Performance Evaluation of a Custom-Designed Contrast Media Injector in a 5-Tesla MRI Environment
Abstract
1. Introduction
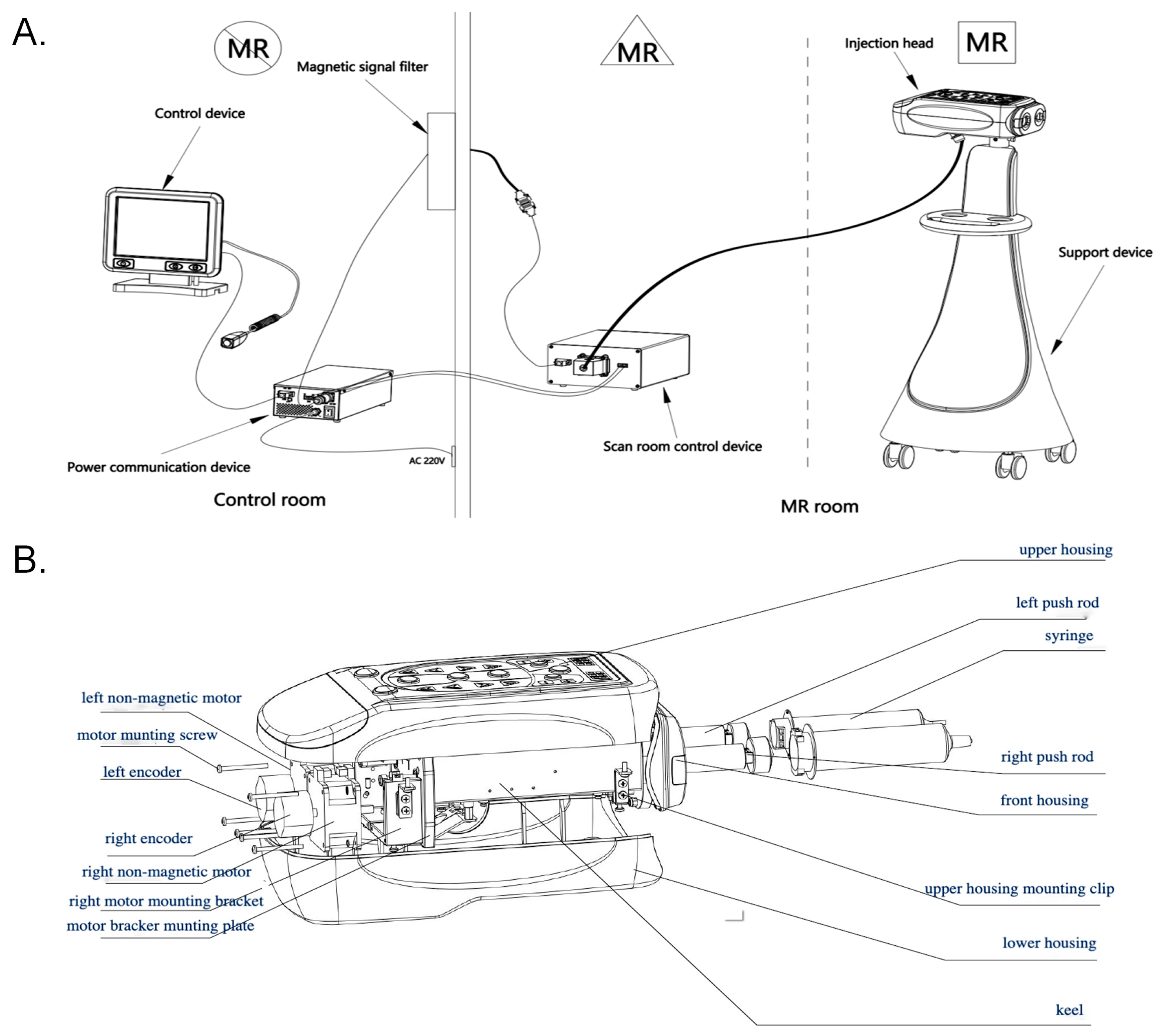
2. Materials and Methods
- Translational Attraction Force: The first objective was to measure and confirm that the translational attraction force exerted on the CMI by the static magnetic field remains below the established safety thresholds.
- Injection Accuracy: The second goal was to assess whether the RF pulses and high magnetic field would affect the CMI’s injection rate accuracy and the total injected volume. Injection flow rates and total injected volume were measured across multiple test conditions to determine if any deviations occurred.
- Image Artifact Evaluation: Finally, we tested whether the CMI would introduce any artifacts on the MR images during contrast agent injection. Standard clinical sequences were used to evaluate image quality, and the presence of any RF-induced artifacts was documented.
