Biological Acoustic Levitation and Its Potential Application for Microgravity Study
Abstract
1. Introduction
2. Acoustic Levitation
2.1. History of Acoustic Levitation
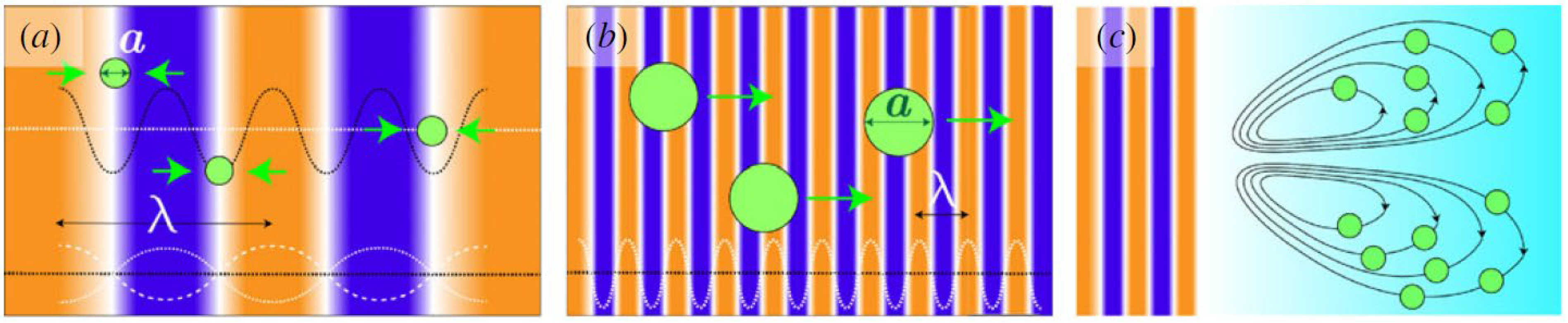
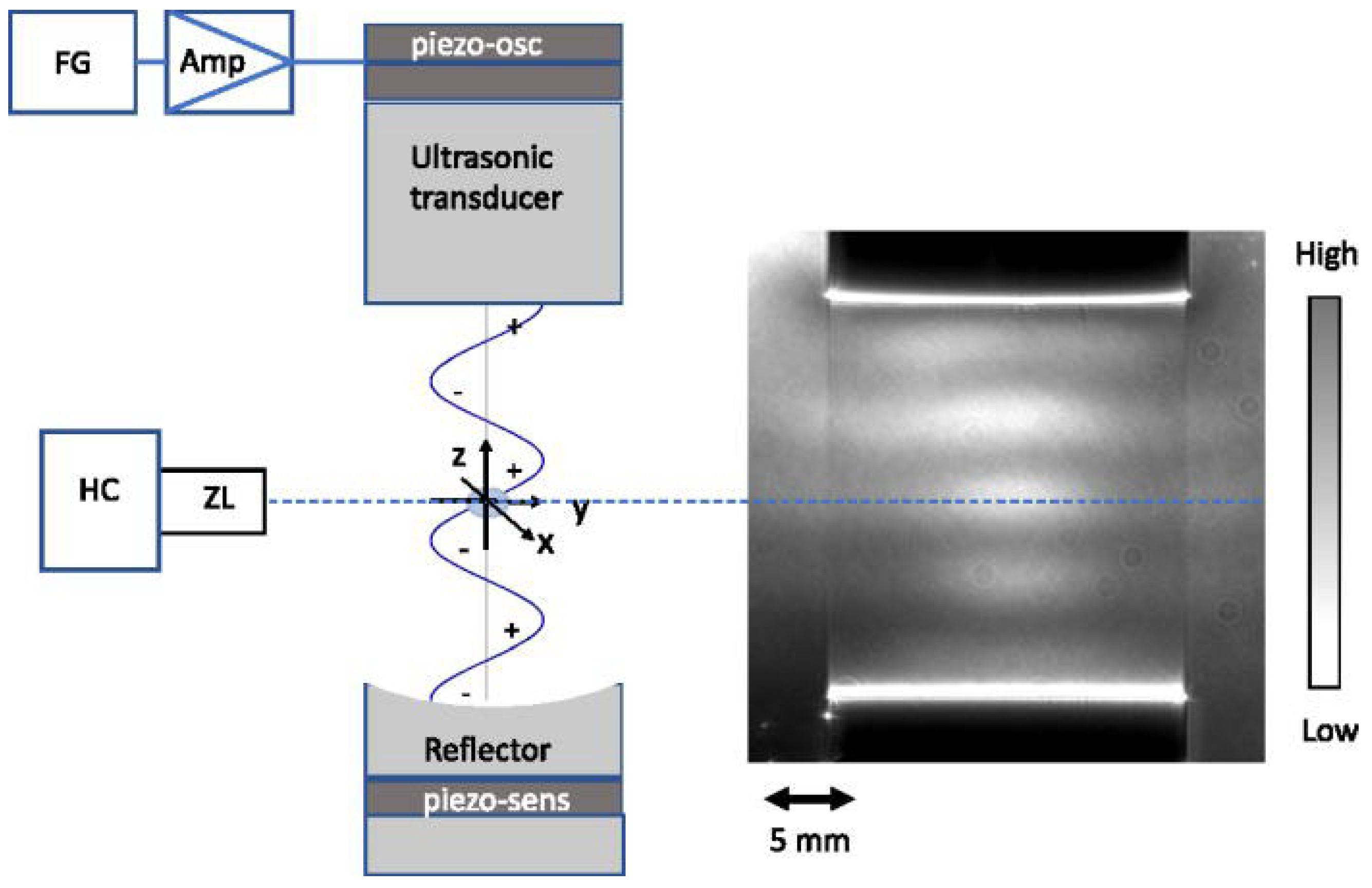
2.2. Concept of Acoustic Levitation
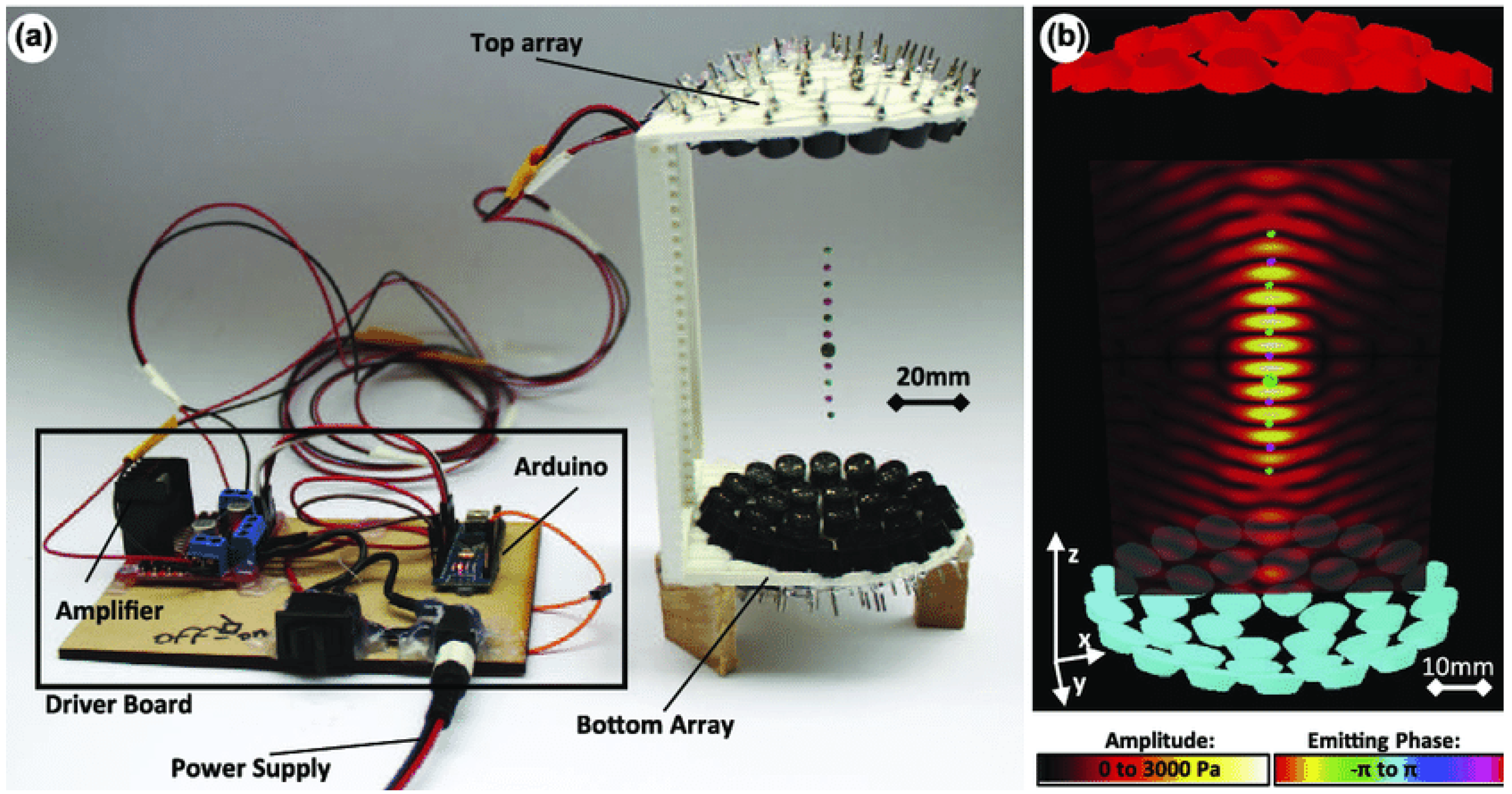
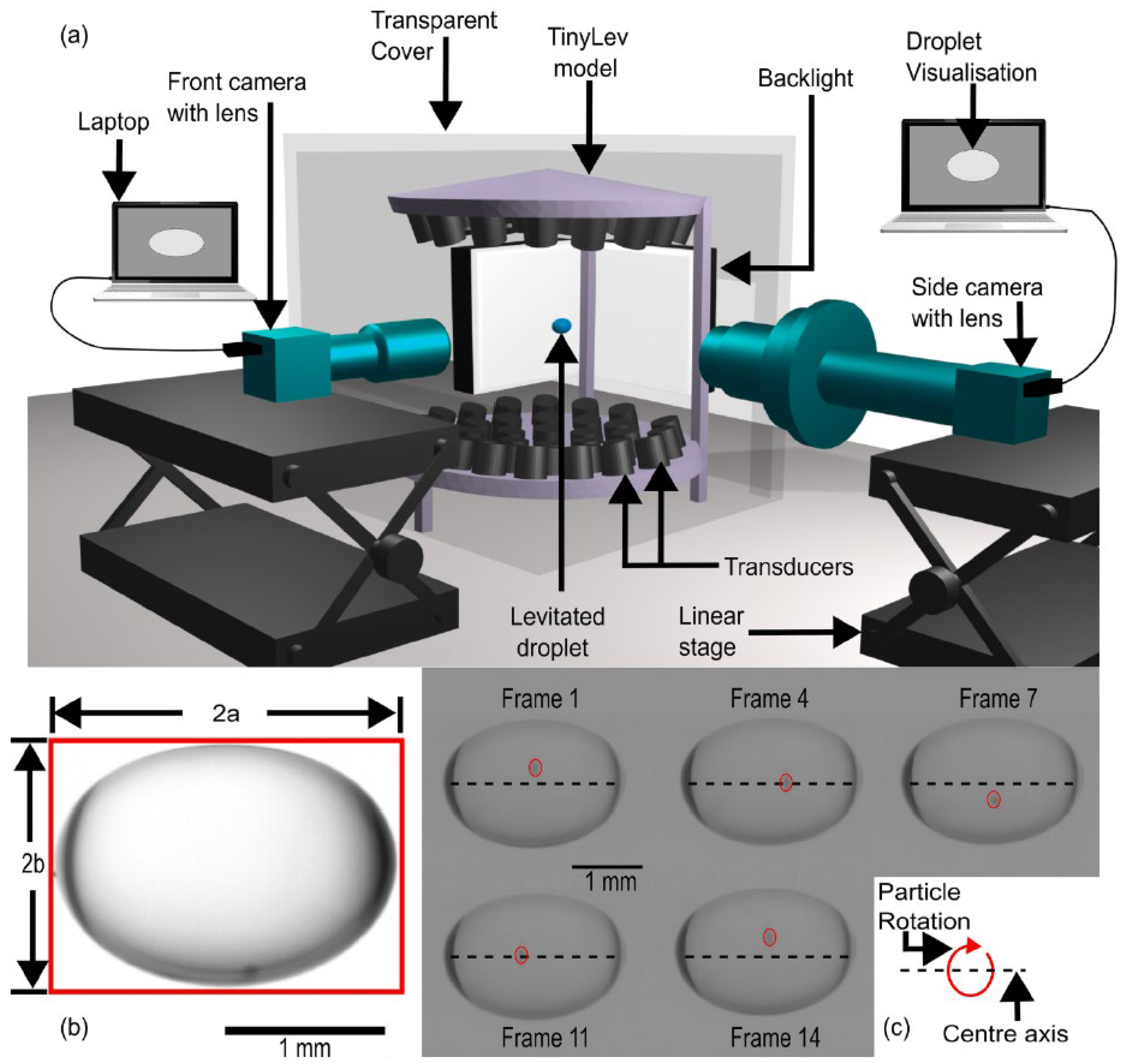
2.3. Acoustic Levitation Platforms
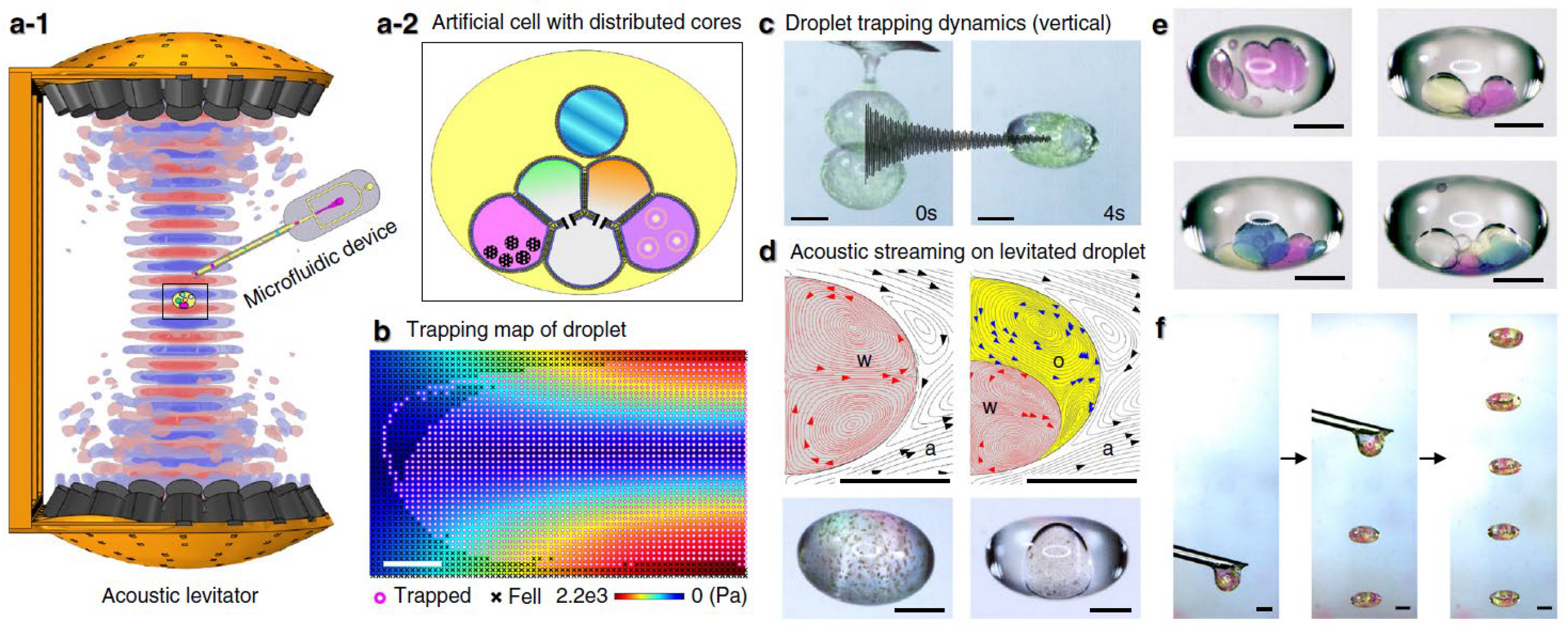
2.4. Acoustic Levitation of Biological Samples
3. Current On-Ground Microgravity Simulation Platforms
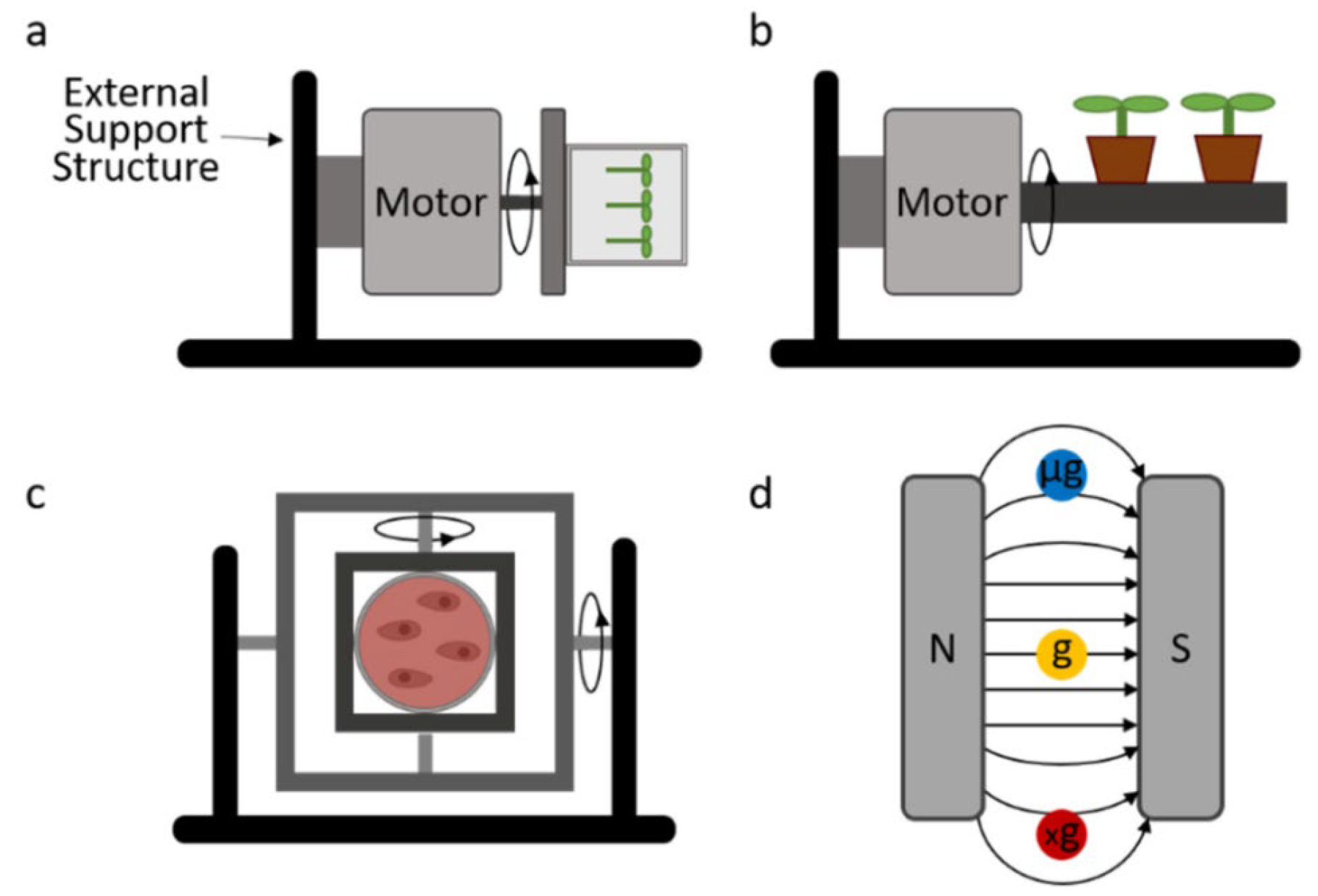
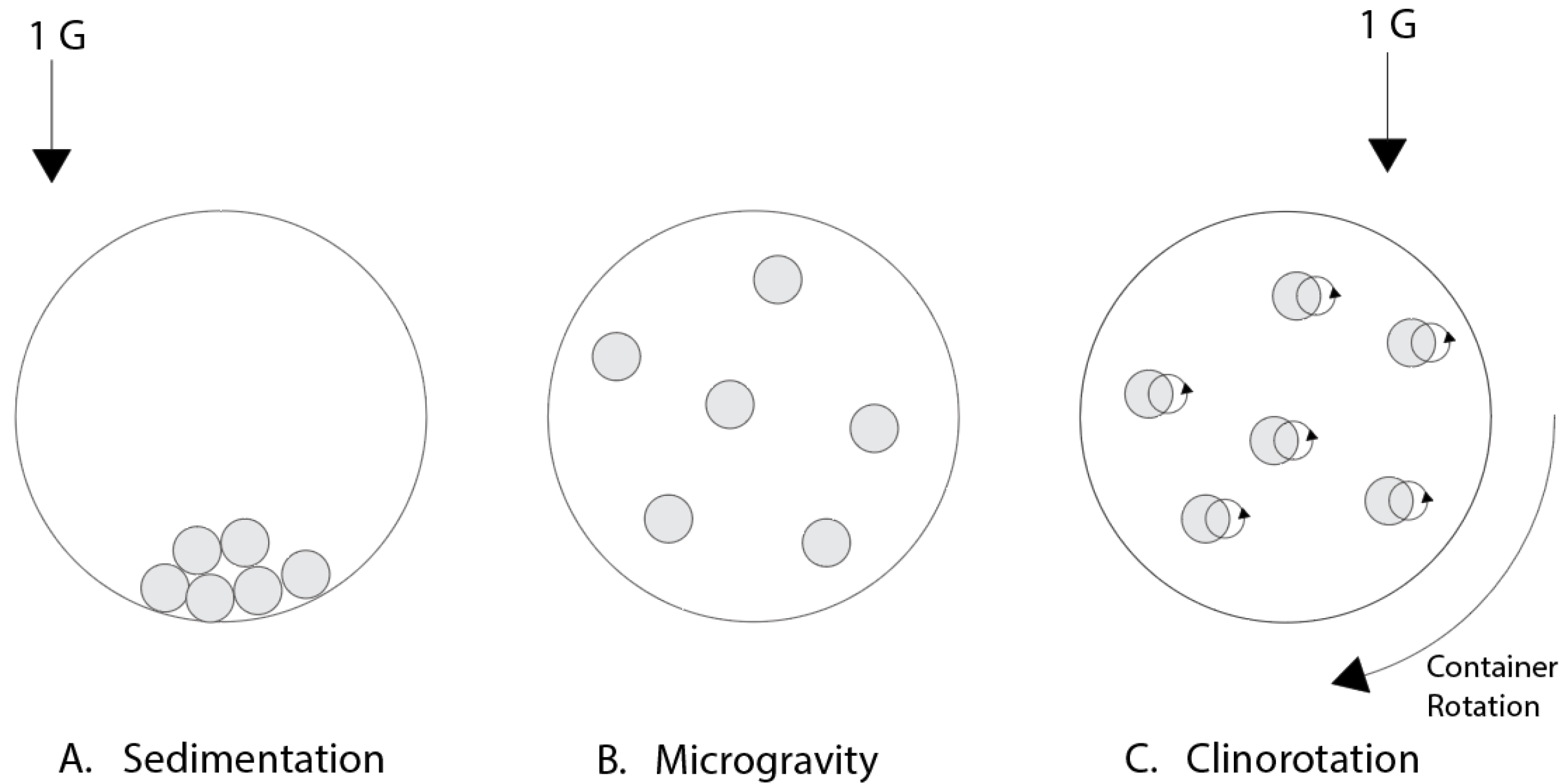
3.1. Clinostat
3.2. Random Positioning Machine (RPM)
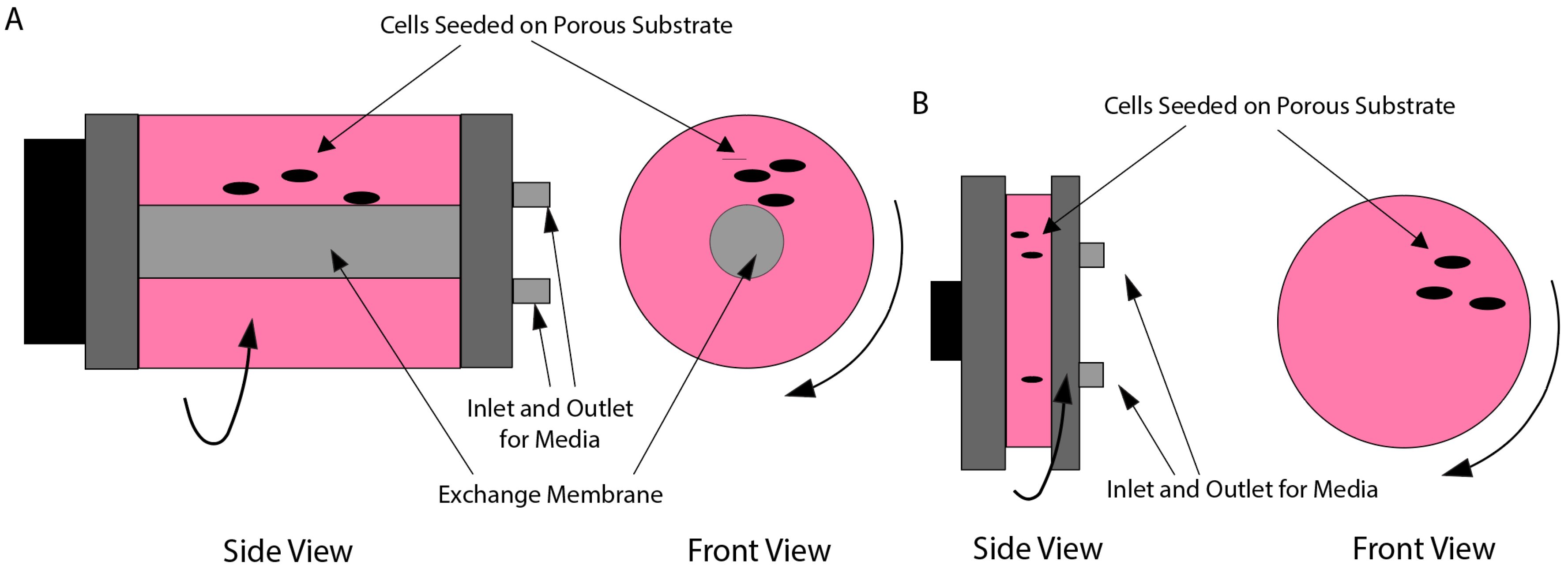
3.3. Rotating Wall Vessels (RWVs)
3.4. Diamagnetic Levitation
4. Acoustic Levitation as a Microgravity Simulation Platform
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARF | Acoustic Radiation Force |
| RPM | Random positioning machine |
| RWV | Rotating wall vessels |
| FG | Function Generator |
| AMP | Amplifier |
| HC | High-Speed Camera |
| ZL | Zoom Lens |
| MSC | Mesenchymal Stem Cell |
| SFX | Serial Femtosecond Crystallography |
| LCP | Liquid-Cubic Phase |
| ISS | International Space Station |
| EMF | Electromagnetic Force |
| YAP | Yes-Associated Protein |
| STLV | Slow-Turning Lateral Vessel |
| HARV | High-Aspect Ratio Vessel |
References
- Santesson, S.; Nilsson, S. Airborne Chemistry: Acoustic Levitation in Chemical Analysis. Anal. Bioanal. Chem. 2004, 378, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Bücks, K.; Müller, H. Über einige Beobachtungen an schwingenden Piezoquarzen und ihrem Schallfeld. Z. Für Physik. 1933, 84, 75–86. [Google Scholar] [CrossRef]
- Beaugnon, E.; Tournier, R. Levitation of Water and Organic Substances in High Static Magnetic Fields. J. Phys. III 1991, 1, 1423. [Google Scholar] [CrossRef]
- Marzo, A.; Barnes, A.; Drinkwater, B.W. TinyLev: A Multi-Emitter Single-Axis Acoustic Levitator. Rev. Sci. Instrum. 2017, 88, 085105. [Google Scholar] [CrossRef]
- Li, L.; Gu, N.; Dong, H.; Li, B.; Kenneth, T.V.G. Analysis of the Effects of Acoustic Levitation to Simulate the Microgravity Environment on the Development of Early Zebrafish Embryos. RSC Adv. 2020, 10, 44593–44600. [Google Scholar] [CrossRef]
