Effects of Experimentally Induced Lower Limb Muscle Fatigue on Healthy Adults’ Gait: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
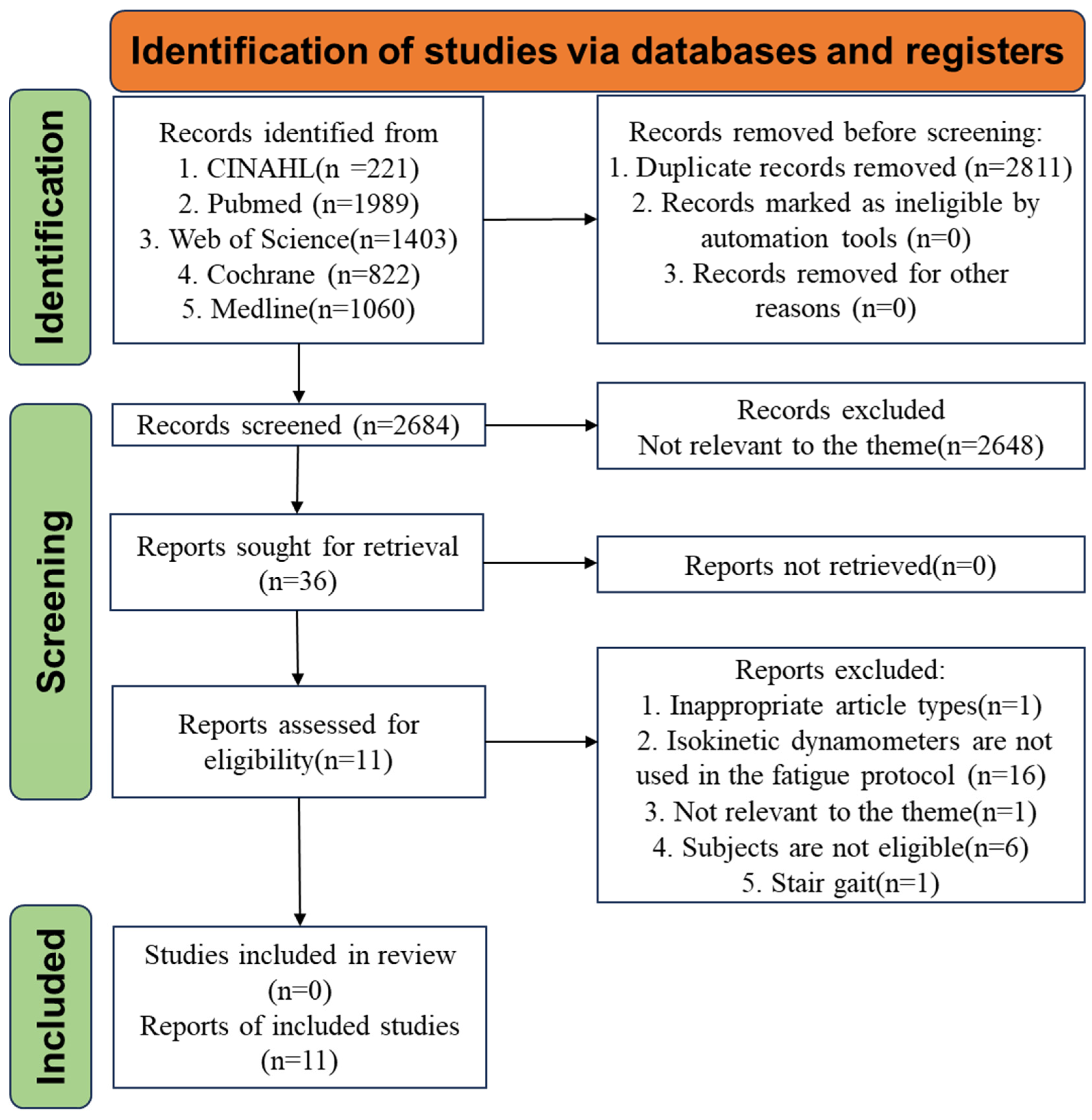
2.3. Studies Selection
2.4. Data Extraction
2.5. Risk of Bias and Study Quality Assessment
| Michael A. Hunt., 2017 [16] | Fui Ling Lew., 2014 [13] | Heather S. Longpré., 2012 [22] | G. H. Murdock., 2011 [23] | Urs Granacher., 2010a [31] | Urs Granacher., 2010b [24] | Prakriti Parijat., 2008 [14] | |
|---|---|---|---|---|---|---|---|
| Participants | 18 | 60 | 20 | 20 | 16 | 14 | 16 |
| Male/Female (N) | 9/9 | 35/25 | 0/20 | 10/10 | 8/8 | 14/0 | 10/6 |
| Age (Mean ± SD in Yrs) | 25.2 | 23.1 ± 1.7 a 24.2 ± 3.0 b 23.5 ± 1.4 c | 18–30 | 19–35 | 24.3 ± 1.4 | 27 ± 3.1 | 24.7 ± 3.9 |
| Gait studies | Level Gait | Level Gait | Level Gait | Level Gait | Level Gait | Level Gait | Level Gait |
| Isokinetic dynamometer | Biodex Medical Systems | Biodex Medical Systems | Biodex Medical Systems | Cybex International Inc. | Cybex International Inc. | Cybex International Inc. | Biodex Medical Systems |
| Fatigue of muscles | Ankle | Ankle, knee, and hip | Knee | Knee | Knee | Ankle | Knee |
| Muscle contraction | Ankle plantar flexion | Hip and knee extension, ankle plantar flexion | Knee flexion and extension | Knee extension | Knee extension | Ankle plantar and dorsiflexion | Knee flexion and extension |
| Type of muscle contraction | Isokinetic contraction | Isotonic contraction | Isotonic contraction | Isokinetic contraction | Isokinetic contraction | Isokinetic contraction | Isokinetic contraction |
| Fatigue regimen | Plantar flexion contractions at 180°/s | Ten lifts/min 60% MVC | 50% MVC | Knee extension at 90°/s | Knee flexion and extension at 60°/s | Ankle plantar and dorsiflexion at 60°/s | Knee flexion and extension at 60°/s |
| Contraction type | Concentric | Concentric | Concentric–concentric | Concentric–concentric | Concentric–concentric | ||
| Unilateral/bilateral | Unilateral | Bilateral | Unilateral | Unilateral | Bilateral | Unilateral | Bilateral |
| Fatigue evaluation | <60% MVC | <70% MVC three times in a row | <75% MVC | <50% MVC | <50% MVC | <50% MVC | <60% MVC |
| Dennis Hamacher., 2016 [32] | F.A. Barbieri., 2014 [25] | Fabio Augusto Barbieri., 2013 [33] | Xingda Qu., 2011 [34] | |
|---|---|---|---|---|
| Participants | 10 | 10 | 20 | 12 |
| Male/Female (N) | NE | 5/5 | 20/0 | 12/0 |
| Age (Mean ± SD in Yrs) | 23.0 ± 4.8 | 27.6 ± 2.7 | 24.7 ± 2.8 | 26.6 ± 2.9 |
| Gait studies | Level Gait | Step gait | Level Gait | Level Gait |
| Fatigue equipment | Cycle ergometer | Sit-to-stand task | Sit-to-stand task | Medical treadmill |
| Fatigue of muscles | Lower limbs | Quadriceps and triceps calves | Lower limbs | Lower limbs |
| Fatigue regimen | Starting workload: 50 watts; Incremental workload: 25 watts; Stage length: 3 min; Additional charge: 3 min by 25 watts; Cadence: 70–80 rpm; | A standard chair (40 cm high, 40 cm wide, 35 cm deep; frequency of 0.5 Hz | A standard chair (43 cm high, 41 cm wide, 42 cm deep; 30 beats/min) | 2 and 10 min; 8 mph |
