Evaluation of Peri-Implant Bone Repair in Ovariectomized Rats Submitted to the Implant Placement Functionalized with Anti-Sclerostin
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics and Calculation of Sample Size
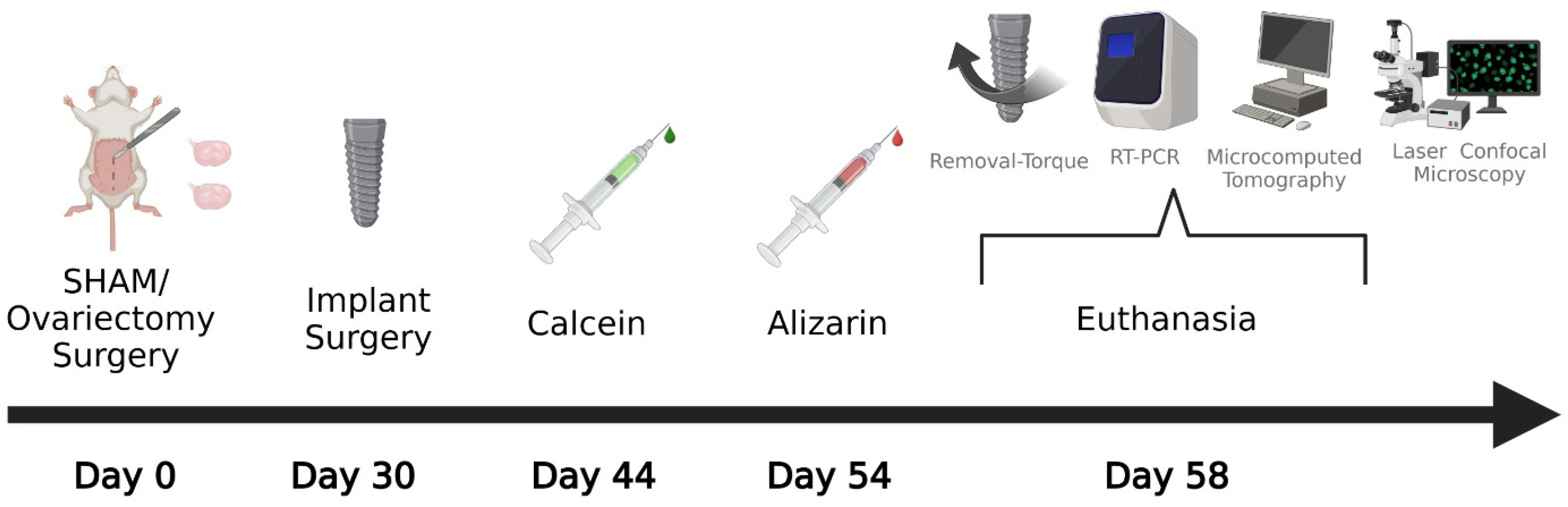
2.2. Experimental Design
2.3. Estrous Cycle
2.4. Ovariectomy
2.5. Functionalization of the Implants
2.6. Implant Surgery
2.7. Euthanasia
