Automatic Blob Detection Method for Cancerous Lesions in Unsupervised Breast Histology Images
Abstract
:1. Introduction
- Augmentation methods are used to deal with data scarcity. Additionally, stain normalization is used to deal with color inconsistencies.
- Morphology operations enhance the image by highlighting important features. The connected components analysis method is used to group components with similar characteristics and assist in separating overlapping and non-overlapping objects.
- The active contours method uses the obtained binary masks from the connected components analysis to highlight and isolate the edges/boundaries of ROIs. Further, the blob detection method is used to resolve undersegmentation from the previous step and identify BC lesions (blobs) from the previously obtained masked images.
2. Related Work
3. Methods and Techniques
3.1. Dataset Preparation and Pre-Processing
3.1.1. Data Augmentation
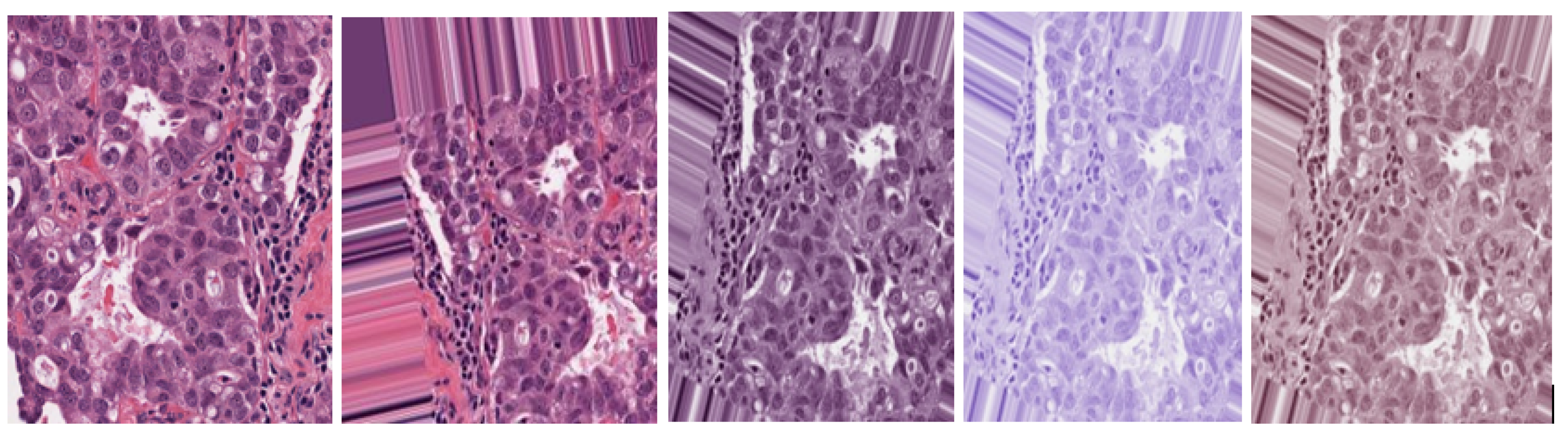