| Fatigue evaluation | RPE: 18 | Below 0.5 Hz or after 30 min | Below 30 beats/min or after 30 min | RPE: 17 |
| Model | Xrcise Cycle Med, Cardiowise®, Pirmasens, Germany | NE | NE | Biodex RTM 600, Shirley, NY, USA |
| First Author and Years | Michael A. Hunt., 2017 [16] | Fui Ling Lew., 2014 [13] | Heather S. Longpré., 2012 [22] | G. H. Murdock., 2011 [23] | Urs Granacher., 2010a [31] | Urs Granacher., 2010b [24] | Prakriti Parijat., 2008 [14] | Dennis Hamacher., 2016 [32] | F.A. Barbieri., 2014 [25] | Fabio Augusto Barbieri., 2013 [33] | Xingda Qu., 2011 [34] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reporting | |||||||||||
| 1. Is the hypothesis/aim objective of the study clearly described? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 2. Are the main outcomes to be measured clearly described in the Introduction or Methods sections? | Y | Y | Y | Y | Y | Y | U | U | Y | U | Y |
| 3. Are the characteristics of the patients included in the study clearly described? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 4. Are the interventions of interest clearly described? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 5. Are the distributions of principal confounders in each group of subjects to be compared clearly described? | Y | Y | N | Y | Y | Y | Y | N | Y | Y | Y |
| 6. Are the main findings of the study clearly described? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 7. Does the study provide estimates of the random variability in the data for the main outcomes? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 8. Have actual probability values been reported for the main outcomes except where the probability value is less than 0.001? | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y |
| External Validity | |||||||||||
| 9. Were the subjects asked to participate in the study representative of the entire population from which they were recruited? | Y | Y | N | Y | Y | N | Y | N | Y | N | N |
| 10. Were those subjects who were prepared to participate representative of the entire population from which they were recruited? | Y | Y | N | Y | Y | N | Y | N | Y | N | N |
| 11. Were the staff, places, and facilities where the patients were treated representative of the treatment the majority of patients receive? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Internal Validity | |||||||||||
| 12. Were the statistical tests used to assess the main outcomes appropriate? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 13. Were the main outcome measures used accurate (valid and reliable)? | Y | Y | Y | Y | Y | Y | U | Y | Y | Y | Y |
| Internal Validity (Confounding, Selection Bias) | |||||||||||
