li, X.; Chen, J. The Differential Expressions and Associations of Intracellular and Extracellular GRP78/Bip with Disease Activity and Progression in Rheumatoid Arthritis. Bioengineering 2025, 12, 58. [Google Scholar] [CrossRef]
- Amin-Wetzel, N.; Saunders, R.A.; Kamphuis, M.J.; Rato, C.; Preissler, S.; Harding, H.P.; Ron, D. A J-Protein Co-chaperone Recruits BiP to Monomerize IRE1 and Repress the Unfolded Protein Response. Cell 2017, 171, 1625–1637. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Z.; Ma, Y.; Liu, G.; Shi, H.; Chen, J.; Dong, L.; Zhao, J.; Zhang, J. Particle-induced osteolysis mediated by endoplasmic reticulum stress in prosthesis loosening. Biomaterials 2013, 34, 2611–2623. [Google Scholar] [CrossRef]
- Liu, G.; Liu, N.; Xu, Y.; Ti, Y.; Chen, J.; Chen, J.; Zhang, J.; Zhao, J. Endoplasmic reticulum stress-mediated inflammatory signaling pathways within the osteolytic periosteum and interface membrane in particle-induced osteolysis. Cell Tissue Res. 2016, 363, 427–447. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ren, Z.; Wang, Y.; Yuan, G.; Hu, J.; Song, L.; Pan, C.; Feng, K.; Liu, Y.; Shao, L.; et al. Kaempferol attenuates particle-induced osteogenic impairment by regulating ER stress via the IRE1alpha-XBP1s pathway. J. Biol. Chem. 2024, 300, 107394. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Im, S.; Kim, H.S.; Lee, D.; Jeong, K.; Ku, J.M.; Nam, T.G. Chemical Chaperones to Inhibit Endoplasmic Reticulum Stress: Implications in Diseases. Drug Des. Dev. Ther. 2022, 16, 4385–4397. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, S.; Chen, B.; Wen, Y.; Zhu, S. Sodium phenylbutyrate antagonizes prostate cancer through the induction of apoptosis and attenuation of cell viability and migration. OncoTargets Ther. 2016, 9, 2825–2833. [Google Scholar] [CrossRef]
- Suaud, L.; Miller, K.; Panichelli, A.E.; Randell, R.L.; Marando, C.M.; Rubenstein, R.C. 4-Phenylbutyrate stimulates Hsp70 expression through the Elp2 component of elongator and STAT-3 in cystic fibrosis epithelial cells. J. Biol. Chem. 2011, 286, 45083–45092. [Google Scholar] [CrossRef] [PubMed]
- de Pablo, S.; Rodriguez-Comas, J.; Diaz-Catalan, D.; Alcarraz-Vizan, G.; Castano, C.; Moreno-Vedia, J.; Montane, J.; Parrizas, M.; Servitja, J.M.; Novials, A. 4-Phenylbutyrate (PBA) treatment reduces hyperglycemia and islet amyloid in a mouse model of type 2 diabetes and obesity. Sci. Rep. 2021, 11, 11878. [Google Scholar] [CrossRef]
- Alqallaf, A.; Cates, D.W.; Render, K.P.; Patel, K.A. Sodium Phenylbutyrate and Taurursodiol: A New Therapeutic Option for the Treatment of Amyotrophic Lateral Sclerosis. Ann. Pharmacother. 2024, 58, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Ebbel, E.N.; Leymarie, N.; Schiavo, S.; Sharma, S.; Gevorkian, S.; Hersch, S.; Matson, W.R.; Costello, C.E. Identification of phenylbutyrate-generated metabolites in Huntington disease patients using parallel liquid chromatography/electrochemical array/mass spectrometry and off-line tandem mass spectrometry. Anal. Biochem. 2010, 399, 152–161. [Google Scholar] [CrossRef]
- Arnold, S.E.; Hendrix, S.; Nicodemus-Johnson, J.; Knowlton, N.; Williams, V.J.; Burns, J.M.; Crane, M.; Mcmanus, A.J.; Vaishnavi, S.N.; Arvanitakis, Z.; et al. Biological effects of sodium phenylbutyrate and taurursodiol in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2024, 10, e12487. [Google Scholar] [CrossRef]