3.1.2. Data Stain Normalization
3.2. Image Enhancement
3.2.1. Thresholding
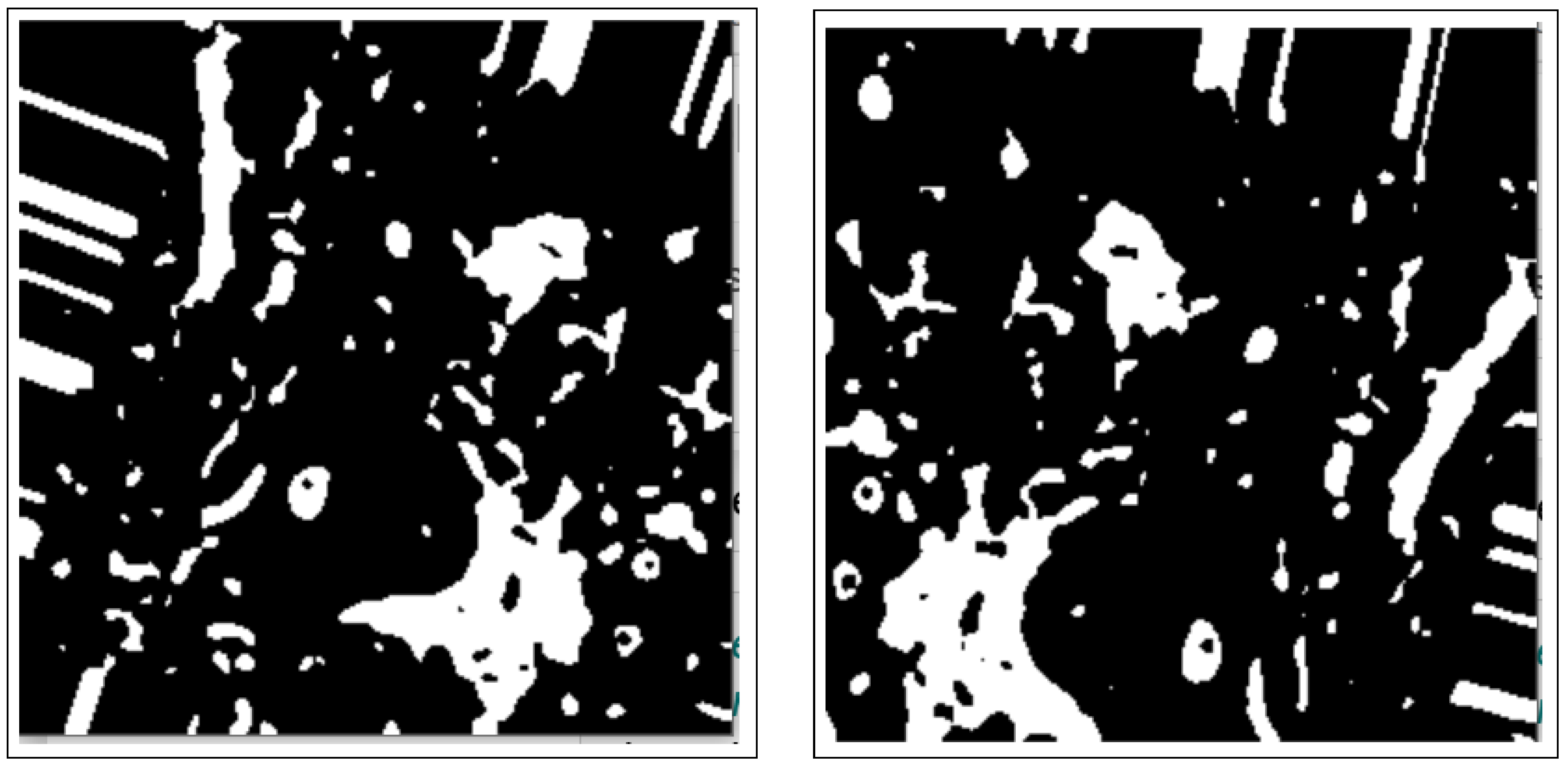
3.2.2. Morphology Operations
3.2.3. Distance Transform
3.3. Segmentation
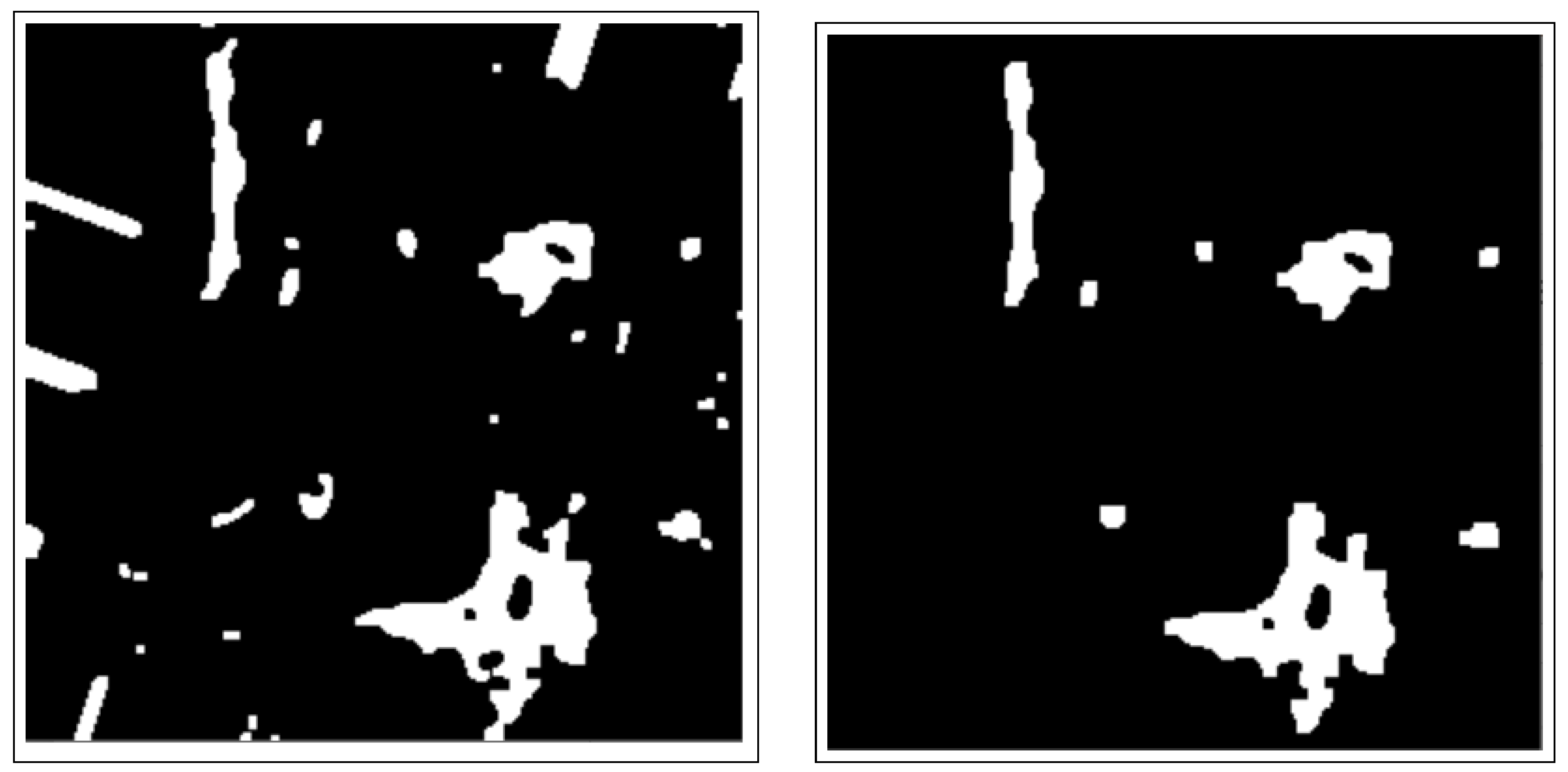
3.3.1. Connected Components Analysis
3.3.2. Active Contours Segmentation
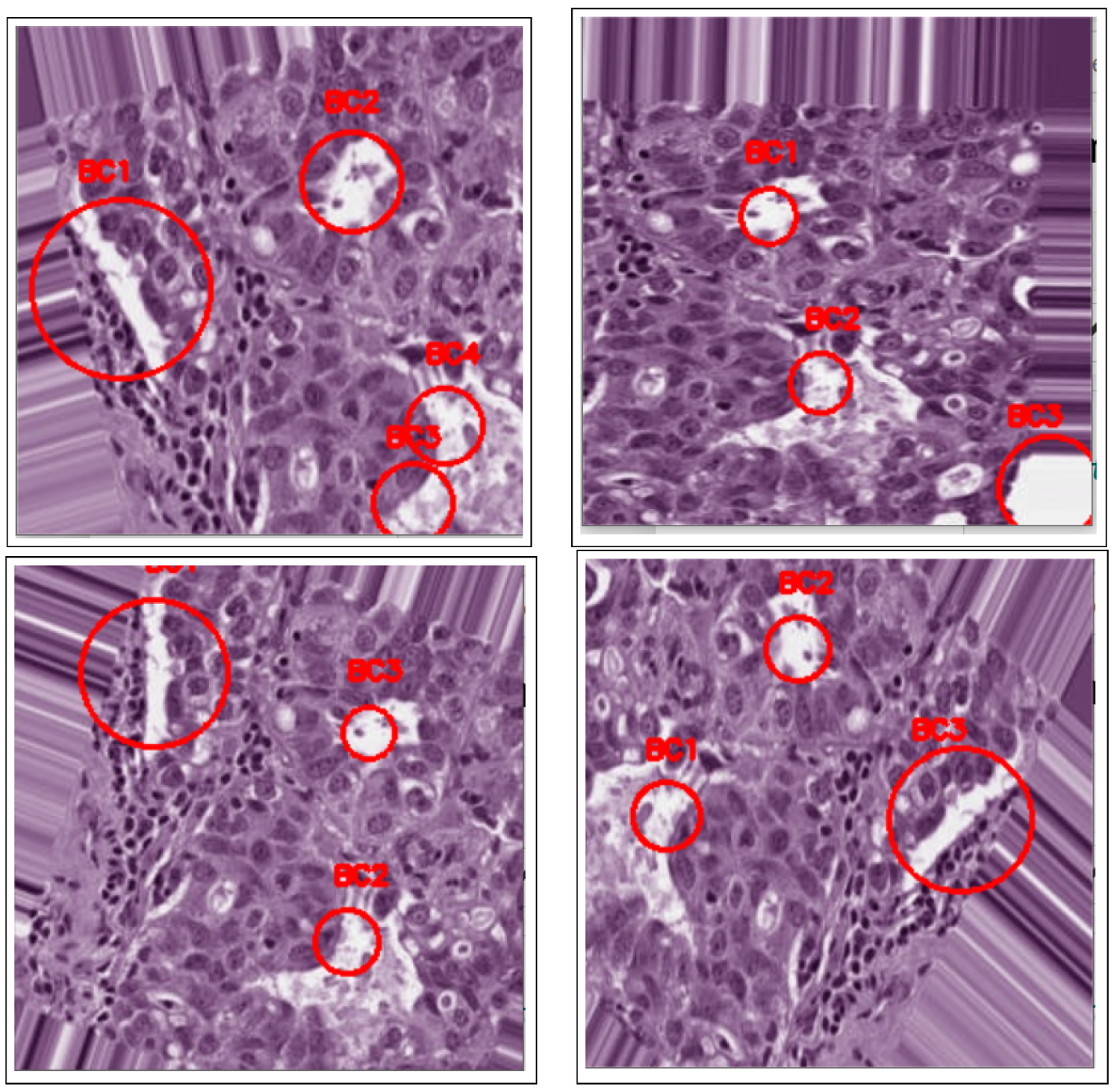
3.4. Detection
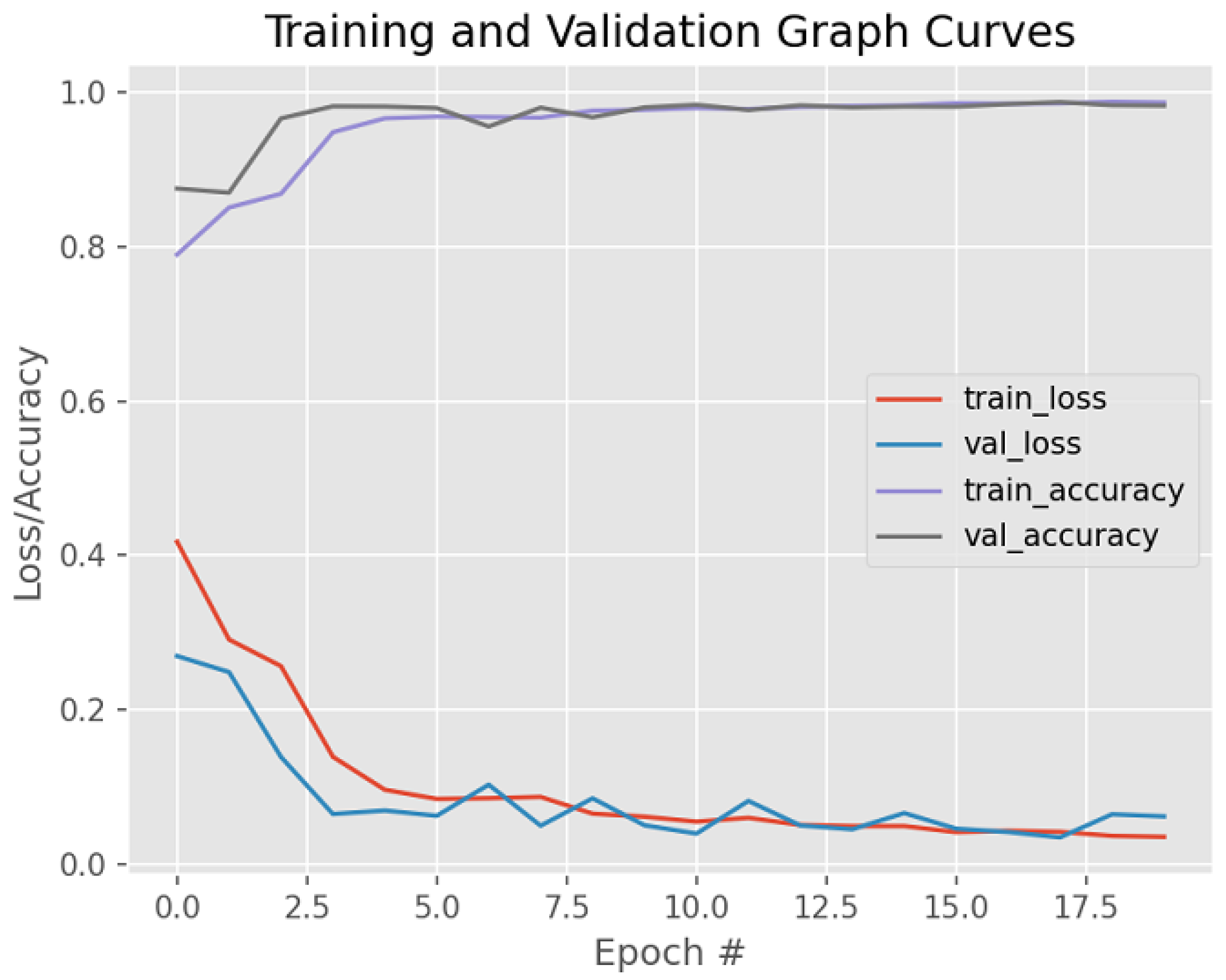
4. Results and Discussion
Limitations
5. Conclusions
6. Future Work
- Data availability and integrity. Most deep learning approaches require huge volumes of data to achieve meaningful performance results. Therefore, publicly available image datasets are necessary, especially histology image datasets, to assist deep learning.
- Regularization methods. These are needed to improve the performance of models. This can be achieved through model hyperparameter tuning, such as optimizing the learning rates, dropout, loss functions, activation functions, and early stopping methods.
