In Vitro Biomechanical Experiment on the Effect of Unilateral Partial Facetectomy Performed by Percutaneous Endoscopy on the Stability of Lumbar Spine
Abstract
1. Introduction
2. Materials and Methods
2.1. Calf Spine Specimen Preparation
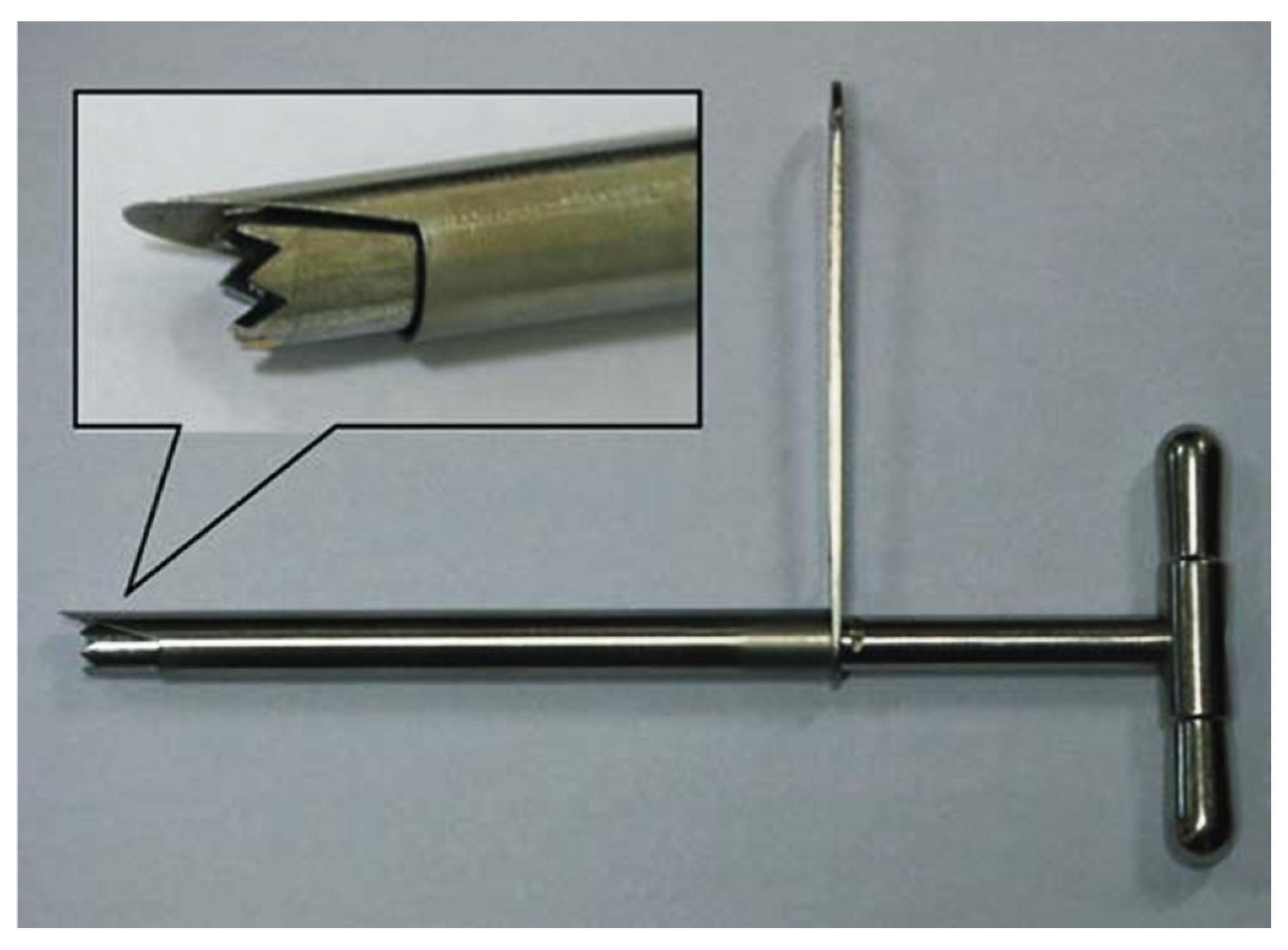
2.2. Insertion Point (IP) Determination
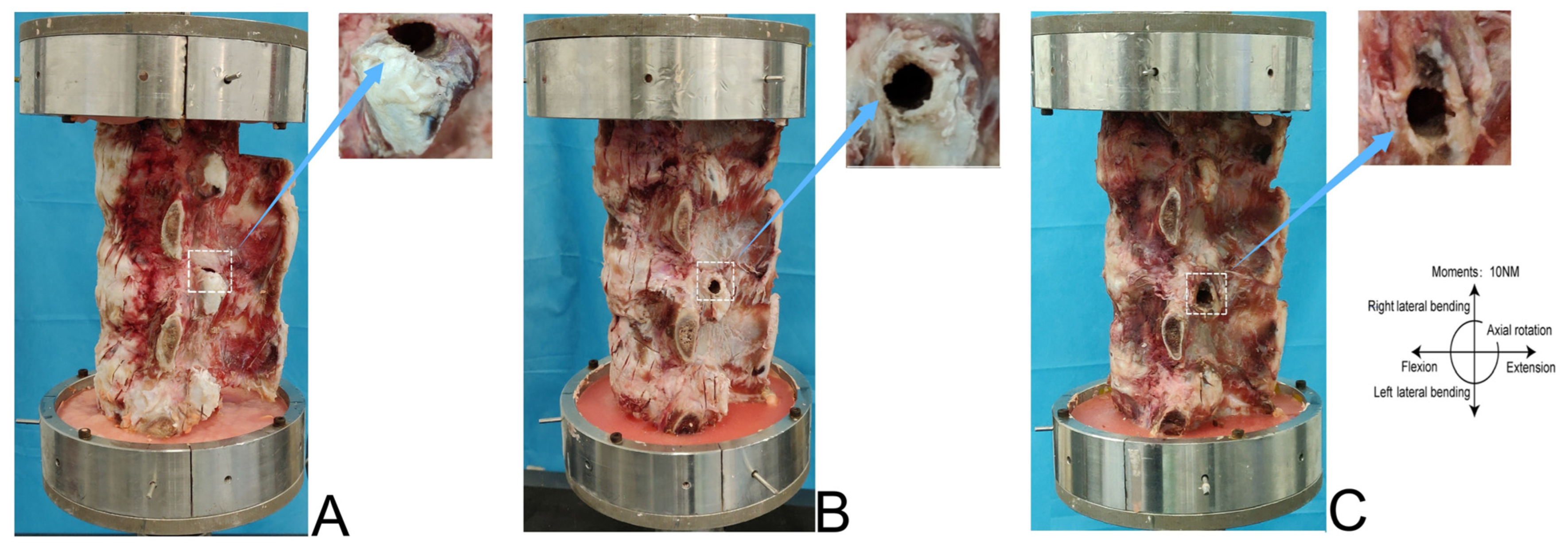
2.3. Facetectomy Simulation and Grouping
Calf Spine Model
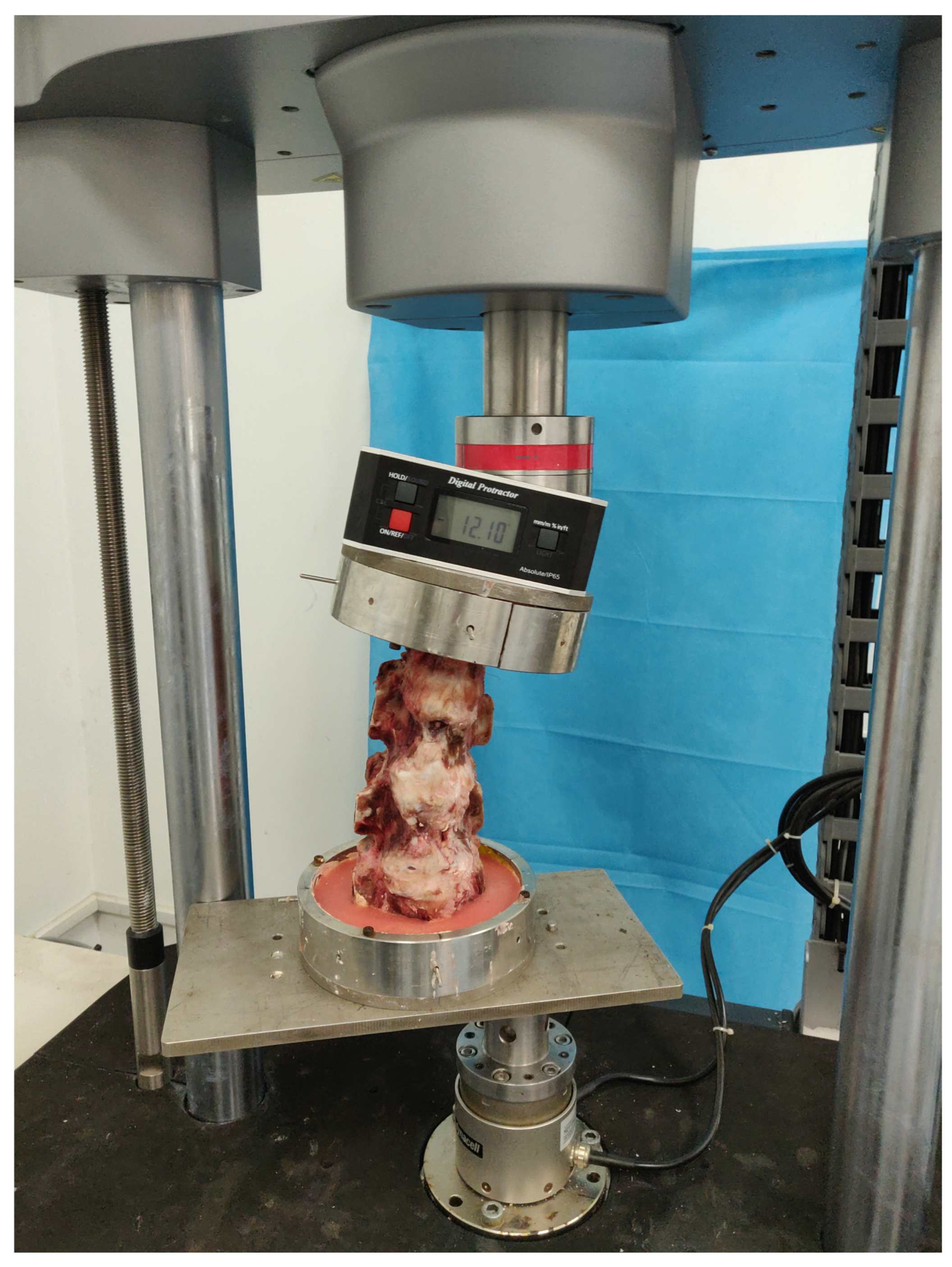
2.4. Model Boundaries and Load Conditions
Calf Spine Model
2.5. Statistical Analysis
3. Results
3.1. Calf Spine Models
3.1.1. L4/5 IMP
3.1.2. ROM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hlaing, S.S.; Puntumetakul, R.; Wanpen, S.; Boucaut, R. Balance Control in Patients with Subacute Non-Specific Low Back Pain, with and without Lumbar Instability: A Cross-Sectional Study. J. Pain Res. 2020, 13, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Tian, W.J.; Liu, H.X.; Guan, P.G. Outcomes of multisegmental transforaminal enlarged decompression plus posterior pedicle screw fixation for multilevel lumbar spinal canal stenosis associated with lumbar instability. Int. J. Surg. 2018, 50, 72–78. [Google Scholar] [CrossRef]
- Gu, G.; Wang, C.; Gu, X.; Zhang, H.; Zhao, Y.; He, S. Percutaneous Transforaminal Endoscopic Discectomy for Adjacent Segment Disease After Lumbar Fusion in Elderly Patients Over 65 Years Old. World Neurosurg. 2018, 112, e830–e836. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, T.; van den Brekel-Dijkstra, K.; Schubert, M.; Miklitz, B. Endoscopic transforaminal discectomy for recurrent lumbar disc herniation: A prospective, cohort evaluation of 262 consecutive cases. Spine 2008, 33, 973–978. [Google Scholar] [CrossRef]