- Cao, H.-L.; Yin, D.-C.; Guo, Y.-Z.; Ma, X.-L.; He, J.; Guo, W.-H.; Xie, X.-Z.; Zhou, B.-R. Rapid Crystallization from Acoustically Levitated Droplets. J. Acoust. Soc. Am. 2012, 131, 3164–3172. [Google Scholar] [CrossRef] [PubMed]
- Vashi, A.; Yadav, A.S.; Nguyen, N.-T.; Sreejith, K.R. Parametric Analysis of Acoustically Levitated Droplet for Potential Microgravity Application. Appl. Acoust. 2023, 213, 109624. [Google Scholar] [CrossRef]
- Grigoriev, A.I.; Oganov, V.S.; Bakulin, A.V.; Poliakov, V.V.; Voronin, L.I.; Morgun, V.V.; Shnaĭder, V.S.; Murashko, L.V.; Novikov, V.E.; LeBlank, A.; et al. Clinical and physiological evaluation of bone changes among astronauts after long-term space flights. Aviakosm. Ekolog. Med. 1998, 32, 21–25. [Google Scholar]
- Levine, B.D.; Zuckerman, J.H.; Pawelczyk, J.A. Cardiac Atrophy after Bed-Rest Deconditioning: A Nonneural Mechanism for Orthostatic Intolerance. Circulation 1997, 96, 517–525. [Google Scholar] [CrossRef]
- Edgerton, V.R.; Zhou, M.Y.; Ohira, Y.; Klitgaard, H.; Jiang, B.; Bell, G.; Harris, B.; Saltin, B.; Gollnick, P.D.; Roy, R.R. Human Fiber Size and Enzymatic Properties after 5 and 11 Days of Spaceflight. J. Appl. Physiol. 1995, 78, 1733–1739. [Google Scholar] [CrossRef]
- Kundt, A. Ueber Eine Neue Art Akustischer Staubfiguren Und Über Die Anwendung Derselben Zur Bestimmung Der Schallgeschwindigkeit in Festen Körpern Und Gasen. Ann. Physik. 1866, 203, 497–523. [Google Scholar] [CrossRef]
- Yosioka, K.; Kawasima, Y. Acoustic Radiation Pressure on a Compressible Sphere. Acta Acust. United Acust. 1955, 5, 167–173. [Google Scholar]
- King, L.V. On the Acoustic Radiation Pressure on Spheres. Proc. R. Soc. Lond. Ser. A 1997, 147, 212–240. [Google Scholar] [CrossRef]
- Gor’kov, L.P. Forces Acting on a Small Particle in an Acoustic Field within an Ideal Fluid. Dokl. Akad. Nauk. SSSR 1961, 140, 88–91. [Google Scholar]
- Nyborg, W.L. Radiation Pressure on a Small Rigid Sphere. J. Acoust. Soc. Am. 1967, 42, 947–952. [Google Scholar] [CrossRef]
- Mohanty, S.; Khalil, I.S.M.; Misra, S. Contactless Acoustic Micro/Nano Manipulation: A Paradigm for Next Generation Applications in Life Sciences. Proc. R. Soc. Lond. Ser. A 2020, 476, 20200621. [Google Scholar] [CrossRef]
- Meng, L.; Cai, F.; Li, F.; Zhou, W.; Niu, L.; Zheng, H. Acoustic Tweezers. J. Phys. D Appl. Phys. 2019, 52, 273001. [Google Scholar] [CrossRef]