- Hybrid image processing/model approaches. Combining various/several image processing methods or model architectures, it would be possible to form a hybrid method that improves the overall evaluation performance. This combination can occur at any step in the model, such as pre-processing, combining various attributes of different models to form one that will enhance the training, extraction, detection, and classification tasks. Additionally, future work could expand, explore, and diagnose other human and animal diseases through image datasets, moving beyond BC histology images.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elston, C.W.; Ellis, I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [PubMed]
- Krithiga, R.; Geetha, P. Breast cancer detection, segmentation and classification on histopathology images analysis: A systematic review. Arch. Comput. Methods Eng. 2021, 28, 2607–2619. [Google Scholar]
- Fox, H. Is H&E morphology coming to an end? J. Clin. Pathol. 2000, 53, 38–40. [Google Scholar] [PubMed]
- Wang, P.; Hu, X.; Li, Y.; Liu, Q.; Zhu, X. Automatic cell nuclei segmentation and classification of breast cancer histopathology images. Signal Process. 2016, 122, 1–13. [Google Scholar]
- Ali, S.; Madabhushi, A. Segmenting multiple overlapping objects via a hybrid active contour model incorporating shape priors: Applications to digital pathology. In Proceedings of the Medical Imaging 2011, Lake Buena Vista, FL, USA, 13–17 February 2011; Volume 7962, pp. 909–921. [Google Scholar]
- Venkataraman, G.; Rycyna, K.; Rabanser, A.; Heinze, G.; Baesens, B.M.; Ananthanarayanan, V.; Paner, G.P.; Barkan, G.A.; Flanigan, R.C.; Wojcik, E.M. Morphometric signature differences in nuclei of Gleason pattern 4 areas in Gleason 7 prostate cancer with differing primary grades on needle biopsy. J. Urol. 2009, 181, 88–94. [Google Scholar]
- Lal, S.; Desouza, R.; Maneesh, M.; Kanfade, A.; Kumar, A.; Perayil, G.; Alabhya, K.; Chanchal, A.K.; Kini, J. A robust method for nuclei segmentation of H&E stained histopathology images. In Proceedings of the 2020 7th International Conference on Signal Processing and Integrated Networks (SPIN), Noida, India, 27–28 February 2020; pp. 453–458. [Google Scholar]
- Kaushal, C.; Singla, A. Automated segmentation technique with self-driven post-processing for histopathological breast cancer images. CAAI Trans. Intell. Technol. 2020, 5, 294–300. [Google Scholar]
- Zebari, D.A.; Zeebaree, D.Q.; Abdulazeez, A.M.; Haron, H.; Hamed, H.N.A. Improved threshold based and trainable fully automated segmentation for breast cancer boundary and pectoral muscle in mammogram images. IEEE Access 2020, 8, 203097–203116. [Google Scholar]
- Kiran, I.; Raza, B.; Ijaz, A.; Khan, M.A. DenseRes-Unet: Segmentation of overlapped/clustered nuclei from multi organ histopathology images. Comput. Biol. Med. 2022, 143, 105267. [Google Scholar]
- Irshad, H.; Veillard, A.; Roux, L.; Racoceanu, D. Methods for nuclei detection, segmentation, and classification in digital histopathology: A review—current status and future potential. IEEE Rev. Biomed. Eng. 2013, 7, 97–114. [Google Scholar]
- Aswathy, M.; Jagannath, M. Detection of breast cancer on digital histopathology images: Present status and future possibilities. Informatics Med. Unlocked 2017, 8, 74–79. [Google Scholar]
- Reshma, V.; Arya, N.; Ahmad, S.S.; Wattar, I.; Mekala, S.; Joshi, S.; Krah, D. Detection of breast cancer using histopathological image classification dataset with deep learning techniques. BioMed Res. Int. 2022, 2022, 8363850. [Google Scholar]
- Xie, J.; Liu, R.; Luttrell, J., IV; Zhang, C. Deep learning based analysis of histopathological images of breast cancer. Front. Genet. 2019, 10, 80. [Google Scholar]
- Gecer, B.; Aksoy, S.; Mercan, E.; Shapiro, L.G.; Weaver, D.L.; Elmore, J.G. Detection and classification of cancer in whole slide breast histopathology images using deep convolutional networks. Pattern Recognit. 2018, 84, 345–356. [Google Scholar] [PubMed]
- Toğaçar, M.; Özkurt, K.B.; Ergen, B.; Cömert, Z. BreastNet: A novel convolutional neural network model through histopathological images for the diagnosis of breast cancer. Phys. A Stat. Mech. Its Appl. 2020, 545, 123592. [Google Scholar]
