Imaging Peripheral Nerves In Vivo with CT Neurogram Using Novel 2,4,6-Tri-Iodinated Lidocaine Contrast Agent
Abstract
1. Introduction
2. Methods and Materials
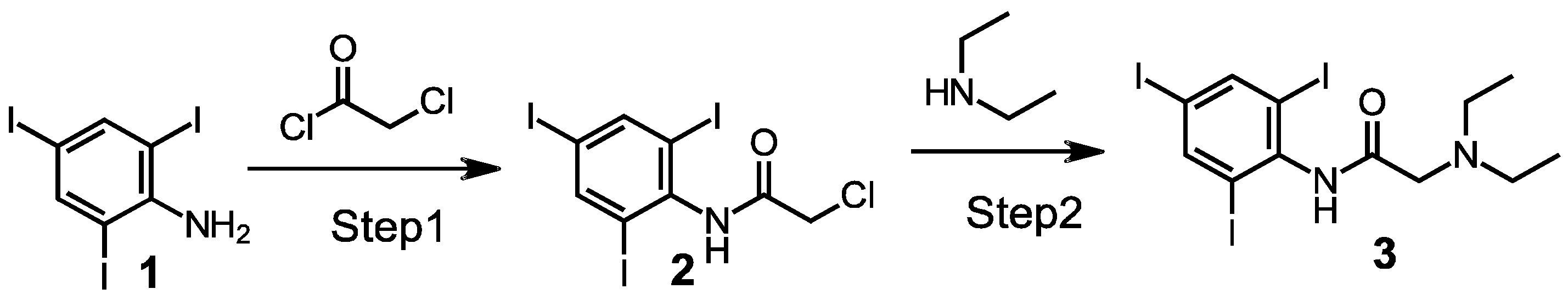
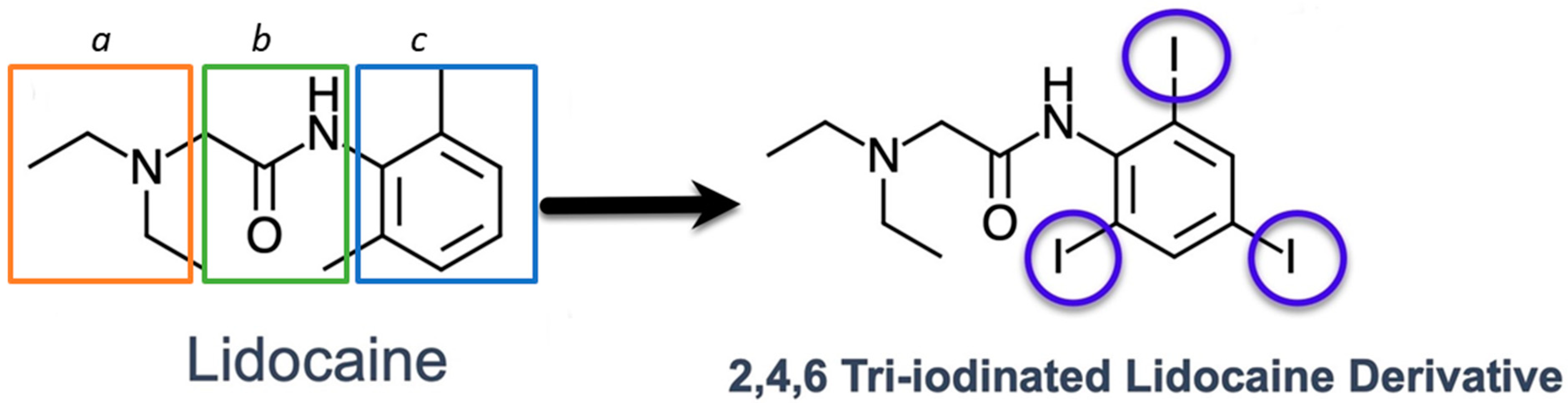
2.1. Synthesis of a 2,4,6, Triiodo-Substituted Lidocaine
2.2. Molecular Docking
2.3. Contrast Testing In Vitro
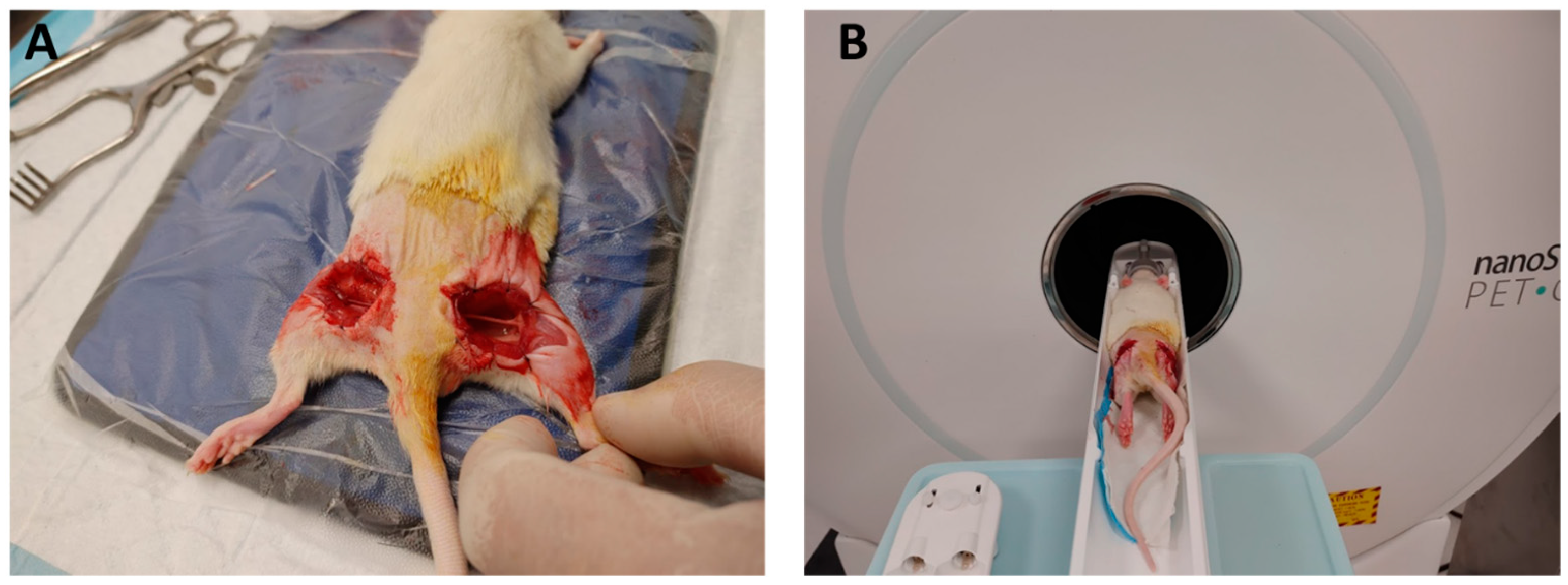
2.4. Animal Models
2.5. In Vivo Imaging Procedure of Exposed Nerve
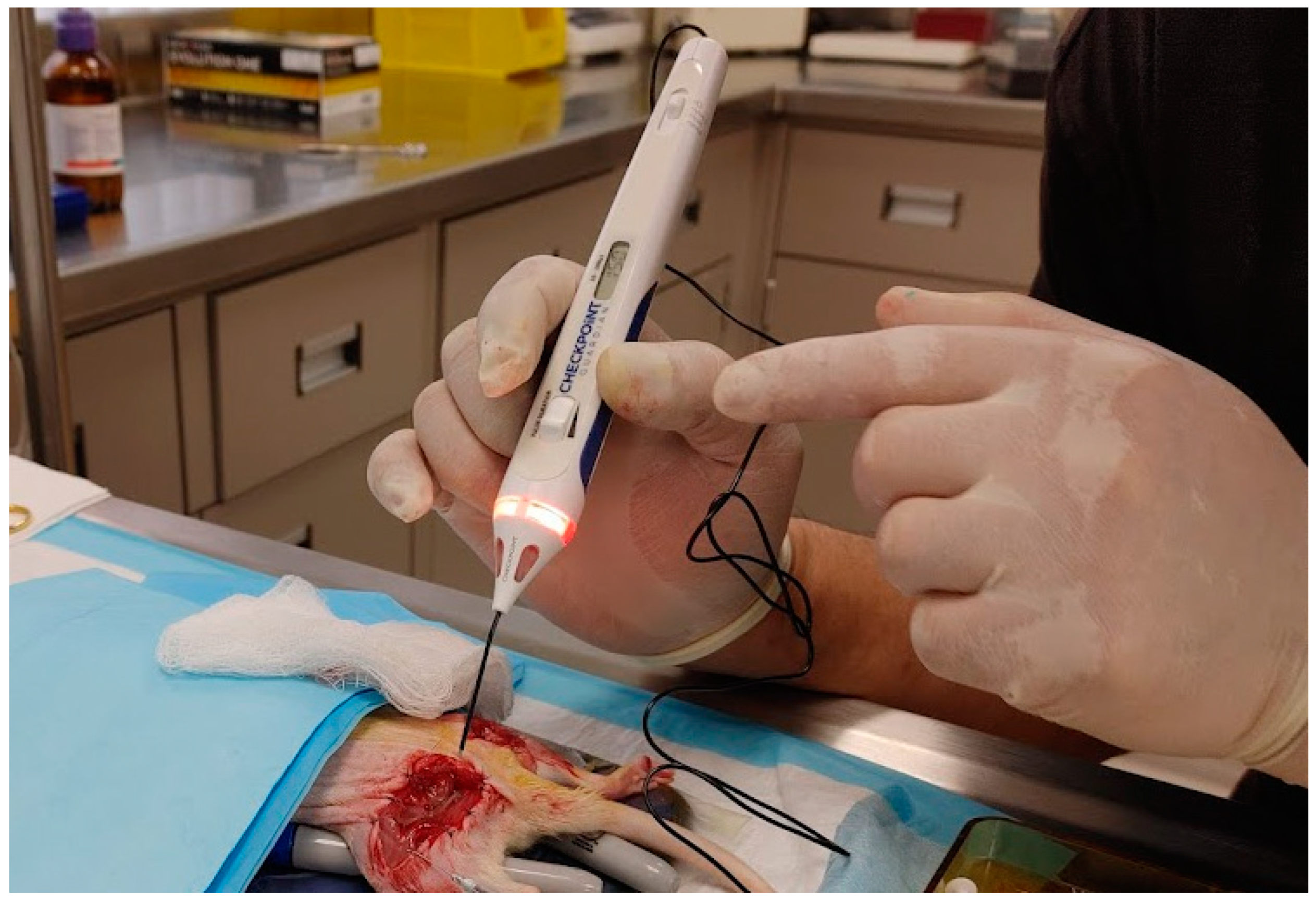
2.6. Electrical Stimulation
2.7. Imaging of Sciatic Nerves Ex Vivo
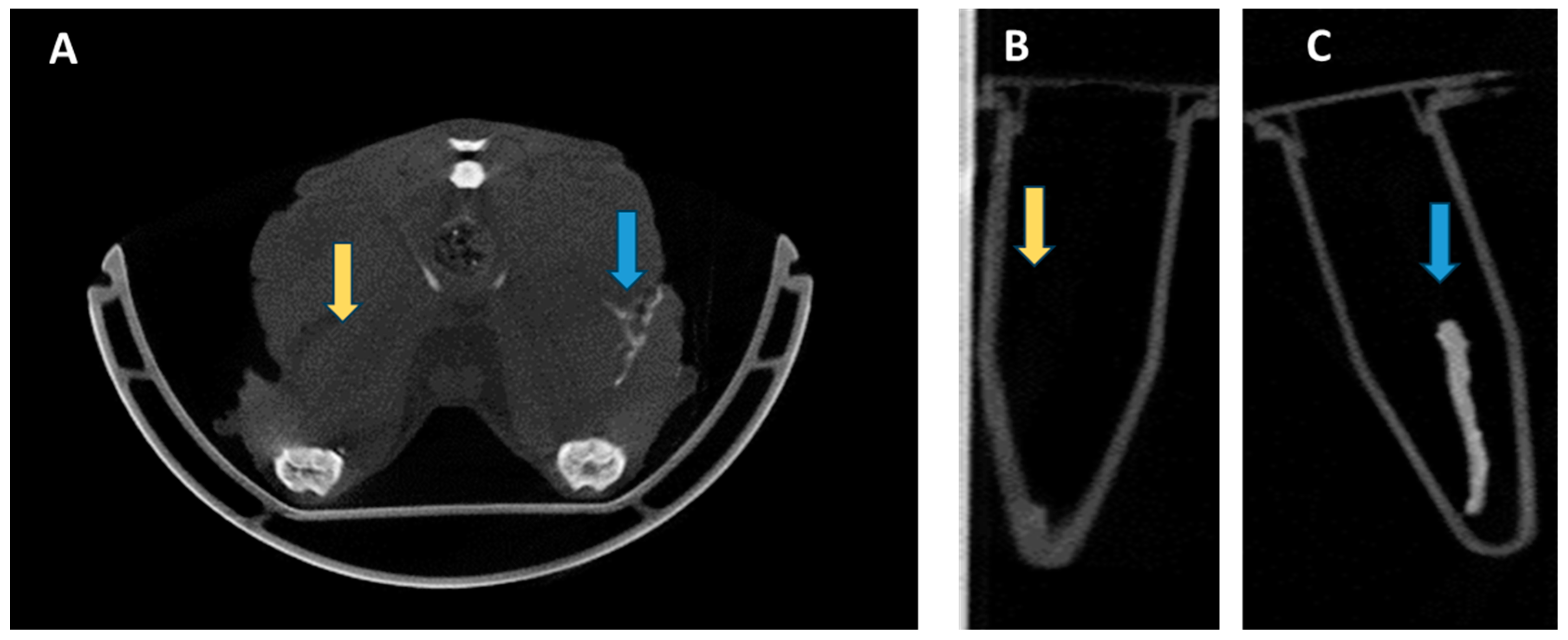
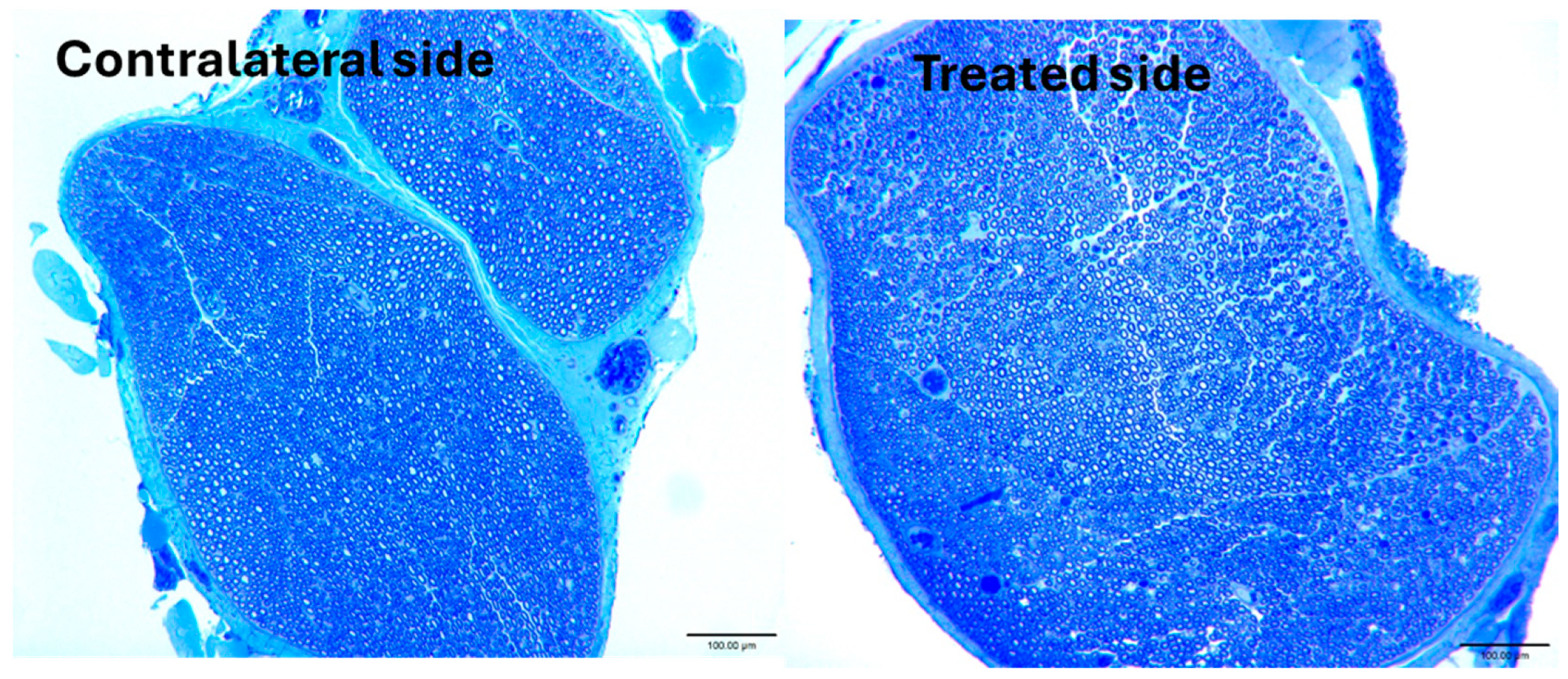
3. Results
Peripheral Nerve Electrical Stimulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otanez, E.S.; Fernandez Trokhimtchouk, T.; Semanate, F.; Palacios, C. Incidence of Facial Nerve Paralysis After Parotidectomy: Our Experience. Cureus 2024, 16, e55045. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patel, N.K.; Krumme, J.; Golladay, G.J. Incidence, Injury Mechanisms, and Recovery of Iatrogenic Nerve Injuries During Hip and Knee Arthroplasty. JAAOS-J. Am. Acad. Orthop. Surg. 2021, 29, e940–e949. