Characterization of Parthanatos in Breast Cancer: Implications for Prognosis and PARP Inhibitor Resistance
Abstract
1. Introduction
2. Methods
2.1. Sample Source and Parthanatos-Related Gene Source
2.2. Gene Expression Analysis
2.3. Analysis of the Correlation and Protein–Protein Interactions (PPI) Between Parthanatos and Other Forms of Cell Death
2.4. Single-Cell Sequencing Data Analysis
2.5. Unsupervised Clustering Based on Parthanatos-Related Genes
2.6. Comparison of Clinical, Genomic, and Transcriptomic Features of Parthanatos-Related Breast Cancer Subtypes
2.7. Identification of Potentially Sensitive Drugs for BC Patients Based on Parthanatos Molecular Classifier
2.8. Parthanatos-Related Breast Cancer Subtypes and Response to PARP Inhibitors
2.9. Statistical Analysis
3. Results
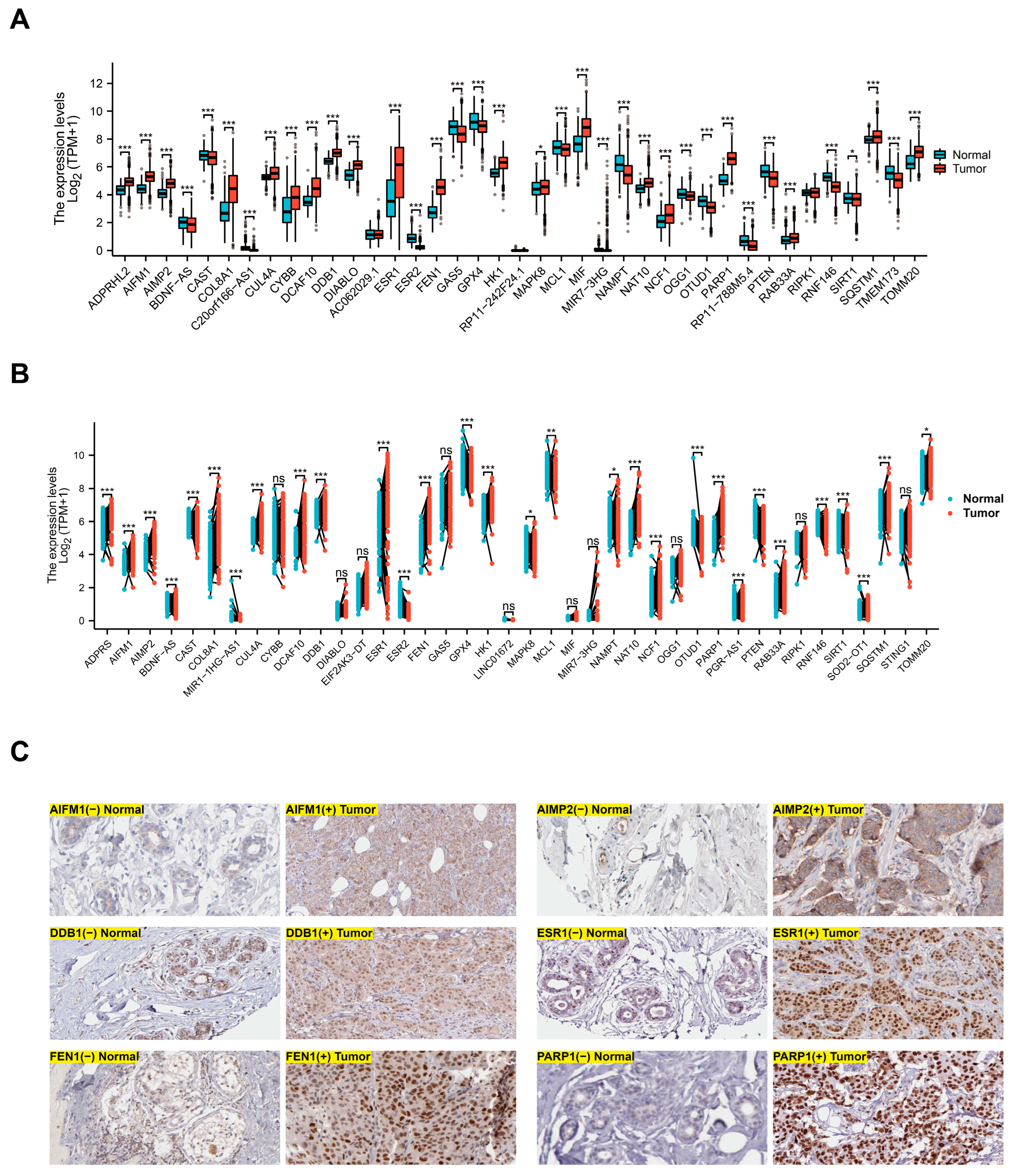
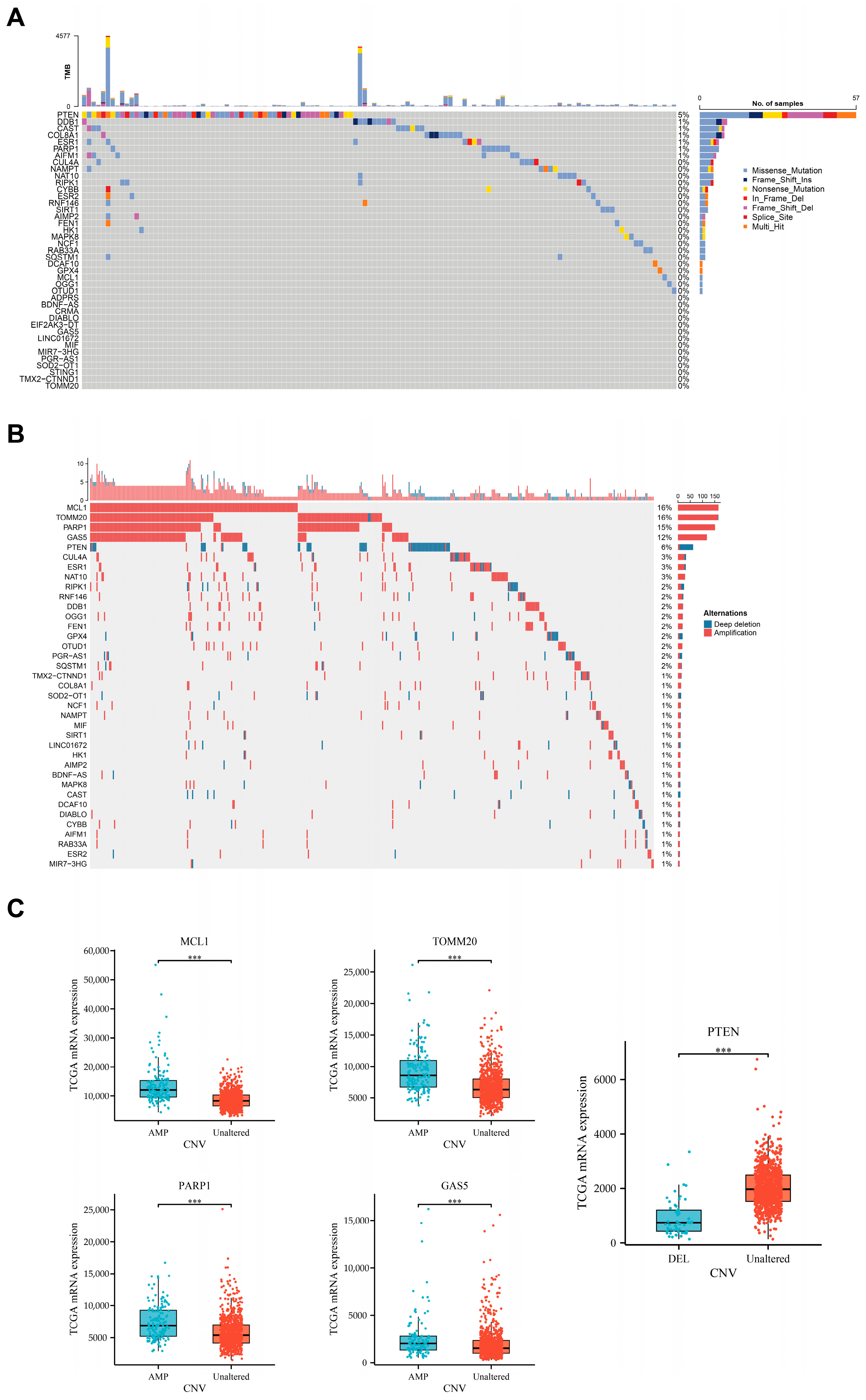
3.1. Differential Expression of Parthanatos-Related Genes in Breast Cancer and Normal Breast Tissue
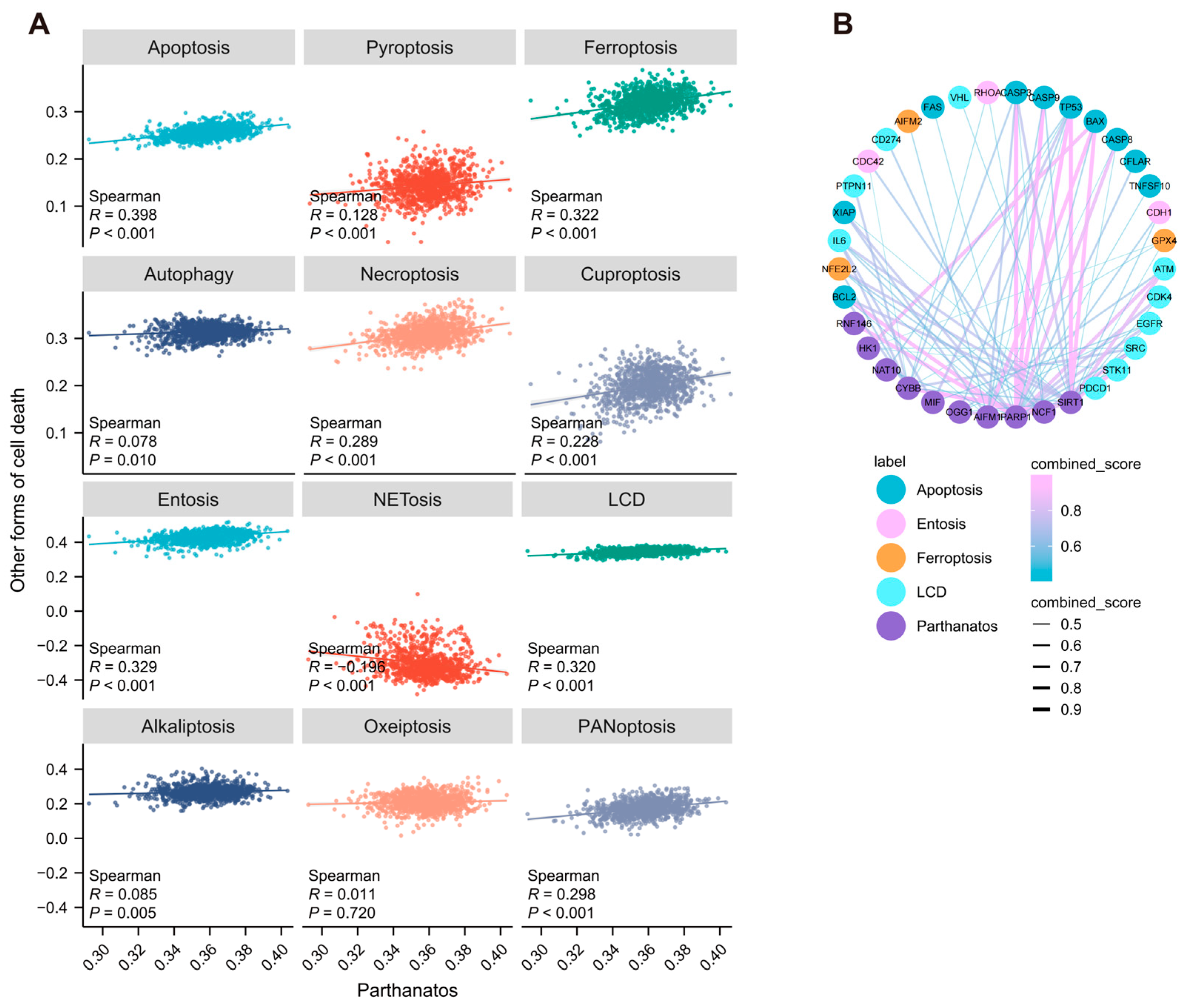
3.2. Correlation Between Parthanatos and Other Forms of Cell Death in Breast Cancer
3.3. Single-Cell Expression Pattern of Parthanatos-Related Genes
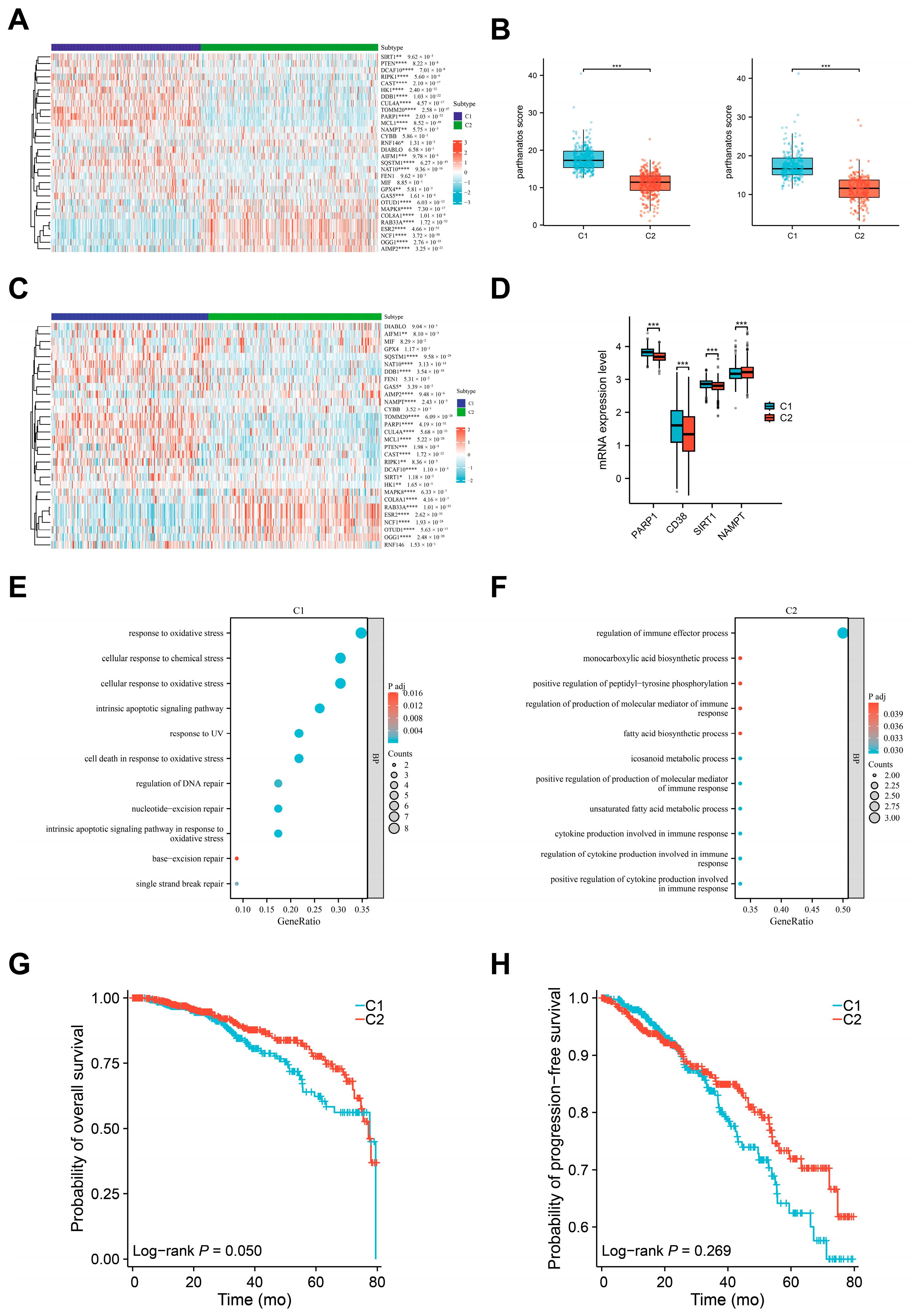
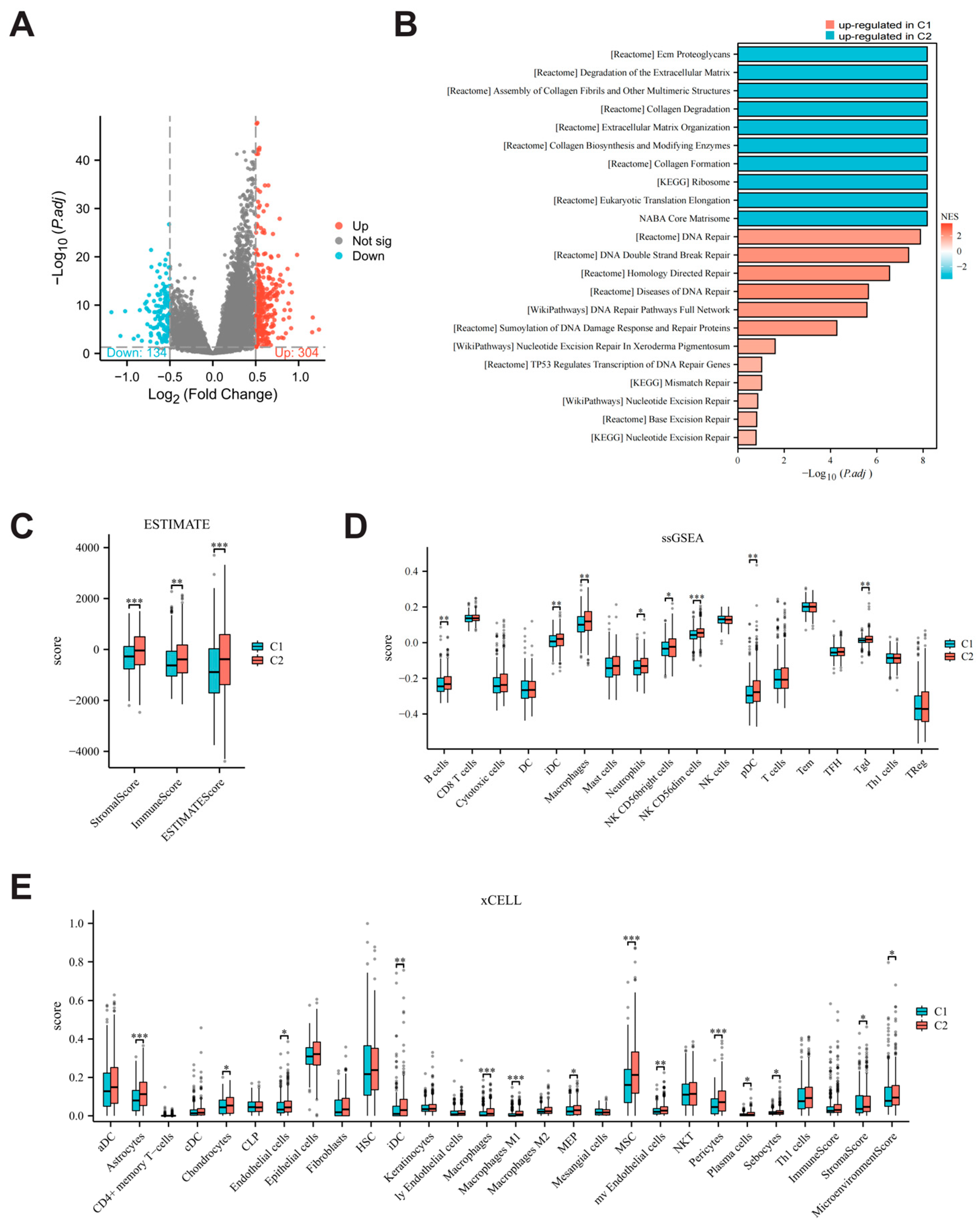
3.4. Defining the Molecular Subtypes of Breast Cancers Based on Parthanatos Activity
3.5. Comparison of Biological Characteristics and Immune Microenvironment Between Parthanatos-Related Breast Cancer Subtypes
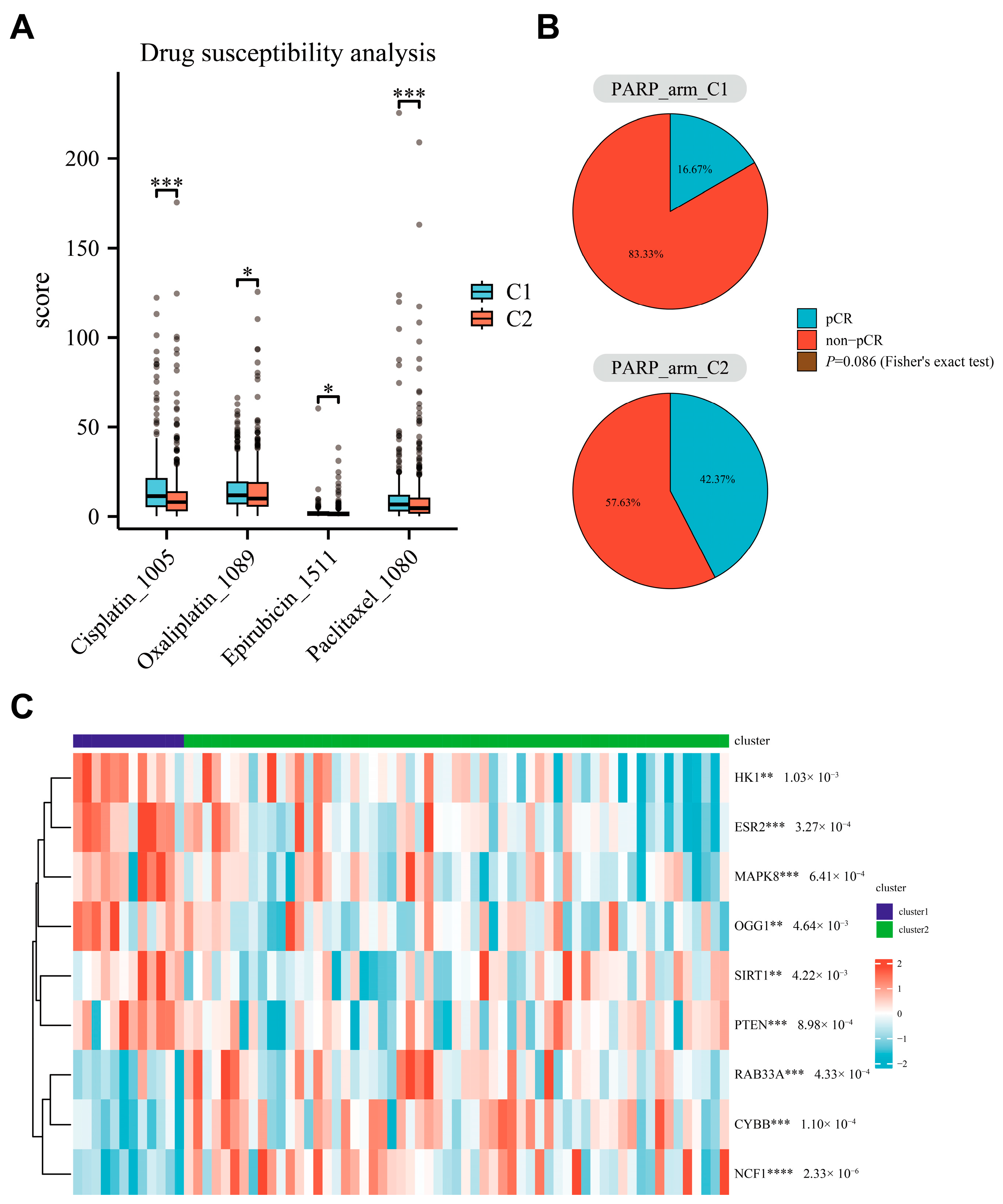
3.6. Relationship Between Parthanatos BC Subtypes and Response to PARP Inhibitors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, F.; Liao, M.; Qin, R.; Zhu, S.; Peng, C.; Fu, L.; Chen, Y.; Han, B. Regulated Cell Death (RCD) in Cancer: Key Pathways and Targeted Therapies. Signal Transduct. Target. Ther. 2022, 7, 286. [Google Scholar] [CrossRef] [PubMed]