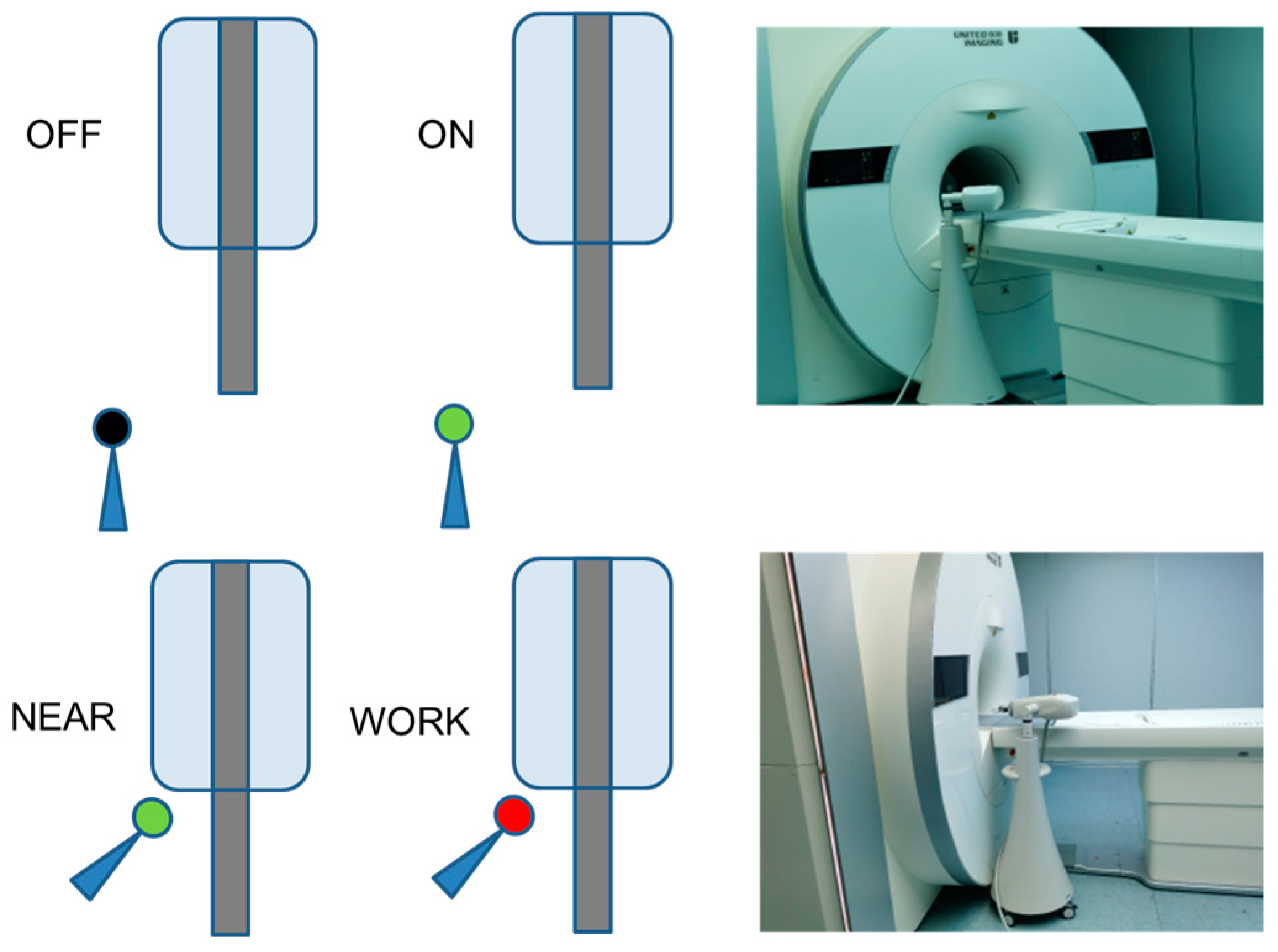
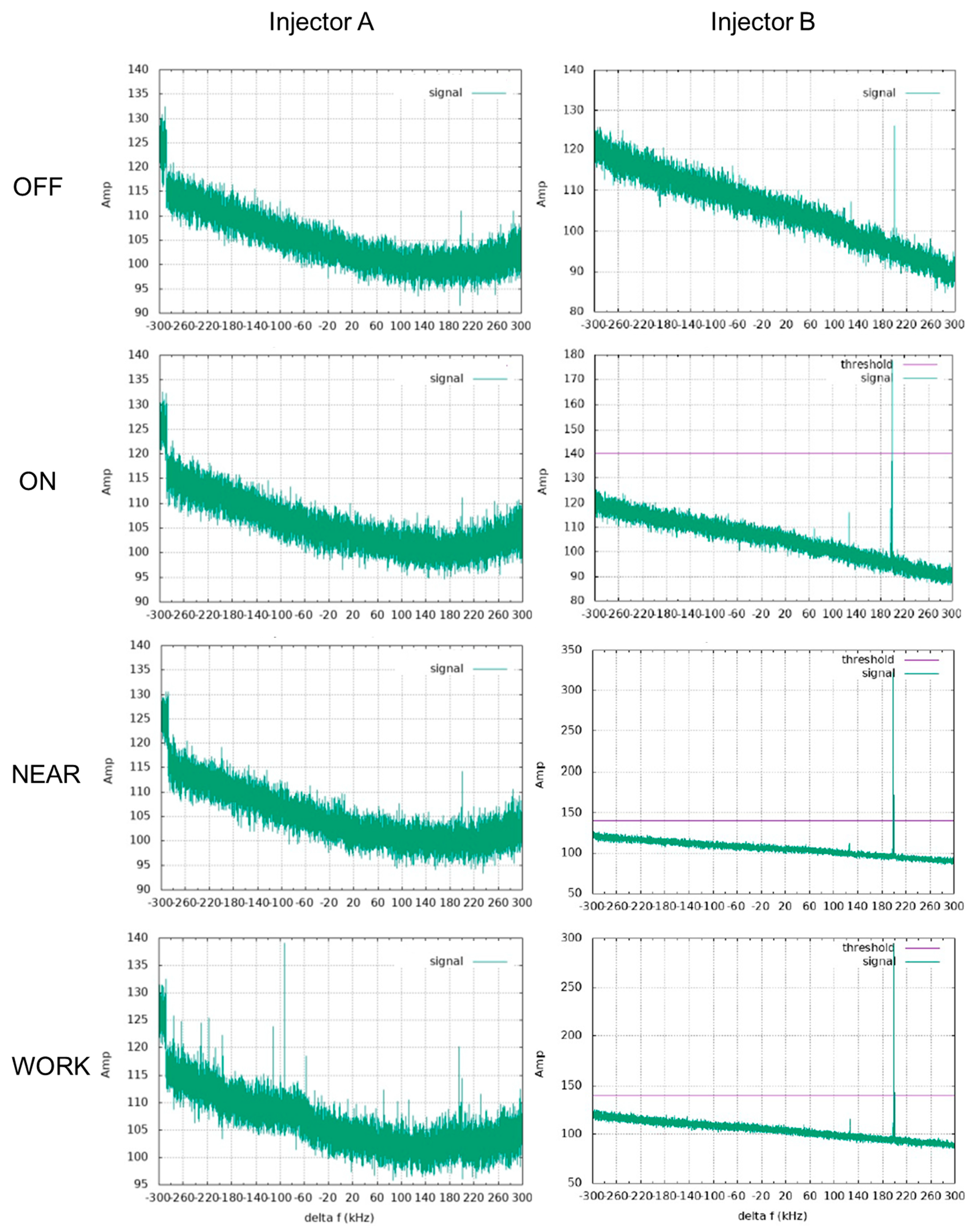
2.1. Measurement of the Translation Attraction Force
2.2. Evaluation of the Accuracy of the Injection
- (1)
- Accuracy of the injection rate
- (2)
- Accuracy of the injection volume
- (3)
- Accuracy of the maximum injection pressure
2.3. Evaluation of the RF-Induced Image Artifacts
- Test 1
- Test 2
- Test 3
- Test 4
- Test 5
2.4. Statistical Analysis
3. Results
3.1. Translation Attraction Force in the 5T MRI Environment
3.2. Accuracy of the Injector
3.2.1. Accuracy of the Injection Rate and Volume
3.2.2. Maximum Injection Pressure
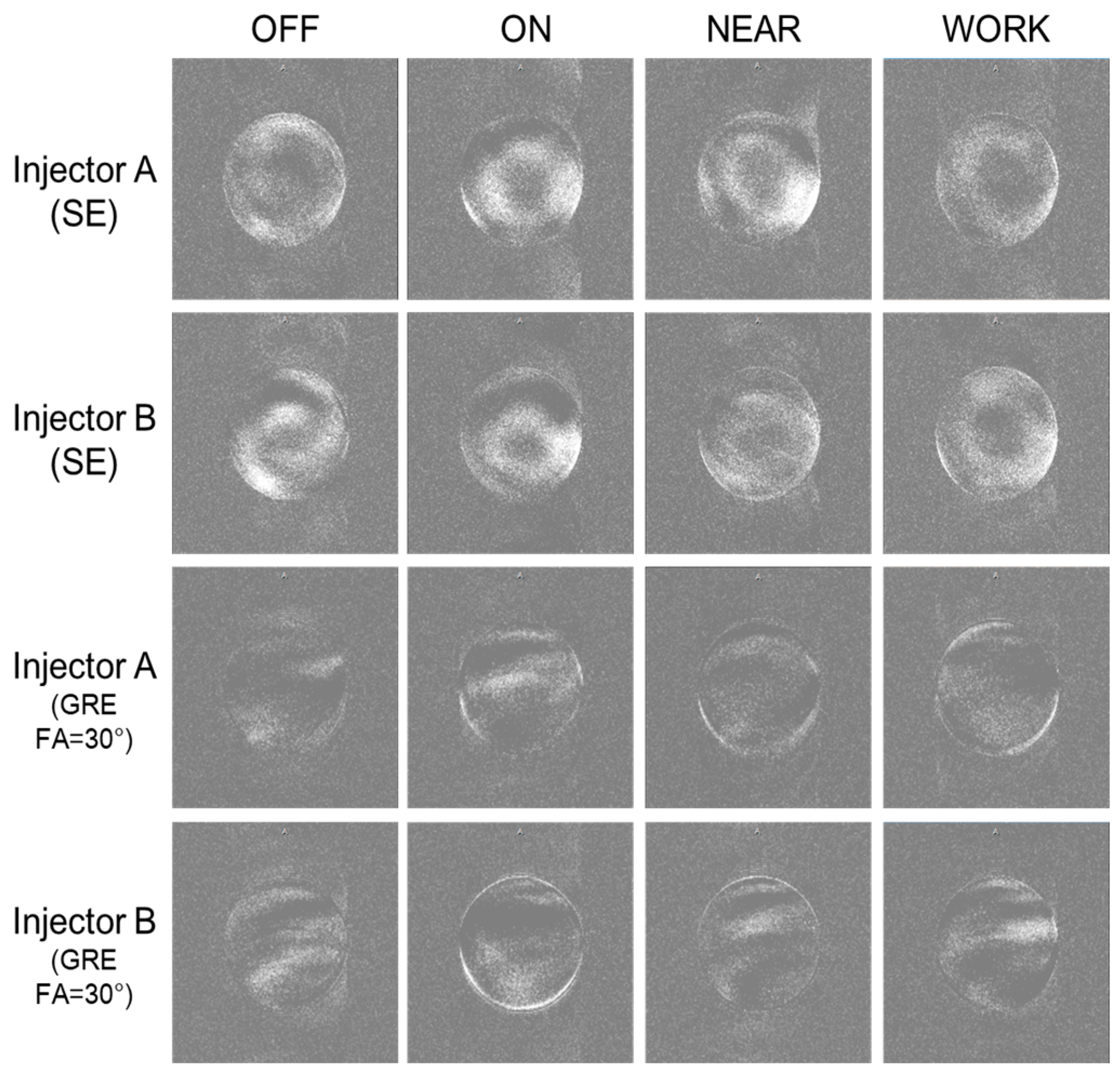
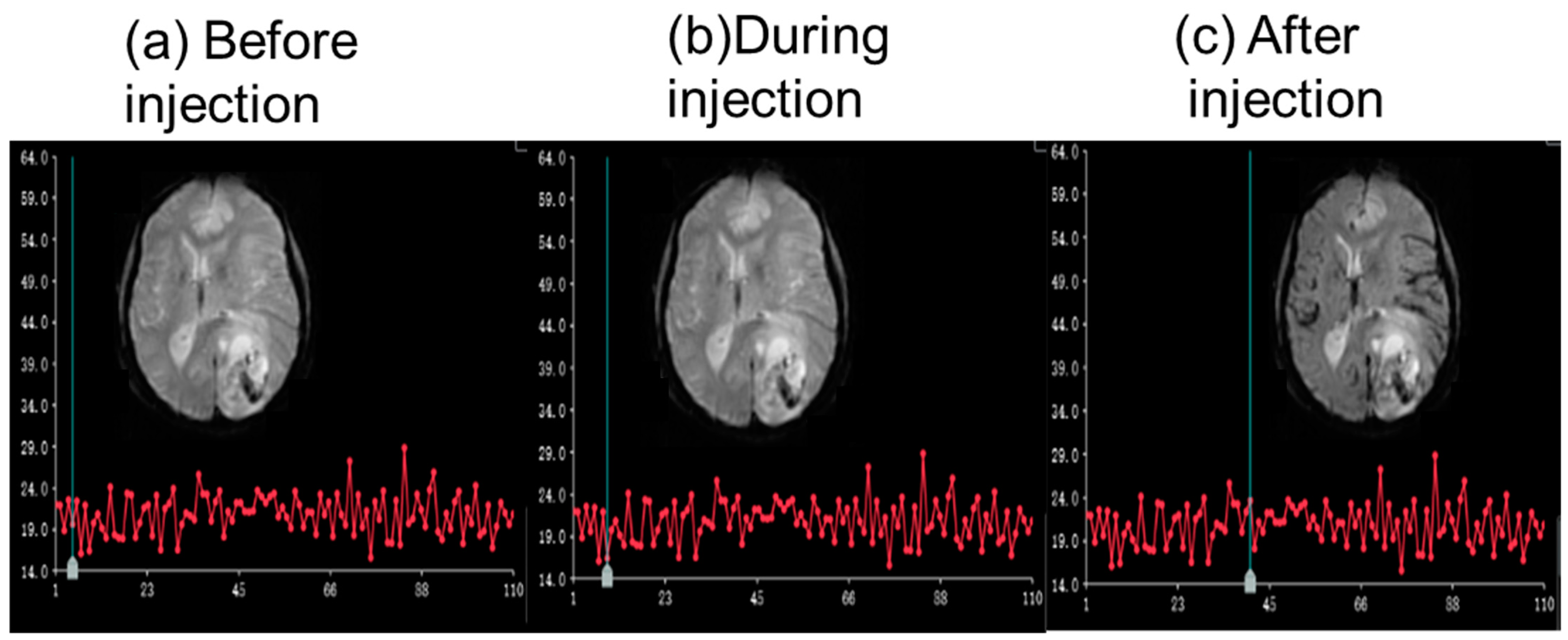
3.2.3. RF Influence of the CMI on MRI Images
- Test 1
- Test 2
- Test 3
- Test 4
- Test 5
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BOLD | Blood oxygen level-dependent |
| CM | Contrast media |
| CMI | Contrast media injector |
| CE | Contrast-enhanced |
| MRI | Magnetic resonance imaging |
| RF | Radiofrequency |
| EMI | Electromagnetic interference |
| SE | Spin echo |
| GRE | Gradient echo |
| DSC | Dynamic susceptibility contrast |
| DCE | Dynamic contrast-enhanced |
References
- Wen, S.W.; Wong, C.H.Y. Aging- and vascular-related pathologies. Microcirculation 2019, 26, e12463. [Google Scholar] [CrossRef] [PubMed]
- Oscanoa, J.A.; Middione, M.J.; Alkan, C.; Yurt, M.; Loecher, M.; Vasanawala, S.S.; Ennis, D.B. Deep Learning-Based Reconstruction for Cardiac MRI: A Review. Bioengineering 2023, 10, 334. [Google Scholar] [CrossRef]