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.J.; Murray, R.S.; Sherman, T.I.; Postma, W.F. Incidence of Nerve Injury After Hip Arthroscopy. J. Am. Acad. Orthop. Surg. 2018, 26, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Sharp, E.; Roberts, M.; Żurada-Zielińska, A.; Zurada, A.; Gielecki, J.; Tubbs, R.S.; Loukas, M. The most commonly injured nerves at surgery: A comprehensive review. Clin. Anat. 2021, 34, 244–262. [Google Scholar] [CrossRef] [PubMed]
- Chui, J.; Murkin, J.M.; Posner, K.L.; Domino, K.B. Perioperative Peripheral Nerve Injury After General Anesthesia: A Qualitative Systematic Review. Anesth. Analg. 2018, 127, 134–143. [Google Scholar] [CrossRef] [PubMed]
- AbuRahma, A.F.; Avgerinos, E.D.; Chang, R.W.; Darling, R.C., 3rd; Duncan, A.A.; Forbes, T.L.; Malas, M.B.; Perler, B.A.; Powell, R.J.; Rockman, C.B.; et al. The Society for Vascular Surgery implementation document for management of extracranial cerebrovascular disease. J. Vasc. Surg. 2022, 75, 26s–98s. [Google Scholar] [CrossRef] [PubMed]
- Mezger, U.; Jendrewski, C.; Bartels, M. Navigation in surgery. Langenbecks Arch. Surg. 2013, 398, 501–514. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luzhansky, I.D.; Sudlow, L.C.; Brogan, D.M.; Wood, M.D.; Berezin, M.Y. Imaging in the repair of peripheral nerve injury. Nanomedicine 2019, 14, 2659–2677. [Google Scholar] [CrossRef]
- Dip, F.; Bregoli, P.; Falco, J.; White, K.P.; Rosenthal, R.J. Nerve autofluorescence in near-ultraviolet light markedly enhances nerve visualization in vivo. Surg. Endosc. 2022, 36, 1999–2005. [Google Scholar] [CrossRef] [PubMed]