- Hadian, K.; Stockwell, B.R. The Therapeutic Potential of Targeting Regulated Non-Apoptotic Cell Death. Nat. Rev. Drug Discov. 2023, 22, 723–742. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, S.; Pioli, C. Multifaceted Role of PARP-1 in DNA Repair and Inflammation: Pathological and Therapeutic Implications in Cancer and Non-Cancer Diseases. Cells 2019, 9, 41. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, L.; Tao, S.; Yao, Y.; Wang, Y.; Wei, Q.; Shao, A.; Deng, Y. Parthanatos and Its Associated Components: Promising Therapeutic Targets for Cancer. Pharmacol. Res. 2021, 163, 105299. [Google Scholar] [CrossRef]
- Lahiri, S.; Hamilton, G.; Moore, G.; Goehring, L.; Huang, T.T.; Jensen, R.B.; Rothenberg, E. BRCA2 Prevents PARPi-Mediated PARP1 Retention to Protect RAD51 Filaments. Nature 2025, 640, 1103–1111. [Google Scholar] [CrossRef]
- Liu, Y.; Stockwell, B.R.; Jiang, X.; Gu, W. P53-Regulated Non-Apoptotic Cell Death Pathways and Their Relevance in Cancer and Other Diseases. Nat. Rev. Mol. Cell Biol. 2025. [Google Scholar] [CrossRef]
- Jin, X.; Jin, W.; Tong, L.; Zhao, J.; Zhang, L.; Lin, N. Therapeutic Strategies of Targeting Non-Apoptotic Regulated Cell Death (RCD) with Small-Molecule Compounds in Cancer. Acta Pharm. Sin. B 2024, 14, 2815–2853. [Google Scholar] [CrossRef]
- Shah, J.; Pueschl, D.; Wubbenhorst, B.; Pluta, J.; Andrea, K.D.; Hubert, A.; Shilan, J.; Zhou, W.; Kraya, A.; Llop-Guevara, A.; et al. Analysis of Matched Primary and Recurrent BRCA1/2 Mutation-Associated Tumors Identifies Recurrence-Specific Drivers. Nat. Commun. 2021, 13, 6728. [Google Scholar] [CrossRef]
- He, R.; Liu, Y.; Fu, W.; He, X.; Liu, S.; Xiao, D.; Tao, Y. Mechanisms and Cross-Talk of Regulated Cell Death and Their Epigenetic Modifications in Tumor Progression. Mol. Cancer 2024, 23, 267. [Google Scholar] [CrossRef]