| 14. Were the patients in different intervention groups (trials and cohort studies), or were the cases and controls (case–control studies) recruited from the same population? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 15. Was there adequate adjustment for confounding in the analyses from which the main findings were drawn? | U | U | U | U | U | U | U | U | U | U | U |
| Power | |||||||||||
| 16. Did the study have sufficient power to detect a clinically important effect where the probability value for a difference being due to chance is less than 5%? | U | U | Y | U | U | U | U | N | Y | N | Y |
| Total Score | |||||||||||
| Score/16 | 14 | 14 | 12 | 13 | 14 | 12 | 12 | 10 | 15 | 11 | 13 |
2.6. Data Analysis
3. Results
3.1. Studies Inclusion and Characteristics
3.2. Quality Assessment
3.3. Fatigue Protocol
3.3.1. Isokinetic Dynamometry
3.3.2. Sit-to-Stand Task
3.3.3. Bicycle Ergometer and Treadmill Tasks
3.4. Fatigue of Muscles
3.5. Biomechanical Methods and Indicators
3.5.1. Kinematic and Kinetic
3.5.2. sEMG
3.5.3. Spatiotemporal Parameters
3.5.4. Dynamic Balance
4. Discussion
4.1. The Impact of Fatigue Protocol
4.2. Effect of Fatigue on Gait
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- THE EPIDEMIOLOGY OF FATIGUE–MORE QUESTIONS THAN ANSWERS-All Databases. Available online: https://webofscience-clarivate-cn-s.vpn.snnu.edu.cn:8081/wos/alldb/full-record/MEDLINE:1583440 (accessed on 6 February 2025).
- Gandevia, S.C. Spinal and Supraspinal Factors in Human Muscle Fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef] [PubMed]
- Stackhouse, S.K.; Stevens, J.E.; Lee, S.C.; Pearce, K.M.; Snyder-Mackler, L.; Binder-Macleod, S.A. Maximum Voluntary Activation in Nonfatigued and Fatigued Muscle of Young and Elderly Individuals. Phys. Ther. 2001, 81, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Rannou, F.; Nybo, L.; Andersen, J.E.; Nordsborg, N.B. Monitoring Muscle Fatigue Progression during Dynamic Exercise. Med. Sci. Sports Exerc. 2019, 51, 1498–1505. [Google Scholar] [CrossRef]
- Enoka, R.M.; Duchateau, J. Translating Fatigue to Human Performance. Med. Sci. Sports Exerc. 2016, 48, 2228–2238. [Google Scholar] [CrossRef] [PubMed]
- Grenier, J.G.; Millet, G.Y.; Peyrot, N.; Samozino, P.; Oullion, R.; Messonnier, L.; Morin, J.-B. Effects of Extreme-Duration Heavy Load Carriage on Neuromuscular Function and Locomotion: A Military-Based Study. PLoS ONE 2012, 7, e43586. [Google Scholar] [CrossRef]
- Paillard, T. Effects of General and Local Fatigue on Postural Control: A Review. Neurosci. Biobehav. Rev. 2012, 36, 162–176. [Google Scholar] [CrossRef]
- Dingwell, J.B.; Cusumano, J.P. Nonlinear Time Series Analysis of Normal and Pathological Human Walking. Chaos Interdiscip. J. Nonlinear Sci. 2000, 10, 848–863. [Google Scholar] [CrossRef]
- Hsiao, H.; Simeonov, P. Preventing Falls from Roofs: A Critical Review. Ergonomics 2001, 44, 537–561. [Google Scholar] [CrossRef]
- Measurement of Human Muscle Fatigue (Topic) – 6210 – All Databases. Available online: https://webofscience.clarivate.cn/wos/alldb/summary/e2505079-c7a8-433c-8bbe-7307a7b2071f-010cdaf441/relevance/1 (accessed on 29 September 2024).