- Dip, F.; Rosenthal, D.; Socolovsky, M.; Falco, J.; De la Fuente, M.; White, K.P.; Rosenthal, R.J. Nerve autofluorescence under near-ultraviolet light: Cutting-edge technology for intra-operative neural tissue visualization in 17 patients. Surg. Endosc. 2022, 36, 4079–4089. [Google Scholar] [CrossRef] [PubMed]
- Pengel, L.H.; Herbert, R.D.; Maher, C.G.; Refshauge, K.M. Acute low back pain: Systematic review of its prognosis. BMJ 2003, 327, 323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saytashev, I.; Yoon, Y.-C.; Vakoc, B.J.; Vasudevan, S.; Hammer, D.X. Improved in vivo optical coherence tomography imaging of animal peripheral nerves using a prism nerve holder. J. Biomed. Opt. 2023, 28, 026002. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.G.; Barth, C.W.; Kitts, C.H.; Mebrat, M.D.; Montaño, A.R.; House, B.J.; McCoy, M.E.; Antaris, A.L.; Galvis, S.N.; McDowall, I.; et al. Near-infrared nerve-binding fluorophores for buried nerve tissue imaging. Sci. Transl. Med. 2020, 12, eaay0712. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.G.; Montaño, A.R.; Masillati, A.M.; Jones, J.A.; Barth, C.W.; Combs, J.R.; Kumarapeli, S.U.; Shams, N.A.; van den Berg, N.S.; Antaris, A.L.; et al. Nerve Visualization using Phenoxazine-Based Near-Infrared Fluorophores to Guide Prostatectomy. Adv. Mater. 