Cardiac Tissue Engineering for Translational Cardiology: From In Vitro Models to Regenerative Therapies
Abstract
1. Introduction
2. Structural and Functional Characteristics of the Human Heart
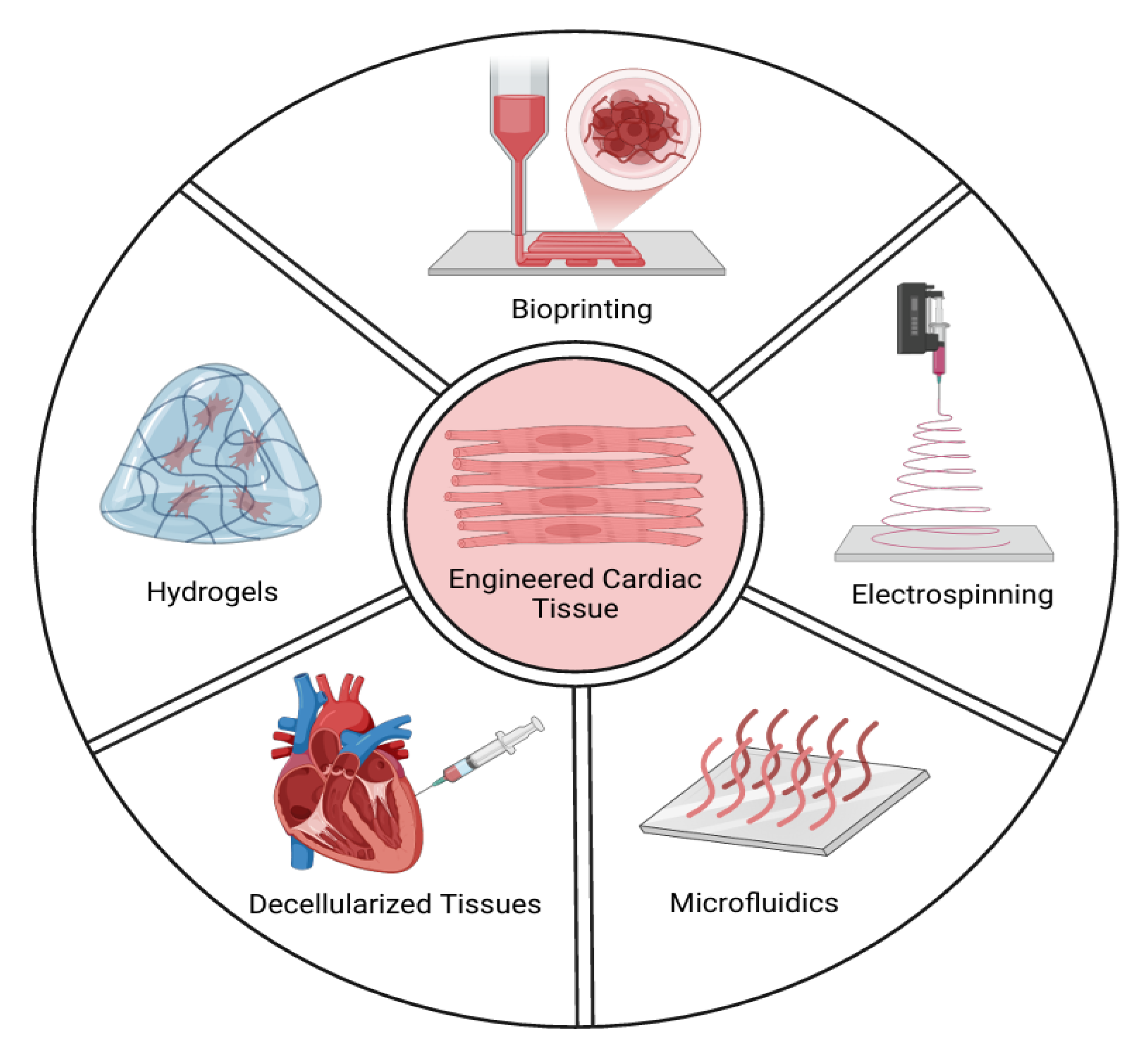
3. Methods for Generating Cardiac Tissue-Engineered Models In Vitro
3.1. Scaffold-Free Systems
3.2. Scaffold-Based Systems
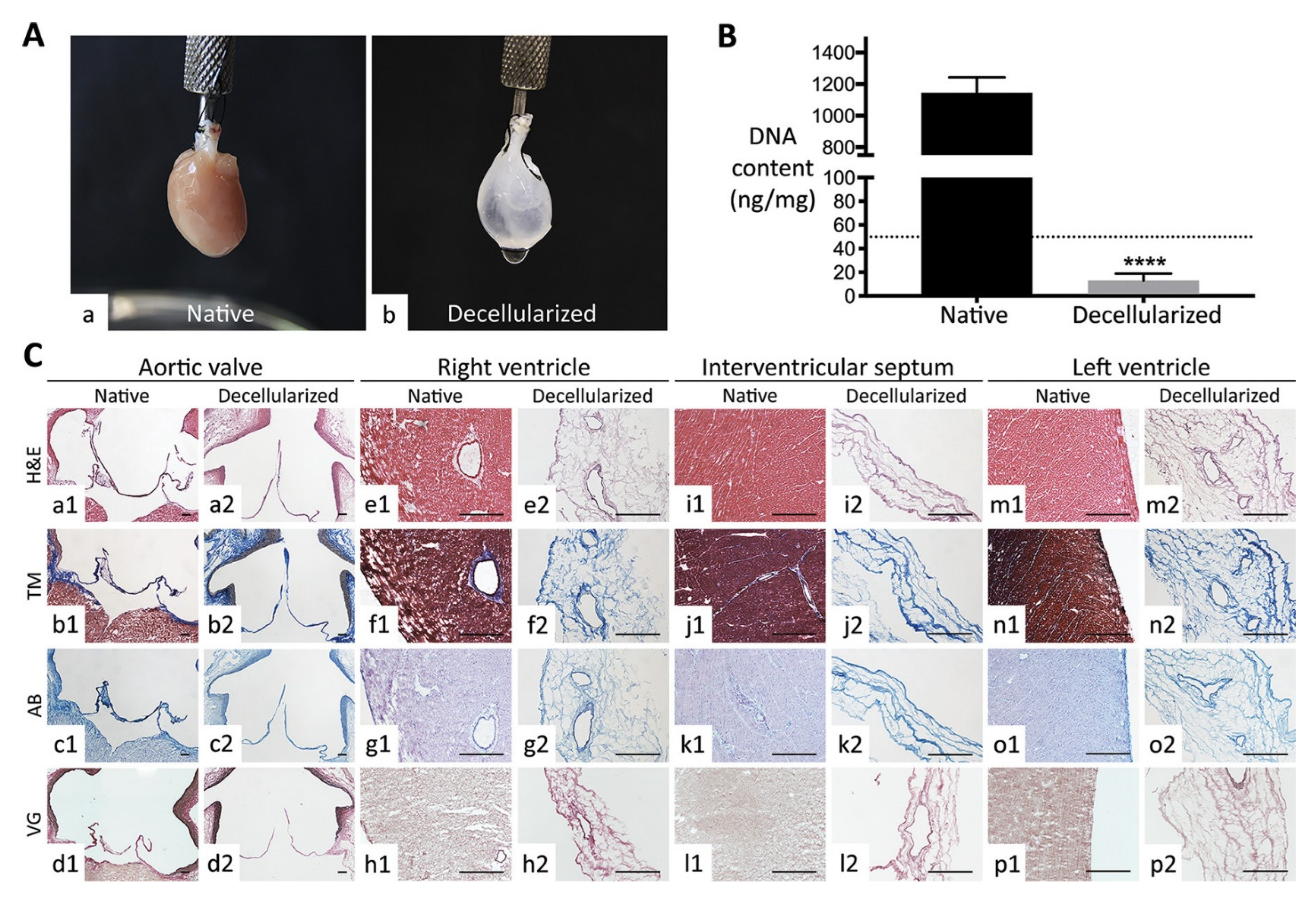
3.3. Cardiac Cell Culture in Decellularized Hearts
3.4. Microfluidics Technology
3.5. Bioprinting Technology
3.6. Electrospinning Technology
3.7. Clinical Relevance of Engineered Cardiac Tissues
4. 3D Modeling Techniques in Cardiac Tissue Engineering
4.1. Spheroids
4.2. Cell Sheets
4.3. Strips
4.4. Patches
4.5. Rings
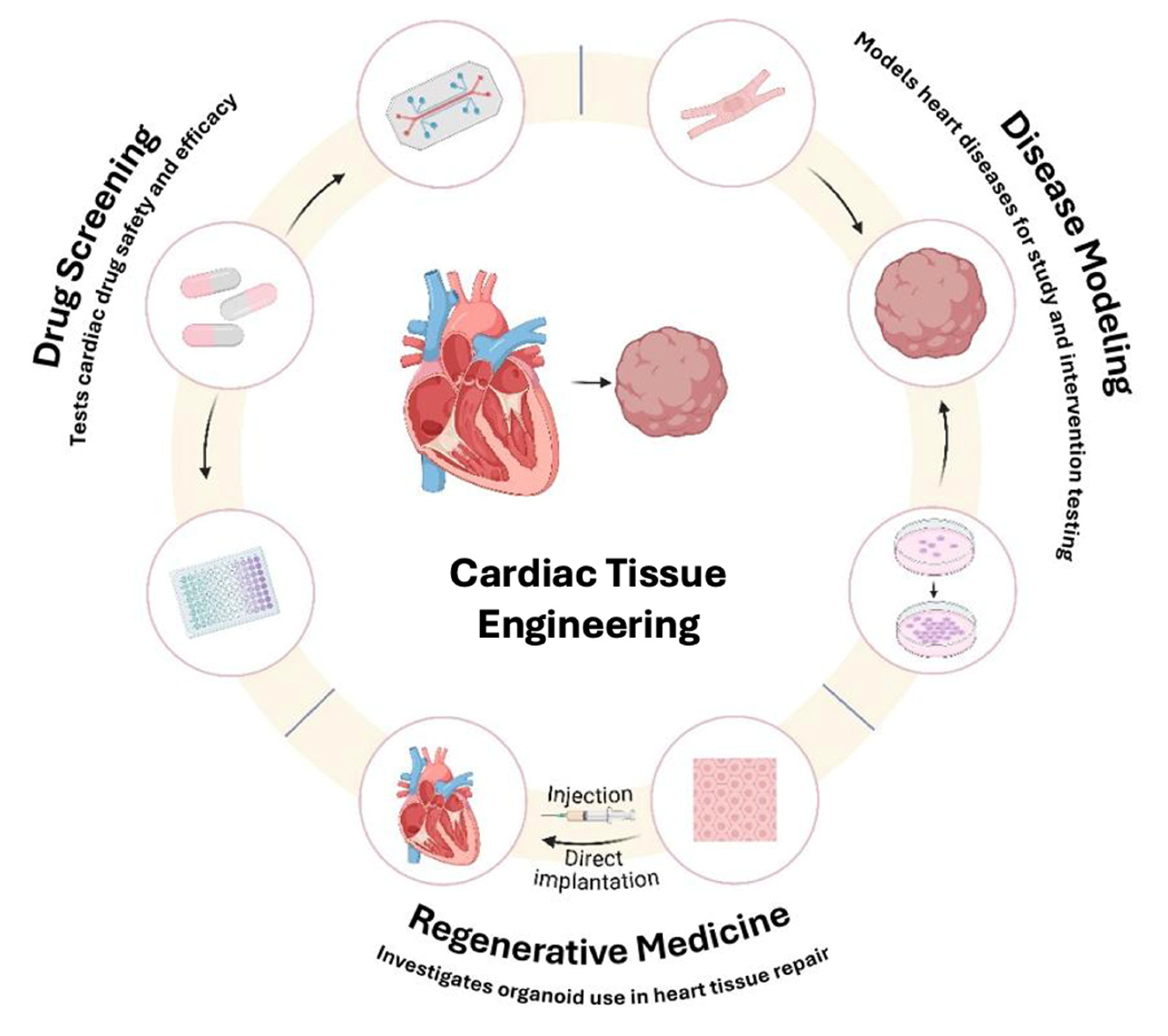
5. Applications in Disease Modeling, Drug Screening, and Toxicity Testing
5.1. Disease Modeling
5.1.1. Congenital Heart Diseases
HLHS
TOF
5.1.2. Ischemic Heart Diseases
5.1.3. Cardiomyopathies
5.2. Drug Screening and Toxicity Testing
6. Therapeutic Applications and Bioengineering Approaches
7. Current Challenges and Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CHD | Congenital Heart Disease |
| iPSCs | Induced Pluripotent Stem Cells |
| BAVD | Bicuspid Aortic Valve Disease |
| CAVD | Calcific Aortic Valve Disease |
| HLHS | Hypoplastic Left Heart Syndrome |
| TOF | Tetralogy of Fallot |
| IHD | Ischemic Heart Disease |
| hiPSC-CMs | Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes |
| hPSC-CMs | Human Pluripotent Stem Cell-Derived Cardiomyocytes |
| MAP4K4 | Mitogen-Activated Protein Kinase Kinase Kinase Kinase 4 |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| BET | Bromodomain and Extra-Terminal |
| DMD | Duchenne Muscular Dystrophy |
| miRNAs | MicroRNA |
| CRISPR/Cas9 | Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated protein 9 |
| e-SiNWs | Electrically Conductive Silicon Nanowires |
| hcFBs | Human Cardiac Fibroblasts |
| hESC-CPCs | Human Embryonic Stem Cell-Derived Cardiac Progenitor Cells |
| FDA | Food and Drug Administration |
| 3D | Three-Dimension |
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 77, 1958–1959. [Google Scholar] [CrossRef]
- Wurie, H.R.; Cappuccio, F.P. Cardiovascular disease in low- and middle-income countries: An urgent priority. Ethn. Health 2012, 17, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, E.C.H.; Amesz, J.H.; Sadeghi, A.H.; de Groot, N.M.S.; Manintveld, O.C.; Taverne, Y.J.H.J. Preclinical Models of Cardiac Disease: A Comprehensive Overview for Clinical Scientists. Cardiovasc. Eng. Technol. 2024, 15, 232–249. [Google Scholar] [CrossRef] [PubMed]
- Tenreiro, M.F.; Louro, A.F.; Alves, P.M.; Serra, M. Next generation of heart regenerative therapies: Progress and promise of cardiac tissue engineering. npj Regen. Med. 2021, 6, 30. [Google Scholar] [CrossRef]
- Litowczenko, J.; Marta, J.; Budych, W.; Staszak, K.; Wieszczycka, K.; Jurga, S.; Tylkowski, B. Milestones and current achievements in development of multifunctional bioscaffolds for medical application. Bioact. Mater. 2021, 6, 2412–2438. [Google Scholar] [CrossRef]
- Furuta, A.; Miyoshi, S.; Itabashi, Y.; Shimizu, T.; Kira, S.; Hayakawa, K.; Nishiyama, N.; Tanimoto, K.; Hagiwara, Y.; Satoh, T.; et al. Pulsatile cardiac tissue grafts using a novel three-dimensional cell sheet manipulation technique functionally integrates with the host heart, in vivo. Circ. Res. 2006, 98, 705–713. [Google Scholar] [CrossRef]
- Nag, A.C. Study of non-muscle cells of the adult mammalian heart: A fine structural analysis and distribution. Cytobios 1980, 28, 41–61. [Google Scholar]
- Achanta, S.; Gorky, J.; Leung, C.; Moss, A.; Robbins, S.; Eisenman, L.; Chen, J.; Tappan, S.; Heal, M.; Farahani, N.; et al. A Comprehensive Integrated Anatomical and Molecular Atlas of Rat Intrinsic Cardiac Nervous System. iScience 2020, 23, 101140. [Google Scholar] [CrossRef]
- Michel, J.-B.; Li, Z.; Lacolley, P. Smooth muscle cells and vascular diseases. Cardiovasc. Res. 2012, 95, 135–137. [Google Scholar] [CrossRef]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef]
