Individualising Galvanic Vestibular Stimulation Further Improves Visuomotor Performance in Parkinson’s Disease
Abstract
1. Introduction
2. Methods
2.1. Study Participants
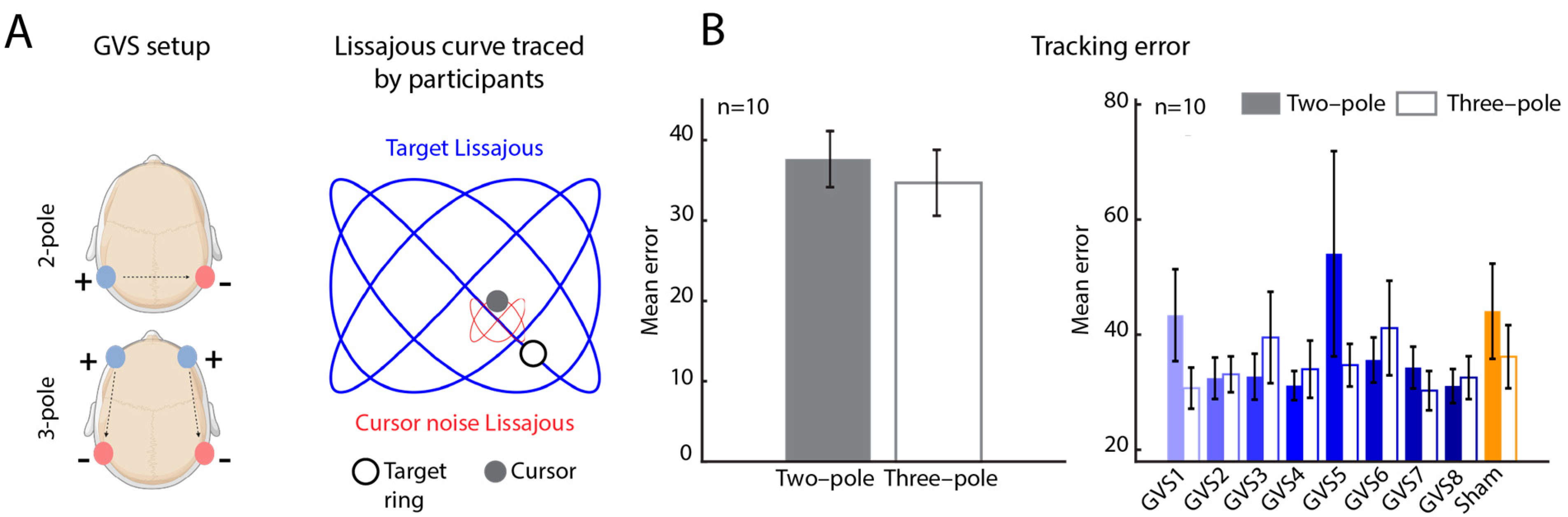
2.2. Galvanic Vestibular Stimulation (GVS)
2.3. Target Tracking Task
2.4. Error Analyses
2.4.1. Tracking Error
2.4.2. Performance Improvement
2.5. Statistical Analyses
2.6. Data Exclusion
3. Results
3.1. Demographics and Clinical Characteristics
3.2. Tracking Error as a Function of Stimulation Parameters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jellinger, K.A. Parkinson’s Disease. In Pathobiology of Human Disease; McManus, L.M., Mitchell, R.N., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 2021–2035. ISBN 978-0-12-386457-4. [Google Scholar]
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s Disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Canadian Chronic Disease Surveillance System (CCDSS). Available online: https://health-infobase.canada.ca/ccdss/data-tool/ (accessed on 13 May 2024).