2024, 36, e2304724. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.E.; Serkova, N.J. Computed Tomography Neurography for Visualization of the In Vivo Nervous System: A Proof of Concept. Hand 2024, 19, 931–935. [Google Scholar] [CrossRef]
- Lenaeus, M.J.; Gamal El-Din, T.M.; Ing, C.; Ramanadane, K.; Pomès, R.; Zheng, N.; Catterall, W.A. Structures of closed and open states of a voltage-gated sodium channel. Proc. Natl. Acad. Sci. USA 2017, 114, E3051–E3060. [Google Scholar] [CrossRef]
- Schöckel, L.; Jost, G.; Seidensticker, P.; Lengsfeld, P.; Palkowitsch, P.; Pietsch, H. Developments in X-Ray Contrast Media and the Potential Impact on Computed Tomography. Investig. Radiol. 2020, 55, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.; Regehr, G.; Naglie, G.; Wright, J.G. Development and validation of diagnostic criteria for carpal tunnel syndrome. J. Hand Surg. Am. 2006, 31, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yan, Y.; Ee, X.; Hunter, D.A.; Akers, W.J.; Wood, M.D.; Berezin, M.Y. Imaging of radicals following injury or acute stress in peripheral nerves with activatable fluorescent probes. Free. Radic. Biol. Med. 2016, 101, 85–92. [Google Scholar] [CrossRef]
- Ding, J.; Tang, S.; Mei, Z.; Wang, L.; Huang, Q.; Hu, H.; Ling, M.; Wu, J. Vina-GPU 2.0: Further accelerating AutoDock Vina and its derivatives with graphics processing units. J. Chem. Inf. Model. 2023, 63, 1982–1998. [Google Scholar] [CrossRef]