- Destgeer, G.; Sung, H.J. Recent Advances in Microfluidic Actuation and Micro-Object Manipulation via Surface Acoustic Waves. Lab Chip 2015, 15, 2722–2738. [Google Scholar] [CrossRef]
- Wiklund, M.; Green, R.; Ohlin, M. Acoustofluidics 14: Applications of Acoustic Streaming in Microfluidic Devices. Lab Chip 2012, 12, 2438–2451. [Google Scholar] [CrossRef]
- Andrade, M.A.B.; Marzo, A.; Adamowski, J.C. Acoustic Levitation in Mid-Air: Recent Advances, Challenges, and Future Perspectives. Appl. Phys. Lett. 2020, 116, 250501. [Google Scholar] [CrossRef]
- Hasegawa, K.; Qiu, L.; Noda, A.; Inoue, S.; Shinoda, H. Electronically Steerable Ultrasound-Driven Long Narrow Air Stream. Appl. Phys. Lett. 2017, 111, 064104. [Google Scholar] [CrossRef]
- Andrade, M.A.B.; Ramos, T.S.; Okina, F.T.A.; Adamowski, J.C. Nonlinear Characterization of a Single-Axis Acoustic Levitator. Rev. Sci. Instrum. 2014, 85, 045125. [Google Scholar] [CrossRef]
- Jackson, D.P.; Chang, M.-H. Acoustic Levitation and the Acoustic Radiation Force. Am. J. Phys. 2021, 89, 383–392. [Google Scholar] [CrossRef]
- Cox, L.; Croxford, A.; Drinkwater, B.W.; Marzo, A. Acoustic Lock: Position and Orientation Trapping of Non-Spherical Sub-Wavelength Particles in Mid-Air Using a Single-Axis Acoustic Levitator. Appl. Phys. Lett. 2018, 113, 054101. [Google Scholar] [CrossRef]
- Li, J.; Jamieson, W.D.; Dimitriou, P.; Xu, W.; Rohde, P.; Martinac, B.; Baker, M.; Drinkwater, B.W.; Castell, O.K.; Barrow, D.A. Building Programmable Multicompartment Artificial Cells Incorporating Remotely Activated Protein Channels Using Microfluidics and Acoustic Levitation. Nat. Commun. 2022, 13, 4125. [Google Scholar] [CrossRef]
- Lin, S. Study on the Multifrequency Langevin Ultrasonic Transducer. Ultrasonics 1995, 33, 445–448. [Google Scholar] [CrossRef]
- Xie, W.J.; Cao, C.D.; Lü, Y.J.; Hong, Z.Y.; Wei, B. Acoustic Method for Levitation of Small Living Animals. Appl. Phys. Lett. 2006, 89, 214102. [Google Scholar] [CrossRef]
- Andrade, M.A.B.; Buiochi, F.; Adamowski, J.C. Finite Element Analysis and Optimization of a Single-Axis Acoustic Levitator. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2010, 57, 469–479. [Google Scholar] [CrossRef]