- Lohrke, J.; Frenzel, T.; Endrikat, J.; Alves, F.C.; Grist, T.M.; Law, M.; Lee, J.M.; Leiner, T.; Li, K.C.; Nikolaou, K.; et al. 25 Years of Contrast-Enhanced MRI: Developments, Current Challenges and Future Perspectives. Adv. Ther. 2016, 33, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Sinan, T.; Al-Khawari, H.; Chishti, F.A.; Al Saeed, O.M.; Sheikh, M. Contrast media extravasation: Manual versus power injector. Med. Princ. Pract. 2005, 14, 107–110. [Google Scholar] [CrossRef]
- Friebe, M. Computed tomography and magnetic resonance imaging contrast media injectors: Technical feature review—What is really needed? Med. Devices 2016, 9, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Indrajit, I.K.; Sivasankar, R.; D’Souza, J.; Pant, R.; Negi, R.S.; Sahu, S.; Hashim, P. Pressure injectors for radiologists: A review and what is new. Indian. J. Radiol. Imaging 2015, 25, 2–10. [Google Scholar] [CrossRef]
- Mann, R.M.; Kuhl, C.K.; Moy, L. Contrast-enhanced MRI for breast cancer screening. J. Magn. Reson. Imaging 2019, 50, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Zhuge, Y.; Ning, H.; Mathen, P.; Cheng, J.Y.; Krauze, A.V.; Camphausen, K.; Miller, R.W. Automated glioma grading on conventional MRI images using deep convolutional neural networks. Med. Phys. 2020, 47, 3044–3053, Erratum in Med. Phys. 2023, 50, 5930–5931. https://doi.org/10.1002/mp.16588. [Google Scholar] [CrossRef]
- Kataoka, M.; Honda, M.; Sagawa, H.; Ohashi, A.; Sakaguchi, R.; Hashimoto, H.; Iima, M.; Takada, M.; Nakamoto, Y. Ultrafast Dynamic Contrast-Enhanced MRI of the Breast: From Theory to Practice. J. Magn. Reson. Imaging 2024, 60, 401–416. [Google Scholar] [CrossRef]
- Zhang, Y.; Sheng, R.; Yang, C.; Dai, Y.; Zeng, M. Higher field reduced FOV diffusion-weighted imaging for abdominal imaging at 5.0 Tesla: Image quality evaluation compared with 3.0 Tesla. Insights Imaging 2023, 14, 171. [Google Scholar] [CrossRef]