- Devalla, H.D.; Passier, R. Cardiac differentiation of pluripotent stem cells and implications for modeling the heart in health and disease. Sci. Transl. Med. 2018, 10, eaah5457. [Google Scholar] [CrossRef] [PubMed]
- Seguret, M.; Vermersch, E.; Jouve, C.; Hulot, J.-S. Cardiac Organoids to Model and Heal Heart Failure and Cardiomyopathies. Biomedicines 2021, 9, 563. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Choi, S.; Alamana, C.; Parker, K.K.; Wu, J.C. Cellular and Engineered Organoids for Cardiovascular Models. Circ. Res. 2022, 130, 1780–1802. [Google Scholar] [CrossRef] [PubMed]
- Rienks, M.; Papageorgiou, A.-P.; Frangogiannis, N.G.; Heymans, S. Myocardial Extracellular Matrix: An Ever-Changing and Diverse Entity. Circ. Res. 2014, 114, 872–888. [Google Scholar] [CrossRef]
- Schwach, V.; Passier, R. Native cardiac environment and its impact on engineering cardiac tissue. Biomater. Sci. 2019, 7, 3566–3580. [Google Scholar] [CrossRef]
- Sahara, M. Recent Advances in Generation of In Vitro Cardiac Organoids. Int. J. Mol. Sci. 2023, 24, 6244. [Google Scholar] [CrossRef]
- Gisone, I.; Cecchettini, A.; Ceccherini, E.; Persiani, E.; Morales, M.A.; Vozzi, F. Cardiac tissue engineering: Multiple approaches and potential applications. Front. Bioeng. Biotechnol. 2022, 10, 980393. [Google Scholar] [CrossRef]
- Voges, H.K.; Mills, R.J.; Elliott, D.A.; Parton, R.G.; Porrello, E.R.; Hudson, J.E. Development of a human cardiac organoid injury model reveals innate regenerative potential. Development 2017, 144, 1118–1127. [Google Scholar] [CrossRef]
- Zhao, D.; Lei, W.; Hu, S. Cardiac organoid—A promising perspective of preclinical model. Stem Cell Res. Ther. 2021, 12, 272. [Google Scholar] [CrossRef]
- Hsieh, P.C.H.; Davis, M.E.; Lisowski, L.K.; Lee, R.T. Endothelial-cardiomyocyte interactions in cardiac development and repair. Annu. Rev. Physiol. 2006, 68, 51–66. [Google Scholar] [CrossRef]
- Murakami, M.; Simons, M. Fibroblast growth factor regulation of neovascularization. Curr. Opin. Hematol. 2008, 15, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Talman, V.; Kivelä, R. Cardiomyocyte—Endothelial Cell Interactions in Cardiac Remodeling and Regeneration. Front. Cardiovasc. Med. 2018, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Gähwiler, E.K.N.; Generali, M.; Hoerstrup, S.P.; Emmert, M.Y. Advances in 3D Organoid Models for Stem Cell-Based Cardiac Regeneration. Int. J. Mol. Sci. 2023, 24, 5188. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, P.; Jahnel, S.M.; Papai, N.; Giesshammer, M.; Deyett, A.; Schmidt, C.; Penc, M.; Tavernini, K.; Grdseloff, N.; Meledeth, C.; et al. Cardioids reveal self-organizing principles of human cardiogenesis. Cell 2021, 184, 3299–3317.e22. [Google Scholar] [CrossRef]
- Lewis-Israeli, Y.R.; Wasserman, A.H.; Gabalski, M.A.; Volmert, B.D.; Ming, Y.; Ball, K.A.; Yang, W.; Zou, J.; Ni, G.; Pajares, N.; et al. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat. Commun. 2021, 12, 5142. [Google Scholar] [CrossRef]
- Pretorius, D.; Kahn-Krell, A.M.; Lou, X.; Fast, V.G.; Berry, J.L.; Kamp, T.J.; Zhang, J. Layer-By-Layer Fabrication of Large and Thick Human Cardiac Muscle Patch Constructs with Superior Electrophysiological Properties. Front. Cell Dev. Biol. 2021, 9, 670504. [Google Scholar] [CrossRef]
- Caleffi, J.T.; Aal, M.C.E.; Gallindo, H.D.O.M.; Caxali, G.H.; Crulhas, B.P.; Ribeiro, A.O.; Souza, G.R.; Delella, F.K. Magnetic 3D cell culture: State of the art and current advances. Life Sci. 2021, 286, 120028. [Google Scholar] [CrossRef]
- Beauchamp, P.; Jackson, C.B.; Ozhathil, L.C.; Agarkova, I.; Galindo, C.L.; Sawyer, D.B.; Suter, T.M.; Zuppinger, C. 3D Co-culture of hiPSC-Derived Cardiomyocytes With Cardiac Fibroblasts Improves Tissue-Like Features of Cardiac Spheroids. Front. Mol. Biosci. 2020, 7, 14. [Google Scholar] [CrossRef]
- Campostrini, G.; Meraviglia, V.; Giacomelli, E.; Van Helden, R.W.J.; Yiangou, L.; Davis, R.P.; Bellin, M.; Orlova, V.V.; Mummery, C.L. Generation, functional analysis and applications of isogenic three-dimensional self-aggregating cardiac microtissues from human pluripotent stem cells. Nat. Protoc. 2021, 16, 2213–2256. [Google Scholar] [CrossRef]