- Bezerra, M.M.; Leão, R.A.; Miranda, L.S.; de Souza, R.O. A brief history behind the most used local anesthetics. Tetrahedron 2020, 76, 131628. [Google Scholar] [CrossRef]
- Balser, J.R.; Nuss, H.B.; Orias, D.W.; Johns, D.C.; Marban, E.; Tomaselli, G.F.; Lawrence, J.H. Local anesthetics as effectors of allosteric gating. Lidocaine effects on inactivation-deficient rat skeletal muscle Na channels. J. Clin. Investig. 1996, 98, 2874–2886. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fozzard, H.A.; Lee, P.J.; Lipkind, G.M. Mechanism of local anesthetic drug action on voltage-gated sodium channels. Curr. Pharm. Des. 2005, 11, 2671–2686. [Google Scholar] [CrossRef] [PubMed]
- Bartolo, N.D.; Reid, S.E.; Krishnan, H.S.; Haseki, A.; Renganathan, M.; Largent-Milnes, T.M.; Norwood, B.A.; Loggia, M.L.; Hooker, J.M. Radiocaine: An Imaging Marker of Neuropathic Injury. ACS Chem. Neurosci. 2022, 13, 3661–3667. [Google Scholar] [CrossRef] [PubMed]
- Buyan, A.; Sun, D.; Corry, B. Protonation state of inhibitors determines interaction sites within voltage-gated sodium channels. Proc. Natl. Acad. Sci. USA 2018, 115, E3135–E3144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.S.; Backman, J.T.; Taavitsainen, P.; Neuvonen, P.J.; Kivistö, K.T. Involvement of CYP1A2 and CYP3A4 in lidocaine N-deethylation and 3-hydroxylation in humans. Drug Metab. Dispos. 