- Rizek, P.; Kumar, N.; Jog, M.S. An Update on the Diagnosis and Treatment of Parkinson Disease. CMAJ Can. Med. Assoc. J. 2016, 188, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Koller, W.C.; Rueda, M.G. Mechanism of Action of Dopaminergic Agents in Parkinson’s Disease. Neurology 1998, 50, S11–S14. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P. Wearing Off, Dyskinesia, and the Use of Continuous Drug Delivery in Parkinson’s Disease. Neurol. Clin. 2013, 31, S17–S35. [Google Scholar] [CrossRef][Green Version]
- Marsden, C.D. Problems with Long-Term Levodopa Therapy for Parkinson’s Disease. Clin. Neuropharmacol. 1994, 17 (Suppl. S2), S32–S44. [Google Scholar] [CrossRef]
- Frey, J.; Cagle, J.; Johnson, K.A.; Wong, J.K.; Hilliard, J.D.; Butson, C.R.; Okun, M.S.; de Hemptinne, C. Past, Present, and Future of Deep Brain Stimulation: Hardware, Software, Imaging, Physiology and Novel Approaches. Front. Neurol. 2022, 13, 825178. [Google Scholar] [CrossRef]
- Hitti, F.L.; Ramayya, A.G.; McShane, B.J.; Yang, A.I.; Vaughan, K.A.; Baltuch, G.H. Long-Term Outcomes Following Deep Brain Stimulation for Parkinson’s Disease. J. Neurosurg. 2019, 132, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Buhmann, C.; Huckhagel, T.; Engel, K.; Gulberti, A.; Hidding, U.; Poetter-Nerger, M.; Goerendt, I.; Ludewig, P.; Braass, H.; Choe, C.; et al. Adverse Events in Deep Brain Stimulation: A Retrospective Long-Term Analysis of Neurological, Psychiatric and Other Occurrences. PLoS ONE 2017, 12, e0178984. [Google Scholar] [CrossRef]
- Government of British Columbia. Deep Brain Stimulation (DBS)—Province of British Columbia. Available online: https://www2.gov.bc.ca/gov/content/health/about-bc-s-health-care-system/partners/health-authorities/bc-health-technology-assessment/health-technology-assessments/deep-brain-stimulation (accessed on 5 May 2024).
- Honey, C.M.; Malhotra, A.K.; Tamber, M.S.; Prud’homme, M.; Mendez, I.; Honey, C.R. Canadian Assessment of Deep Brain Stimulation Access: The Canada Study. Can. J. Neurol. Sci. 2018, 45, 553–558. [Google Scholar] [CrossRef]
- Madrid, J.; Benninger, D.H. Non-Invasive Brain Stimulation for Parkinson’s Disease: Clinical Evidence, Latest Concepts and Future Goals: A Systematic Review. J. Neurosci. Methods 2021, 347, 108957. [Google Scholar] [CrossRef]
- Kobayashi, M. Effect of Slow Repetitive TMS of the Motor Cortex on Ipsilateral Sequential Simple Finger Movements and Motor Skill Learning. Restor. Neurol. Neurosci. 2010, 28, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.X.D.; Mai, P.T.; Chang, Y.-J.; Hsieh, T.-H. Effects of Transcranial Direct Current Stimulation Alone and in Combination with Rehabilitation Therapies on Gait and Balance among Individuals with Parkinson’s Disease: A Systematic Review and Meta-Analysis. J. Neuroeng. Rehabil. 2024, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Del Felice, A.; Castiglia, L.; Formaggio, E.; Cattelan, M.; Scarpa, B.; Manganotti, P.; Tenconi, E.; Masiero, S. Personalized Transcranial Alternating Current Stimulation (tACS) and Physical Therapy to Treat Motor and Cognitive Symptoms in Parkinson’s Disease: A Randomized Cross-over Trial. NeuroImage Clin. 2019, 22, 101768. [Google Scholar] [CrossRef] [PubMed]
- Muksuris, K.; Scarisbrick, D.M.; Mahoney, J.J.; Cherkasova, M.V. Noninvasive Neuromodulation in Parkinson’s Disease: Insights from Animal Models. J. Clin. Med. 2023, 12, 5448. [Google Scholar] [CrossRef]
- Stiles, L.; Smith, P.F. The Vestibular–Basal Ganglia Connection: Balancing Motor Control. Brain Res. 2015, 1597, 180–188. [Google Scholar] [CrossRef]