- Dilawari, A.; Buturla, J.; Osgood, C.; Gao, X.; Chen, W.; Ricks, T.K.; Schaefer, T.; Avasarala, S.; Turcu, F.R.; Pathak, A.; et al. US Food and Drug Administration Approval Summary: Capivasertib With Fulvestrant for Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Locally Advanced or Metastatic Breast Cancer With PIK3CA/AKT1/PTEN Alterations. J. Clin. Oncol. 2024, 42, 4103–4113. [Google Scholar] [CrossRef]
- Duan, J.-L.; Chen, W.; Xie, J.-J.; Zhang, M.-L.; Nie, R.-C.; Liang, H.; Mei, J.; Han, K.; Xiang, Z.-C.; Wang, F.-W.; et al. A Novel Peptide Encoded by N6-Methyladenosine Modified CircMAP3K4 Prevents Apoptosis in Hepatocellular Carcinoma. Mol. Cancer 2022, 21, 93. [Google Scholar] [CrossRef] [PubMed]
- Chehade, C.H.; Gebrael, G.; Sayegh, N.; Ozay, Z.I.; Narang, A.; Crispino, T.; Golan, T.; Litton, J.K.; Swami, U.; Moore, K.N.; et al. A Pan-tumor Review of the Role of Poly(Adenosine Diphosphate Ribose) Polymerase Inhibitors. CA A Cancer J. Clin. 2025, 75, 141–167. [Google Scholar] [CrossRef] [PubMed]
- Pal, B.; Chen, Y.; Vaillant, F.; Capaldo, B.D.; Joyce, R.; Song, X.; Bryant, V.L.; Penington, J.S.; Stefano, L.D.; Ribera, N.T.; et al. A Single-cell RNA Expression Atlas of Normal, Preneoplastic and Tumorigenic States in the Human Breast. EMBO J. 2021, 40, e107333. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.M.; Yau, C.; Wulfkuhle, J.; Brown-Swigart, L.; Gallagher, R.I.; Lee, P.R.E.; Zhu, Z.; Magbanua, M.J.; Sayaman, R.; O’Grady, N.; et al. Redefining Breast Cancer Subtypes to Guide Treatment Prioritization and Maximize Response: Predictive Biomarkers across 10 Cancer Therapies. Cancer Cell 2022, 40, 609–623.e6. [Google Scholar] [CrossRef]