- Tiwari, S.; Gupta, P.; Singh, A.; Chaturvedi, S.; Wahajuddin, M.; Mishra, A.; Singh, S. 4-Phenylbutyrate Mitigates the Motor Impairment and Dopaminergic Neuronal Death During Parkinson’s Disease Pathology via Targeting VDAC1 Mediated Mitochondrial Function and Astrocytes Activation. Neurochem. Res. 2022, 47, 3385–3401. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.H.; Yue, Z.S.; Zheng, W.J.; Shen, H.F.; Zeng, L.R.; Hu, Z.Q.; Xiong, Z.F. 4-Phenylbutyric acid presents therapeutic effect on osteoarthritis via inhibiting cell apoptosis and inflammatory response induced by endoplasmic reticulum stress. Biotechnol. Appl. Biochem. 2018, 65, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, E.G.; Jeong, J.H.; Yoo, W.H. 4-Phenylbutyric acid, a potent endoplasmic reticulum stress inhibitor, attenuates the severity of collagen-induced arthritis in mice via inhibition of proliferation and inflammatory responses of synovial fibroblasts. Kaohsiung J. Med. Sci. 2021, 37, 604–615. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, N.; Shi, T.; Zhou, G.; Wang, Z.; Gan, J.; Guo, T.; Qian, H.; Bao, N.; Zhao, J. ER Stress Mediates TiAl6V4 Particle-Induced Peri-Implant Osteolysis by Promoting RANKL Expression in Fibroblasts. PLoS ONE 2015, 10, e0137774. [Google Scholar] [CrossRef]
- Zhang, L.; Bao, D.; Li, P.; Lu, Z.; Pang, L.; Chen, Z.; Guo, H.; Gao, Z.; Jin, Q. Particle-induced SIRT1 downregulation promotes osteoclastogenesis and osteolysis through ER stress regulation. Biomed. Pharmacother. 2018, 104, 300–306. [Google Scholar] [CrossRef]
- Mostoufi, A.; Baghgoli, R.; Fereidoonnezhad, M. Synthesis, cytotoxicity, apoptosis and molecular docking studies of novel phenylbutyrate derivatives as potential anticancer agents. Comput. Biol. Chem. 2019, 80, 128–137. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, L.; Yang, Y.; Yu, Q. Sodium 4-phenylbutyrate induces apoptosis of human lung carcinoma cells through activating JNK pathway. J. Cell. Biochem. 2004, 93, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.N.; Smith, C.E.; Draper, L.V.; Watne, R.M.; Wommack, A.J.; Vaughan, R.A. Physiological 4-phenylbutyrate promotes mitochondrial biogenesis and metabolism in C2C12 myotubes. Biochimie 2024, 219, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Sanaei, M.; Kavoosi, F.; Poursadgh, S.I. Effect of Sodium Butyrate and Epigallocatechin-3-Gallate on the Genes Expression of Intrinsic Apoptotic Pathway on PA-TU-8902, CFPAC-1, and CAPAN-1 Human Pancreatic Cancer Cell Lines: Epi-drugs and Intrinsic Apoptotic Pathway in Pancreatic Cancer. Galen 2022, 11, e2248. [Google Scholar] [CrossRef]
- Asa’Ad, F.; Thomsen, P.; Kunrath, M.F. The Role of Titanium Particles and Ions in the Pathogenesis of Peri-Implantitis. J. Bone Metab. 2022, 29, 145–154. [Google Scholar] [CrossRef]
- Ivanovski, S.; Bartold, P.M.; Huang, Y.S. The role of foreign body response in peri-implantitis: What is the evidence? Periodontology 2000 2022, 90, 176–185. [Google Scholar] [CrossRef]
- Billi, F.; Campbell, P. Nanotoxicology of metal wear particles in total joint arthroplasty: A review of current concepts. J. Appl. Biomater. Biomech. 2010, 8, 1–6. [Google Scholar]
- Deans, C.F.; Buckner, B.C.; Garvin, K.L. Wear, Osteolysis, and Aseptic Loosening Following Total Hip Arthroplasty in Young Patients with Highly Cross-Linked Polyethylene: A Review of Studies with a Follow-Up of over 15 Years. J. Clin. Med. 2023, 12, 6615. [Google Scholar] [CrossRef]
- Marmotti, A.; Messina, D.; Cykowska, A.; Beltramo, C.; Bellato, E.; Colombero, D.; Agati, G.; Mangiavini, L.; Bruzzone, M.; Dettoni, F.; et al. Periprosthetic osteolysis: A narrative review. J. Biol. Regul. Homeost. Agents 2020, 34, 405–417. [Google Scholar]
- Veronesi, F.; Tschon, M.; Fini, M. Gene Expression in Osteolysis: Review on the Identification of Altered Molecular Pathways in Preclinical and Clinical Studies. Int. J. Mol. Sci. 2017, 18, 499. [Google Scholar] [CrossRef]
- Hodges, N.A.; Sussman, E.M.; Stegemann, J.P. Aseptic and septic prosthetic joint loosening: Impact of biomaterial wear on immune cell function, inflammation, and infection. Biomaterials 2021, 278, 121127. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Gong, G.; Liu, X.; Yin, J. Mechanism of regulating macrophages/osteoclasts in attenuating wear particle-induced aseptic osteolysis. Front. Immunol. 2023, 14, 1274679. [Google Scholar] [CrossRef] [PubMed]