- Bai, Y.; Yeung, E.; Lui, C.; Ong, C.S.; Pitaktong, I.; Huang, C.; Inoue, T.; Matsushita, H.; Ma, C.; Hibino, N. A Net Mold-based Method of Scaffold-free Three-Dimensional Cardiac Tissue Creation. J. Vis. Exp. 2018, 138, 58252. [Google Scholar] [CrossRef]
- Miller, K.L.; Xiang, Y.; Yu, C.; Pustelnik, J.; Wu, J.; Ma, X.; Matsui, T.; Imahashi, K.; Chen, S. Rapid 3D BioPrinting of a human iPSC-derived cardiac micro-tissue for high-throughput drug testing. Organs-on-a-Chip 2021, 3, 100007. [Google Scholar] [CrossRef]
- Gjorevski, N.; Sachs, N.; Manfrin, A.; Giger, S.; Bragina, M.E.; Ordóñez-Morán, P.; Clevers, H.; Lutolf, M.P. Designer matrices for intestinal stem cell and organoid culture. Nature 2016, 539, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Jabri, A.; Khan, J.; Taftafa, B.; Alsharif, M.; Mhannayeh, A.; Chinnappan, R.; Alzhrani, A.; Kazmi, S.; Mir, M.S.; Alsaud, A.W.; et al. Bioengineered Organoids Offer New Possibilities for Liver Cancer Studies: A Review of Key Milestones and Challenges. Bioengineering 2024, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Khademhosseini, A.; Eng, G.; Yeh, J.; Kucharczyk, P.A.; Langer, R.; Vunjak-Novakovic, G.; Radisic, M. Microfluidic patterning for fabrication of contractile cardiac organoids. Biomed. Microdevices 2007, 9, 149–157. [Google Scholar] [CrossRef]
- Whitehead, A.K.; Barnett, H.H.; Caldorera-Moore, M.E.; Newman, J.J. Poly (ethylene glycol) hydrogel elasticity influences human mesenchymal stem cell behavior. Regen. Biomater. 2018, 5, 167–175. [Google Scholar] [CrossRef]
- Nih, L.R.; Sideris, E.; Carmichael, S.T.; Segura, T. Injection of Microporous Annealing Particle (MAP) Hydrogels in the Stroke Cavity Reduces Gliosis and Inflammation and Promotes NPC Migration to the Lesion. Adv. Mater. 2017, 29, 1606471. [Google Scholar] [CrossRef]
- Madihally, S.V.; Matthew, H.W. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Lin, C.C.; Anseth, K.S. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef]
- Ding, M.; Andersson, H.; Martinsson, S.; Sabirsh, A.; Jonebring, A.; Wang, Q.-D.; Plowright, A.T.; Drowley, L. Aligned nanofiber scaffolds improve functionality of cardiomyocytes differentiated from human induced pluripotent stem cell-derived cardiac progenitor cells. Sci. Rep. 2020, 10, 13575. [Google Scholar] [CrossRef]
- Sridharan, D.; Palaniappan, A.; Blackstone, B.N.; Powell, H.M.; Khan, M. Electrospun Aligned Coaxial Nanofibrous Scaffold for Cardiac Repair. In Wound Regeneration; Das, H., Ed.; Springer: New York, NY, USA, 2021; Volume 2193, pp. 129–140. [Google Scholar] [CrossRef]
- Ahn, S.; Ardoña, H.A.M.; Lind, J.U.; Eweje, F.; Kim, S.L.; Gonzalez, G.M.; Liu, Q.; Zimmerman, J.F.; Pyrgiotakis, G.; Zhang, Z.; et al. Mussel-inspired 3D fiber scaffolds for heart-on-a-chip toxicity studies of engineered nanomaterials. Anal. Bioanal. Chem. 2018, 410, 6141–6154. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Nassiri, S.; Witherel, C.E.; Anfang, R.R.; Ng, J.; Nakazawa, K.R.; Yu, T.; Vunjak-Novakovic, G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 2015, 37, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Sadtler, K.; Singh, A.; Wolf, M.; Wang, X.; Pardoll, D.M.; Elisseeff, J.H. Design, clinical translation and immunological response of biomaterials in regenerative medicine. Nat. Rev. Mater. 2016, 1, 16040. [Google Scholar] [CrossRef]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants—A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef]
- Iyer, R.K.; Chiu, L.L.Y.; Reis, L.A.; Radisic, M. Engineered cardiac tissues. Curr. Opin. Biotechnol. 2011, 22, 706–714. [Google Scholar] [CrossRef]
- Dal Sasso, E.; Menabò, R.; Agrillo, D.; Arrigoni, G.; Franchin, C.; Giraudo, C.; Filippi, A.; Borile, G.; Ascione, G.; Zanella, F.; et al. RegenHeart: A Time-Effective, Low-Concentration, Detergent-Based Method Aiming for Conservative Decellularization of the Whole Heart Organ. ACS Biomater. Sci. Eng. 2020, 6, 5493–5506. [Google Scholar] [CrossRef]
- Lu, T.-Y.; Lin, B.; Kim, J.; Sullivan, M.; Tobita, K.; Salama, G.; Yang, L. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat. Commun. 2013, 4, 2307. [Google Scholar] [CrossRef]