- Qiao, X.; Sun, J.; Ren, P.; Guo, H.; Xu, H.; Bao, C.; Jiang, C. Integrated Single-Cell Sequencing, Spatial Transcriptome Sequencing and Bulk RNA Sequencing Highlights the Molecular Characteristics of Parthanatos in Gastric Cancer. Aging 2024, 16, 5471–5500. [Google Scholar] [CrossRef]
- Berghe, T.V.; Linkermann, A.; Jouan-Lanhouet, S.; Walczak, H.; Vandenabeele, P. Regulated Necrosis: The Expanding Network of Non-Apoptotic Cell Death Pathways. Nat. Rev. Mol. Cell Biol. 2014, 15, 135–147. [Google Scholar] [CrossRef]
- Conrad, M.; Angeli, J.P.F.; Vandenabeele, P.; Stockwell, B.R. Regulated Necrosis: Disease Relevance and Therapeutic Opportunities. Nat. Rev. Drug Discov. 2016, 15, 348–366. [Google Scholar] [CrossRef]
- Zou, Y.; Xie, J.; Zheng, S.; Liu, W.; Tang, Y.; Tian, W.; Deng, X.; Wu, L.; Zhang, Y.; Wong, C.-W.; et al. Leveraging Diverse Cell-Death Patterns to Predict the Prognosis and Drug Sensitivity of Triple-Negative Breast Cancer Patients after Surgery. Int. J. Surg. 2022, 107, 106936. [Google Scholar] [CrossRef]
- Yi, M.; Nissley, D.V.; McCormick, F.; Stephens, R.M. SsGSEA Score-Based Ras Dependency Indexes Derived from Gene Expression Data Reveal Potential Ras Addiction Mechanisms with Possible Clinical Implications. Sci. Rep. 2020, 10, 10258. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein–Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2022, 51, D638–D646. [Google Scholar] [CrossRef]
- Renz, M.; Sarwat, M.; Ding, J.; Zhang, B.; Wang, X.; Zhou, C. TSNE: Trajectory Similarity Network Embedding. In Proceedings of the 30th International Conference on Advances in Geographic Information Systems, Seattle, WA, USA, 1–4 November 2022; pp. 1–4. [Google Scholar] [CrossRef]
- Wilkerson, M.D.; Hayes, D.N. ConsensusClusterPlus: A Class Discovery Tool with Confidence Assessments and Item Tracking. Bioinformatics 2010, 26, 1572–1573. [Google Scholar] [CrossRef] [PubMed]