- Bijukumar, D.R.; Salunkhe, S.; Zheng, G.; Barba, M.; Hall, D.J.; Pourzal, R.; Mathew, M.T. Wear particles induce a new macrophage phenotype with the potential to accelerate material corrosion within total hip replacement interfaces. Acta Biomater. 2020, 101, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Catelas, I.; Petit, A.; Zukor, D.J.; Marchand, R.; Yahia, L.; Huk, O.L. Induction of macrophage apoptosis by ceramic and polyethylene particles in vitro. Biomaterials 1999, 20, 625–630. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Z.; Xiao, W.; Gu, Z.; Xiao, G.; Yuan, F.; Chen, F.; Pei, Y.; Li, H.; Su, L. 4-Phenylbutyrate Prevents Endoplasmic Reticulum Stress-Mediated Apoptosis Induced by Heatstroke in the Intestines of Mice. Shock 2020, 54, 102–109. [Google Scholar] [CrossRef]
- Hajimohammadi, S.; Rameshrad, M.; Karimi, G. Exploring the therapeutic effects of sulforaphane: An in-depth review on endoplasmic reticulum stress modulation across different disease contexts. Inflammopharmacology 2024, 32, 2185–2201. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, H.; Wei, S.; Wang, Z.; Ji, G. Inhibition of ER stress-related IRE1alpha/CREB/NLRP1 pathway promotes the apoptosis of human chronic myelogenous leukemia cell. Mol. Immunol. 2018, 101, 377–385. [Google Scholar] [CrossRef]
- Stein, D.; Slobodnik, Z.; Tam, B.; Einav, M.; Akabayov, B.; Berstein, S.; Toiber, D. 4-phenylbutyric acid-Identity crisis; can it act as a translation inhibitor? Aging Cell 2022, 21, e13738. [Google Scholar] [CrossRef]
- Kapuy, O.; Marton, M.; Banhegyi, G.; Vinod, P.K. Multiple system-level feedback loops control life-and-death decisions in endoplasmic reticulum stress. FEBS Lett. 2020, 594, 1112–1123. [Google Scholar] [CrossRef]
- Kim, I.; Xu, W.; Reed, J.C. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008, 7, 1013–1030. [Google Scholar] [CrossRef]
- Xu, C.; Bailly-Maitre, B.; Reed, J.C. Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Investig. 2005, 115, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Ruiz, J.R.; Bettigole, S.E.; Glimcher, L.H. Tumorigenic and Immunosuppressive Effects of Endoplasmic Reticulum Stress in Cancer. Cell 2017, 168, 692–706. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Kaufman, R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature 2008, 454, 455–462. [Google Scholar] [CrossRef]
- Patel, V.A.; Lee, D.J.; Longacre-Antoni, A.; Feng, L.; Lieberthal, W.; Rauch, J.; Ucker, D.S.; Levine, J.S. Apoptotic and necrotic cells as sentinels of local tissue stress and inflammation: Response pathways initiated in nearby viable cells. Autoimmunity 2009, 42, 317–321. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buque, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef]
- Garg, A.D.; Agostinis, P. Cell death and immunity in cancer: From danger signals to mimicry of pathogen defense responses. Immunol. Rev. 2017, 280, 126–148. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, M.; Zhu, R.; Zhou, J.; Ni, L.; Wang, Z.; Liu, N.; Zhu, F.; Shi, T.; Deng, Z.; et al. Hydrogen sulfide protects against particle-induced inflammatory response and osteolysis via SIRT1 pathway in prosthesis loosening. FASEB J. 2020, 34, 3743–3754. [Google Scholar] [CrossRef]
- Deng, Z.; Jin, J.; Wang, Z.; Wang, Y.; Gao, Q.; Zhao, J. The metal nanoparticle-induced inflammatory response is regulated by SIRT1 through NF-kappaB deacetylation in aseptic loosening. Int. J. Nanomed. 2017, 12, 3617–3636. [Google Scholar] [CrossRef]
- Wang, W.; Wu, C.; Tian, B.; Liu, X.; Zhai, Z.; Qu, X.; Jiang, C.; Ouyang, Z.; Mao, Y.; Tang, T.; et al. The inhibition of RANKL-induced osteoclastogenesis through the suppression of p38 signaling pathway by naringenin and attenuation of titanium-particle-induced osteolysis. Int. J. Mol. Sci. 2014, 15, 21913–21934. [Google Scholar] [CrossRef]