- Tsujino, S.; Shinoda, A.; Tomizaki, T. On-Demand Droplet Loading of Ultrasonic Acoustic Levitator and Its Application for Protein Crystallography Experiments. Appl. Phys. Lett. 2019, 114, 213702. [Google Scholar] [CrossRef]
- Lewis, G.K., Jr.; Olbricht, W.L. Design and Characterization of a High-Power Ultrasound Driver with Ultralow-Output Impedance. Rev. Sci. Instrum. 2009, 80, 114704. [Google Scholar] [CrossRef]
- Weber, J.K.R.; Rey, C.A.; Neuefeind, J.; Benmore, C.J. Acoustic Levitator for Structure Measurements on Low Temperature Liquid Droplets. Rev. Sci. Instrum. 2009, 80, 083904. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.A.B.; Pérez, N.; Adamowski, J.C. Particle Manipulation by a Non-Resonant Acoustic Levitator. Appl. Phys. Lett. 2015, 106, 014101. [Google Scholar] [CrossRef]
- Jeger-Madiot, N.; Arakelian, L.; Setterblad, N.; Bruneval, P.; Hoyos, M.; Larghero, J.; Aider, J.-L. Self-Organization and Culture of Mesenchymal Stem Cell Spheroids in Acoustic Levitation. Sci. Rep. 2021, 11, 8355. [Google Scholar] [CrossRef]
- Wang, X.; Tian, L.; Ren, Y.; Zhao, Z.; Du, H.; Zhang, Z.; Drinkwater, B.W.; Mann, S.; Han, X. Chemical Information Exchange in Organized Protocells and Natural Cell Assemblies with Controllable Spatial Positions. Small 2020, 16, 1906394. [Google Scholar] [CrossRef] [PubMed]
- Kepa, M.W.; Tomizaki, T.; Sato, Y.; Ozerov, D.; Sekiguchi, H.; Yasuda, N.; Aoyama, K.; Skopintsev, P.; Standfuss, J.; Cheng, R.; et al. Acoustic Levitation and Rotation of Thin Films and Their Application for Room Temperature Protein Crystallography. Sci. Rep. 2022, 12, 5349. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Tsujino, S.; Tomizaki, T. Ultrasonic Acoustic Levitation for Fast Frame Rate X-Ray Protein Crystallography at Room Temperature. Sci. Rep. 2016, 6, 25558. [Google Scholar] [CrossRef]
- Rucktooa, P.; Cheng, R.K.Y.; Segala, E.; Geng, T.; Errey, J.C.; Brown, G.A.; Cooke, R.M.; Marshall, F.H.; Doré, A.S. Towards High Throughput GPCR Crystallography: In Meso Soaking of Adenosine A2A Receptor Crystals. Sci. Rep. 2018, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Kiss, J.Z.; Wolverton, C.; Wyatt, S.E.; Hasenstein, K.H.; van Loon, J.J. Comparison of Microgravity Analogs to Spaceflight in Studies of Plant Growth and Development. Front. Plant Sci. 2019, 10, 1577. [Google Scholar] [CrossRef]