- Smith, P.F. Recent Developments in the Understanding of the Interactions between the Vestibular System, Memory, the Hippocampus, and the Striatum. Front. Neurol. 2022, 13, 986302. [Google Scholar] [CrossRef]
- Lee, S.; Liu, A.; McKeown, M.J. Current Perspectives on Galvanic Vestibular Stimulation in the Treatment of Parkinson’s Disease. Expert Rev. Neurother. 2021, 21, 405–418. [Google Scholar] [CrossRef]
- Kuatsjah, E.; Khoshnam, M.; Menon, C. Investigation on the Effect of Noisy Galvanic Vestibular Stimulation on Fine Motor Skills during a Visuomotor Task in Healthy Participants. PLoS ONE 2019, 14, e0216214. [Google Scholar] [CrossRef]
- Mahmud, M.; Hadi, Z.; Prendergast, M.; Ciocca, M.; Saad, A.R.; Pondeca, Y.; Tai, Y.; Scott, G.; Seemungal, B.M. The Effect of Galvanic Vestibular Stimulation on Postural Balance in Parkinson’s Disease: A Systematic Review and Meta-Analysis. J. Neurol. Sci. 2022, 442, 120414. [Google Scholar] [CrossRef]
- Kataoka, H.; Okada, Y.; Kiriyama, T.; Kita, Y.; Nakamura, J.; Morioka, S.; Shomoto, K.; Ueno, S. Can Postural Instability Respond to Galvanic Vestibular Stimulation in Patients with Parkinson’s Disease? J. Mov. Disord. 2016, 9, 40–43. [Google Scholar] [CrossRef]
- Khoshnam, M.; Häner, D.M.C.; Kuatsjah, E.; Zhang, X.; Menon, C. Effects of Galvanic Vestibular Stimulation on Upper and Lower Extremities Motor Symptoms in Parkinson’s Disease. Front. Neurosci. 2018, 12, 633. [Google Scholar] [CrossRef]
- Dlugaiczyk, J.; Gensberger, K.D.; Straka, H. Galvanic Vestibular Stimulation: From Basic Concepts to Clinical Applications. J. Neurophysiol. 2019, 121, 2237–2255. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Maeda, T.; Krebs, H.I. Headset Design to Accommodate Four-Pole Galvanic Vestibular Stimulation. In Proceedings of the 2016 6th IEEE International Conference on Biomedical Robotics and Biomechatronics (BioRob), Singapore, 26–29 June 2016; pp. 1335–1339. [Google Scholar]
- Aoyama, K.; Iizuka, H.; Ando, H.; Maeda, T. Four-Pole Galvanic Vestibular Stimulation Causes Body Sway About Three Axes|Scientific Reports. Available online: https://www.nature.com/articles/srep10168 (accessed on 5 May 2024).
- Séverac Cauquil, A.; Martinez, P.; Ouaknine, M.; Tardy-Gervet, M.-F. Orientation of the Body Response to Galvanic Stimulation as a Function of the Inter-Vestibular Imbalance. Exp. Brain Res. 2000, 133, 501–505. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Lee, S.-B.; Kang, J.-J.; Oh, S.-Y. Optimal Design of Galvanic Vestibular Stimulation for Patients with Vestibulopathy and Cerebellar Disorders. Brain Sci. 2023, 13, 1333. [Google Scholar] [CrossRef] [PubMed]
- Mulavara, A.P.; Fiedler, M.J.; Kofman, I.S.; Wood, S.J.; Serrador, J.M.; Peters, B.; Cohen, H.S.; Reschke, M.F.; Bloomberg, J.J. Improving Balance Function Using Vestibular Stochastic Resonance: Optimizing Stimulus Characteristics. Exp. Brain Res. 2011, 210, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism. Neurology 1967, 17, 427. [Google Scholar] [CrossRef]
- Menon, A.; Vigneswaran, M.; Zhang, T. The Effects of two-pole and three-pole Galvanic Vestibular Stimulation on Motor Performance in Parkinson’s Disease. In Proceedings of the School of Biomedical Engineering Research Day, Vancouver, BC, Canada, 8 June 2022. [Google Scholar]
- Forbes, P.A.; Kwan, A.; Rasman, B.G.; Mitchell, D.E.; Cullen, K.E.; Blouin, J.-S. Neural Mechanisms Underlying High-Frequency Vestibulocollic Reflexes in Humans and Monkeys. J. Neurosci. 2020, 40, 1874–1887. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Yekutieli, D.; Benjamini, Y. Resampling-Based False Discovery Rate Controlling Multiple Test Procedures for Correlated Test Statistics. J. Stat. Plan. Inference 1999, 82, 171–196. [Google Scholar] [CrossRef]