- Goodman, S.B.; Gibon, E.; Gallo, J.; Takagi, M. Macrophage Polarization and the Osteoimmunology of Periprosthetic Osteolysis. Curr. Osteoporos. Rep. 2022, 20, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.B.; Gallo, J.; Gibon, E.; Takagi, M. Diagnosis and management of implant debris-associated inflammation. Expert Rev. Med. Devices 2020, 17, 41–56. [Google Scholar] [CrossRef] [PubMed]
 ) were identified according to histomorphological criteria. Original magnification: ×40. Scale bar: 500 μm. (C,D) Histological analysis of calvaria stained with hematoxylin and eosin (HE). The infiltration of inflammatory cells (
) were identified according to histomorphological criteria. Original magnification: ×40. Scale bar: 500 μm. (C,D) Histological analysis of calvaria stained with hematoxylin and eosin (HE). The infiltration of inflammatory cells ( ) and osteoclasts (
) and osteoclasts ( ) in calvaria was observed. Original magnification: ×200. Scale bar 100 μm. (E,F) Histological analysis of calvaria stained with tartrate-resistant acid phosphatase (TRAP). Osteoclasts (
) in calvaria was observed. Original magnification: ×200. Scale bar 100 μm. (E,F) Histological analysis of calvaria stained with tartrate-resistant acid phosphatase (TRAP). Osteoclasts ( ) were identified according to histomorphological criteria. Original magnification: ×40. Scale bar 500 μm. The quantitative data (B,D,F) are derived from unbiased histomorphometric analysis of multiple fields and samples (n = 5 mice per group). The images are representative fields, and the quantification reflects the average of multiple fields and samples. Data are represented as the means ± S.E.M from three independent experiments. *: p < 0.05, **: p < 0.01 versus Control. #: p < 0.05, ##: p < 0.01 versus TiNPs.
) were identified according to histomorphological criteria. Original magnification: ×40. Scale bar 500 μm. The quantitative data (B,D,F) are derived from unbiased histomorphometric analysis of multiple fields and samples (n = 5 mice per group). The images are representative fields, and the quantification reflects the average of multiple fields and samples. Data are represented as the means ± S.E.M from three independent experiments. *: p < 0.05, **: p < 0.01 versus Control. #: p < 0.05, ##: p < 0.01 versus TiNPs.
 ) were identified according to histomorphological criteria. Original magnification: ×40. Scale bar: 500 μm. (C,D) Histological analysis of calvaria stained with hematoxylin and eosin (HE). The infiltration of inflammatory cells (
) were identified according to histomorphological criteria. Original magnification: ×40. Scale bar: 500 μm. (C,D) Histological analysis of calvaria stained with hematoxylin and eosin (HE). The infiltration of inflammatory cells ( ) and osteoclasts (
) and osteoclasts ( ) in calvaria was observed. Original magnification: ×200. Scale bar 100 μm. (E,F) Histological analysis of calvaria stained with tartrate-resistant acid phosphatase (TRAP). Osteoclasts (
) in calvaria was observed. Original magnification: ×200. Scale bar 100 μm. (E,F) Histological analysis of calvaria stained with tartrate-resistant acid phosphatase (TRAP). Osteoclasts ( ) were identified according to histomorphological criteria. Original magnification: ×40. Scale bar 500 μm. The quantitative data (B,D,F) are derived from unbiased histomorphometric analysis of multiple fields and samples (n = 5 mice per group). The images are representative fields, and the quantification reflects the average of multiple fields and samples. Data are represented as the means ± S.E.M from three independent experiments. *: p < 0.05, **: p < 0.01 versus Control. #: p < 0.05, ##: p < 0.01 versus TiNPs.