- Ng, S.L.J.; Narayanan, K.; Gao, S.; Wan, A.C.A. Lineage restricted progenitors for the repopulation of decellularized heart. Biomaterials 2011, 32, 7571–7580. [Google Scholar] [CrossRef]
- Guyette, J.P.; Charest, J.M.; Mills, R.W.; Jank, B.J.; Moser, P.T.; Gilpin, S.E.; Gershlak, J.R.; Okamoto, T.; Gonzalez, G.; Milan, D.J.; et al. Bioengineering Human Myocardium on Native Extracellular Matrix. Circ. Res. 2016, 118, 56–72. [Google Scholar] [CrossRef]
- Min, S.; Kim, S.; Sim, W.S.; Choi, Y.S.; Joo, H.; Park, J.-H.; Lee, S.-J.; Kim, H.; Lee, M.J.; Jeong, I.; et al. Versatile human cardiac tissues engineered with perfusable heart extracellular microenvironment for biomedical applications. Nat. Commun. 2024, 15, 2564. [Google Scholar] [CrossRef]
- Weng, K.-C.; Kurokawa, Y.K.; Hajek, B.S.; Paladin, J.A.; Shirure, V.S.; George, S.C. Human Induced Pluripotent Stem-Cardiac-Endothelial-Tumor-on-a-Chip to Assess Anticancer Efficacy and Cardiotoxicity. Tissue Eng. Part C Methods 2020, 26, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Rafatian, N.; Feric, N.T.; Cox, B.J.; Aschar-Sobbi, R.; Wang, E.Y.; Aggarwal, P.; Zhang, B.; Conant, G.; Ronaldson-Bouchard, K.; et al. A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling. Cell 2019, 176, 913–927.e18. [Google Scholar] [CrossRef] [PubMed]
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivì, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.E.-S.; Seliktar, D.; et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 2018, 8, 13532. [Google Scholar] [CrossRef] [PubMed]
- Joddar, B.; Natividad-Diaz, S.L.; Padilla, A.E.; Esparza, A.A.; Ramirez, S.P.; Chambers, D.R.; Ibaroudene, H. Engineering approaches for cardiac organoid formation and their characterization. Transl. Res. 2022, 250, 46–67. [Google Scholar] [CrossRef]
- El Khoury, R.; Nagiah, N.; Mudloff, J.A.; Thakur, V.; Chattopadhyay, M.; Joddar, B. 3D Bioprinted Spheroidal Droplets for Engineering the Heterocellular Coupling between Cardiomyocytes and Cardiac Fibroblasts. Cyborg Bionic Syst. 2021, 2021, 9864212. [Google Scholar] [CrossRef]
- Arai, K.; Murata, D.; Verissimo, A.R.; Mukae, Y.; Itoh, M.; Nakamura, A.; Morita, S.; Nakayama, K. Fabrication of scaffold-free tubular cardiac constructs using a Bio-3D printer. PLoS ONE 2018, 13, e0209162. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, L.; Liu, Y.; Zhang, H.; Song, Y.; Park, J.-H.; Dashnyam, K.; Lee, J.-H.; Khalak, F.A.-H.; Riester, O.; et al. 3D Bioprinting tissue analogs: Current development and translational implications. J. Tissue Eng. 2023, 14, 20417314231187113. [Google Scholar] [CrossRef]
- Leach, M.K.; Feng, Z.-Q.; Tuck, S.J.; Corey, J.M. Electrospinning Fundamentals: Optimizing Solution and Apparatus Parameters. J. Vis. Exp. 2011, 47, 2494. [Google Scholar] [CrossRef]
- Lobo, A.O.; Afewerki, S.; De Paula, M.M.M.; Ghannadian, P.; Marciano, F.R.; Zhang, Y.S.; Webster, T.J.; Khademhosseini, A. Electrospun nanofiber blend with improved mechanical and biological performance. Int. J. Nanomed. 2018, 13, 7891–7903. [Google Scholar] [CrossRef]
- Nagiah, N.; El Khoury, R.; Othman, M.H.; Akimoto, J.; Ito, Y.; Roberson, D.A.; Joddar, B. Development and Characterization of Furfuryl-Gelatin Electrospun Scaffolds for Cardiac Tissue Engineering. ACS Omega 2022, 7, 13894–13905. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nature reviews. Cardiology 2014, 11, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Kraehenbuehl, T.P.; Langer, R.; Ferreira, L.S. Three-dimensional biomaterials for the study of human pluripotent stem cells. Nat. Methods 2011, 8, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Vunjak-Novakovic, G.; Lui, K.O.; Tandon, N.; Chien, K.R. Bioengineering heart muscle: A paradigm for regenerative medicine. Annu. Rev. Biomed. Eng. 2011, 13, 245–267. [Google Scholar] [CrossRef]
- Zuppinger, C. 3D Cardiac Cell Culture: A Critical Review of Current Technologies and Applications. Front. Cardiovasc. Med. 2019, 6, 87. [Google Scholar] [CrossRef]
- Polonchuk, L.; Chabria, M.; Badi, L.; Hoflack, J.-C.; Figtree, G.; Davies, M.J.; Gentile, C. Cardiac spheroids as promising in vitro models to study the human heart microenvironment. Sci. Rep. 2017, 7, 7005. [Google Scholar] [CrossRef]
