Scoping Review of Machine Learning Techniques in Marker-Based Clinical Gait Analysis
Abstract
1. Introduction
Related Literature Reviews
- (1)
- Provide a focused review of all machine learning techniques, not exclusively deep learning, used in the analysis of gold standard marker-based 3DGA for supervised and unsupervised learning.
- (2)
- Trends in the use of different ML techniques and comprehensive reporting of the strengths and deficiencies of each method.
- (3)
- Discussion of clinical relevance and opportunities for future research.
2. Materials and Methods
2.1. Eligibility Criteria
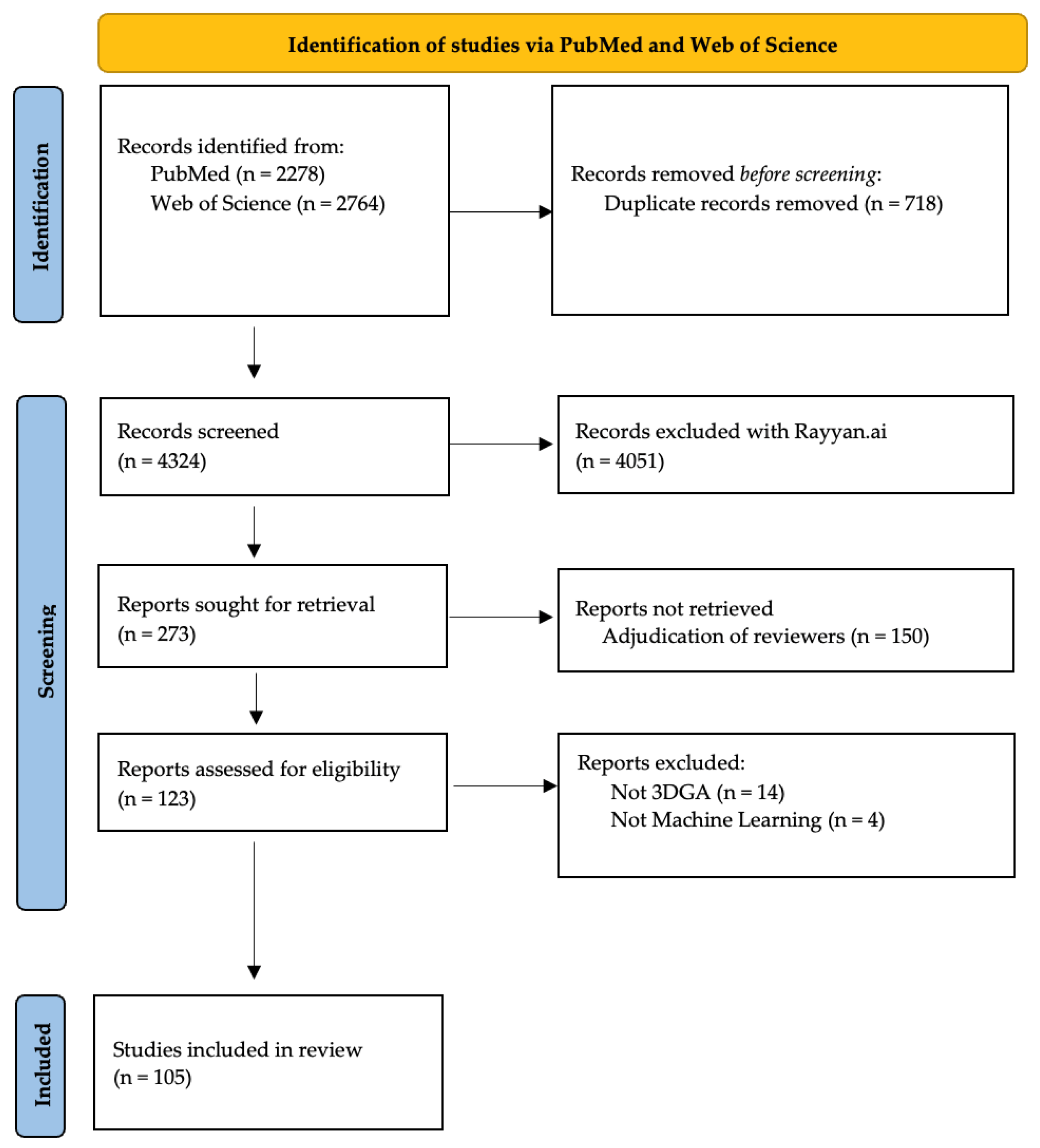
2.2. Selection of Evidence
- Use of marker-based 3D gait analysis data.
- Analysis of data by machine learning techniques.
- Leveraging of machine learning techniques to make predictions or classifications.