- Ferranti, F.; Del Bianco, M.; Pacelli, C. Advantages and Limitations of Current Microgravity Platforms for Space Biology Research. Appl. Sci. 2021, 11, 68. [Google Scholar] [CrossRef]
- Böhmer, M.; Schleiff, E. Microgravity Research in Plants. EMBO Rep. 2019, 20, e48541. [Google Scholar] [CrossRef] [PubMed]
- Knight, T.A. On the Direction of the Radicle and Germen during the Vegetation of Seeds. By Thomas Andrew Knight, Esq. F. R. S. In a Letter to the Right Hon. Sir Joseph Banks, K.B.P.R.S. Proc. R. Soc. Lond. 1832, 1, 218–220. [Google Scholar] [CrossRef]
- von Sachs, J. Uber Ausschliessung der geotropischen und heliotropischen Kr mmungen wahrend des Wachsens. Arb. Bot. Inst. Wurzbg. 1879, 2, 209–225. [Google Scholar]
- Cogoli, M. The Fast Rotating Clinostat: A History of Its Use in Gravitational Biology and a Comparison of Ground-Based and Flight Experiment Results. ASGSB Bull. 1992, 5, 59–67. [Google Scholar]
- Häder, D.-P.; Hemmersbach, R.; Lebert, M. Gravity and the Behavior of Unicellular Organisms. In Developmental and Cell Biology Series; Cambridge University Press: Cambridge, UK, 2005; ISBN 978-0-521-82052-3. [Google Scholar]
- Klaus, D.M.; Todd, P.; Schatz, A. Functional Weightlessness during Clinorotation of Cell Suspensions. Adv. Space Res. 1998, 21, 1315–1318. [Google Scholar] [CrossRef] [PubMed]
- Dedolph, R.R.; Dipert, M.H. The Physical Basis of Gravity Stimulus Nullification by Clinostat Rotation. Plant Physiol. 1971, 47, 756–764. [Google Scholar] [CrossRef]
- United Nations Office for Outer Space Affairs. Teacher’s Guide to Plant Experiments; United Nations: Vienna, Austria, 2013; Available online: https://www.unoosa.org/oosa/oosadoc/data/documents/2013/stspace/stspace63_0.html (accessed on 25 February 2025).
- Thompson, M.; Woods, K.; Newberg, J.; Oxford, J.T.; Uzer, G. Low-Intensity Vibration Restores Nuclear YAP Levels and Acute YAP Nuclear Shuttling in Mesenchymal Stem Cells Subjected to Simulated Microgravity. NPJ Microgravity 2020, 6, 35. [Google Scholar] [CrossRef]
- Zhou, Y.; Lv, W.; Peng, X.; Cheng, Y.; Tu, Y.; Song, G.; Luo, Q. Simulated Microgravity Attenuates Skin Wound Healing by Inhibiting Dermal Fibroblast Migration via F-Actin/YAP Signaling Pathway. J. Cell. Physiol. 2023, 238, 2751–2764. [Google Scholar] [CrossRef]