- Mohanakurup, V.; Parambil Gangadharan, S.M.; Goel, P.; Verma, D.; Alshehri, S.; Kashyap, R.; Malakhil, B. Breast cancer detection on histopathological images using a composite dilated Backbone Network. Comput. Intell. Neurosci. 2022, 2022, 8517706. [Google Scholar]
- Araújo, T.; Aresta, G.; Castro, E.; Rouco, J.; Aguiar, P.; Eloy, C.; Polónia, A.; Campilho, A. Classification of breast cancer histology images using convolutional neural networks. PLoS ONE 2017, 12, e0177544. [Google Scholar]
- Vesal, S.; Ravikumar, N.; Davari, A.; Ellmann, S.; Maier, A. Classification of breast cancer histology images using transfer learning. In Proceedings of the Image Analysis and Recognition: 15th International Conference, ICIAR 2018, Póvoa de Varzim, Portugal, 27–29 June 2018; pp. 812–819. [Google Scholar]
- Feng, Y.; Zhang, L.; Yi, Z. Breast cancer cell nuclei classification in histopathology images using deep neural networks. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 179–191. [Google Scholar]
- Sohail, A.; Khan, A.; Nisar, H.; Tabassum, S.; Zameer, A. Mitotic nuclei analysis in breast cancer histopathology images using deep ensemble classifier. Med. Image Anal. 2021, 72, 102121. [Google Scholar]
- Macenko, M.; Niethammer, M.; Marron, J.S.; Borland, D.; Woosley, J.T.; Guan, X.; Schmitt, C.; Thomas, N.E. A method for normalizing histology slides for quantitative analysis. In Proceedings of the 2009 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Boston, MA, USA, 28 June–1 July 2009; pp. 1107–1110. [Google Scholar]
- Buda, M.; Maki, A.; Mazurowski, M.A. A systematic study of the class imbalance problem in convolutional neural networks. Neural Netw. 2018, 106, 249–259. [Google Scholar]
- Golatkar, A.; Anand, D.; Sethi, A. Classification of breast cancer histology using deep learning. In Proceedings of the Image Analysis and Recognition: 15th International Conference, ICIAR 2018, Póvoa de Varzim, Portugal, 27–29 June 2018; pp. 837–844. [Google Scholar]
- Yu, C.; Chen, H.; Li, Y.; Peng, Y.; Li, J.; Yang, F. Breast cancer classification in pathological images based on hybrid features. Multimed. Tools Appl. 2019, 78, 21325–21345. [Google Scholar]
- Liu, X.; Liu, J.; Feng, Z.; Xu, X.; Tang, J. Mass classification in mammogram with semi-supervised relief based feature selection. In Proceedings of the Fifth International Conference on Graphic and Image Processing (ICGIP 2013), Hong Kong, China, 26–27 October 2013; Volume 9069, pp. 252–256. [Google Scholar]
- George, K.; Faziludeen, S.; Sankaran, P. Breast cancer detection from biopsy images using nucleus guided transfer learning and belief based fusion. Comput. Biol. Med. 2020, 124, 103954. [Google Scholar]
- Lowe, D.G. Distinctive image features from scale-invariant keypoints. Int. J. Comput. Vis. 2004, 60, 91–110. [Google Scholar]
- Sornapudi, S.; Stanley, R.J.; Stoecker, W.V.; Almubarak, H.; Long, R.; Antani, S.; Thoma, G.; Zuna, R.; Frazier, S.R. Deep learning nuclei detection in digitized histology images by superpixels. J. Pathol. Informatics 2018, 9, 5. [Google Scholar]
- Dinh, T.L.; Kwon, S.G.; Lee, S.H.; Kwon, K.R. Breast tumor cell nuclei segmentation in histopathology images using efficientunet++ and multi-organ transfer learning. J. Korea Multimed. Soc. 2021, 24, 1000–1011. [Google Scholar]
- Sandler, M.; Howard, A.; Zhu, M.; Zhmoginov, A.; Chen, L.C. Mobilenetv2: Inverted residuals and linear bottlenecks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–22 June 2018; pp. 4510–4520. [Google Scholar]
- Rashmi, R.; Prasad, K.; Udupa, C.B.K. Breast histopathological image analysis using image processing techniques for diagnostic purposes: A methodological review. J. Med. Syst. 2022, 46, 7. [Google Scholar]
- Veta, M.; Huisman, A.; Viergever, M.A.; van Diest, P.J.; Pluim, J.P. Marker-controlled watershed segmentation of nuclei in H&E stained breast cancer biopsy images. In Proceedings of the 2011 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Chicago, IL, USA, 30 March–2 April 2011; pp. 618–621. [Google Scholar]
- Natarajan, V.A.; Kumar, M.S.; Patan, R.; Kallam, S.; Mohamed, M.Y.N. Segmentation of nuclei in histopathology images using fully convolutional deep neural architecture. In Proceedings of the 2020 International Conference on Computing and Information Technology (ICCIT-1441), Tabuk, Saudi Arabia, 9–10 September 2020; pp. 1–7. [Google Scholar]