2000, 28, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, R.; Fukami, T.; Nakajima, M.; Yokoi, T. Prilocaine- and lidocaine-induced methemoglobinemia is caused by human carboxylesterase-, CYP2E1-, and CYP3A4-mediated metabolic activation. Drug Metab. Dispos. 2013, 41, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Gamal El-Din, T.M.; Lenaeus, M.J.; Zheng, N.; Catterall, W.A. Fenestrations control resting-state block of a voltage-gated sodium channel. Proc. Natl. Acad. Sci. USA 2018, 115, 13111–13116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perisinakis, K.; Tzedakis, A.; Spanakis, K.; Papadakis, A.E.; Hatzidakis, A.; Damilakis, J. The effect of iodine uptake on radiation dose absorbed by patient tissues in contrast enhanced CT imaging: Implications for CT dosimetry. Eur. Radiol. 2018, 28, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Wilson, V.J.; Yoshida, M. Monosynaptic inhibition of neck motoneurons by the medial vestibular nucleus. Exp. Brain Res. 1969, 9, 365–380. [Google Scholar] [CrossRef]
- Nakashima, M.; Kawai, T.; Matsumoto, K.; Kawaguchi, T.; Kitera, N.; Watanabe, S.; Itoh, T.; Hiwatashi, A. Delineation of the brachial plexus by contrast-enhanced photon-counting detector CT and virtual monoenergetic images. Eur. J. Radiol. 2025, 184, 111964. [Google Scholar] [CrossRef] [PubMed]