- Zuppinger, C. 3D culture for cardiac cells. Biochim. Biophys. Acta 2016, 1863 Pt B, 1873–1881. [Google Scholar] [CrossRef]
- Bates, R. Spheroids and cell survival. Crit. Rev. Oncol./Hematol. 2000, 36, 61–74. [Google Scholar] [CrossRef]
- Beauchamp, P.; Moritz, W.; Kelm, J.M.; Ullrich, N.D.; Agarkova, I.; Anson, B.D.; Suter, T.M.; Zuppinger, C. Development and Characterization of a Scaffold-Free 3D Spheroid Model of Induced Pluripotent Stem Cell-Derived Human Cardiomyocytes. Tissue Eng. Part C Methods 2015, 21, 852–861. [Google Scholar] [CrossRef]
- Giacomelli, E.; Meraviglia, V.; Campostrini, G.; Cochrane, A.; Cao, X.; Van Helden, R.W.J.; Krotenberg Garcia, A.; Mircea, M.; Kostidis, S.; Davis, R.P.; et al. Human-iPSC-Derived Cardiac Stromal Cells Enhance Maturation in 3D Cardiac Microtissues and Reveal Non-cardiomyocyte Contributions to Heart Disease. Cell Stem Cell 2020, 26, 862–879.e11. [Google Scholar] [CrossRef]
- Gallet, R.; Tseliou, E.; Dawkins, J.; Middleton, R.; Valle, J.; Angert, D.; Reich, H.; Luthringer, D.; Kreke, M.; Smith, R.; et al. Intracoronary Delivery of Self-Assembling Heart-Derived Microtissues (Cardiospheres) for Prevention of Adverse Remodeling in a Pig Model of Convalescent Myocardial Infarction. Circ. Cardiovasc. Interv. 2015, 8, e002391. [Google Scholar] [CrossRef]
- Shimizu, T.; Yamato, M.; Isoi, Y.; Akutsu, T.; Setomaru, T.; Abe, K.; Kikuchi, A.; Umezu, M.; Okano, T. Fabrication of Pulsatile Cardiac Tissue Grafts Using a Novel 3-Dimensional Cell Sheet Manipulation Technique and Temperature-Responsive Cell Culture Surfaces. Circ. Res. 2002, 90, e40. [Google Scholar] [CrossRef] [PubMed]
- Sekine, H.; Shimizu, T.; Sakaguchi, K.; Dobashi, I.; Wada, M.; Yamato, M.; Kobayashi, E.; Umezu, M.; Okano, T. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat. Commun. 2013, 4, 1399. [Google Scholar] [CrossRef] [PubMed]
- Feric, N.T.; Pallotta, I.; Singh, R.; Bogdanowicz, D.R.; Gustilo, M.M.; Chaudhary, K.W.; Willette, R.N.; Chendrimada, T.P.; Xu, X.; Graziano, M.P.; et al. Engineered Cardiac Tissues Generated in the Biowire II: A Platform for Human-Based Drug Discovery. Toxicol. Sci. 2019, 172, 89–97. [Google Scholar] [CrossRef]
- Hansen, A.; Eder, A.; Bönstrup, M.; Flato, M.; Mewe, M.; Schaaf, S.; Aksehirlioglu, B.; Schwörer, A.; Uebeler, J.; Eschenhagen, T. Development of a Drug Screening Platform Based on Engineered Heart Tissue. Circ. Res. 2010, 107, 35–44. [Google Scholar] [CrossRef]
- Serrao, G.W.; Turnbull, I.C.; Ancukiewicz, D.; Kim, D.E.; Kao, E.; Cashman, T.J.; Hadri, L.; Hajjar, R.J.; Costa, K.D. Myocyte-Depleted Engineered Cardiac Tissues Support Therapeutic Potential of Mesenchymal Stem Cells. Tissue Eng. Part A 2012, 18, 1322–1333. [Google Scholar] [CrossRef]
- Zhang, J. Engineered Tissue Patch for Cardiac Cell Therapy. Curr. Treat. Options Cardiovasc. Med. 2015, 17, 37. [Google Scholar] [CrossRef]
- Shadrin, I.Y.; Allen, B.W.; Qian, Y.; Jackman, C.P.; Carlson, A.L.; Juhas, M.E.; Bursac, N. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat. Commun. 2017, 8, 1825. [Google Scholar] [CrossRef]
- Tiburcy, M.; Hudson, J.E.; Balfanz, P.; Schlick, S.; Meyer, T.; Chang Liao, M.-L.; Levent, E.; Raad, F.; Zeidler, S.; Wingender, E.; et al. Defined Engineered Human Myocardium With Advanced Maturation for Applications in Heart Failure Modeling and Repair. Circulation 2017, 135, 1832–1847. [Google Scholar] [CrossRef]
- Takeda, M.; Miyagawa, S.; Ito, E.; Harada, A.; Mochizuki-Oda, N.; Matsusaki, M.; Akashi, M.; Sawa, Y. Development of a drug screening system using three-dimensional cardiac tissues containing multiple cell types. Sci. Rep. 2021, 11, 5654. [Google Scholar] [CrossRef]
- Colman, M.A. Arrhythmia mechanisms and spontaneous calcium release: Bi-directional coupling between re-entrant and focal excitation. PLoS Comput. Biol. 2019, 15, e1007260. [Google Scholar] [CrossRef]
- Eschenhagen, T.; Zimmermann, W.H. Engineering Myocardial Tissue. Circ. Res. 2005, 97, 1220–1231. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, L.; Yu, L.; Minami, I.; Miyagawa, S.; Hörning, M.; Dong, J.; Qiao, J.; Qu, X.; Hua, Y.; et al. Circulating re-entrant waves promote maturation of hiPSC-derived cardiomyocytes in self-organized tissue ring. Commun. Biol. 2020, 3, 122. [Google Scholar] [CrossRef] [PubMed]