- Bentley, T.A.; Haslam, R.A. Identification of Risk Factors and Countermeasures for Slip, Trip and Fall Accidents during the Delivery of Mail. Appl. Ergon. 2001, 32, 127–134. [Google Scholar] [CrossRef]
- Rapp Van Roden, E.A.; George, J.; Milan, L.T.; Bove, R.T. Evaluation of Injury Patterns and Accident Modality in Step Ladder-Related Injuries. Appl. Ergon. 2021, 96, 103492. [Google Scholar] [CrossRef]
- Lew, F.L.; Qu, X. Effects of Multi-Joint Muscular Fatigue on Biomechanics of Slips. J. Biomech. 2014, 47, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Parijat, P.; Lockhart, T.E. Effects of Quadriceps Fatigue on the Biomechanics of Gait and Slip Propensity. Gait Posture 2008, 28, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.M.-S.; Close, J.C.T.; Wong, A.K.W.; Lord, S.R. Falls Incidence, Risk Factors, and Consequences in Chinese Older People: A Systematic Review. J. Am. Geriatr. Soc. 2011, 59, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.A.; Hatfield, G.L. Ankle and Knee Biomechanics during Normal Walking Following Ankle Plantarflexor Fatigue. J. Electromyogr. Kinesiol. 2017, 35, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Hirata, R.P.; Erbs, A.W.; Gadsboll, E.; Winther, R.; Christensen, S.H.; Simonsen, M.B. A 3-Dimensional Gait Analysis of the Effects of Fatigue-Induced Reduced Foot Adductor Muscle Strength on the Walking of Healthy Subjects. J. Appl. Biomech. 2022, 38, 271–279. [Google Scholar] [CrossRef]
- Boksem, M.A.S.; Meijman, T.F.; Lorist, M.M. Effects of Mental Fatigue on Attention: An ERP Study. Cogn. Brain Res. 2005, 25, 107–116. [Google Scholar] [CrossRef]
- Lorist, M.M. Impact of Top-down Control during Mental Fatigue. Brain Res. 2008, 1232, 113–123. [Google Scholar] [CrossRef]
- Mizuno, K.; Tanaka, M.; Yamaguti, K.; Kajimoto, O.; Kuratsune, H.; Watanabe, Y. Mental Fatigue Caused by Prolonged Cognitive Load Associated with Sympathetic Hyperactivity. Behav. Brain Funct. 2011, 7, 17. [Google Scholar] [CrossRef]
- Rocha dos Santos, P.C.; Barbieri, F.A.; Zijdewind, I.; Bucken Gobbi, L.T.; Lamoth, C.; Hortobagyi, T. Effects of Experimentally Induced Fatigue on Healthy Older Adults’ Gait: A Systematic Review. PLoS ONE 2019, 14, e0226939. [Google Scholar] [CrossRef]
- Longpré, H.S.; Potvin, J.R.; Maly, M.R. Biomechanical Changes at the Knee after Lower Limb Fatigue in Healthy Young Women. Clin. Biomech. 2013, 28, 441–447. [Google Scholar] [CrossRef]
- Murdock, G.H.; Hubley-Kozey, C.L. Effect of a High Intensity Quadriceps Fatigue Protocol on Knee Joint Mechanics and Muscle Activation during Gait in Young Adults. Eur. J. Appl. Physiol. 2012, 112, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Granacher, U.; Gruber, M.; Förderer, D.; Strass, D.; Gollhofer, A. Effects of Ankle Fatigue on Functional Reflex Activity during Gait Perturbations in Young and Elderly Men. Gait Posture 2010, 32, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, F.A.; Gobbi, L.T.B.; Lee, Y.J.; Pijnappels, M.; Van Dieën, J.H. Effect of Triceps Surae and Quadriceps Muscle Fatigue on the Mechanics of Landing in Stepping down in Ongoing Gait. Ergonomics 2014, 57, 934–942. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moher, D. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Int. J. Surg. 2010, 8, 658, Erratum in Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- De Cock, C.; Milne-Ives, M.; Van Velthoven, M.H.; Alturkistani, A.; Lam, C.; Meinert, E. Effectiveness of Conversational Agents (Virtual Assistants) in Health Care: Protocol for a Systematic Review. JMIR Res. Protoc. 2020, 9, e16934. [Google Scholar] [CrossRef]