2.3. Synthesis of Results
3. Results
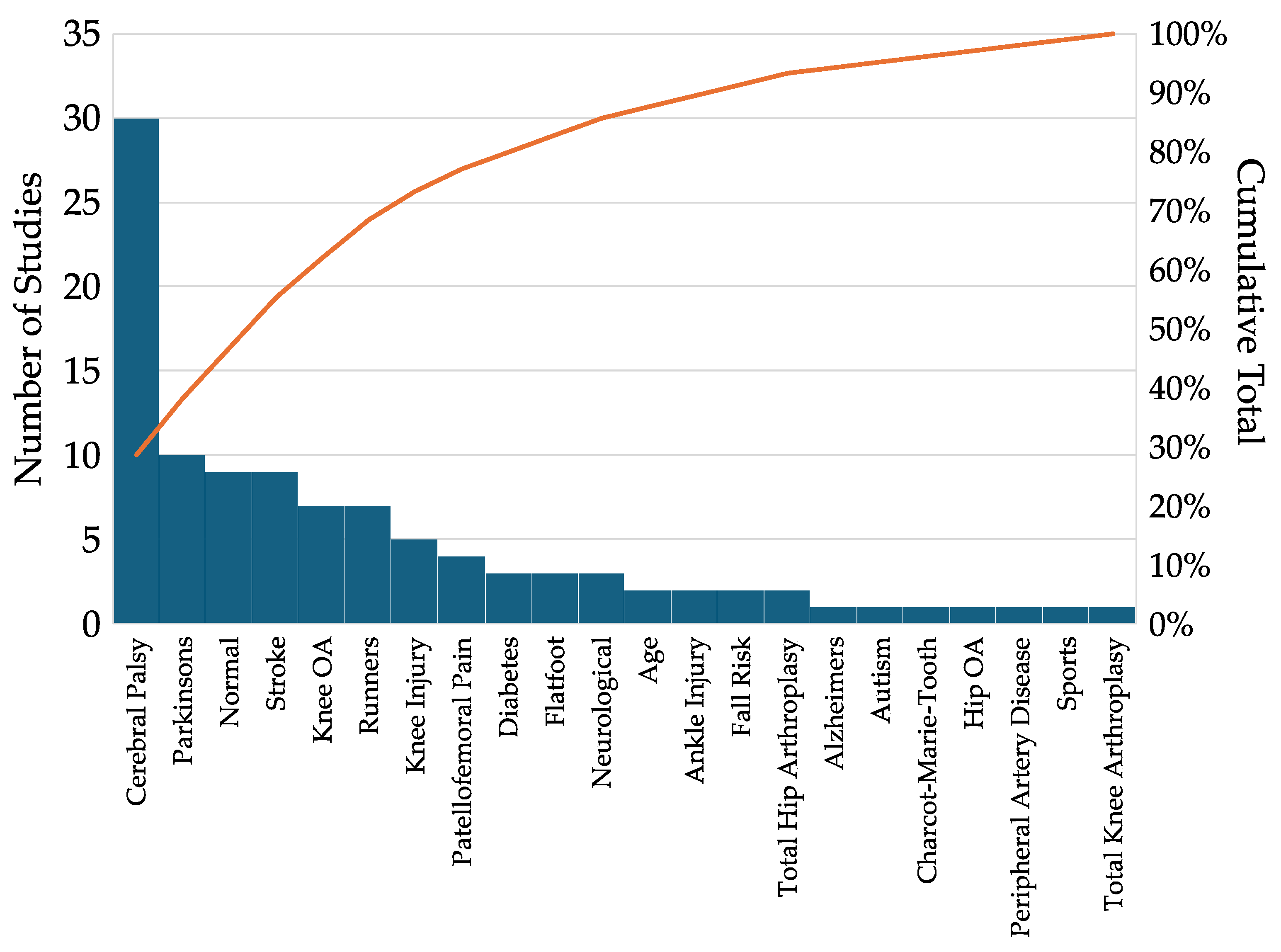
3.1. Distribution of Clinical Conditions
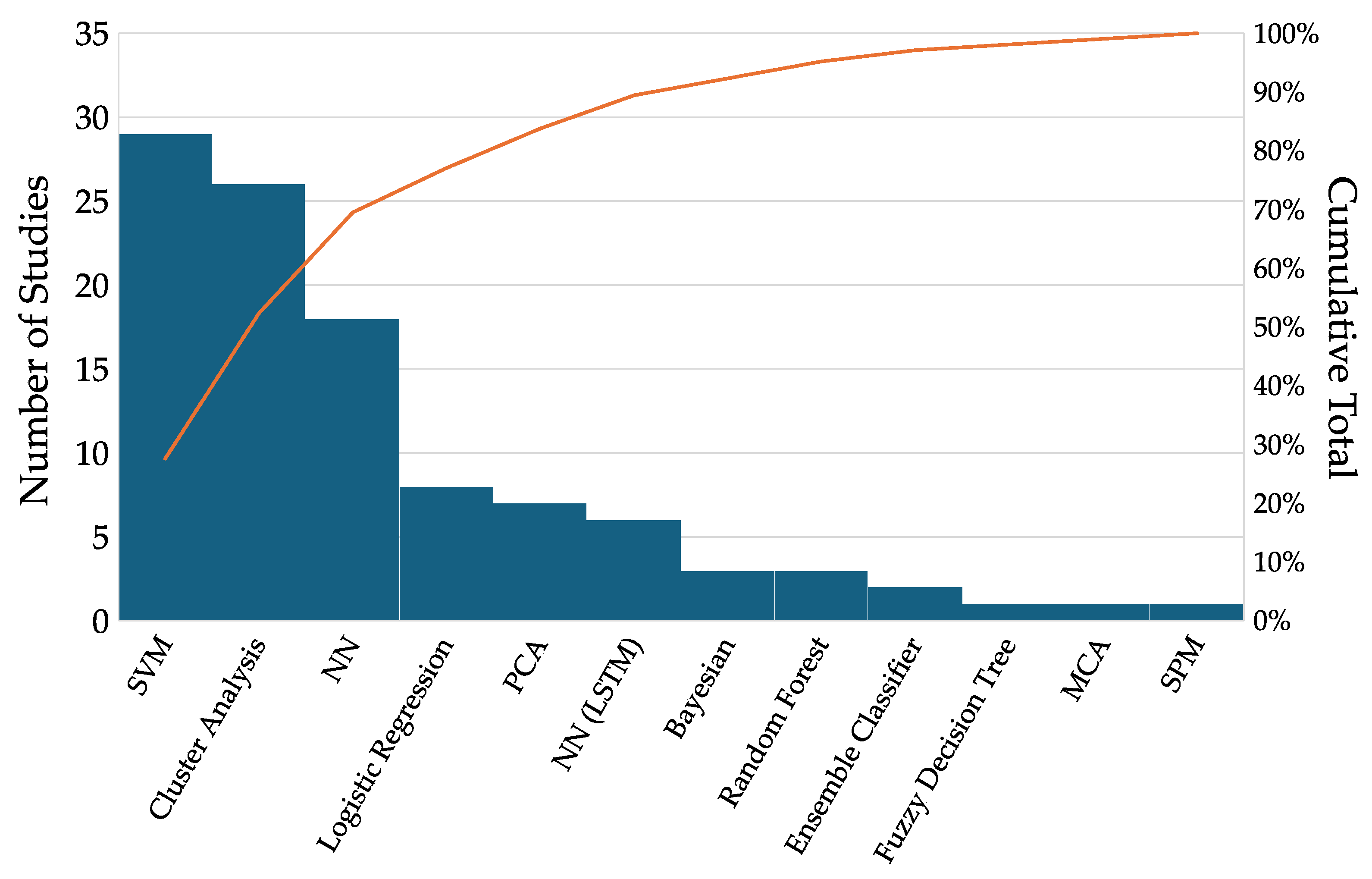
3.2. Machine Learning Techniques
3.3. Support Vector Machines (SVMs, n = 29)
3.4. Cluster Analysis (n = 26)
3.5. Neural Networks (NNs, n = 18)
3.6. Long Short-Term Memory NN (NN (LSTM), n = 6)
3.7. Other Machine Learning Techniques (n = 26)
3.8. Explainable AI (XAI)
3.9. Shapley Additive Explanations (ShAPs)
3.10. Local Interpretable Model-Agnostic Explanations (LIMEs)
3.11. Layer-Wise Relevance Propagation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3DGA | 3D Gait Analysis |
| AI | Artificial Intelligence |
| ML | Machine Learning |
| DL | Deep Learning |
| SVM | Support Vector Machine |
| NN | Neural Network |
| RNN | Recurrent Neural Network |
| LSTM | Long Short-Term Memory |
| XAI | Explainable Artificial Intelligence |
| ShAP | Shapley Additive Explanation |
| LIME | Local Interpretable Model Explanation |
| LRP | Layer-Wise Relevance Propagation |
Appendix A
Appendix A.1. PubMed Search Terms
Appendix A.2. Web of Science Search Terms
References
- States, R.A.; Salem, Y.; Krzak, J.J.; Godwin, E.M.; McMulkin, M.L.; Kaplan, S.L. Three-Dimensional Instrumented Gait Analysis for Children with Cerebral Palsy: An Evidence-Based Clinical Practice Guideline. Pediatr. Phys. Ther. Off. Publ. Sect. Pediatr. Am. Phys. Ther. Assoc. 2024, 36, 182–206. [Google Scholar] [CrossRef]
- Schwartz, M.H.; Ries, A.J.; Georgiadis, A.G.; Kainz, H. Demonstrating the Utility of Instrumented Gait Analysis in the Treatment of Children with Cerebral Palsy. PLoS ONE 2024, 19, e0301230. [Google Scholar] [CrossRef]
- Amene, J.; Krzak, J.J.; Kruger, K.M.; Killen, L.; Graf, A.; Altiok, H.; Smith, P.A.; Harris, G.F. Kinematic Foot Types in Youth with Pes Planovalgus Secondary to Cerebral Palsy. Gait Posture 2019, 68, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Kruger, K.M.; Graf, A.; Flanagan, A.; McHenry, B.D.; Altiok, H.; Smith, P.A.; Harris, G.F.; Krzak, J.J. Segmental Foot and Ankle Kinematic Differences between Rectus, Planus, and Cavus Foot Types. J. Biomech. 2019, 94, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Eskofier, B.M.; Federolf, P.; Kugler, P.F.; Nigg, B.M. Marker-Based Classification of Young-Elderly Gait Pattern Differences via Direct PCA Feature Extraction and SVMs. Comput. Methods Biomech. Biomed. Engin. 2013, 16, 435–442. [Google Scholar] [CrossRef]
- Chia, K.; Fischer, I.; Thomason, P.; Graham, H.; Sangeux, M. A Decision Support System to Facilitate Identification of Musculoskeletal Impairments and Propose Recommendations Using Gait Analysis in Children with Cerebral Palsy. Front. Bioeng. Biotechnol. 2020, 8, 529415. [Google Scholar] [CrossRef]
- Rao, S.; Dietz, F.; Yack, H.J. Kinematics and Kinetics During Gait in Symptomatic and Asymptomatic Limbs of Children with Myelomeningocele. J. Pediatr. Orthop. 2012, 32, 106. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Bian, G.; Hou, Z.; Zhao, J.; Su, G.; Zhou, H.; Peng, L.; Wang, W. Simultaneous Recognition and Assessment of Post-Stroke Hemiparetic Gait by Fusing Kinematic, Kinetic, and Electrophysiological Data. IEEE Trans. NEURAL Syst. Rehabil. Eng. 2018, 26, 856–864. [Google Scholar] [CrossRef]
- Filtjens, B.; Ginis, P.; Nieuwboer, A.; Afzal, M.R.; Spildooren, J.; Vanrumste, B.; Slaets, P. Modelling and Identification of Characteristic Kinematic Features Preceding Freezing of Gait with Convolutional Neural Networks and Layer-Wise Relevance Propagation. BMC Med. Inform. Decis. Mak. 2021, 21, 341. [Google Scholar] [CrossRef]
- Pradhan, A.; Chester, V.; Padhiar, K. Classification of Autism and Control Gait in Children Using Multisegment Foot Kinematic Features. Bioengineering 2022, 9, 552. [Google Scholar] [CrossRef]
- Kokkotis, C.; Moustakidis, S.; Tsatalas, T.; Ntakolia, C.; Chalatsis, G.; Konstadakos, S.; Hantes, M.E.; Giakas, G.; Tsaopoulos, D. Leveraging Explainable Machine Learning to Identify Gait Biomechanical Parameters Associated with Anterior Cruciate Ligament Injury. Sci. Rep. 2022, 12, 6647. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Visscher, R.M.S.; Viehweger, E.; Singh, N.B.; Taylor, W.R.; Vogl, F. A Deep-Learning Approach for Automatically Detecting Gait-Events Based on Foot-Marker Kinematics in Children with Cerebral Palsy-Which Markers Work Best for Which Gait Patterns? PLoS ONE 2022, 17, e0275878. [Google Scholar] [CrossRef] [PubMed]