- Li, X.R.; Yu, J.; Zhang, W.; Gao, G.M.; Han, L.; Chen, L.; Nong, L.M. Biomechanical Model Study of the Effect of Partial Facetectomy on Lumbar Stability Under Percutaneous Endoscopy. World Neurosurg. 2020, 139, e255–e264. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Z.; Zhou, R.; Zhu, Q.; Ji, W.; Long, Y.; Wang, J. The Effects of Orientation of Lumbar Facet Joints on the Facet Joint Contact Forces: An In Vitro Biomechanical Study. Spine 2018, 43, E216–E220. [Google Scholar] [CrossRef]
- Zeng, Z.L.; Zhu, R.; Wu, Y.C.; Zuo, W.; Yu, Y.; Wang, J.J.; Cheng, L.M. Effect of Graded Facetectomy on Lumbar Biomechanics. J. Healthc. Eng. 2017, 2017, 7981513. [Google Scholar] [CrossRef] [PubMed]
- Kiapour, A.; Ambati, D.; Hoy, R.W.; Goel, V.K. Effect of graded facetectomy on biomechanics of Dynesys dynamic stabilization system. Spine 2012, 37, E581–E589. [Google Scholar] [CrossRef]
- Bermel, E.A.; Barocas, V.H.; Ellingson, A.M. The role of the facet capsular ligament in providing spinal stability. Comput. Methods Biomech. Biomed. Eng. 2018, 21, 712–721. [Google Scholar] [CrossRef]
- Haher, T.R.; O’Brien, M.; Dryer, J.W.; Nucci, R.; Zipnick, R.; Leone, D.J. The role of the lumbar facet joints in spinal stability. Identification of alternative paths of loading. Spine 1994, 19, 2667–2670; discussion 2671. [Google Scholar] [CrossRef]
- Campos, W.K.; Linhares, M.N.; Sarda, J.; Santos, A.R.S.; Lin, K.; Latini, A.; Walz, R. Predictors of Pain Recurrence After Lumbar Facet Joint Injections. Front. Neurosci. 2019, 13, 958. [Google Scholar] [CrossRef]
- Schmidt, H.; Galbusera, F.; Rohlmann, A.; Zander, T.; Wilke, H.J. Effect of multilevel lumbar disc arthroplasty on spine kinematics and facet joint loads in flexion and extension: A finite element analysis. Eur. Spine J. 2012, 21 (Suppl. S5), 663–674. [Google Scholar] [CrossRef]