- Mayakonda, A.; Koeffler, H.P. Maftools: Efficient Analysis, Visualization and Summarization of MAF Files from Large-Scale Cohort Based Cancer Studies. bioRXiv 2016. [Google Scholar] [CrossRef]
- Irizarry, R.A.; Wang, C.; Zhou, Y.; Speed, T.P. Gene Set Enrichment Analysis Made Simple. Stat. Methods Méd. Res. 2009, 18, 565–575. [Google Scholar] [CrossRef]
- Yoshihara, K.; Shahmoradgoli, M.; Martínez, E.; Vegesna, R.; Kim, H.; Torres-Garcia, W.; Treviño, V.; Shen, H.; Laird, P.W.; Levine, D.A.; et al. Inferring Tumour Purity and Stromal and Immune Cell Admixture from Expression Data. Nat. Commun. 2013, 4, 2612. [Google Scholar] [CrossRef]
- Angel, A.; Naom, L.; Nabet-Levy, S.; Aran, D. XCell 2.0: Robust Algorithm for Cell Type Proportion Estimation Predicts Response to Immune Checkpoint Blockade. bioRXiv 2024. [Google Scholar] [CrossRef]
- Maeser, D.; Gruener, R.F.; Huang, R.S. OncoPredict: An R Package for Predicting in Vivo or Cancer Patient Drug Response and Biomarkers from Cell Line Screening Data. Brief. Bioinform. 2021, 22, bbab260. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, W.; Wang, Y. PARP-1 and Its Associated Nucleases in DNA Damage Response. DNA Repair. 2019, 81, 102651. [Google Scholar] [CrossRef]
- Geeleher, P.; Cox, N.J.; Huang, R.S. Clinical Drug Response Can Be Predicted Using Baseline Gene Expression Levels and in Vitro Drug Sensitivity in Cell Lines. Genome Biol. 2014, 15, R47. [Google Scholar] [CrossRef]
- Dörsam, B.; Seiwert, N.; Foersch, S.; Stroh, S.; Nagel, G.; Begaliew, D.; Diehl, E.; Kraus, A.; McKeague, M.; Minneker, V.; et al. PARP-1 Protects against Colorectal Tumor Induction, but Promotes Inflammation-Driven Colorectal Tumor Progression. Proc. Natl. Acad. Sci. USA 2018, 115, E4061–E4070. [Google Scholar] [CrossRef]