- Samadi Kohnehshahri, F.; Merlo, A.; Mazzoli, D.; Bò, M.C.; Stagni, R. Machine Learning Applied to Gait Analysis Data in Cerebral Palsy and Stroke: A Systematic Review. Gait Posture 2024, 111, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Slijepcevic, D.; Horst, F.; Lapuschkin, S.; Horsak, B.; Raberger, A.-M.; Kranzl, A.; Samek, W.; Breiteneder, C.; Schöllhorn, W.I.; Zeppelzauer, M. Explaining Machine Learning Models for Clinical Gait Analysis. ACM Trans. Comput. Healthc. 2021, 3, 1–27. [Google Scholar] [CrossRef]
- Khan, A.; Galarraga, O.; Garcia-Salicetti, S.; Vigneron, V. Deep Learning for Quantified Gait Analysis: A Systematic Literature Review. IEEE Access 2024, 12, 138932–138957. [Google Scholar] [CrossRef]
- Khera, P.; Kumar, N. Role of Machine Learning in Gait Analysis: A Review. J. Med. Eng. Technol. 2020, 44, 441–467. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Begg, R.; Kamruzzaman, J. A Machine Learning Approach for Automated Recognition of Movement Patterns Using Basic, Kinetic and Kinematic Gait Data. J. Biomech. 2005, 38, 401–408. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Liu, L. Feature Extraction via KPCA for Classification of Gait Patterns. Hum. Mov. Sci. 2007, 26, 393–411. [Google Scholar] [CrossRef]
- Osis, S.; Kobsar, D.; Leigh, R.; Macaulay, C.; Ferber, R. An Expert System Feedback Tool Improves the Reliability of Clinical Gait Kinematics for Older Adults with Lower Limb Osteoarthritis. Gait Posture 2017, 58, 261–267. [Google Scholar] [CrossRef]
- Phinyomark, A.; Osis, S.; Hettinga, B.; Kobsar, D.; Ferber, R. Gender Differences in Gait Kinematics for Patients with Knee Osteoarthritis. BMC Musculoskelet. Disord. 2016, 17, 157. [Google Scholar] [CrossRef] [PubMed]
- Nuesch, C.; Valderrabano, V.; Huber, C.; von Tscharner, V.; Pagenstert, G. Gait Patterns of Asymmetric Ankle Osteoarthritis Patients. Clin. Biomech. 2012, 27, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Laroche, D.; Tolambiya, A.; Morisset, C.; Maillefert, J.; French, R.; Ornetti, P.; Thomas, E. A Classification Study of Kinematic Gait Trajectories in Hip Osteoarthritis. Comput. Biol. Med. 2014, 55, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Park, J.; Jang, S.; Cho, J. Novel Method of Classification in Knee Osteoarthritis: Machine Learning Application Versus Logistic Regression Model. Ann. Rehabil. Med.-ARM 2020, 44, 415–427. [Google Scholar] [CrossRef]
- Bin Kwon, S.; Han, H.; Lee, M.; Kim, H.; Ku, Y.; Ro, D. Machine Learning-Based Automatic Classification of Knee Osteoarthritis Severity Using Gait Data and Radiographic Images. IEEE Access 2020, 8, 120597–120603. [Google Scholar] [CrossRef]
- Salazar, A.J.; De Castro, O.C.; Bravo, R.J. Novel Approach for Spastic Hemiplegia Classification through the Use of Support Vector Machines. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Piscataway, NJ, USA, 12 July 2004; Volume 2006, pp. 466–469. [Google Scholar] [CrossRef]
- Kamruzzaman, J.; Begg, R. Support Vector Machines and Other Pattern Recognition Approaches to the Diagnosis of Cerebral Palsy Gait. IEEE Trans. Biomed. Eng. 2006, 53, 2479–2490. [Google Scholar] [CrossRef]
- Maurer, C.; Stief, F.; Jonas, A.; Kovac, A.; Groneberg, D.A.; Meurer, A.; Ohlendorf, D. Influence of the Lower Jaw Position on the Running Pattern. PLoS ONE 2015, 10, e0135712. [Google Scholar] [CrossRef]
- Fukuchi, R.; Eskofier, B.; Duarte, M.; Ferber, R. Support Vector Machines for Detecting Age-Related Changes in Running Kinematics. J. Biomech. 2011, 44, 540–542. [Google Scholar] [CrossRef]
- Liu, J.; Powers, C. Classification of Runners with High versus Low Hip Adduction Based on Measures of Pelvis and Femur Morphology. Med. Sci. Sports Exerc. 2022, 54, 590–597. [Google Scholar] [CrossRef]
- Suda, E.; Watari, R.; Matias, A.; Sacco, I. Recognition of Foot-Ankle Movement Patterns in Long-Distance Runners with Different Experience Levels Using Support Vector Machines. Front. Bioeng. Biotechnol. 2020, 8, 576. [Google Scholar] [CrossRef]
- Clermont, C.; Phinyomark, A.; Osis, S.; Ferber, R. Classification of Higher- and Lower-Mileage Runners Based on Running Kinematics. J. Sport Health Sci. 2019, 8, 249–257. [Google Scholar] [CrossRef]
- Sarbaz, Y.; Abedi, B. Presenting A New Decision Support System for Screening Parkinson’s Disease Patients Using Symlet Wavelet. Biomed. Eng.-Appl. Basis Commun. 2019, 31, 1950026. [Google Scholar] [CrossRef]
- Vidya, B.; Sasikumar, P. Gait Based Parkinson’s Disease Diagnosis and Severity Rating Using Multi-Class Support Vector Machine. Appl. Soft Comput. 2021, 113, 107939. [Google Scholar] [CrossRef]