- Ebenbichler, G.R.; Leitgeb, J.; Amtmann, G.; Konig, F.; Schernthaner, M.; Resch, K.L.; Kainberger, F. Degeneration and Instability and the Relation to Patients’ Function Late After Lumbar Disc Surgery: Data from a 12-Year Follow-Up. Am. J. Phys. Med. Rehabil. 2016, 95, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Yee, T.J.; Terman, S.W.; La Marca, F.; Park, P. Comparison of adjacent segment disease after minimally invasive or open transforaminal lumbar interbody fusion. J. Clin. Neurosci. 2014, 21, 1796–1801. [Google Scholar] [CrossRef]
- Ahuja, S.; Moideen, A.N.; Dudhniwala, A.G.; Karatsis, E.; Papadakis, L.; Varitis, E. Lumbar stability following graded unilateral and bilateral facetectomy: A finite element model study. Clin. Biomech. 2020, 75, 105011. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Lim, T.J.; Park, J. A biomechanical study of the instrumented and adjacent lumbar levels after In-Space interspinous spacer insertion. J. Neurosurg. Spine 2010, 12, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.J.; Mathes, B.; Midderhoff, S.; Graf, N. Development of a scoliotic spine model for biomechanical in vitro studies. Clin. Biomech. 2015, 30, 182–187. [Google Scholar] [CrossRef]
- Mas, Y.; Gracia, L.; Ibarz, E.; Gabarre, S.; Pena, D.; Herrera, A. Finite element simulation and clinical follow-up of lumbar spine biomechanics with dynamic fixations. PLoS ONE 2017, 12, e0188328. [Google Scholar] [CrossRef]
- Hsieh, Y.Y.; Chen, C.H.; Tsuang, F.Y.; Wu, L.C.; Lin, S.C.; Chiang, C.J. Removal of fixation construct could mitigate adjacent segment stress after lumbosacral fusion: A finite element analysis. Clin. Biomech. 2017, 43, 115–120. [Google Scholar] [CrossRef]
- Tang, S.; Rebholz, B.J. Does lumbar microdiscectomy affect adjacent segmental disc degeneration? A finite element study. J. Surg. Res. 2013, 182, 62–67. [Google Scholar] [CrossRef]
- Adams, M.A.; Freeman, B.J.; Morrison, H.P.; Nelson, I.W.; Dolan, P. Mechanical initiation of intervertebral disc degeneration. Spine 2000, 25, 1625–1636. [Google Scholar] [CrossRef]