- Galia, A.; Calogero, A.E.; Condorelli, R.; Fraggetta, F.; Corte, A.L.; Ridolfo, F.; Bosco, P.; Castiglione, R.; Salemi, M. PARP-1 Protein Expression in Glioblastoma Multiforme. Eur. J. Histochem. 2011, 56, 9. [Google Scholar] [CrossRef]
- Li, D.; Bi, F.-F.; Chen, N.-N.; Cao, J.-M.; Sun, W.-P.; Zhou, Y.-M.; Li, C.-Y.; Yang, Q. A Novel Crosstalk between BRCA1 and Poly (ADP-Ribose) Polymerase 1 in Breast Cancer. Cell Cycle 2014, 13, 3442–3449. [Google Scholar] [CrossRef] [PubMed]
- Do, K.; Chen, A.P. Molecular Pathways: Targeting PARP in Cancer Treatment. Clin. Cancer Res. 2013, 19, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, S.A.; Dawson, T.M.; Dawson, V.L. Mitochondrial and Nuclear Cross Talk in Cell Death. Ann. N. Y. Acad. Sci. 2008, 1147, 233–241. [Google Scholar] [CrossRef]
- Qiao, W.; Pan, L.; Kou, C.; Li, K.; Yang, M. Prognostic and Clinicopathological Value of Poly (Adenosine Diphosphate-Ribose) Polymerase Expression in Breast Cancer: A Meta-Analysis. PLoS ONE 2017, 12, e0172413. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Yin, S.-J.; Gong, Y.-Y.; Li, Z.-R.; Yang, Q.; Fan, Y.-X.; Zhou, T.; Meng, R.; Wang, P.; He, G.-H. Parp1 as a Prognostic Biomarker for Human Cancers: A Meta-Analysis. Biomark. Med. 2021, 15, 1563–1578. [Google Scholar] [CrossRef]
- Ma, D.; Lu, B.; Feng, C.; Wang, C.; Wang, Y.; Luo, T.; Feng, J.; Jia, H.; Chi, G.; Luo, Y.; et al. Deoxypodophyllotoxin Triggers Parthanatos in Glioma Cells via Induction of Excessive ROS. Cancer Lett. 2016, 371, 194–204. [Google Scholar] [CrossRef]
- Kwa, M.; Adams, S. Prognostic and Predictive Value of Tumor-Infiltrating Lymphocytes in Breast Cancer. Curr. Breast Cancer Rep. 2016, 8, 1–13. [Google Scholar] [CrossRef]
- Ono, M.; Tsuda, H.; Shimizu, C.; Yamamoto, S.; Shibata, T.; Yamamoto, H.; Hirata, T.; Yonemori, K.; Ando, M.; Tamura, K.; et al. Tumor-Infiltrating Lymphocytes Are Correlated with Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer. Breast Cancer Res. Treat. 2011, 132, 793–805. [Google Scholar] [CrossRef]