- Lv, W.; Peng, X.; Tu, Y.; Shi, Y.; Song, G.; Luo, Q. YAP Inhibition Alleviates Simulated Microgravity-Induced Mesenchymal Stem Cell Senescence via Targeting Mitochondrial Dysfunction. Antioxidants 2023, 12, 990. [Google Scholar] [CrossRef]
- Wubshet, N.H.; Cai, G.; Chen, S.J.; Sullivan, M.; Reeves, M.; Mays, D.; Harrison, M.; Varnado, P.; Yang, B.; Arreguin-Martinez, E.; et al. Cellular Mechanotransduction of Human Osteoblasts in Microgravity. NPJ Microgravity 2024, 10, 35. [Google Scholar] [CrossRef]
- Hoson, T.; Kamisaka, S.; Masuda, Y.; Yamashita, M. Changes in Plant Growth Processes under Microgravity Conditions Simulated by a Three-Dimensional Clinostat. Bot. Mag. 1992, 105, 53–70. [Google Scholar] [CrossRef]
- Hoson, T.; Kamisaka, S.; Masuda, Y.; Yamashita, M.; Buchen, B. Evaluation of the Three-Dimensional Clinostat as a Simulator of Weightlessness. Planta 1997, 203, S187–S197. [Google Scholar] [CrossRef] [PubMed]
- Borst, A.G.; van Loon, J.J.W.A. Technology and Developments for the Random Positioning Machine, RPM. Microgravity Sci. Technol. 2008, 21, 287. [Google Scholar] [CrossRef]
- Mesland, D.A.M. Novel Ground-Based Facilities for Research in the Effects of Weight. ESA Microgravity News 1996, 9, 5–10. [Google Scholar]
- van Loon, J.J. Some History and Use of the Random Positioning Machine, RPM, in Gravity Related Research. Adv. Space Res. 2007, 39, 1161–1165. [Google Scholar] [CrossRef]
- Grimm, D.; Wehland, M.; Pietsch, J.; Aleshcheva, G.; Wise, P.; van Loon, J.; Ulbrich, C.; Magnusson, N.E.; Infanger, M.; Bauer, J. Growing Tissues in Real and Simulated Microgravity: New Methods for Tissue Engineering. Tissue Eng. Part B Rev. 2014, 20, 555–566. [Google Scholar] [CrossRef]
- Kraft, T.; Loon, J.; Kiss, J. Plastid Position in Arabidopsis Columella Cells Is Similar in Microgravity and on a Random-Positioning Machine. Planta 2000, 211, 415–422. [Google Scholar] [CrossRef]
- Krause, L.; Braun, M.; Hauslage, J.; Hemmersbach, R. Analysis of Statoliths Displacement in Chara Rhizoids for Validating the Microgravity-Simulation Quality of Clinorotation Modes. Microgravity Sci. Technol. 2018, 30, 229–236. [Google Scholar] [CrossRef]
- Martin, Y.; Vermette, P. Bioreactors for Tissue Mass Culture: Design, Characterization, and Recent Advances. Biomaterials 2005, 26, 7481–7503. [Google Scholar] [CrossRef]