- Denteneer, L.; Stassijns, G.; De Hertogh, W.; Truijen, S.; Van Daele, U. Inter- and Intrarater Reliability of Clinical Tests Associated With Functional Lumbar Segmental Instability and Motor Control Impairment in Patients With Low Back Pain: A Systematic Review. Arch. Phys. Med. Rehabil. 2017, 98, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Manni, T.; Bonetti, F.; Villafane, J.H.; Vanti, C. A literature review of clinical tests for lumbar instability in low back pain: Validity and applicability in clinical practice. Chiropr. Man. Therap 2015, 23, 14. [Google Scholar] [CrossRef]
- Hasegawa, K.; Shimoda, H.; Kitahara, K.; Sasaki, K.; Homma, T. What are the reliable radiological indicators of lumbar segmental instability? J. Bone Joint Surg. Br. 2011, 93, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Sairyo, K.; Chikawa, T.; Nagamachi, A. State-of-the-art transforaminal percutaneous endoscopic lumbar surgery under local anesthesia: Discectomy, foraminoplasty, and ventral facetectomy. J. Orthop. Sci. 2018, 23, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Phillips, F.M.; Tzermiadianos, M.N.; Voronov, L.I.; Havey, R.M.; Carandang, G.; Renner, S.M.; Rosler, D.M.; Ochoa, J.A.; Patwardhan, A.G. Effect of the Total Facet Arthroplasty System after complete laminectomy-facetectomy on the biomechanics of implanted and adjacent segments. Spine J. 2009, 9, 96–102. [Google Scholar] [CrossRef]
- Okuda, S.; Miyauchi, A.; Oda, T.; Haku, T.; Yamamoto, T.; Iwasaki, M. Surgical complications of posterior lumbar interbody fusion with total facetectomy in 251 patients. J. Neurosurg. Spine 2006, 4, 304–309. [Google Scholar] [CrossRef]
- Erbulut, D.U. Biomechanical effect of graded facetectomy on asymmetrical finite element model of the lumbar spin. Turk. Neurosurg. 2014, 24, 923–928. [Google Scholar] [CrossRef]