- Pantelidou, C.; Sonzogni, O.; Taveira, M.D.O.; Mehta, A.K.; Kothari, A.; Wang, D.; Visal, T.; Li, M.K.; Pinto, J.; Castrillon, J.A.; et al. PARP Inhibitor Efficacy Depends on CD8+ T-Cell Recruitment via Intratumoral STING Pathway Activation in BRCA-Deficient Models of Triple-Negative Breast Cancer. Cancer Discov. 2019, 9, 722–737. [Google Scholar] [CrossRef]
- Loi, S.; Sirtaine, N.; Piette, F.; Salgado, R.; Viale, G.; Eenoo, F.V.; Rouas, G.; Francis, P.; Crown, J.P.A.; Hitre, E.; et al. Prognostic and Predictive Value of Tumor-Infiltrating Lymphocytes in a Phase III Randomized Adjuvant Breast Cancer Trial in Node-Positive Breast Cancer Comparing the Addition of Docetaxel to Doxorubicin With Doxorubicin-Based Chemotherapy: BIG 02-98. J. Clin. Oncol. 2013, 31, 860–867. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.-A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Gonçalves, A.; Lee, K.-H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef]
- Abraham, J.E.; Pinilla, K.; Dayimu, A.; Grybowicz, L.; Demiris, N.; Harvey, C.; Drewett, L.M.; Lucey, R.; Fulton, A.; Roberts, A.N.; et al. The PARTNER Trial of Neoadjuvant Olaparib with Chemotherapy in Triple-Negative Breast Cancer. Nature 2024, 629, 1142–1148. [Google Scholar] [CrossRef]
- Tutt, A.; Robson, M.; Garber, J.E.; Domchek, S.M.; Audeh, M.W.; Weitzel, J.N.; Friedlander, M.; Arun, B.; Loman, N.; Schmutzler, R.K.; et al. Oral Poly(ADP-Ribose) Polymerase Inhibitor Olaparib in Patients with BRCA1 or BRCA2 Mutations and Advanced Breast Cancer: A Proof-of-Concept Trial. Lancet Lond. Engl. 2010, 376, 235–244. [Google Scholar] [CrossRef]