- Christian, J.; Kroll, J.; Strutzenberger, G.; Alexander, N.; Ofner, M.; Schwameder, H. Computer Aided Analysis of Gait Patterns in Patients with Acute Anterior Cruciate Ligament Injury. Clin. Biomech. 2016, 33, 55–60. [Google Scholar] [CrossRef]
- Lai, D.T.H.; Levinger, P.; Begg, R.K.; Gilleard, W.; Palaniswami, M. Identification of Patellofemoral Pain Syndrome Using a Support Vector Machine Approach. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; Volume 2007, pp. 3144–3147. [Google Scholar] [CrossRef]
- Lai, D.T.H.; Levinger, P.; Begg, R.K.; Gilleard, W.L.; Palaniswami, M. Automatic Recognition of Gait Patterns Exhibiting Patellofemoral Pain Syndrome Using a Support Vector Machine Approach. IEEE Trans. Inf. Technol. Biomed. Publ. IEEE Eng. Med. Biol. Soc. 2009, 13, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Lai, D.; Schache, A.; Morris, M. Classification of Gait Disorders Following Traumatic Brain Injury. J. Head Trauma Rehabil. 2015, 30, E13–E23. [Google Scholar] [CrossRef] [PubMed]
- Boyle, A.; Ross, G.B.; Graham, R.B. Machine Learning and Deep Neural Network Architectures for 3D Motion Capture Datasets. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 20–24 July 2020; Volume 2020, pp. 4827–4830. [Google Scholar] [CrossRef]
- Horst, F.; Eekhoff, A.; Newell, K.; Schollhorn, W. Intra-Individual Gait Patterns across Different Time-Scales as Revealed by Means of a Supervised Learning Model Using Kernel-Based Discriminant Regression. PLoS ONE 2017, 12, e0179738. [Google Scholar] [CrossRef]
- Balazia, M.; Sojka, P. Gait Recognition from Motion Capture Data. ACM Trans. Multimed. Comput. Commun. Appl. 2018, 14, 1–18. [Google Scholar] [CrossRef]
- Horst, F.; Kramer, F.; Schafer, B.; Eekhoff, A.; Hegen, P.; Nigg, B.; Schollhorn, W. Daily Changes of Individual Gait Patterns Identified by Means of Support Vector Machines. Gait Posture 2016, 49, 309–314. [Google Scholar] [CrossRef]
- Krzak, J.; Corcos, D.; Damiano, D.; Graf, A.; Hedeker, D.; Smith, P.; Harris, G. Kinematic Foot Types in Youth with Equinovarus Secondary to Hemiplegia. Gait Posture 2015, 41, 402–408. [Google Scholar] [CrossRef]
- Toro, B.; Nester, C.; Farren, P. Cluster Analysis for the Extraction of Sagittal Gait Patterns in Children with Cerebral Palsy. Gait Posture 2007, 25, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Braatz, F.; Metaxiotis, D.; Armbrust, P.; Dreher, T.; Doderlein, L.; Mikut, R. Gait Analysis May Help to Distinguish Hereditary Spastic Paraplegia from Cerebral Palsy. Gait Posture 2011, 33, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Domagalska-Szopa, M.; Szopa, A. Gait Pattern Differences Among Children with Bilateral Cerebral Palsy. Front. Neurol. 2019, 10, 183. [Google Scholar] [CrossRef]
- Kuntze, G.; Nettel-Aguirre, A.; Ursulak, G.; Robu, I.; Bowal, N.; Goldstein, S.; Emery, C.A. Multi-Joint Gait Clustering for Children and Youth with Diplegic Cerebral Palsy. PLoS ONE 2018, 13, e0205174. [Google Scholar] [CrossRef]
- Darbandi, H.; Baniasad, M.; Baghdadi, S.; Khandan, A.; Vafaee, A.; Farahmand, F. Automatic Classification of Gait Patterns in Children with Cerebral Palsy Using Fuzzy Clustering Method. Clin. Biomech. Bristol Avon 2020, 73, 189–194. [Google Scholar] [CrossRef]
- Carriero, A.; Zavatsky, A.; Stebbins, J.; Theologis, T.; Shefelbine, S. Determination of Gait Patterns in Children with Spastic Diplegic Cerebral Palsy Using Principal Components. Gait Posture 2009, 29, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Pauk, J.; Minta-Bielecka, K. Gait Patterns Classification Based on Cluster and Bicluster Analysis. Biocybern. Biomed. Eng. 2016, 36, 391–396. [Google Scholar] [CrossRef]
- Rozumalski, A.; Schwartz, M.H. Crouch Gait Patterns Defined Using K-Means Cluster Analysis Are Related to Underlying Clinical Pathology. Gait Posture 2009, 30, 155–160. [Google Scholar] [CrossRef]
- Abbasi, L.; Rojhani-Shirazi, Z.; Razeghi, M.; Raeisi-Shahraki, H. Kinematic Cluster Analysis of the Crouch Gait Pattern in Children with Spastic Diplegic Cerebral Palsy Using Sparse K-Means Method. Clin. Biomech. 2021, 81, 105248. [Google Scholar] [CrossRef]
- Armand, S.; Watelain, E.; Mercier, M.; Lensel, G.; Lepoutre, F.-X. Identification and Classification of Toe-Walkers Based on Ankle Kinematics, Using a Data-Mining Method. Gait Posture 2006, 23, 240–248. [Google Scholar] [CrossRef]