- Downs, S.H.; Black, N. The Feasibility of Creating a Checklist for the Assessment of the Methodological Quality Both of Randomised and Non-Randomised Studies of Health Care Interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Gao, P.; Zhu, J.; Tuo, H.; Lin, Q.; Jing, F.; Liu, W. The Effect of Mobile Phone Task and Age on Gait: A Systematic Review and Meta-Analysis. Front. Physiol. 2023, 14, 1163655. [Google Scholar] [CrossRef]
- Bruyneel, A.-V.; Reinmann, A.; Gafner, S.C.; Sandoz, J.-D.; Duclos, N.C. Does Texting While Walking Affect Spatiotemporal Gait Parameters in Healthy Adults, Older People, and Persons with Motor or Cognitive Disorders? A Systematic Review and Meta-Analysis. Gait Posture 2023, 100, 284–301. [Google Scholar] [CrossRef]
- Granacher, U.; Wolf, I.; Wehrle, A.; Bridenbaugh, S.; Kressig, R.W. Effects of Muscle Fatigue on Gait Characteristics under Single and Dual-Task Conditions in Young and Older Adults. J. Neuroeng. Rehabil. 2010, 7, 56. [Google Scholar] [CrossRef]
- Hamacher, D.; Törpel, A.; Hamacher, D.; Schega, L. The Effect of Physical Exhaustion on Gait Stability in Young and Older Individuals. Gait Posture 2016, 48, 137–139. [Google Scholar] [CrossRef]
- Barbieri, F.A.; Dos Santos, P.C.R.; Vitório, R.; Van Dieën, J.H.; Gobbi, L.T.B. Effect of Muscle Fatigue and Physical Activity Level in Motor Control of the Gait of Young Adults. Gait Posture 2013, 38, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Yeo, J.C. Effects of Load Carriage and Fatigue on Gait Characteristics. J. Biomech. 2011, 44, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Ariel De Lima, D.; Helito, C.P.; Lima, F.R.A.D.; Leite, J.A.D. Surgical Indications for Anterior Cruciate Ligament Reconstruction Combined with Extra-Articular Lateral Tenodesis or Anterolateral Ligament Reconstruction. Rev. Bras. Ortop. (Engl. Ed.) 2018, 53, 661–667. [Google Scholar] [CrossRef]
- Pássaro, A.D.C.; Marques, A.P.; Sacco, I.D.C.N.; Amadio, A.C.; Bacarin, T.D.A. Mecanismos de Ativação Agonista e Antagonista No Joelho de Indivíduos Com Reconstrução de Ligamento Cruzado Anterior: Estudo Cinético e Eletromiográfico. Acta Ortop. Bras. 2008, 16, 117–121. [Google Scholar] [CrossRef]
- Başdelioğlu, K.; Meriç, G.; Pündük, Z.; Akseki, D.; Atik, A.; Sargın, S. Outcomes of Isokinetic Tests and Functional Assessment of Anterior Cruciate Ligament Reconstruction: Transtibial versus Single Anatomic Femoral Tunnel Technique. Acta Orthop. Traumato. 2019, 53, 86–91. [Google Scholar] [CrossRef]
- Claudio, A.C.D.J.; Da Silva, L.Z.R.; Fonseca, L.G.; De Camargo, C.C.; Machado, A.F.; Micheletti, J.K.; Menossi, B.R.D.S. Isokinetic Testing Protocol-Based Discharge Criteria after Anterior Ligament Reconstruction: A Systematic Review. Isokinet. Exerc. Sci. 2024, 32, 85–107. [Google Scholar] [CrossRef]