- Cosottini, M.; Roccatagliata, L. Neuroimaging at 7 T: Are we ready for clinical transition? Eur. Radiol. Exp. 2021, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Krug, R.; Stehling, C.; Kelley, D.A.; Majumdar, S.; Link, T.M. Imaging of the musculoskeletal system in vivo using ultra-high field magnetic resonance at 7 T. Investig. Radiol. 2009, 44, 613–618. [Google Scholar] [CrossRef]
- Shi, Z.; Zhao, X.; Zhu, S.; Miao, X.; Zhang, Y.; Han, S.; Wang, B.; Zhang, B.; Ye, X.; Dai, Y.; et al. Time-of-Flight Intracranial MRA at 3 T versus 5 T versus 7 T: Visualization of Distal Small Cerebral Arteries. Radiology 2022, 305, E72. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, W.; Xu, D.; Yu, H.; Mei, H.; Song, X.; Xu, H. Stability and repeatability of diffusion-weighted imaging (DWI) of normal pancreas on 5.0 Tesla magnetic resonance imaging (MRI). Sci. Rep. 2023, 13, 11954. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Tarakameh, A.; Adriany, G.; Metzger, G.J.; Lagore, R.L.; Jungst, S.; DelaBarre, L.; Van de Moortele, P.F.; Ugurbil, K.; Atalar, E.; Eryaman, Y. Improving radiofrequency power and specific absorption rate management with bumped transmit elements in ultra-high field MRI. Magn. Reson. Med. 2020, 84, 3485–3493. [Google Scholar] [CrossRef]
- Fagan, A.J.; Bitz, A.K.; Björkman-Burtscher, I.M.; Collins, C.M.; Kimbrell, V.; Raaijmakers, A.J.E.; ISMRM Safety Committee. 7T MR Safety. J. Magn. Reson. Imaging 2021, 53, 333–346. [Google Scholar] [CrossRef]
- Qian, X.; Wang, S.; Wu, Y.; Miao, X.; Chen, Y.; Lu, H.; Wang, R.; Wang, D.; Wang, F.; Zhang, S.; et al. Late Gadolinium Enhancement of Nonischemic Cardiomyopathy at 5.0 T versus 3.0 T: A Crossover Design Study. Radiol. Cardiothorac. Imaging 2024, 6, e240035. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Yang, C.; Sheng, R.; Dai, Y.; Zeng, M. Renal imaging at 5 T versus 3 T: A comparison study. Insights Imaging 2022, 13, 155. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Yu, D.; Yang, Y.; Li, Z.; Wang, X.; Yang, Y.; Cheng, C.; Zou, C.; Gan, J. Comparative analysis of hepatic fat quantification across 5 T, 3 T and 1.5 T: A study on consistency and feasibility. Eur. J. Radiol. 2024, 180, 111709. [Google Scholar] [CrossRef]
- Jost, G.; Endrikat, J.; Pietsch, H. The Impact of Injector-Based Contrast Agent Administration on Bolus Shape and Magnetic Resonance Angiography Image Quality. Magn. Reson. Insights 2017, 10, 1178623X17705894. [Google Scholar] [CrossRef]
- Shellock, F.G.; Tkach, J.A.; Ruggieri, P.M.; Masaryk, T.J.; Rasmussen, P.A. Aneurysm clips: Evaluation of magnetic field interactions and translational attraction by use of “long-bore” and “short-bore” 3.0-T MR imaging systems. AJNR Am. J. Neuroradiol. 2003, 24, 463–471. [Google Scholar] [PubMed]
- Dula, A.N.; Shellock, F.G. Evaluation of a power injection system in the 7-Tesla MRI environment. Int. J. Imaging Syst. Technol. 2015, 25, 50–55. [Google Scholar] [CrossRef]
- Omidvari, N.; Topping, G.; Cabello, J.; Paul, S.; Schwaiger, M.; Ziegler, S.I. MR-compatibility assessment of MADPET4: A study of interferences between an SiPM-based PET insert and a 7 T MRI system. Phys. Med. Biol. 2018, 63, 095002. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, C.J.; Fan, A.P. BOLD signal physiology: Models and applications. Neuroimage 2019, 187, 116–127. [Google Scholar] [CrossRef]
- Shin, K.E.; Ahn, K.J.; Choi, H.S.; Jung, S.L.; Kim, B.S.; Jeon, S.S.; Hong, Y.G. DCE and DSC MR perfusion imaging in the differentiation of recurrent tumour from treatment-related changes in patients with glioma. Clin. Radiol. 2014, 69, e264-72. [Google Scholar] [CrossRef]