- Schwarz, R.P.; Goodwin, T.J.; Wolf, D.A. Cell Culture for Three-Dimensional Modeling in Rotating-Wall Vessels: An Application of Simulated Microgravity. J. Tissue Cult. Methods 1992, 14, 51–57. [Google Scholar] [CrossRef]
- Klaus, D.M. Clinostats and Bioreactors. Gravit. Space Biol. Bull. 2001, 14, 55–64. [Google Scholar]
- Gardner, J.K.; Herbst-Kralovetz, M.M. Three-Dimensional Rotating Wall Vessel-Derived Cell Culture Models for Studying Virus-Host Interactions. Viruses 2016, 8, 304. [Google Scholar] [CrossRef]
- Goodwin, T.J.; McCarthy, M.; Osterrieder, N.; Cohrs, R.J.; Kaufer, B.B. Three-Dimensional Normal Human Neural Progenitor Tissue-Like Assemblies: A Model of Persistent Varicella-Zoster Virus Infection. PLoS Pathog. 2013, 9, e1003512. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liu, W.; Zhao, S.; Zhao, Y.; Dai, J. Advances in Microgravity Directed Tissue Engineering. Adv. Healthc. Mater. 2023, 12, 2202768. [Google Scholar] [CrossRef] [PubMed]
- Herranz, R.; Larkin, O.J.; Dijkstra, C.E.; Hill, R.J.; Anthony, P.; Davey, M.R.; Eaves, L.; van Loon, J.J.; Medina, F.J.; Marco, R. Microgravity Simulation by Diamagnetic Levitation: Effects of a Strong Gradient Magnetic Field on the Transcriptional Profile of Drosophila Melanogaster. BMC Genom. 2012, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Valles, J.M.; Maris, H.J.; Seidel, G.M.; Tang, J.; Yao, W. Magnetic Levitation-Based Martian and Lunar Gravity Simulator. Adv. Space Res. 2005, 36, 114–118. [Google Scholar] [CrossRef]
- Maret, G.; Dransfeld, K. Biomolecules and Polymers in High Steady Magnetic Fields. In Strong and Ultrastrong Magnetic Fields and Their Applications; Herlach, F., Ed.; Springer: Berlin/Heidelberg, Germany, 1985; pp. 143–204. ISBN 978-3-540-38962-0. [Google Scholar]
- Valiron, O.; Peris, L.; Rikken, G.; Schweitzer, A.; Saoudi, Y.; Remy, C.; Job, D. Cellular Disorders Induced by High Magnetic Fields. J. Magn. Reason. Imaging 2005, 22, 334–340. [Google Scholar] [CrossRef]
- Vashi, A.; Sreejith, K.R.; Nguyen, N.-T. Lab-on-a-Chip Technologies for Microgravity Simulation and Space Applications. Micromachines 2023, 14, 116. [Google Scholar] [CrossRef]
- Benmore, C.J.; Weber, J.K.R. Amorphization of Molecular Liquids of Pharmaceutical Drugs by Acoustic Levitation. Phys. Rev. X 2011, 1, 011004. [Google Scholar] [CrossRef]