- Kaufman, B.; Shapira-Frommer, R.; Schmutzler, R.K.; Audeh, M.W.; Friedlander, M.; Balmaña, J.; Mitchell, G.; Fried, G.; Stemmer, S.M.; Hubert, A.; et al. Olaparib Monotherapy in Patients with Advanced Cancer and a Germline BRCA1/2 Mutation. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 33, 244–250. [Google Scholar] [CrossRef]
- Gelmon, K.A.; Tischkowitz, M.; Mackay, H.; Swenerton, K.; Robidoux, A.; Tonkin, K.; Hirte, H.; Huntsman, D.; Clemons, M.; Gilks, B.; et al. Olaparib in Patients with Recurrent High-Grade Serous or Poorly Differentiated Ovarian Carcinoma or Triple-Negative Breast Cancer: A Phase 2, Multicentre, Open-Label, Non-Randomised Study. Lancet Oncol. 2011, 12, 852–861. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Liu, Q.; Du, W.; Chen, L.; Qi, F.; Zhang, R.; Hoang, B.H.; Geller, D.S.; Yang, R.; Wang, J.; et al. Characterization of Parthanatos in Breast Cancer: Implications for Prognosis and PARP Inhibitor Resistance. Bioengineering 2025, 12, 586. https://doi.org/10.3390/bioengineering12060586
Tang J, Liu Q, Du W, Chen L, Qi F, Zhang R, Hoang BH, Geller DS, Yang R, Wang J, et al. Characterization of Parthanatos in Breast Cancer: Implications for Prognosis and PARP Inhibitor Resistance. Bioengineering. 2025; 12(6):586. https://doi.org/10.3390/bioengineering12060586
Chicago/Turabian StyleTang, Junjie, Qian Liu, Wei Du, Linxi Chen, Feiyang Qi, Ranxin Zhang, Bang H. Hoang, David S. Geller, Rui Yang, Jichuan Wang, and et al. 2025. "Characterization of Parthanatos in Breast Cancer: Implications for Prognosis and PARP Inhibitor Resistance" Bioengineering 12, no. 6: 586. https://doi.org/10.3390/bioengineering12060586
APA StyleTang, J., Liu, Q., Du, W., Chen, L., Qi, F., Zhang, R., Hoang, B. H., Geller, D. S., Yang, R., Wang, J., & Hu, L. (2025). Characterization of Parthanatos in Breast Cancer: Implications for Prognosis and PARP Inhibitor Resistance. Bioengineering, 12(6), 586. https://doi.org/10.3390/bioengineering12060586