- Xue, P.; Zhang, J.; Ma, L.; Liu, M.; Gu, Y.; Huang, J. Structure-aware registration network for liver DCE-CT images. IEEE J. Biomed. Health Inform. 2024, 28, 2163–2174. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiao, L.; Liu, Y.; Leong, A.T.; Wu, E.X. Electromagnetic interference elimination via active sensing and deep learning prediction for radiofrequency shielding-free MRI. NMR Biomed. 2024, 37, e4956. [Google Scholar] [CrossRef]



| Parameters | SE | GRE | DSC | DCE |
|---|---|---|---|---|
| TR (ms) | 1500 | 800 | 1600 | 2.77 |
| TE (ms) | 30 | 25 | 32.5 | 1.03 |
| FOV (mm2) | 300 × 300 | 300 × 300 | 230 × 230 | 230 × 230 |
| FA (°) | 180 | 30/0 | 90 | 10 |
| Gap (%) | 0 | 0 | 20 | 0 |
| Thickness (mm) | 5 | 5 | 5 | 5 |
| Slice number | 1 | 1 | 19 | 24 |
| Resolution (mm2) | 1.17 × 1.17 | 1.56 × 1.56 | 1.80 × 1.80 | 1.60 × 1.60 |
| BW (kHz) | 1000 | 1000 | 1630 | 800 |
| Accelerating factor | 1 | 1 | 2 | 2 |
| Number of Dynamic scans | 1 | 1 | 50 | 50 |
| Syringe | Target Rate (mL/s) | Target Volume (mL) | Injection Round | Time (s) | Volume (mL) | Actual Flow Rate (mL/s) | Average Actual Rate (mL/s) | Deviation of the Actual Rate (mL/s) |
|---|---|---|---|---|---|---|---|---|
| CM | 0.1 | 60 | 1st | 602.0 | 59.27 | 0.1 | 0.1 | 0 |
| 2nd | 602.6 | 59.32 | 0.1 | |||||
| 3rd | 604.3 | 59.45 | 0.1 | |||||
| saline | 0.1 | 60 | 1st | 603.4 | 59.43 | 0.1 | 0.1 | 0 |
| 2nd | 603.2 | 59.25 | 0.1 | |||||
| 3rd | 603.7 | 59.60 | 0.1 | |||||
| CM | 5 | 50 | 1st | 10.2 | 49.35 | 4.89 | 4.89 | −0.11 |
| 2nd | 10.1 | 49.13 | 4.91 | |||||
| 3rd | 10.2 | 49.25 | 4.88 | |||||