- Guatemala-Sanchez, V.R.; Peregrina-Barreto, H.; Lopez-Armas, G. Nuclei segmentation on histopathology images of breast carcinoma. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Mexico, 1–5 November 2021; pp. 2622–2628. [Google Scholar]
- Xie, L.; Qi, J.; Pan, L.; Wali, S. Integrating deep convolutional neural networks with marker-controlled watershed for overlapping nuclei segmentation in histopathology images. Neurocomputing 2020, 376, 166–179. [Google Scholar]
- Mahanta, L.B.; Hussain, E.; Das, N.; Kakoti, L.; Chowdhury, M. IHC-Net: A fully convolutional neural network for automated nuclear segmentation and ensemble classification for Allred scoring in breast pathology. Appl. Soft Comput. 2021, 103, 107136. [Google Scholar]
- Niaz, A.; Memon, A.A.; Rana, K.; Joshi, A.; Soomro, S.; Kang, J.S.; Choi, K.N. Inhomogeneous image segmentation using hybrid active contours model with application to breast tumor detection. IEEE Access 2020, 8, 186851–186861. [Google Scholar]
- Kaladevi, P.; Kanimozhi, N.; Nirmala, B.; Sivasankari, R. Morpho-contour exponential estimation algorithm for predicting breast tumor growth from MRI imagery. Int. J. Inf. Technol. 2024, 1–16. [Google Scholar]
- Xu, Y.; Wu, T.; Gao, F.; Charlton, J.R.; Bennett, K.M. Improved small blob detection in 3D images using jointly constrained deep learning and Hessian analysis. Sci. Rep. 2020, 10, 326. [Google Scholar]
- Majanga, V.; Viriri, S. Automatic blob detection for dental caries. Appl. Sci. 2021, 11, 9232. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, F.; Wu, T.; Bennett, K.M.; Charlton, J.R.; Sarkar, S. U-net with optimal thresholding for small blob detection in medical images. In Proceedings of the 2019 IEEE 15th International Conference on Automation Science and Engineering (CASE), Vancouver, BC, Canada, 22–26 August 2019; pp. 1761–1767. [Google Scholar]
- Ingle, S.; Vidhate, A.; Chaudhari, S. Automatic pectoral muscles and artefacts removal in mammogram images for improved breast cancer diagnosis. Int. J. Bioinform. Res. Appl. 2024, 20, 627–647. [Google Scholar] [CrossRef]
- Kumar, T.S.; Sridhar, G.; Manju, D.; Subhash, P.; Nagaraju, G. Breast Cancer Classification and Predicting Class Labels Using ResNet50. J. Electr. Syst. 2023, 19. [Google Scholar] [CrossRef]
- Majanga, V.; Viriri, S. Dental images’ segmentation using threshold connected component analysis. Comput. Intell. Neurosci. 2021, 2021, 2921508. [Google Scholar] [CrossRef]
- Chlap, P.; Min, H.; Vandenberg, N.; Dowling, J.; Holloway, L.; Haworth, A. A review of medical image data augmentation techniques for deep learning applications. J. Med. Imaging Radiat. Oncol. 2021, 65, 545–563. [Google Scholar]
- Goceri, E. Medical image data augmentation: Techniques, comparisons and interpretations. Artif. Intell. Rev. 2023, 56, 12561–12605. [Google Scholar]
- Hussain, Z.; Gimenez, F.; Yi, D.; Rubin, D. Differential data augmentation techniques for medical imaging classification tasks. In Proceedings of the AMIA Annual Symposium Proceedings, Washington, DC, USA, 4–8 November 2017; American Medical Informatics Association: Bethesda, MD, USA, 2017; Volume 2017, p. 979. [Google Scholar]
- Garcea, F.; Serra, A.; Lamberti, F.; Morra, L. Data augmentation for medical imaging: A systematic literature review. Comput. Biol. Med. 2023, 152, 106391. [Google Scholar]
- Hoque, M.Z.; Keskinarkaus, A.; Nyberg, P.; Seppänen, T. Stain normalization methods for histopathology image analysis: A comprehensive review and experimental comparison. Inf. Fusion 2024, 102, 101997. [Google Scholar]
- Veta, M.; Pluim, J.P.; Van Diest, P.J.; Viergever, M.A. Breast cancer histopathology image analysis: A review. IEEE Trans. Biomed. Eng. 2014, 61, 1400–1411. [Google Scholar]
- Elmoataz, A.; Schüpp, S.; Clouard, R.; Herlin, P.; Bloyet, D. Using active contours and mathematical morphology tools for quantification of immunohistochemical images. Signal Process. 1998, 71, 215–226. [Google Scholar]
- Osher, S.; Sethian, J.A. Fronts propagating with curvature-dependent speed: Algorithms based on Hamilton-Jacobi formulations. J. Comput. Phys. 1988, 79, 12–49. [Google Scholar] [CrossRef]
- Sethian, J.A. Level Set Methods: Evolving Interfaces in Geometry, Fluid Mechanics, Computer Vision, and Materials Science; Cambridge Monographs on Applied and Computational Mathematics; Cambridge University Press: Cambridge, UK, 1996; Volume 3. [Google Scholar]