- Qu, X. Effects of Lower-Limb Muscular Fatigue on Stair Gait. J. Biomech. 2015, 48, 4059–4064. [Google Scholar] [CrossRef]
- Lewek, M.D.; Rudolph, K.S.; Snyder-Mackler, L. Quadriceps Femoris Muscle Weakness and Activation Failure in Patients with Symptomatic Knee Osteoarthritis. J. Orthop. Res. 2004, 22, 110–115. [Google Scholar] [CrossRef]
- Hubley-Kozey, C.; Deluzio, K.; Dunbar, M. Muscle Co-Activation Patterns during Walking in Those with Severe Knee Osteoarthritis. Clin. Biomech. 2008, 23, 71–80. [Google Scholar] [CrossRef]
- Petterson, S.C.; Barrance, P.; Buchanan, T.; Binder-Macleod, S.; Snyder-Mackler, L. Mechanisms Underlying Quadriceps Weakness in Knee Osteoarthritis. Med. Sci. Sports Exercise 2008, 40, 422–427. [Google Scholar] [CrossRef]
- Chaffin, D.B.; Andersson, G.B.J.; Martin, B.J. Occupational Biomechanics; Wiley–Blackwell: New York, NY, USA, 1999; ISBN 978-0-471-24697-8. [Google Scholar]
- Hinman, R.S.; Bennell, K.L.; Metcalf, B.R.; Crossley, K.M. Delayed Onset of Quadriceps Activity and Altered Knee Joint Kinematics during Stair Stepping in Individuals with Knee Osteoarthritis. Arch. Phys. Med. Rehabil. 2002, 83, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Pincivero, D.M.; Gandhi, V.; Timmons, M.K.; Coelho, A.J. Quadriceps Femoris Electromyogram during Concentric, Isometric and Eccentric Phases of Fatiguing Dynamic Knee Extensions. J. Biomech. 2006, 39, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Kinematic and EMG Patterns during Slow, Free, and Fast Walking–PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/6491818/ (accessed on 11 February 2025).
- Marino, G.; Scano, A.; Beltrame, G.; Brambilla, C.; Marazzi, A.; Aparo, F.; Molinari Tosatti, L.; Gatti, R.; Portinaro, N. Influence of Backpack Carriage and Walking Speed on Muscle Synergies in Healthy Children. Bioengineering 2024, 11, 173. [Google Scholar] [CrossRef]
- Cheng, A.J.; Rice, C.L. Fatigue and Recovery of Power and Isometric Torque Following Isotonic Knee Extensions. J. Appl. Physiol. (1985) 2005, 99, 1446–1452. [Google Scholar] [CrossRef]
- Callahan, D.M.; Foulis, S.A.; Kent-Braun, J.A. Age-Related Fatigue Resistance in the Knee Extensor Muscles Is Specific to Contraction Mode. Muscle Nerve 2009, 39, 692–702. [Google Scholar] [CrossRef]
- Is There a “Normal” Profile of EMG Activity in Gait?–All Databases. Available online: https://webofscience.clarivate.cn/wos/alldb/full-record/MEDLINE:3796061 (accessed on 10 October 2024).
- Barbieri, F.A.; Lee, Y.-J.; Gobbi, L.T.B.; Pijnappels, M.; Van Dieen, J.H. The Effect of Muscle Fatigue on the Last Stride before Stepping down a Curb. Gait Posture 2013, 37, 542–546. [Google Scholar] [CrossRef]
- Granata, K.P.; Wilson, S.E.; Massimini, A.K.; Gabriel, R. Active Stiffness of the Ankle in Response to Inertial and Elastic Loads. J. Electromyogr. Kinesiol. 2004, 14, 599–609. [Google Scholar] [CrossRef]
- Electromyographic Changes of Agonist and Antagonist Calf Muscles During Maximum Isometric Induced Fatigue-All Databases. Available online: https://webofscience-clarivate-cn-s.vpn.snnu.edu.cn:8081/wos/alldb/full-record/WOS:000175927700010 (accessed on 11 February 2025).