| saline | 5 | 100 | 1st | 20.2 | 99.38 | 4.94 | 4.95 | −0.05 |
| 2nd | 20.1 | 99.21 | 4.96 | |||||
| 3rd | 20.2 | 99.36 | 4.94 | |||||
| CM | 10 | 50 | 1st | 5.2 | 49.43 | 9.73 | 9.70 | −0.3 |
| 2nd | 5.2 | 49.28 | 9.68 | |||||
| 3rd | 5.2 | 49.51 | 9.69 | |||||
| saline | 10 | 100 | 1st | 10.2 | 99.36 | 9.84 | 9.84 | −0.16 |
| 2nd | 10.2 | 99.41 | 9.84 | |||||
| 3rd | 10.2 | 99.25 | 9.83 |
| Syringe | Target Rate (mL/s) | Target Volume (mL) | Injection Round | Actual Volume (mL) | Average Actual Volume (mL) | Deviation of the Actual Volume (mL) |
|---|---|---|---|---|---|---|
| CM | 0.1 | 1 | 1st | 1.09 | 1.06 | 0.06 |
| 2nd | 1.04 | |||||
| 3rd | 1.05 | |||||
| 5 | 30 | 1st | 29.63 | 29.66 | −0.34 | |
| 2nd | 29.71 | |||||
| 3rd | 29.64 | |||||
| 10 | 60 | 1st | 59.38 | 59.39 | −0.61 | |
| 2nd | 59.35 | |||||
| 3rd | 59.44 | |||||
| saline | 0.1 | 1 | 1st | 21.06 | 1.04 | 0.04 |
| 2nd | 1.03 | |||||
| 3rd | 1.04 | |||||
| 5 | 55 | 1st | 553 | 553 | 0.03 | |
| 2nd | 551 | |||||
| 3rd | 556 | |||||
| 10 | 110 | 1st | 109.30 | 109.32 | −0.68 | |
| 2nd | 109.34 | |||||
| 3rd | 109.31 |
| Sequence | Status | Injector A (Center) | Injector A (Background) | Injector B (Center) | Injector B (Background) |
|---|---|---|---|---|---|
| SE | OFF | 15.27 ± 15.47 | 3.18 ± 4.68 | 12.85 ± 13.23 | 3.20 ± 4.70 |
| ON | 13.88 ± 15.56 | 3.30 ± 4.80 | 15.32 ± 15.67 | 3.19 ± 4.79 | |
| NEAR | 12.51 ± 12.89 | 3.18 ± 4.65 | 16.7 ± 17.0 | 3.20 ± 4.70 | |
| WORK | 14.6 ± 14.4 | 3.0 ± 4.5 | 10.73 ± 12.02 | 3.33 ± 4.83 | |
| GRE (FA = 30°) | OFF | 6.70 ± 8.45 | 2.54 ± 3.66 | 4.31 ± 6.72 | 2.32 ± 3.48 |
| ON | 5.40 ± 8.19 | 2.40 ± 3.59 | 6.50 ± 8.67 | 2.46 ± 3.62 | |
| NEAR | 4.54 ± 6.99 | 2.38 ± 3.49 | 4.38 ± 6.57 | 2.28 ± 3.34 | |