- Claves, J.L.; Wise, S.W.; Hopper, K.D.; Tully, D.; Ten Have, T.R.; Weaver, J. Evaluation of contrast densities in the diagnosis of carotid stenosis by CT angiography. AJR Am. J. Roentgenol. 1997, 169, 569–573. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, R.; Perez, R.; Brogan, D.M.; Berezin, M.Y.; McCarthy, J.E. Imaging Peripheral Nerves In Vivo with CT Neurogram Using Novel 2,4,6-Tri-Iodinated Lidocaine Contrast Agent. Bioengineering 2025, 12, 422. https://doi.org/10.3390/bioengineering12040422
Tang R, Perez R, Brogan DM, Berezin MY, McCarthy JE. Imaging Peripheral Nerves In Vivo with CT Neurogram Using Novel 2,4,6-Tri-Iodinated Lidocaine Contrast Agent. Bioengineering. 2025; 12(4):422. https://doi.org/10.3390/bioengineering12040422
Chicago/Turabian StyleTang, Rui, Ron Perez, David M. Brogan, Mikhail Y. Berezin, and James E. McCarthy. 2025. "Imaging Peripheral Nerves In Vivo with CT Neurogram Using Novel 2,4,6-Tri-Iodinated Lidocaine Contrast Agent" Bioengineering 12, no. 4: 422. https://doi.org/10.3390/bioengineering12040422
APA StyleTang, R., Perez, R., Brogan, D. M., Berezin, M. Y., & McCarthy, J. E. (2025). Imaging Peripheral Nerves In Vivo with CT Neurogram Using Novel 2,4,6-Tri-Iodinated Lidocaine Contrast Agent. Bioengineering, 12(4), 422. https://doi.org/10.3390/bioengineering12040422