- Lee, S.; Smith, P.F.; Lee, W.H.; McKeown, M.J. Frequency-Specific Effects of Galvanic Vestibular Stimulation on Response-Time Performance in Parkinson’s Disease. Front. Neurol. 2021, 12, 758122. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J. Electrophysiological and Motoric Effects of Galvanic Vestibular Stimulation in Normal and Parkinson’s Disease Subjects. Master’s Thesis, University of British Columbia, Vancouver, BC, Canada, 2013. [Google Scholar]
- Liu, A.; Bi, H.; Li, Y.; Lee, S.; Cai, J.; Mi, T.; Garg, S.; Kim, J.L.; Zhu, M.; Chen, X.; et al. Galvanic Vestibular Stimulation Improves Subnetwork Interactions in Parkinson’s Disease. J. Healthc. Eng. 2021, 2021, e6632394. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, D.; McKeown, M.J. Galvanic Vestibular Stimulation (GVS) Effects on Impaired Interhemispheric Connectivity in Parkinson’s Disease. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Seogwipo, Republic of Korea, 11–15 July 2017; pp. 2109–2113. [Google Scholar]
- Kazemi, A.; Mirian, M.S.; Lee, S.; McKeown, M.J. Galvanic Vestibular Stimulation Effects on EEG Biomarkers of Motor Vigor in Parkinson’s Disease. Front. Neurol. 2021, 12, 759149. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.; Nouri, S.; Mirian, M.S.; Lee, S.; McKeown, M.J. Predicting Motor Vigor from EEG Phase Amplitude Coupling (PAC) in Parkinson’s Disease: Effects of Dopaminergic Medication and Non-Invasive Modulation. medRxiv 2024. [Google Scholar] [CrossRef]
- Lee, S.C.; Dorfman, B.E.; Razek, O.A. Vestibular System Anatomy. Available online: https://emedicine.medscape.com/article/883956-overview?form=fpf (accessed on 9 May 2025).

| Characteristic | |
|---|---|
| Age, years | |
| Mean (SD) | 74.5 (6.6) |
| Gender, n | |
| Female | 4 |
| Male | 8 |
| Side affected, n | |
| Left | 10 |
| Right | 2 |
| MoCA score | |
| Mean (SD) | 26.4 (4.2) |
| UPDRS III score (total) | |
| Mean (SD) | 46.6 (9.8) |
| Stimulus | Improvement with Individualised GVS (%) | Improvement with GVS over Sham (%) |
|---|---|---|
| GVS1 | 47 | 12 |
| GVS2 | 28 | 19 |
| GVS3 | 37 | 13 |
| GVS4 | 27 | 23 |
| GVS5 | 87 | 7 |
| GVS6 | 45 | 4 |
| GVS7 | 25 | 21 |
| GVS8 | 24 | 25 |
| SHAM | 51 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menon, A.; Vigneswaran, M.; Zhang, T.; Sreenivasan, V.; Kim, C.; McKeown, M.J. Individualising Galvanic Vestibular Stimulation Further Improves Visuomotor Performance in Parkinson’s Disease. Bioengineering 2025, 12, 523. https://doi.org/10.3390/bioengineering12050523
Menon A, Vigneswaran M, Zhang T, Sreenivasan V, Kim C, McKeown MJ. Individualising Galvanic Vestibular Stimulation Further Improves Visuomotor Performance in Parkinson’s Disease. Bioengineering. 2025; 12(5):523. https://doi.org/10.3390/bioengineering12050523
Chicago/Turabian StyleMenon, Anjali, Madhini Vigneswaran, Tina Zhang, Varsha Sreenivasan, Christina Kim, and Martin J. McKeown. 2025. "Individualising Galvanic Vestibular Stimulation Further Improves Visuomotor Performance in Parkinson’s Disease" Bioengineering 12, no. 5: 523. https://doi.org/10.3390/bioengineering12050523
APA StyleMenon, A., Vigneswaran, M., Zhang, T., Sreenivasan, V., Kim, C., & McKeown, M. J. (2025). Individualising Galvanic Vestibular Stimulation Further Improves Visuomotor Performance in Parkinson’s Disease. Bioengineering, 12(5), 523. https://doi.org/10.3390/bioengineering12050523