| WORK | 5.77 ± 9.02 | 2.47 ± 3.59 | 5.22 ± 7.93 | 2.32 ± 3.54 |
| Sequence | Status | Injector A | Injector B |
|---|---|---|---|
| DCE | OFF | 21.11 ± 0.67 | 23.61 ± 0.46 |
| ON | 20.96 ± 0.58 | 23.49 ± 0.46 | |
| NEAR | 20.92 ± 0.63 | 23.46 ± 0.51 | |
| WORK | 21.01 ± 0.60 | 23.48 ± 0.45 | |
| DSC | OFF | 41.02 ± 0.99 | 48.84 ± 1.28 |
| ON | 41.23 ± 0.90 | 48.96 ± 1.15 | |
| NEAR | 40.86 ± 0.82 | 48.15 ± 1.23 | |
| WORK | 41.26 ± 0.95 | 48.32 ± 1.48 | |
| GRE (FA = 0°) | OFF | 42.35 ± 4.12 | 55.45 ± 6.99 |
| ON | 42.35 ± 4.14 | 56.00 ± 7.79 | |
| NEAR | 42.42 ± 4.14 | 56.4 ± 8.3 | |
| WORK | 42.55 ± 4.18 | 56.9 ± 9.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Sun, W.; Wang, Z.; Wang, W.; Liao, R.; Ruan, Z.; Li, H.; Xu, H.; Topgaard, D. Performance Evaluation of a Custom-Designed Contrast Media Injector in a 5-Tesla MRI Environment. Bioengineering 2025, 12, 566. https://doi.org/10.3390/bioengineering12060566
Hu Y, Sun W, Wang Z, Wang W, Liao R, Ruan Z, Li H, Xu H, Topgaard D. Performance Evaluation of a Custom-Designed Contrast Media Injector in a 5-Tesla MRI Environment. Bioengineering. 2025; 12(6):566. https://doi.org/10.3390/bioengineering12060566
Chicago/Turabian StyleHu, Yuannan, Wenbo Sun, Zhusha Wang, Wei Wang, Rufang Liao, Zhao Ruan, Huan Li, Haibo Xu, and Daniel Topgaard. 2025. "Performance Evaluation of a Custom-Designed Contrast Media Injector in a 5-Tesla MRI Environment" Bioengineering 12, no. 6: 566. https://doi.org/10.3390/bioengineering12060566
APA StyleHu, Y., Sun, W., Wang, Z., Wang, W., Liao, R., Ruan, Z., Li, H., Xu, H., & Topgaard, D. (2025). Performance Evaluation of a Custom-Designed Contrast Media Injector in a 5-Tesla MRI Environment. Bioengineering, 12(6), 566. https://doi.org/10.3390/bioengineering12060566