- Kichenassamy, S.; Kumar, A.; Olver, P.; Tannenbaum, A.; Yezzi, A. Gradient flows and geometric active contour models. In Proceedings of the IEEE International Conference on Computer Vision, Cambridge, MA, USA, 20–23 June 1995; pp. 810–815. [Google Scholar]
- Caselles, V.; Kimmel, R.; Sapiro, G. Geodesic active contours. Int. J. Comput. Vis. 1997, 22, 61–79. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Hjort, N.L. Goodness-of-fit processes for logistic regression: Simulation results. Stat. Med. 2002, 21, 2723–2738. [Google Scholar] [CrossRef] [PubMed]






| Authors | Type of Image | Pre-Processing/Image Enhancement Methods | Segmentation Method | Detection Method | Accuracy |
|---|---|---|---|---|---|
| Reshma et al. [13] | Unsupervised images | Median filters, top- and bottom-hat filtering, grayscaling | Fourier transform | Speeded Up Robust Features (SURF) method | 85.17% |
| Kiran et al. [10] | Unsupervised images | Color deconvolution, data augmentation | Binary thresholding, marker-controlled watershed algorithm | Dense Res-U-Net model | 90.03% |
| Araujo et al. [18] | Supervised images | Macenko normalization, augmentation | CNN | Support vector machine (SVM) | 95.6% |
| Isohail et al. [21] | Unsupervised images | Macenko normalization, mean standard deviation-based normalization | Masked R-CNN | Deep High Ensemble Mitotic Classifier (DHE-Mit-Classifier) | 77% |
| Yu et al. [25] | Unsupervised images | Color deconvolution | Speeded Up Robust Features (SURF), gray-level co-occurrence matrix (GLCM), and local binary patterns (LBP) | Support vector machine (SVM) | 96.7% |
| George et al. [27] | Supervised images | Macenko normalization | Laplacian of Gaussian (LoG)–blob detection algorithm | CNN (transfer learning) | 96.3% |
| Sornapudi et al. [29] | Unsupervised images | Gaussian and median filters, linear transformation | Simple linear iterative clustering (SLIC) super-pixel algorithm | CNN (transfer learning) | 95.70% |
| Veta et al. [33] | Unsupervised images | Color deconvolution, opening and closing morphology operations | Fast radial symmetry transform | Marker-controlled watershed | 81.5% |
| Natarajan et al. [34] | Unsupervised images | Color and illumination normalization | LinkNet encoder–decoder architecture | LinkNet + Freeman chain coding (post-processing) | 97.2% |
| Niaz [38] | Unsupervised images | None | Chan–Vese (CV) method, local binary fitting (LBF) method, local image fitting (LIF) method, variational level set with bias correction (VLSBC) method | Weighted length regularization by place (WLRP) method | 98.39% |
| Xu et al. [40] | Unsupervised glomerulus images | Difference of Gradient (DoG), Hessian analysis | U-Net probability map | Hessian convexity map + U-Net probability map (blob detection) | 96.3% |
| Majanga et al. [41] | Unsupervised dental images | Grayscaling, Gaussian blurring | Thresholding, erosion, dilation morphology, connected components analysis | Active contours, blob detection + convexity thresholding | 97.0% |
| Proposed Method | Unsupervised breast histology images | Augmentation, Macenko normalization, binary thresholding, erosion, dilation, opening and closing morphology operations, distance transformation | Connected components analysis (CCA) method, active contours (AC) method | Blob detection on (CCA+AC) outputs | 98.82% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majanga, V.; Mnkandla, E.; Wang, Z.; Moulla, D.K. Automatic Blob Detection Method for Cancerous Lesions in Unsupervised Breast Histology Images. Bioengineering 2025, 12, 364. https://doi.org/10.3390/bioengineering12040364
Majanga V, Mnkandla E, Wang Z, Moulla DK. Automatic Blob Detection Method for Cancerous Lesions in Unsupervised Breast Histology Images. Bioengineering. 2025; 12(4):364. https://doi.org/10.3390/bioengineering12040364
Chicago/Turabian StyleMajanga, Vincent, Ernest Mnkandla, Zenghui Wang, and Donatien Koulla Moulla. 2025. "Automatic Blob Detection Method for Cancerous Lesions in Unsupervised Breast Histology Images" Bioengineering 12, no. 4: 364. https://doi.org/10.3390/bioengineering12040364
APA StyleMajanga, V., Mnkandla, E., Wang, Z., & Moulla, D. K. (2025). Automatic Blob Detection Method for Cancerous Lesions in Unsupervised Breast Histology Images. Bioengineering, 12(4), 364. https://doi.org/10.3390/bioengineering12040364