- Qian, J.; Yu, S.S.; Liu, J.J.; Chen, L.; Jing, J.H. Biomechanics changes of lumbar spine caused by foraminotomy via percutaneous transforaminal endoscopic lumbar discectomy. Zhonghua Yi Xue Za Zhi 2018, 98, 1013–1018. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Xu, W.; Xi, Z.; Xie, L. Reducing the extent of facetectomy may decrease morbidity in failed back surgery syndrome. BMC Musculoskelet. Disord. 2019, 20, 369. [Google Scholar] [CrossRef]
- Lee, K.K.; Teo, E.C.; Qiu, T.X.; Yang, K. Effect of facetectomy on lumbar spinal stability under sagittal plane loadings. Spine 2004, 29, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Feng, F.; Chen, Z.; He, L.; Yang, B.; Chen, R.; Wu, W.; Liu, B.; Dong, J.; Shu, T.; et al. Percutaneous transforaminal full endoscopic decompression for the treatment of lumbar spinal stenosis. BMC Musculoskelet. Disord. 2020, 21, 546. [Google Scholar] [CrossRef]
- Wang, Y.B.; Chen, S.L.; Cao, C.; Zhang, K.; Liu, L.M.; Gao, Y.Z. Percutaneous Transforaminal Endoscopic Discectomy and Fenestration Discectomy to Treat Posterior Ring Apophyseal Fractures: A Retrospective Cohort Study. Orthop. Surg. 2020, 12, 1092–1099. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, L.; Dong, J.; Xie, P.; Liu, B.; Wang, Q.; Chen, R.; Feng, F.; Yang, B.; Shu, T.; et al. Percutaneous transforaminal endoscopic discectomy compared with microendoscopic discectomy for lumbar disc herniation: 1-year results of an ongoing randomized controlled trial. J. Neurosurg. Spine 2018, 28, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, T. Percutaneous endoscopic discectomy. J. Neurosurg. 1993, 79, 967–968. [Google Scholar]
- Schubert, M.; Hoogland, T. Endoscopic transforaminal nucleotomy with foraminoplasty for lumbar disk herniation. Oper. Orthop. Traumatol. 2005, 17, 641–661. [Google Scholar] [CrossRef]
- Szkoda-Poliszuk, K.; Zak, M.; Pezowicz, C. Finite element analysis of the influence of three-joint spinal complex on the change of the intervertebral disc bulge and height. Int. J. Numer. Method. Biomed. Eng. 2018, 34, e3107. [Google Scholar] [CrossRef]