- Van Der Linde, D.; Konings, E.E.M.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.M.; Roos-Hesselink, J.W. Birth Prevalence of Congenital Heart Disease Worldwide. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Sala, L.; Gnecchi, M.; Schwartz, P.J. Long QT Syndrome Modelling with Cardiomyocytes Derived from Human-induced Pluripotent Stem Cells. Arrhythmia Electrophysiol. Rev. 2019, 8, 105–110. [Google Scholar] [CrossRef]
- Sleiman, Y.; Souidi, M.; Kumar, R.; Yang, E.; Jaffré, F.; Zhou, T.; Bernardin, A.; Reiken, S.; Cazorla, O.; Kajava, A.V.; et al. Modeling polymorphic ventricular tachycardia at rest using patient-specific induced pluripotent stem cell-derived cardiomyocytes. EBioMedicine 2020, 60, 103024. [Google Scholar] [CrossRef]
- Zhang, M.; D’Aniello, C.; Verkerk, A.O.; Wrobel, E.; Frank, S.; Ward-van Oostwaard, D.; Piccini, I.; Freund, C.; Rao, J.; Seebohm, G.; et al. Recessive cardiac phenotypes in induced pluripotent stem cell models of Jervell and Lange-Nielsen syndrome: Disease mechanisms and pharmacological rescue. Proc. Natl. Acad. Sci. USA 2014, 111, E5383–E5392. [Google Scholar] [CrossRef]
- Hinson, J.T.; Chopra, A.; Nafissi, N.; Polacheck, W.J.; Benson, C.C.; Swist, S.; Gorham, J.; Yang, L.; Schafer, S.; Sheng, C.C.; et al. HEART DISEASE. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science 2015, 349, 982–986. [Google Scholar] [CrossRef]
- Theodoris, C.V.; Li, M.; White, M.P.; Liu, L.; He, D.; Pollard, K.S.; Bruneau, B.G.; Srivastava, D. Human Disease Modeling Reveals Integrated Transcriptional and Epigenetic Mechanisms of NOTCH1 Haploinsufficiency. Cell 2015, 160, 1072–1086. [Google Scholar] [CrossRef]
- Ang, Y.-S.; Rivas, R.N.; Ribeiro, A.J.S.; Srivas, R.; Rivera, J.; Stone, N.R.; Pratt, K.; Mohamed, T.M.A.; Fu, J.-D.; Spencer, C.I.; et al. Disease Model of GATA4 Mutation Reveals Transcription Factor Cooperativity in Human Cardiogenesis. Cell 2016, 167, 1734–1749.e22. [Google Scholar] [CrossRef]
- Krane, M.; Dreßen, M.; Santamaria, G.; My, I.; Schneider, C.M.; Dorn, T.; Laue, S.; Mastantuono, E.; Berutti, R.; Rawat, H.; et al. Sequential Defects in Cardiac Lineage Commitment and Maturation Cause Hypoplastic Left Heart Syndrome. Circulation 2021, 144, 1409–1428. [Google Scholar] [CrossRef]
- Rufaihah, A.J.; Chen, C.K.; Yap, C.H.; Mattar, C.N.Z. Mending a broken heart: In vitro, in vivo and in silico models of congenital heart disease. Dis. Models Mech. 2021, 14, dmm047522. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Frias, J.; Horenstein, M.S.; Guillaume, M. Tetralogy of Fallot. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK513288/ (accessed on 24 February 2025).
- Grunert, M.; Appelt, S.; Schönhals, S.; Mika, K.; Cui, H.; Cooper, A.; Cyganek, L.; Guan, K.; Sperling, S.R. Induced pluripotent stem cells of patients with Tetralogy of Fallot reveal transcriptional alterations in cardiomyocyte differentiation. Sci. Rep. 2020, 10, 10921. [Google Scholar] [CrossRef] [PubMed]
- Kritzmire, S.M.; Cossu, A.E. Hypoplastic Left Heart Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK554576/ (accessed on 26 February 2025).
- Crucean, A.; Alqahtani, A.; Barron, D.J.; Brawn, W.J.; Richardson, R.V.; O’Sullivan, J.; Anderson, R.H.; Henderson, D.J.; Chaudhry, B. Re-evaluation of hypoplastic left heart syndrome from a developmental and morphological perspective. Orphanet J. Rare Dis. 2017, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhang, Y.-Y.; Meng, M.-Y.; Hou, Z.-L.; Meng, P.; Zhao, Y.-Y.; Gao, H.; Tang, J.; Liu, Z.; Yang, L.-L.; et al. Generation of human iPSC line from a patient with Tetralogy of Fallot, YAHKMUi001-A, carrying a mutation in TBX1 gene. Stem Cell Res. 2020, 42, 101687. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Social Security Cardiovascular Disability Criteria. Cardiovascular Disability: Updating the Social Security Listings; National Academies Press: Washington, DC, USA, 2010. Available online: http://www.ncbi.nlm.nih.gov/books/NBK209975/ (accessed on 26 February 2025).