- Moyer, B.E.; Chambers, A.J.; Redfern, M.S.; Cham, R. Gait Parameters as Predictors of Slip Severity in Younger and Older Adults. Ergonomics 2006, 49, 329–343. [Google Scholar] [CrossRef]
- Helbostad, J.L.; Leirfall, S.; Moe-Nilssen, R.; Sletvold, O. Physical Fatigue Affects Gait Characteristics in Older Persons. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2007, 62, 1010–1015. [Google Scholar] [CrossRef]
- Ribeiro, F.; Mota, J.; Oliveira, J. Effect of Exercise-Induced Fatigue on Position Sense of the Knee in the Elderly. Eur. J. Appl. Physiol. 2007, 99, 379–385. [Google Scholar] [CrossRef]
- Lin, D.; Nussbaum, M.A.; Seol, H.; Singh, N.B.; Madigan, M.L.; Wojcik, L.A. Acute Effects of Localized Muscle Fatigue on Postural Control and Patterns of Recovery during Upright Stance: Influence of Fatigue Location and Age. Eur. J. Appl. Physiol. 2009, 106, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Speeding Up or Slowing Down?: Gait Adaptations to Preserve Gait Stability in Response to Balance Perturbations–PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/22464635/ (accessed on 17 February 2025).
- Hof, A.L.; van Bockel, R.M.; Schoppen, T.; Postema, K. Control of Lateral Balance in Walking. Experimental Findings in Normal Subjects and above-Knee Amputees. Gait Posture 2007, 25, 250–258. [Google Scholar] [CrossRef]
- Marimon, X.; Mengual, I.; López-de-Celis, C.; Portela, A.; Rodríguez-Sanz, J.; Herráez, I.A.; Pérez-Bellmunt, A. Kinematic Analysis of Human Gait in Healthy Young Adults Using IMU Sensors: Exploring Relevant Machine Learning Features for Clinical Applications. Bioengineering 2024, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Quijoux, F.; Vienne-Jumeau, A.; Bertin-Hugault, F.; Zawieja, P.; Lefèvre, M.; Vidal, P.-P.; Ricard, D. Center of Pressure Displacement Characteristics Differentiate Fall Risk in Older People: A Systematic Review with Meta-Analysis. Ageing Res. Rev. 2020, 62, 101117. [Google Scholar] [CrossRef]
- Rajachandrakumar, R.; Mann, J.; Schinkel-Ivy, A.; Mansfield, A. Exploring the Relationship between Stability and Variability of the Centre of Mass and Centre of Pressure. Gait Posture 2018, 63, 254–259. [Google Scholar] [CrossRef]

| PICOS | Description |
|---|---|
| Participant | The study will include healthy adults aged between 18 and 59. Participants should have no history of lower extremity diseases, disorders, or injuries that could affect their mobility or physical function. |
| Intervention | The non-invasive protocols for inducing lower limb muscle fatigue in a laboratory environment, such as isometric contractions, dynamic exercises, or repetitive tasks, allow for controlled and reproducible investigation of fatigue-related effects. |
| Comparison | Comparison of gait characteristics before and after fatigue. |
| Outcome | Lower limb joint biomechanical characteristics and balance indicators. |
| Study Design | Original intervention studies, including non-randomised controlled trials (non-RCTs). |
| Database | Search Strategy |
|---|---|
| CINAHL | ((MH “Gait” OR “Gait Analysis”) AND (MH “Fatigue” OR “Muscle Fatigue”)) AND ((MH “Gait” OR “Walking Pattern”) AND (MH “Fatigue” OR “Muscle Fatigue” OR “Fatigue Assessment”)) |
| Pubmed | (“gait” OR “gait analysis”) AND (“fatigue” OR “muscle fatigue”) AND (“gait” OR “gait analysis” OR “walking pattern”) AND (“fatigue” OR “muscle fatigue” OR “fatigue assessment”) |