- Schmidt, S.; Bohm, H.; Dussa, C.; Bienias, K.; Fujak, A. Characteristic 3D Foot Motion Patterns during Gait of Patients with Charcot-Marie-Tooth Identified by Cluster Analysis*. Gait Posture 2023, 104, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Sawacha, Z.; Sartor, C.D.; Yi, L.C.; Guiotto, A.; Spolaor, F.; Sacco, I.C.N. Clustering Classification of Diabetic Walking Abnormalities: A New Approach Taking into Account Intralimb Coordination Patterns. Gait Posture 2020, 79, 33–40. [Google Scholar] [CrossRef]
- Sawacha, Z.; Guarneri, G.; Avogaro, A.; Cobelli, C. A New Classification of Diabetic Gait Pattern Based on Cluster Analysis of Biomechanical Data. J. Diabetes Sci. Technol. 2010, 4, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Okamura, K.; Hasegawa, M.; Ikeda, T.; Fukuda, K.; Egawa, K.; Kanai, S. Classification of Medial Longitudinal Arch Kinematics during Running and Characteristics of Foot Muscle Morphology in Novice Runners with Pronated Foot. Gait Posture 2022, 93, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Böhm, H.; Oestreich, C.; Rethwilm, R.; Federolf, P.; Döderlein, L.; Fujak, A.; Dussa, C.U. Cluster Analysis to Identify Foot Motion Patterns in Children with Flexible Flatfeet Using Gait Analysis-A Statistical Approach to Detect Decompensated Pathology? Gait Posture 2019, 71, 151–156. [Google Scholar] [CrossRef]
- Young-Shand, K.; Roy, P.; Dunbar, M.; Abidi, S.; Wilson, J. Gait Biomechanics Phenotypes among Total Knee Arthroplasty Candidates by Machine Learning Cluster Analysis. J. Orthop. Res. 2023, 41, 335–344. [Google Scholar] [CrossRef]
- Leporace, G.; Gonzalez, F.; Metsavaht, L.; Motta, M.; Carpes, F.; Chahla, J.; Luzo, M. Are There Different Gait Profiles in Patients with Advanced Knee Osteoarthritis? A Machine Learning Approach. Clin. Biomech. 2021, 88, 105447. [Google Scholar] [CrossRef]
- Phinyomark, A.; Osis, S.; Hettinga, B.; Ferber, R. Kinematic Gait Patterns in Healthy Runners: A Hierarchical Cluster Analysis. J. Biomech. 2015, 48, 3897–3904. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.-H.; Kim, S.-J.; Choi, M.-T. Pathological Gait Clustering in Post-Stroke Patients Using Motion Capture Data. Gait Posture 2022, 94, 210–216. [Google Scholar] [CrossRef]
- Mulroy, S.; Gronley, J.; Weiss, W.; Newsam, C.; Perry, J. Use of Cluster Analysis for Gait Pattern Classification of Patients in the Early and Late Recovery Phases Following Stroke. Gait Posture 2003, 18, 114–125. [Google Scholar] [CrossRef]
- Chantraine, F.; Schreiber, C.; Pereira, J.; Kaps, J.; Dierick, F. Classification of Stiff-Knee Gait Kinematic Severity after Stroke Using Retrospective k-Means Clustering Algorithm. J. Clin. Med. 2022, 11, 6270. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Ng, H.; Yap, T.; Ho, C. Gait Analysis and Classification on Subjects with Parkinson’s Disease. J. Teknol. 2015, 77, 79–85. [Google Scholar]
- Serrao, M.; Chini, G.; Bergantino, M.; Sarnari, D.; Casali, C.; Conte, C.; Ranavolo, A.; Marcotulli, C.; Rinaldi, M.; Coppola, G.; et al. Identification of Specific Gait Patterns in Patients with Cerebellar Ataxia, Spastic Paraplegia, and Parkinson’s Disease: A Non-Hierarchical Cluster Analysis. Hum. Mov. Sci. 2018, 57, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Shin, H.; Jung, M. Joint Motion Pattern Classification by Cluster Analysis of Kinematic, Demographic, and Subjective Variables. Appl. Ergon. 2013, 44, 636–642. [Google Scholar] [CrossRef]
- Visani, G.; Bagli, E.; Chesani, F.; Poluzzi, A.; Capuzzo, D. Statistical Stability Indices for LIME: Obtaining Reliable Explanations for Machine Learning Models. J. Oper. Res. Soc. 2022, 73, 91–101. [Google Scholar] [CrossRef]
- Barton, G.; Lisboa, P.; Lees, A.; Attfield, S. Gait Quality Assessment Using Self-Organising Artificial Neural Networks. Gait Posture 2007, 25, 374–379. [Google Scholar] [CrossRef]
- Barton, G.J.; Hawken, M.B.; Scott, M.A.; Schwartz, M.H. Leaving Hip Rotation out of a Conventional 3D Gait Model Improves Discrimination of Pathological Gait in Cerebral Palsy: A Novel Neural Network Analysis. Gait Posture 2019, 70, 48–52. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y. Application of Supervised Machine Learning Algorithms in the Classification of Sagittal Gait Patterns of Cerebral Palsy Children with Spastic Diplegia. Comput. Biol. Med. 2019, 106, 33–39. [Google Scholar] [CrossRef]
- Ferrari, A.; Bergamini, L.; Guerzoni, G.; Calderara, S.; Bicocchi, N.; Vitetta, G.; Borghi, C.; Neviani, R.; Ferrari, A. Gait-Based Diplegia Classification Using LSMT Networks. J. Healthc. Eng. 2019, 2019, 3796898. [Google Scholar] [CrossRef]