- Talman, V.; Ruskoaho, H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 2016, 365, 563–581. [Google Scholar] [CrossRef]
- Häkli, M.; Kreutzer, J.; Mäki, A.-J.; Välimäki, H.; Lappi, H.; Huhtala, H.; Kallio, P.; Aalto-Setälä, K.; Pekkanen-Mattila, M. Human induced pluripotent stem cell-based platform for modeling cardiac ischemia. Sci. Rep. 2021, 11, 4153. [Google Scholar] [CrossRef]
- Richards, D.J.; Coyle, R.C.; Tan, Y.; Jia, J.; Wong, K.; Toomer, K.; Menick, D.R.; Mei, Y. Inspiration from heart development: Biomimetic development of functional human cardiac organoids. Biomaterials 2017, 142, 112–123. [Google Scholar] [CrossRef]
- Forte, E.; Furtado, M.B.; Rosenthal, N. The interstitium in cardiac repair: Role of the immune-stromal cell interplay. Nature Reviews. Cardiology 2018, 15, 601–616. [Google Scholar] [CrossRef]
- Blinova, K.; Schocken, D.; Patel, D.; Daluwatte, C.; Vicente, J.; Wu, J.C.; Strauss, D.G. Clinical Trial in a Dish: Personalized Stem Cell-Derived Cardiomyocyte Assay Compared With Clinical Trial Results for Two QT-Prolonging Drugs. Clin. Transl. Sci. 2019, 12, 687–697. [Google Scholar] [CrossRef]
- da Rocha, A.M.; Campbell, K.; Mironov, S.; Jiang, J.; Mundada, L.; Guerrero-Serna, G.; Jalife, J.; Herron, T.J. hiPSC-CM Monolayer Maturation State Determines Drug Responsiveness in High Throughput Pro-Arrhythmia Screen. Sci. Rep. 2017, 7, 13834. [Google Scholar] [CrossRef]
- Gaballah, M.; Penttinen, K.; Kreutzer, J.; Mäki, A.-J.; Kallio, P.; Aalto-Setälä, K. Cardiac Ischemia On-a-Chip: Antiarrhythmic Effect of Levosimendan on Ischemic Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Cells 2022, 11, 1045. [Google Scholar] [CrossRef] [PubMed]
- Kandaswamy, E.; Zuo, L. Recent Advances in Treatment of Coronary Artery Disease: Role of Science and Technology. Int. J. Mol. Sci. 2018, 19, 424. [Google Scholar] [CrossRef] [PubMed]
- Garbern, J.C.; Lee, R.T. Cardiac stem cell therapy and the promise of heart regeneration. Cell Stem Cell 2013, 12, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, N.J.; Kant, R.J.; Minor, A.J.; Coulombe, K.L.K. Optimizing Blended Collagen-Fibrin Hydrogels for Cardiac Tissue Engineering with Human iPSC-derived Cardiomyocytes. ACS Biomater. Sci. Eng. 2019, 5, 887–899. [Google Scholar] [CrossRef]
- Li, J.; Minami, I.; Shiozaki, M.; Yu, L.; Yajima, S.; Miyagawa, S.; Shiba, Y.; Morone, N.; Fukushima, S.; Yoshioka, M.; et al. Human Pluripotent Stem Cell-Derived Cardiac Tissue-like Constructs for Repairing the Infarcted Myocardium. Stem Cell Rep. 2017, 9, 1546–1559. [Google Scholar] [CrossRef]
- Ong, C.S.; Fukunishi, T.; Zhang, H.; Huang, C.Y.; Nashed, A.; Blazeski, A.; DiSilvestre, D.; Vricella, L.; Conte, J.; Tung, L.; et al. Biomaterial-Free Three-Dimensional Bioprinting of Cardiac Tissue using Human Induced Pluripotent Stem Cell Derived Cardiomyocytes. Sci. Rep. 2017, 7, 4566. [Google Scholar] [CrossRef]
- Sasaki, D.; Matsuura, K.; Seta, H.; Haraguchi, Y.; Okano, T.; Shimizu, T. Contractile force measurement of human induced pluripotent stem cell-derived cardiac cell sheet-tissue. PLoS ONE 2018, 13, e0198026. [Google Scholar] [CrossRef]
- Long, C.; Li, H.; Tiburcy, M.; Rodriguez-Caycedo, C.; Kyrychenko, V.; Zhou, H.; Zhang, Y.; Min, Y.-L.; Shelton, J.M.; Mammen, P.P.A.; et al. Correction of diverse muscular dystrophy mutations in human engineered heart muscle by single-site genome editing. Sci. Adv. 2018, 4, eaap9004. [Google Scholar] [CrossRef]
- Marini, V.; Marino, F.; Aliberti, F.; Giarratana, N.; Pozzo, E.; Duelen, R.; Cortés Calabuig, Á.; La Rovere, R.; Vervliet, T.; Torella, D.; et al. Long-term culture of patient-derived cardiac organoids recapitulated Duchenne muscular dystrophy cardiomyopathy and disease progression. Front. Cell Dev. Biol. 2022, 10, 878311. [Google Scholar] [CrossRef]
- Fiedler, L.R.; Chapman, K.; Xie, M.; Maifoshie, E.; Jenkins, M.; Golforoush, P.A.; Bellahcene, M.; Noseda, M.; Faust, D.; Jarvis, A.; et al. MAP4K4 Inhibition Promotes Survival of Human Stem Cell-Derived Cardiomyocytes and Reduces Infarct Size In Vivo. Cell Stem Cell 2019, 24, 579–591.e12. [Google Scholar] [CrossRef]
- Mills, R.J.; Humphrey, S.J.; Fortuna, P.R.J.; Lor, M.; Foster, S.R.; Quaife-Ryan, G.A.; Johnston, R.L.; Dumenil, T.; Bishop, C.; Rudraraju, R.; et al. BET inhibition blocks inflammation-induced cardiac dysfunction and SARS-CoV-2 infection. Cell 2021, 184, 2167–2182.e22. [Google Scholar] [CrossRef]
- Richards, D.J.; Li, Y.; Kerr, C.M.; Yao, J.; Beeson, G.C.; Coyle, R.C.; Chen, X.; Jia, J.; Damon, B.; Wilson, R.; et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat. Biomed. Eng. 2020, 4, 446–462. [Google Scholar] [CrossRef]
- Sallam, K.; Thomas, D.; Gaddam, S.; Lopez, N.; Beck, A.; Beach, L.; Rogers, A.J.; Zhang, H.; Chen, I.Y.; Ameen, M.; et al. Modeling Effects of Immunosuppressive Drugs on Human Hearts Using Induced Pluripotent Stem Cell-Derived Cardiac Organoids and Single-Cell RNA Sequencing. Circulation 2022, 145, 1367–1369. [Google Scholar] [CrossRef]
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B.; American Heart Association; et al. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006, 113, 1807–1816. [Google Scholar] [CrossRef]
- Kohama, Y.; Higo, S.; Masumura, Y.; Shiba, M.; Kondo, T.; Ishizu, T.; Higo, T.; Nakamura, S.; Kameda, S.; Tabata, T.; et al. Adeno-associated virus-mediated gene delivery promotes S-phase entry-independent precise targeted integration in cardiomyocytes. Sci. Rep. 2020, 10, 15348. [Google Scholar] [CrossRef]
- Mosqueira, D.; Mannhardt, I.; Bhagwan, J.R.; Lis-Slimak, K.; Katili, P.; Scott, E.; Hassan, M.; Prondzynski, M.; Harmer, S.C.; Tinker, A.; et al. CRISPR/Cas9 editing in human pluripotent stem cell-cardiomyocytes highlights arrhythmias, hypocontractility, and energy depletion as potential therapeutic targets for hypertrophic cardiomyopathy. Eur. Heart J. 2018, 39, 3879–3892. [Google Scholar] [CrossRef]
- Pérez-Serra, A.; Toro, R.; Sarquella-Brugada, G.; de Gonzalo-Calvo, D.; Cesar, S.; Carro, E.; Llorente-Cortes, V.; Iglesias, A.; Brugada, J.; Brugada, R.; et al. Genetic basis of dilated cardiomyopathy. Int. J. Cardiol. 2016, 224, 461–472. [Google Scholar] [CrossRef]
- Al-Wahaibi, K.; Al-Wahshi, Y.; Mohamed Elfadil, O. Myocardial Injury Is Associated with Higher Morbidity and Mortality in Patients with 2019 Novel Coronavirus Disease (COVID-19). SN Compr. Clin. Med. 2020, 2, 2514–2520. [Google Scholar] [CrossRef]