- Abbas, J.; Slon, V.; Stein, D.; Peled, N.; Hershkovitz, I.; Hamoud, K. In the quest for degenerative lumbar spinal stenosis etiology: The Schmorl’s nodes model. BMC Musculoskelet. Disord. 2017, 18, 164. [Google Scholar] [CrossRef]
- Li, Z.; Liu, F.; Liu, S.; Chen, Z.; Jiang, C.; Feng, Z.; Jiang, X. Miniopen Transforaminal Lumbar Interbody Fusion with Unilateral Fixation: A Comparison between Ipsilateral and Contralateral Reherniation. Biomed. Res. Int. 2016, 2016, 7261027. [Google Scholar] [CrossRef]
- Zehr, J.D.; Barrett, J.M.; Fewster, K.M.; Laing, A.C.; Callaghan, J.P. Strain of the facet joint capsule during rotation and translation range-of-motion tests: An in vitro porcine model as a human surrogate. Spine J. 2020, 20, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Widmer, J.; Cornaz, F.; Scheibler, G.; Spirig, J.M.; Snedeker, J.G.; Farshad, M. Biomechanical contribution of spinal structures to stability of the lumbar spine-novel biomechanical insights. Spine J. 2020, 20, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
| Grouping | Control | A | B | C | F Value | p Value |
|---|---|---|---|---|---|---|
| Flexion | 572.48 ± 23.38 | 694.91 ± 29.65 | 654.64 ± 15.99 | 729.48 ± 24.22 a | 54.926 | p < 0.001 |
| Extension | 627.53 ± 41.22 | 941.51 ± 39.62 a | 714.05 ± 41.35 | 1000.23 ± 52.32 a | 66.921 | p < 0.001 |
| Left lateral flexion | 556.37 ± 43.42 | 682.89 ± 51.74 | 638.08 ± 33.68 | 715.79 ± 46.29 a | 78.891 | p < 0.001 |
| Right lateral flexion | 578.47 ± 52.12 | 705.20 ± 55.48 | 664.88 ± 43.15 | 738.40 ± 46.53 a | 68.212 | p < 0.001 |
| Left axial rotation | 685.28 ± 37.42 | 794.08 ± 48.26 | 742.27 ± 33.82 | 1123.47 ± 33.58 a | 88.651 | p < 0.001 |
| Right axial rotation | 663.78 ± 46.96 | 786.45 ± 45.92 | 725.11 ± 47.15 | 1047.60 ± 51.62 a | 85.621 | p < 0.001 |