- Ding, X.; Li, P.; Lin, S.-C.S.; Stratton, Z.S.; Nama, N.; Guo, F.; Slotcavage, D.; Mao, X.; Shi, J.; Costanzo, F.; et al. Surface Acoustic Wave Microfluidics. Lab Chip 2013, 13, 3626–3649. [Google Scholar] [CrossRef]
- Guo, F.; Li, P.; French, J.B.; Mao, Z.; Zhao, H.; Li, S.; Nama, N.; Fick, J.R.; Benkovic, S.J.; Huang, T.J. Controlling Cell–Cell Interactions Using Surface Acoustic Waves. Proc. Natl. Acad. Sci. USA 2015, 112, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, Y.; Zhang, T.; Shung, K.K.; Zhu, B. Recent Advancements in Ultrasound Transducer: From Material Strategies to Biomedical Applications. BME Front. 2022, 2022, 9764501. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Chen, Y.; Qiao, B.; Fan, S.; Wang, Y.; Li, X. Enhancing Acoustic Levitation Capacity through Array Geometry Optimization. Appl. Acoust. 2024, 222, 110040. [Google Scholar] [CrossRef]


| Platform | Advantages | Disadvantages | References |
|---|---|---|---|
| Clinostat |
|
| [42,43,44,45,46,47,48,49,50,51,52] |
| Random positioning machine (RPM) |
|
| [53,54,55,56,57,58,59,60] |
| Rotating wall vessel (RWV) |
|
| [61,62,63,64,65,66] |
| Diamagnetic Levitation |
|
| [67,68,69,70] |
| Acoustic Levitation |
|
| [5,6,7,25,71,72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boudreaux, T.; Freyhof, L.; Riehl, B.D.; Kim, E.; Pedrigi, R.M.; Lim, J.Y. Biological Acoustic Levitation and Its Potential Application for Microgravity Study. Bioengineering 2025, 12, 458. https://doi.org/10.3390/bioengineering12050458
Boudreaux T, Freyhof L, Riehl BD, Kim E, Pedrigi RM, Lim JY. Biological Acoustic Levitation and Its Potential Application for Microgravity Study. Bioengineering. 2025; 12(5):458. https://doi.org/10.3390/bioengineering12050458
Chicago/Turabian StyleBoudreaux, Taylor, Luke Freyhof, Brandon D. Riehl, Eunju Kim, Ryan M. Pedrigi, and Jung Yul Lim. 2025. "Biological Acoustic Levitation and Its Potential Application for Microgravity Study" Bioengineering 12, no. 5: 458. https://doi.org/10.3390/bioengineering12050458
APA StyleBoudreaux, T., Freyhof, L., Riehl, B. D., Kim, E., Pedrigi, R. M., & Lim, J. Y. (2025). Biological Acoustic Levitation and Its Potential Application for Microgravity Study. Bioengineering, 12(5), 458. https://doi.org/10.3390/bioengineering12050458