- Filtjens, B.; Nieuwboer, A.; D’cruz, N.; Spildooren, J.; Slaets, P.; Vanrumste, B. A Data-Driven Approach for Detecting Gait Events during Turning in People with Parkinson’s Disease and Freezing of Gait. Gait Posture 2020, 80, 130–136. [Google Scholar] [CrossRef]
- Filtjens, B.; Ginis, P.; Nieuwboer, A.; Slaets, P.; Vanrumste, B. Automated Freezing of Gait Assessment with Marker-Based Motion Capture and Multi-Stage Spatial-Temporal Graph Convolutional Neural Networks. J. Neureng. Rehabil. 2022, 19, 48. [Google Scholar] [CrossRef]
- Ertugrul, O.; Kaya, Y.; Tekin, R.; Almali, M. Detection of Parkinson’s Disease by Shifted One Dimensional Local Binary Patterns from Gait. Expert Syst. Appl. 2016, 56, 156–163. [Google Scholar] [CrossRef]
- Varrecchia, T.; Castiglia, S.F.; Ranavolo, A.; Conte, C.; Tatarelli, A.; Coppola, G.; Di Lorenzo, C.; Draicchio, F.; Pierelli, F.; Serrao, M. An Artificial Neural Network Approach to Detect Presence and Severity of Parkinson’s Disease via Gait Parameters. PLoS ONE 2021, 16, e0244396. [Google Scholar] [CrossRef]
- Rueangsirarak, W.; Zhang, J.; Aslam, N.; Ho, E.; Shum, H. Automatic Musculoskeletal and Neurological Disorder Diagnosis with Relative Joint Displacement From Human Gait. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 2387–2396. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Clifford, G.D.; Genias, I.; Bernhard, D.; Esper, C.D.; Factor, S.A.; McKay, J.L. An Explainable Spatial-Temporal Graphical Convolutional Network to Score Freezing of Gait in Parkinsonian Patients. MedRxiv Prepr. Serv. Health Sci. 2023, 23, 1766. [Google Scholar] [CrossRef]
- Kaczmarczyk, K.; Wit, A.; Krawczyk, M.; Zaborski, J. Gait Classification in Post-Stroke Patients Using Artificial Neural Networks. Gait Posture 2009, 30, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, P.; Karunakar, A.; Anitha, H.; Pradhan, N. Classification of Gait Signals into Different Neurodegenerative Diseases Using Statistical Analysis and Recurrence Quantification Analysis. Pattern Recognit. Lett. 2020, 139, 10–16. [Google Scholar] [CrossRef]
- Jeon, S.; Lee, K.M.; Koo, S. Anomalous Gait Feature Classification From 3-D Motion Capture Data. IEEE J. Biomed. Health Inform. 2022, 26, 696–703. [Google Scholar] [CrossRef]
- Miller, A. Gait Event Detection Using a Multilayer Neural Network. Gait Posture 2009, 29, 542–545. [Google Scholar] [CrossRef]
- Horst, F.; Lapuschkin, S.; Samek, W.; Muller, K.; Schollhorn, W. Explaining the Unique Nature of Individual Gait Patterns with Deep Learning. Sci. Rep. 2019, 9, 2391. [Google Scholar] [CrossRef]
- Zeng, W.; Ma, L.; Yuan, C.; Liu, F.; Wang, Q.; Wang, Y.; Zhang, Y. Classification of Asymptomatic and Osteoarthritic Knee Gait Patterns Using Gait Analysis via Deterministic Learning. Artif. Intell. Rev. 2019, 52, 449–467. [Google Scholar] [CrossRef]
- Zeng, W.; Ismail, S.; Lim, Y.; Smith, R.; Pappas, E. Classification of Gait Patterns Using Kinematic and Kinetic Features, Gait Dynamics and Neural Networks in Patients with Unilateral Anterior Cruciate Ligament Deficiency. Neural Process. Lett. 2019, 50, 887–909. [Google Scholar] [CrossRef]
- Al-Ramini, A.; Hassan, M.; Fallahtafti, F.; Takallou, M.A.; Rahman, H.; Qolomany, B.; Pipinos, I.I.; Alsaleem, F.; Myers, S.A. Machine Learning-Based Peripheral Artery Disease Identification Using Laboratory-Based Gait Data. Sensors 2022, 22, 7432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, S.; Liu, J.; Fan, Q.; Zhou, Y. A Combined Low-Rank Matrix Completion and CDBN-LSTM Based Classification Model for Lower Limb Motion Function. IEEE Access 2020, 8, 205436–205443. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, C.; Zheng, B.; Guo, Q.; Yu, Y.; Zhang, D.; Wulamu, A. Spatiotemporal and Kinematic Characteristics Augmentation Using Dual-GAN for Ankle Instability Detection. Math. Biosci. Eng. 2022, 19, 10037–10059. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, C.; Zheng, B.; Guo, Q.; Zhang, Z.; Wulamu, A.; Zhang, D. Synthesizing Foot and Ankle Kinematic Characteristics for Lateral Collateral Ligament Injuries Detection. IEEE Access 2020, 8, 188429–188440. [Google Scholar] [CrossRef]
- De Laet, T.; Papageorgiou, E.; Nieuwenhuys, A.; Desloovere, K. Does Expert Knowledge Improve Automatic Probabilistic Classification of Gait Joint Motion Patterns in Children with Cerebral Palsy? PLoS ONE 2017, 12, e0178378. [Google Scholar] [CrossRef]
- Bisele, M.; Bencsik, M.; Lewis, M.; Barnett, C. Optimisation of a Machine Learning Algorithm in Human Locomotion Using Principal Component and Discriminant Function Analyses. PLoS ONE 2017, 12, e0183990. [Google Scholar] [CrossRef]