) were identified according to histomorphological criteria. Original magnification: ×40. Scale bar 500 μm. The quantitative data (B,D,F) are derived from unbiased histomorphometric analysis of multiple fields and samples (n = 5 mice per group). The images are representative fields, and the quantification reflects the average of multiple fields and samples. Data are represented as the means ± S.E.M from three independent experiments. *: p < 0.05, **: p < 0.01 versus Control. #: p < 0.05, ##: p < 0.01 versus TiNPs.
 ) and fibroblasts (
) and fibroblasts ( ) were identified according to histomorphological criteria. Original magnification: 400×. Scale bar: 50 μm. The quantitative data (B,D) are derived from unbiased histomorphometric analysis of multiple fields and samples (n = 5 mice per group). The images are representative fields, and the quantification reflects the average of multiple fields and samples. Data are represented as the means ± S.E.M from three independent experiments. *: p < 0.05, **: p < 0.01 versus Control. #: p < 0.05, ##: p < 0.01 versus TiNPs.
) were identified according to histomorphological criteria. Original magnification: 400×. Scale bar: 50 μm. The quantitative data (B,D) are derived from unbiased histomorphometric analysis of multiple fields and samples (n = 5 mice per group). The images are representative fields, and the quantification reflects the average of multiple fields and samples. Data are represented as the means ± S.E.M from three independent experiments. *: p < 0.05, **: p < 0.01 versus Control. #: p < 0.05, ##: p < 0.01 versus TiNPs.
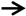 ) and fibroblasts (
) and fibroblasts ( ) were identified according to histomorphological criteria. Original magnification: 400×. Scale bar: 50 μm. The quantitative data (B,D) are derived from unbiased histomorphometric analysis of multiple fields and samples (n = 5 mice per group). The images are representative fields, and the quantification reflects the average of multiple fields and samples. Data are represented as the means ± S.E.M from three independent experiments. *: p < 0.05, **: p < 0.01 versus Control. #: p < 0.05, ##: p < 0.01 versus TiNPs.