- Lee, S.-G.; Kim, J.; Oh, M.-S.; Ryu, B.; Kang, K.-R.; Baek, J.; Lee, J.-M.; Choi, S.-O.; Kim, C.-Y.; Chung, H.M. Development and validation of dual-cardiotoxicity evaluation method based on analysis of field potential and contractile force of human iPSC-derived cardiomyocytes/multielectrode assay platform. Biochem. Biophys. Res. Commun. 2021, 555, 67–73. [Google Scholar] [CrossRef]
- Kamp, T.J.; Hamdan, M.H.; January, C.T. Chloroquine or Hydroxychloroquine for COVID-19: Is Cardiotoxicity a Concern? J. Am. Heart Assoc. 2020, 9, e016887. [Google Scholar] [CrossRef]
- Scuderi, G.J.; Butcher, J. Naturally Engineered Maturation of Cardiomyocytes. Front. Cell Dev. Biol. 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- McKinley, K.L.; Longaker, M.T.; Naik, S. Emerging frontiers in regenerative medicine. Science 2023, 380, 796–798. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Gregorich, Z.R.; Zhu, W.; Mattapally, S.; Oduk, Y.; Lou, X.; Kannappan, R.; Borovjagin, A.V.; Walcott, G.P.; Pollard, A.E.; et al. Large Cardiac Muscle Patches Engineered From Human Induced-Pluripotent Stem Cell-Derived Cardiac Cells Improve Recovery From Myocardial Infarction in Swine. Circulation 2018, 137, 1712–1730. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, M.; Di Pasquale, E. Toward Cardiac Regeneration: Combination of Pluripotent Stem Cell-Based Therapies and Bioengineering Strategies. Front. Bioeng. Biotechnol. 2020, 8, 455. [Google Scholar] [CrossRef]
- Varzideh, F.; Pahlavan, S.; Ansari, H.; Halvaei, M.; Kostin, S.; Feiz, M.-S.; Latifi, H.; Aghdami, N.; Braun, T.; Baharvand, H. Human cardiomyocytes undergo enhanced maturation in embryonic stem cell-derived organoid transplants. Biomaterials 2019, 192, 537–550. [Google Scholar] [CrossRef]
- Tan, Y.; Coyle, R.C.; Barrs, R.W.; Silver, S.E.; Li, M.; Richards, D.J.; Lin, Y.; Jiang, Y.; Wang, H.; Menick, D.R.; et al. Nanowired human cardiac organoid transplantation enables highly efficient and effective recovery of infarcted hearts. Sci. Adv. 2023, 9, eadf2898. [Google Scholar] [CrossRef]
- Li, Q.; Nan, K.; Le Floch, P.; Lin, Z.; Sheng, H.; Blum, T.S.; Liu, J. Cyborg Organoids: Implantation of Nanoelectronics via Organogenesis for Tissue-Wide Electrophysiology. Nano Lett. 2019, 19, 5781–5789. [Google Scholar] [CrossRef]



| Device/Model & Author | Specific Application | Stage of Validation | Data Variability & Performance Metrics |
|---|---|---|---|
| iPSC-derived Cardiomyocytes [92] | Modeling Hypoplastic Left Heart Syndrome (HLHS) | In vitro | Moderate reproducibility; downregulation of MESP1, TNNT2, GJA1; delayed GATA4 expression. Functional immaturity and altered calcium handling observed across patient-specific lines. |
| Cardiac Progenitor Cells [91] | Studying genetic disruption in early cardiogenesis (HLHS) | In vitro | Consistent gene expression changes across HLHS samples; impaired growth factor response; premature cell cycle exit and apoptosis uniformly observed. |
| TOF-specific Cardiomyocytes [94] | Modeling Tetralogy of Fallot (TOF) and identifying causal mutations | In vitro | Low interline variability; reproducible effects of TP53, FBLN2, DAAM2 mutations on myocardial maturation and WNT signaling. Robust gene-disease correlation. |
| TOF-specific iPSCs [97] | Exploring TBX1 mutation effects on neural crest migration in TOF | In vitro | The YAHKMUi001-A cell line can be used as a tool for in vitro modeling of TOF for etiology research or gene therapy. |
| Device Name/Model | Specific Application | Stage of Validation | Data Variability and Performance Metrics |
|---|---|---|---|
| 3D Cardiac Organoids [10] | Modeling myocardial infarction under hypoxic conditions | In vitro | Mimicked infarction features: fibrosis, altered calcium handling |
| hiPSC-CMs under hypoxia [105] | IHD modeling and levosimendan drug testing | In vitro | Hypoxia slowed Ca²⁺ transients, caused sarcomere disarray; drug showed an antiarrhythmic effect |
| Engineered Cardiac Patches [108,109,110,111] | Myocardial regeneration after ischemia (fibrin/collagen hydrogels, cell sheets, bioprinted structures) | Preclinical/in vivo | Structural integration in animal models; clinical trials underway in some cases |
| iPSC-CMs [112] | DMD cardiomyopathy modeling and CRISPR correction | In vitro | Restored dystrophin expression and improved contractility after 50% correction |
| 3D DMD Cardiac Organoids [113] | Identification of dysregulated miRNAs in DMD | In vitro | Identified 5 miRNAs enriched in disease state; supports molecular mechanism exploration |
| iPSC-CMs + H2O2 injury [114] | Drug screening post-ischemia; MAP4K4 gene targeting | In vitro | Identified a small molecule increasing post-injury survival, validated via gene silencing |
| Human Cardiac Organoids [115] | COVID-19 cytokine storm modeling and drug screening | In vitro & animal model | Diastolic dysfunction replicated; BET inhibitors reversed damage |
| iPSC-CMs [116] | Cardiotoxicity testing of doxorubicin in the ischemic heart disease context | In vitro | Reduced contraction amplitude, actin disorganization |
| hPSC-derived Cardiac Organoids [117] | Cardiotoxicity evaluation of immunosuppressants (tacrolimus, sirolimus) | In vitro | Sirolimus reduced fibrosis in cardiac clusters; therapeutic potential for heart failure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabri, A.; Taftafa, B.; Mhannayeh, A.; Alsharif, M.; Abbad, T.; Ahmed, S.; Alshehri, E.A.; Elsalti, A.; Khan, J.; Mir, T.A.; et al. Cardiac Tissue Engineering for Translational Cardiology: From In Vitro Models to Regenerative Therapies. Bioengineering 2025, 12, 518. https://doi.org/10.3390/bioengineering12050518
Jabri A, Taftafa B, Mhannayeh A, Alsharif M, Abbad T, Ahmed S, Alshehri EA, Elsalti A, Khan J, Mir TA, et al. Cardiac Tissue Engineering for Translational Cardiology: From In Vitro Models to Regenerative Therapies. Bioengineering. 2025; 12(5):518. https://doi.org/10.3390/bioengineering12050518
Chicago/Turabian StyleJabri, Abdullah, Bader Taftafa, Abdulaziz Mhannayeh, Mohamed Alsharif, Tasnim Abbad, Sana Ahmed, Eman A. Alshehri, Abdulrahman Elsalti, Jibran Khan, Tanveer Ahmad Mir, and et al. 2025. "Cardiac Tissue Engineering for Translational Cardiology: From In Vitro Models to Regenerative Therapies" Bioengineering 12, no. 5: 518. https://doi.org/10.3390/bioengineering12050518
APA StyleJabri, A., Taftafa, B., Mhannayeh, A., Alsharif, M., Abbad, T., Ahmed, S., Alshehri, E. A., Elsalti, A., Khan, J., Mir, T. A., & Yaqinuddin, A. (2025). Cardiac Tissue Engineering for Translational Cardiology: From In Vitro Models to Regenerative Therapies. Bioengineering, 12(5), 518. https://doi.org/10.3390/bioengineering12050518