- Mc Ardle, R.; Galna, B.; Donaghy, P.; Thomas, A.; Rochester, L. Do Alzheimer’s and Lewy Body Disease Have Discrete Pathological Signatures of Gait? Alzheimers Dement. 2019, 15, 1367–1377. [Google Scholar] [CrossRef]
- White, H.; Uhl, T.L.; Augsburger, S. Do Three Different Passive Assessments of Quadriceps Spasticity Relate to the Functional Activity of Walking for Children Diagnosed with Cerebral Palsy? Neurosci. J. 2015, 2015, 872015. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, O.; Fabre, J.M.; Wood, R.H.; Garcia, S.U.; Ivey, K.M.; McCann, E.D. Classification of Older Adults with/without a Fall History Using Machine Learning Methods. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; Volume 2015, pp. 6760–6763. [Google Scholar] [CrossRef]
- Foucher, K.C. Preoperative Gait Mechanics Predict Clinical Response to Total Hip Arthroplasty. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2017, 35, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Kaptein, R.G.; Wezenberg, D.; IJmker, T.; Houdijk, H.; Beek, P.J.; Lamoth, C.J.C.; Daffertshofer, A. Shotgun Approaches to Gait Analysis: Insights & Limitations. J. Neuroeng. Rehabil. 2014, 11, 120. [Google Scholar] [CrossRef]
- Galarraga, C.O.A.; Vigneron, V.; Dorizzi, B.; Khouri, N.; Desailly, E. Predicting Postoperative Gait in Cerebral Palsy. Gait Posture 2017, 52, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Pedoia, V.; Haefeli, J.; Morioka, K.; Teng, H.-L.; Nardo, L.; Souza, R.B.; Ferguson, A.R.; Majumdar, S. MRI and Biomechanics Multidimensional Data Analysis Reveals R(2)-R(1ρ) as an Early Predictor of Cartilage Lesion Progression in Knee Osteoarthritis. J. Magn. Reson. Imaging JMRI 2018, 47, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Osis, S.T.; Hettinga, B.A.; Ferber, R. Predicting Ground Contact Events for a Continuum of Gait Types: An Application of Targeted Machine Learning Using Principal Component Analysis. Gait Posture 2016, 46, 86–90. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Gao, L.; Hassan, E.; Li, H.; Li, S.; Liao, F. Quantitative Analysis of Gait Coordination Based on Gait Events in Children with Cerebral Palsy. Am. J. Phys. Med. Rehabil. 2012, 91, 671–680. [Google Scholar] [CrossRef]
- Phinyomark, A.; Hettinga, B.; Osis, S.; Ferber, R. Do Intermediate- and Higher-Order Principal Components Contain Useful Information to Detect Subtle Changes in Lower Extremity Biomechanics during Running? Hum. Mov. Sci. 2015, 44, 91–101. [Google Scholar] [CrossRef]
- Dillmann, U.; Holzhoffer, C.; Johann, Y.; Bechtel, S.; Gräber, S.; Massing, C.; Spiegel, J.; Behnke, S.; Bürmann, J.; Louis, A.K. Principal Component Analysis of Gait in Parkinson’s Disease: Relevance of Gait Velocity. Gait Posture 2014, 39, 882–887. [Google Scholar] [CrossRef]
- Quan, W.; Zhou, H.; Xu, D.; Li, S.; Baker, J.; Gu, Y. Competitive and Recreational Running Kinematics Examined Using Principal Components Analysis. Healthcare 2021, 9, 1321. [Google Scholar] [CrossRef]
- Robbins, S.; Morelli, M.; Martineau, P.; St-Onge, N.; Boily, M.; Dimentberg, R.; Antoniou, J. A Comparison of Muscle Activation and Knee Mechanics during Gait between Patients with Non-Traumatic and Post-Traumatic Knee Osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1033–1042. [Google Scholar] [CrossRef]
- Bonnefoy-Mazure, A.; Sagawa, Y.; Lascombes, P.; De Coulon, G.; Armand, S. Identification of Gait Patterns in Individuals with Cerebral Palsy Using Multiple Correspondence Analysis. Res. Dev. Disabil. 2013, 34, 2684–2693. [Google Scholar] [CrossRef] [PubMed]
- Goulermas, J.; Findlow, A.; Nester, C.; Howard, D.; Bowker, P. Automated Design of Robust Discriminant Analysis Classifier for Foot Pressure Lesions Using Kinematic Data. IEEE Trans. Biomed. Eng. 2005, 52, 1549–1562. [Google Scholar] [CrossRef]
- MacWilliams, B.A.; Carroll, K.L.; Stotts, A.K.; Kerr, L.M.; Schwartz, M.H. Discrimination between Hereditary Spastic Paraplegia and Cerebral Palsy Based on Gait Analysis Data: A Machine Learning Approach. Gait Posture 2022, 98, 34–38. [Google Scholar] [CrossRef]
- Van Gestel, L.; De Laet, T.; Di Lello, E.; Bruyninckx, H.; Molenaers, G.; Van Campenhout, A.; Aertbelien, E.; Schwartz, M.; Wambacq, H.; De Cock, P.; et al. Probabilistic Gait Classification in Children with Cerebral Palsy: A Bayesian Approach. Res. Dev. Disabil. 2011, 32, 2542–2552. [Google Scholar] [CrossRef]
- Armand, S.; Watelain, E.; Roux, E.; Mercier, M.; Lepoutre, F. Linking Clinical Measurements and Kinematic Gait Patterns of Toe-Walking Using Fuzzy Decision Trees. Gait Posture 2007, 25, 475–484. [Google Scholar] [CrossRef]