) were identified according to histomorphological criteria. Original magnification: 400×. Scale bar: 50 μm. The quantitative data (B,D) are derived from unbiased histomorphometric analysis of multiple fields and samples (n = 5 mice per group). The images are representative fields, and the quantification reflects the average of multiple fields and samples. Data are represented as the means ± S.E.M from three independent experiments. *: p < 0.05, **: p < 0.01 versus Control. #: p < 0.05, ##: p < 0.01 versus TiNPs.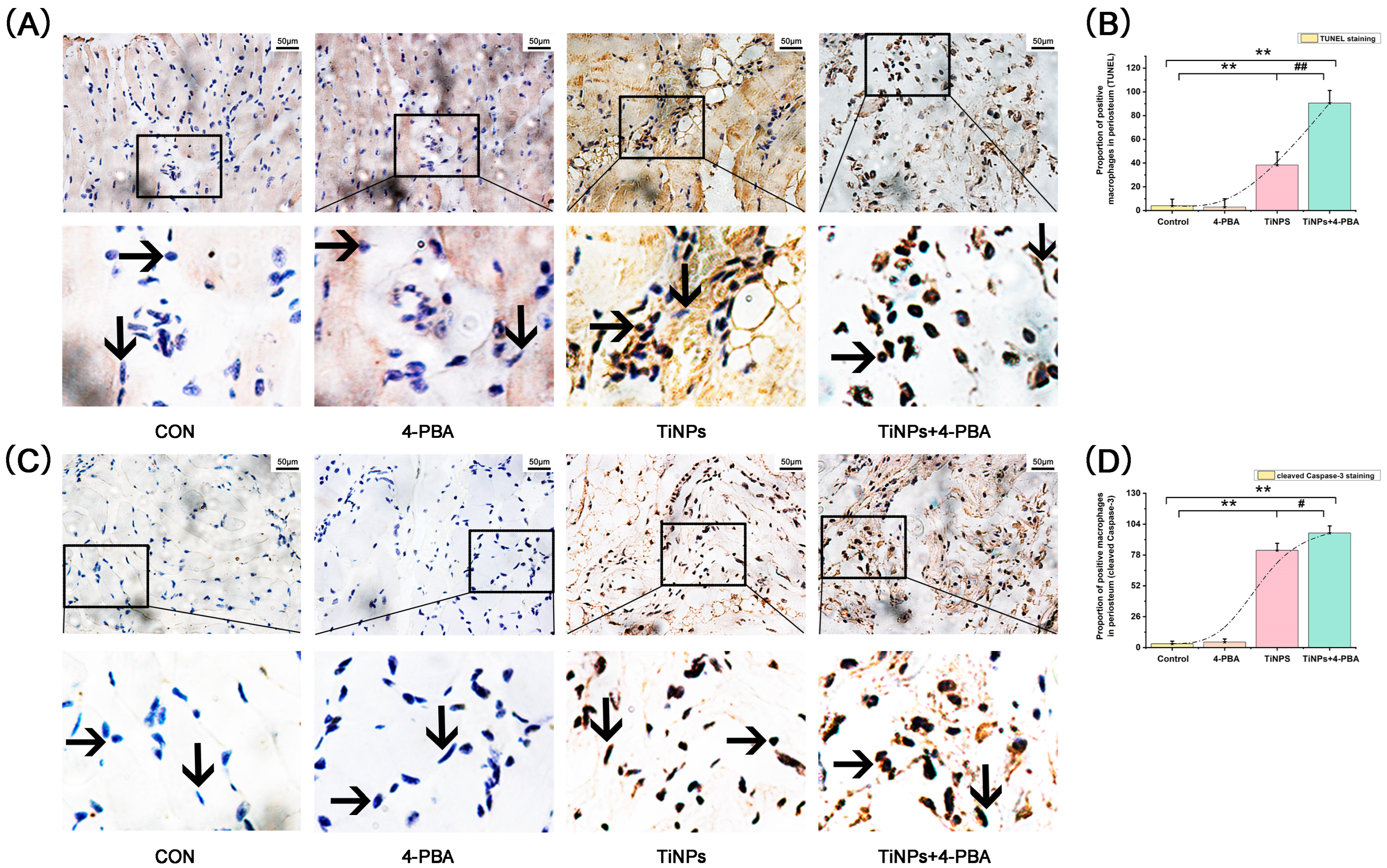
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Gong, H.; Bai, T.; Fu, Y.; Li, X.; Lu, J.; Zhao, J.; Chen, J. Pharmacological Intervention with 4-Phenylbutyrate Ameliorates TiAl6V4 Nanoparticles-Induced Inflammatory Osteolysis by Promoting Macrophage Apoptosis. Bioengineering 2025, 12, 701. https://doi.org/10.3390/bioengineering12070701
Liu G, Gong H, Bai T, Fu Y, Li X, Lu J, Zhao J, Chen J. Pharmacological Intervention with 4-Phenylbutyrate Ameliorates TiAl6V4 Nanoparticles-Induced Inflammatory Osteolysis by Promoting Macrophage Apoptosis. Bioengineering. 2025; 12(7):701. https://doi.org/10.3390/bioengineering12070701
Chicago/Turabian StyleLiu, Guoyin, Haiyang Gong, Tianting Bai, Yahui Fu, Xin Li, Junhao Lu, Jianning Zhao, and Jianmin Chen. 2025. "Pharmacological Intervention with 4-Phenylbutyrate Ameliorates TiAl6V4 Nanoparticles-Induced Inflammatory Osteolysis by Promoting Macrophage Apoptosis" Bioengineering 12, no. 7: 701. https://doi.org/10.3390/bioengineering12070701
APA StyleLiu, G., Gong, H., Bai, T., Fu, Y., Li, X., Lu, J., Zhao, J., & Chen, J. (2025). Pharmacological Intervention with 4-Phenylbutyrate Ameliorates TiAl6V4 Nanoparticles-Induced Inflammatory Osteolysis by Promoting Macrophage Apoptosis. Bioengineering, 12(7), 701. https://doi.org/10.3390/bioengineering12070701