| Grouping | Control | A | B | C | F Value | p Value |
|---|---|---|---|---|---|---|
| Flexion | 10.23 ± 2.09 | 11.44 ± 3.29 | 11.55 ± 2.12 | 12.68 ± 3.11 | 2.052 | p > 0.05 |
| Extension | 7.20 ± 1.77 | 7.85 ± 1.64 | 7.65 ± 0.84 | 8.15 ± 1.14 | 1.986 | p > 0.05 |
| Left lateral flexion | 8.33 ± 1.27 | 8.95 ± 1.19 | 8.90 ± 1.11 | 10.45 ± 1.53 | 1.999 | p > 0.05 |
| Right lateral flexion | 8.85 ± 1.25 | 9.47 ± 1.34 | 8.95 ± 1.18 | 10.15 ± 1.20 | 1.879 | p > 0.05 |
| Left axial rotation | 4.80 ± 0.75 | 5.35 ± 0.68 | 5.20 ± 0.76 | 9.23 ± 1.50 a | 10.598 | p < 0.001 |
| Right axial rotation | 5.85 ± 0.84 | 6.35 ± 0.67 | 6.55 ± 0.75 | 10.15 ± 1.44 a | 12.368 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Tu, X.; Li, J.; Wu, J.; Nong, L. In Vitro Biomechanical Experiment on the Effect of Unilateral Partial Facetectomy Performed by Percutaneous Endoscopy on the Stability of Lumbar Spine. Bioengineering 2025, 12, 414. https://doi.org/10.3390/bioengineering12040414
Ma T, Tu X, Li J, Wu J, Nong L. In Vitro Biomechanical Experiment on the Effect of Unilateral Partial Facetectomy Performed by Percutaneous Endoscopy on the Stability of Lumbar Spine. Bioengineering. 2025; 12(4):414. https://doi.org/10.3390/bioengineering12040414
Chicago/Turabian StyleMa, Tao, Xiaoshuang Tu, Junyang Li, Jingwei Wu, and Luming Nong. 2025. "In Vitro Biomechanical Experiment on the Effect of Unilateral Partial Facetectomy Performed by Percutaneous Endoscopy on the Stability of Lumbar Spine" Bioengineering 12, no. 4: 414. https://doi.org/10.3390/bioengineering12040414
APA StyleMa, T., Tu, X., Li, J., Wu, J., & Nong, L. (2025). In Vitro Biomechanical Experiment on the Effect of Unilateral Partial Facetectomy Performed by Percutaneous Endoscopy on the Stability of Lumbar Spine. Bioengineering, 12(4), 414. https://doi.org/10.3390/bioengineering12040414