- Dindorf, C.; Teufl, W.; Taetz, B.; Becker, S.; Bleser, G.; Frohlich, M. Feature Extraction and Gait Classification in Hip Replacement Patients on the Basis of Kinematic Waveform Data. Biomed. Hum. Kinet. 2021, 13, 177–186. [Google Scholar] [CrossRef]
- Kwon, S.B.; Ro, D.H.; Song, M.K.; Han, H.-S.; Lee, M.C.; Kim, H.C. Identifying Key Gait Features Associated with the Radiological Grade of Knee Osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1755–1760. [Google Scholar] [CrossRef]
- Sung, J.; Han, S.; Park, H.; Hwang, S.; Lee, S.; Park, J.; Youn, I. Classification of Stroke Severity Using Clinically Relevant Symmetric Gait Features Based on Recursive Feature Elimination with Cross-Validation. IEEE Access 2022, 10, 119437–119447. [Google Scholar] [CrossRef]
- Wang, S.; Nguyen, T.; Bhatt, T. Trip-Related Fall Risk Prediction Based on Gait Pattern in Healthy Older Adults: A Machine-Learning Approach. Sensors 2023, 23, 5536. [Google Scholar] [CrossRef]
- Honert, E.; Pataky, T. Timing of Gait Events Affects Whole Trajectory Analyses: A Statistical Parametric Mapping Sensitivity Analysis of Lower Limb Biomechanics. J. Biomech. 2021, 119, 110329. [Google Scholar] [CrossRef]
- Osborne, J.W.; Costello, A.B. Sample size and subject to item ratio in principal components analysis. Pract. Assess. Res. Eval. 2004, 9, 11. [Google Scholar] [CrossRef]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 5th ed.; Allyn & Bacon/Pearson Education: Boston, MA, USA; p. xxvii, 980. 2007; ISBN 978-0-205-45938-4. [Google Scholar]
- Vabalas, A.; Gowen, E.; Poliakoff, E.; Casson, A.J. Machine Learning Algorithm Validation with a Limited Sample Size. PLoS ONE 2019, 14, e0224365. [Google Scholar] [CrossRef]
- Alwosheel, A.; van Cranenburgh, S.; Chorus, C.G. Is Your Dataset Big Enough? Sample Size Requirements When Using Artificial Neural Networks for Discrete Choice Analysis. J. Choice Model. 2018, 28, 167–182. [Google Scholar] [CrossRef]
- AI Act|Shaping Europe’s Digital Future. Available online: https://digital-strategy.ec.europa.eu/en/policies/regulatory-framework-ai (accessed on 22 April 2025).
- US Food and Drug Administration. Artificial Intelligence and Machine Learning in Software as a Medical Device; FDA: Silver Spring, MD, USA, 2025. [Google Scholar]
- D’Souza, S.; Siebert, T.; Fohanno, V. A Comparison of Lower Body Gait Kinematics and Kinetics between Theia 3D Markerless and Marker-Based Models in Healthy Subjects and Clinical Patients. Sci. Rep. 2024, 14, 29154. [Google Scholar] [CrossRef] [PubMed]
- Wren, T.A.L.; Isakov, P.; Rethlefsen, S.A. Comparison of Kinematics between Theia Markerless and Conventional Marker-Based Gait Analysis in Clinical Patients. Gait Posture 2023, 104, 9–14. [Google Scholar] [CrossRef]
- Slijepcevic, D.; Zeppelzauer, M.; Unglaube, F.; Kranzl, A.; Breiteneder, C.; Horsak, B. Explainable Machine Learning in Human Gait Analysis: A Study on Children with Cerebral Palsy. IEEE Access 2023, 11, 65906–65923. [Google Scholar] [CrossRef]
- Özateş, M.E.; Yaman, A.; Salami, F.; Campos, S.; Wolf, S.I.; Schneider, U. Identification and Interpretation of Gait Analysis Features and Foot Conditions by Explainable AI. Sci. Rep. 2024, 14, 5998. [Google Scholar] [CrossRef]
- Ahmed, S.; Nielsen, I.E.; Tripathi, A.; Siddiqui, S.; Ramachandran, R.P.; Rasool, G. Transformers in Time-Series Analysis: A Tutorial. Circuits Syst. Signal Process. 2023, 42, 7433–7466. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dibbern, K.N.; Krzak, M.G.; Olivas, A.; Albert, M.V.; Krzak, J.J.; Kruger, K.M. Scoping Review of Machine Learning Techniques in Marker-Based Clinical Gait Analysis. Bioengineering 2025, 12, 591. https://doi.org/10.3390/bioengineering12060591
Dibbern KN, Krzak MG, Olivas A, Albert MV, Krzak JJ, Kruger KM. Scoping Review of Machine Learning Techniques in Marker-Based Clinical Gait Analysis. Bioengineering. 2025; 12(6):591. https://doi.org/10.3390/bioengineering12060591
Chicago/Turabian StyleDibbern, Kevin N., Maddalena G. Krzak, Alejandro Olivas, Mark V. Albert, Joseph J. Krzak, and Karen M. Kruger. 2025. "Scoping Review of Machine Learning Techniques in Marker-Based Clinical Gait Analysis" Bioengineering 12, no. 6: 591. https://doi.org/10.3390/bioengineering12060591
APA StyleDibbern, K. N., Krzak, M. G., Olivas, A., Albert, M. V., Krzak, J. J., & Kruger, K. M. (2025). Scoping Review of Machine Learning Techniques in Marker-Based Clinical Gait Analysis. Bioengineering, 12(6), 591. https://doi.org/10.3390/bioengineering12060591