| Web of Science | ((gait OR “gait analysis”) AND (fatigue OR “muscle fatigue”)) and ((gait OR “gait analysis” OR “walking pattern”) AND (fatigue OR “muscle fatigue” OR “fatigue assessment”)) |
| Cochrane | (“gait” OR “gait analysis”) AND (“fatigue” OR “muscle fatigue”) AND (“gait” OR “gait analysis” OR “walking pattern”) AND (“fatigue” OR “muscle fatigue” OR “fatigue assessment”) |
| Medline | (“gait” [Mesh] OR “gait analysis” [Mesh] OR “gait” OR “walking pattern”) AND (“fatigue” [Mesh] OR “muscle fatigue” [Mesh] OR “fatigue” OR “fatigue assessment”) |
| Michael A. Hunt., 2017 [16] | Fui Ling Lew., 2014 [13] | Heather S. Longpré., 2012 [22] | G. H. Murdock., 2011 [23] | Urs Granacher., 2010a [31] | Urs Granacher., 2010b [24] | Prakriti Parijat., 2008 [14] | Dennis Hamacher., 2016 [32] | F.A. Barbieri., 2014 [25] | Fabio Augusto Barbieri., 2013 [33] | Xingda Qu., 2011 [34] | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental setup | A walkway (10 m) | A walkway (12 × 1.5 m) | NE | A walkway (6 m) | A walkway (10 m) | NE | A walkway (15.5 × 1.5 m) | NE | 10 cm height platform | A walkway (8 m) | NE |
| Biomechanical methods | Kinematics, kinetics and sEMG | Kinematics and kinetics | Kinematics, kinetics and sEMG | Kinematics, kinetics and sEMG | Kinetics | Kinematics and sEMG | Kinematics and kinetics | Inertial sensor | Kinematics and kinetics | Kinematics and kinetics | Kinematics |
| Study | Joint Moment | Joint Angle | sEMG | Spatiotemporal Parameters | Other Parameters |
|---|---|---|---|---|---|
| Michael A. Hunt., 2017 [16] | Knee extension 一 Knee flexion ↓ Plantar flexion ↓ | Knee flexion ↑ | MG, LG ↓ | GV 一 SF ↑ | |
| Fui Ling Lew., 2014 [13] | Knee 一 Plantar flexion ↑ | SD ↑ RCOF 一 | |||
| Heather S. Longpré., 2012 [22] | Knee extension ↓ Knee flexion 一 | Knee 一 | BF 一, RF ↓ | GV 一 SL 一 | |
| G. H. Murdock., 2011 [23] | Knee flexion ↓ Knee external Rotation ↓ | Knee Adduction ↑ Knee 一 | VM, RF ↓ | ||
| Urs Granacher., 2010a [31] | GV ↓ SL ↓ SLV 一 | ||||
| Urs Granacher., 2010b [24] | TA ↓, SO 一 | Angular velocity (plantar–dorsiflexion) ↑ | |||
| Prakriti Parijat., 2008 [14] | Knee extension ↓ | Knee flexion ↑ Plantar flexion ↑ | GV ↓ | RCOF ↑ HCV ↑ | |
| Dennis Hamacher., 2016 [32] | LDS ↑ | ||||
| F.A. Barbieri., 2014 [25] | SW ↑ STL 一 SDN 一 | ||||
| Fabio Augusto Barbieri., 2013 [33] | GV ↑ SW ↑ SL ↑ SDN ↓ | ||||
| Xingda Qu., 2011 [34] | Trunk ROM ↑, Hip ROM ↑, Knee ROM ↑ | SW ↑ SL 一 SLV ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Ma, W.; Zhu, W.; Zhai, L.; Sun, Y. Effects of Experimentally Induced Lower Limb Muscle Fatigue on Healthy Adults’ Gait: A Systematic Review. Bioengineering 2025, 12, 225. https://doi.org/10.3390/bioengineering12030225
Wang L, Ma W, Zhu W, Zhai L, Sun Y. Effects of Experimentally Induced Lower Limb Muscle Fatigue on Healthy Adults’ Gait: A Systematic Review. Bioengineering. 2025; 12(3):225. https://doi.org/10.3390/bioengineering12030225
Chicago/Turabian StyleWang, Liangsen, Wenyue Ma, Wenfei Zhu, Lin Zhai, and Yuliang Sun. 2025. "Effects of Experimentally Induced Lower Limb Muscle Fatigue on Healthy Adults’ Gait: A Systematic Review" Bioengineering 12, no. 3: 225. https://doi.org/10.3390/bioengineering12030225
APA StyleWang, L., Ma, W., Zhu, W., Zhai, L., & Sun, Y. (2025). Effects of Experimentally Induced Lower Limb Muscle Fatigue on Healthy Adults’ Gait: A Systematic Review. Bioengineering, 12(3), 225. https://doi.org/10.3390/bioengineering12030225