2.8. Biomechanical Analysis (Removal Torque)
2.9. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.10. Microcomputed Tomography (Micro-CT)
2.11. Laser Confocal Microscopy
2.12. Statistical Analysis
3. Results
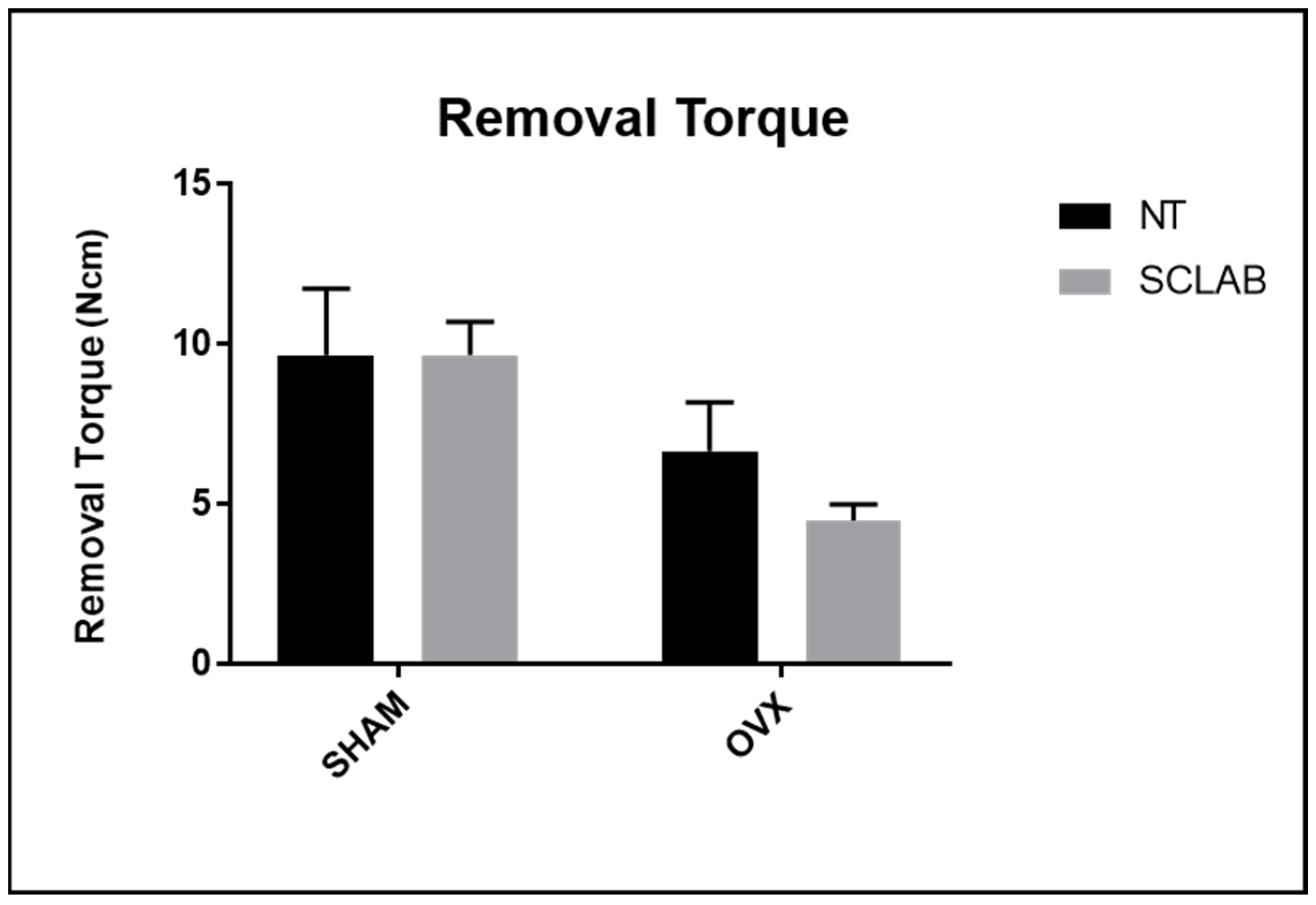
3.1. Removal Torque
3.2. RT-PCR
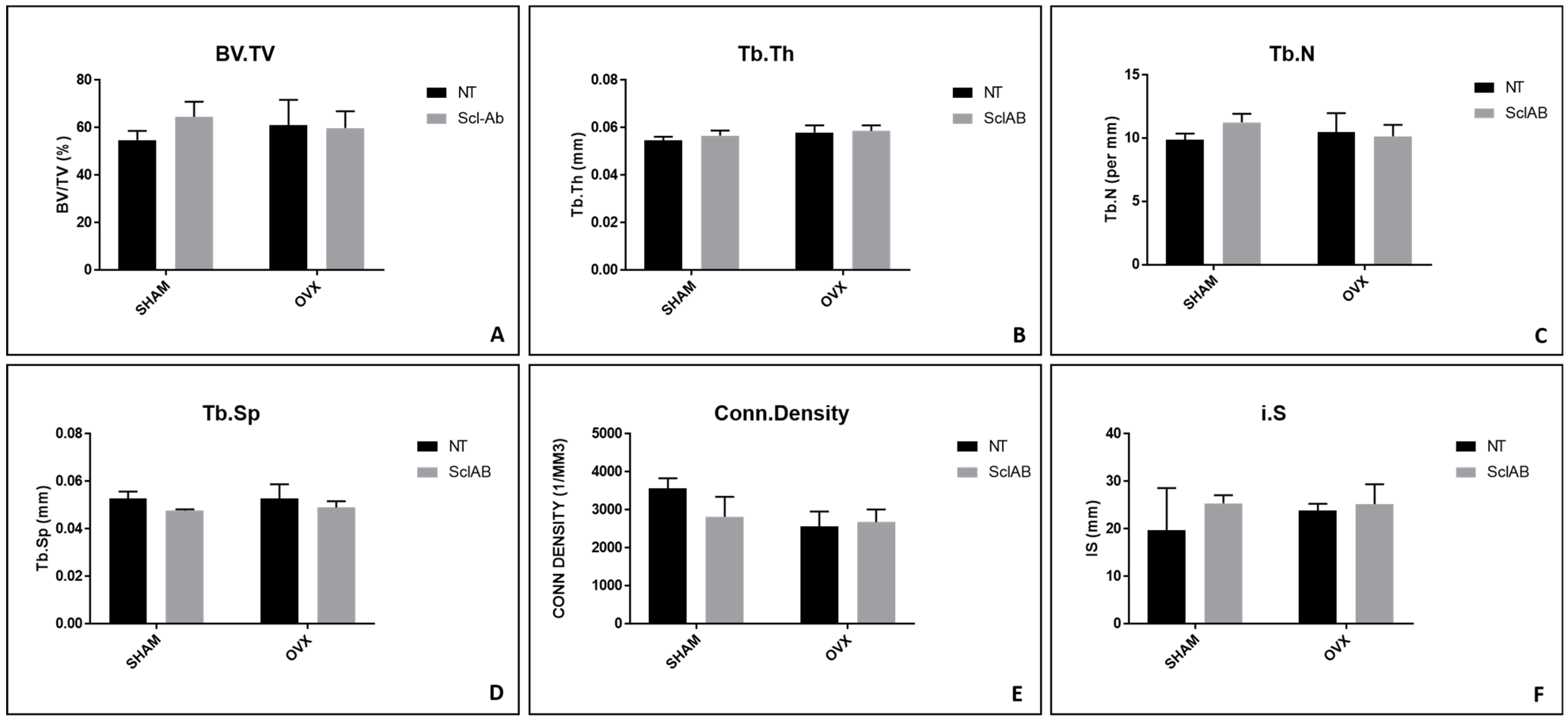
3.3. Micro-CT
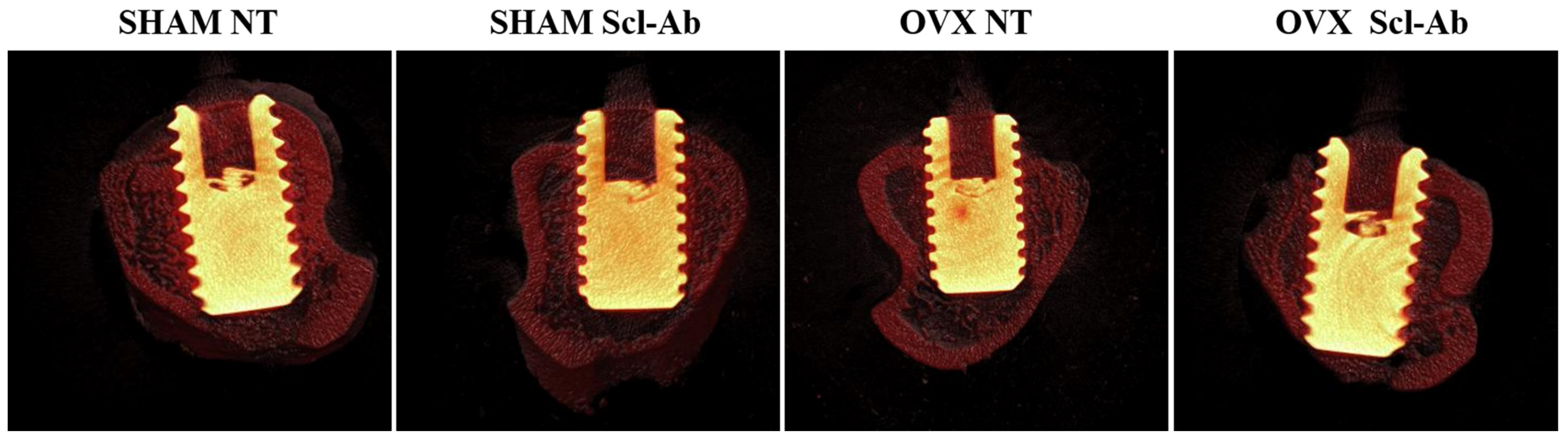
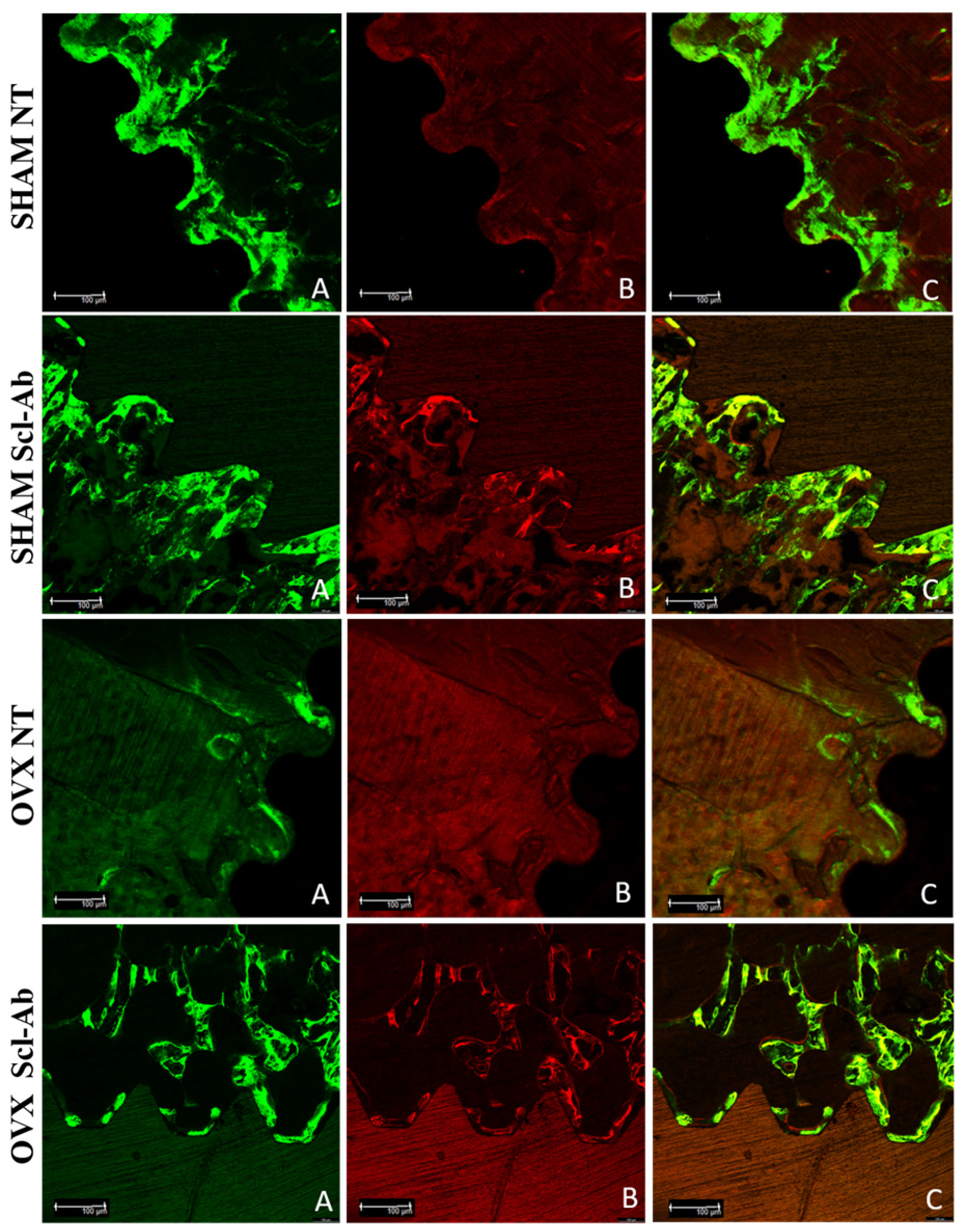
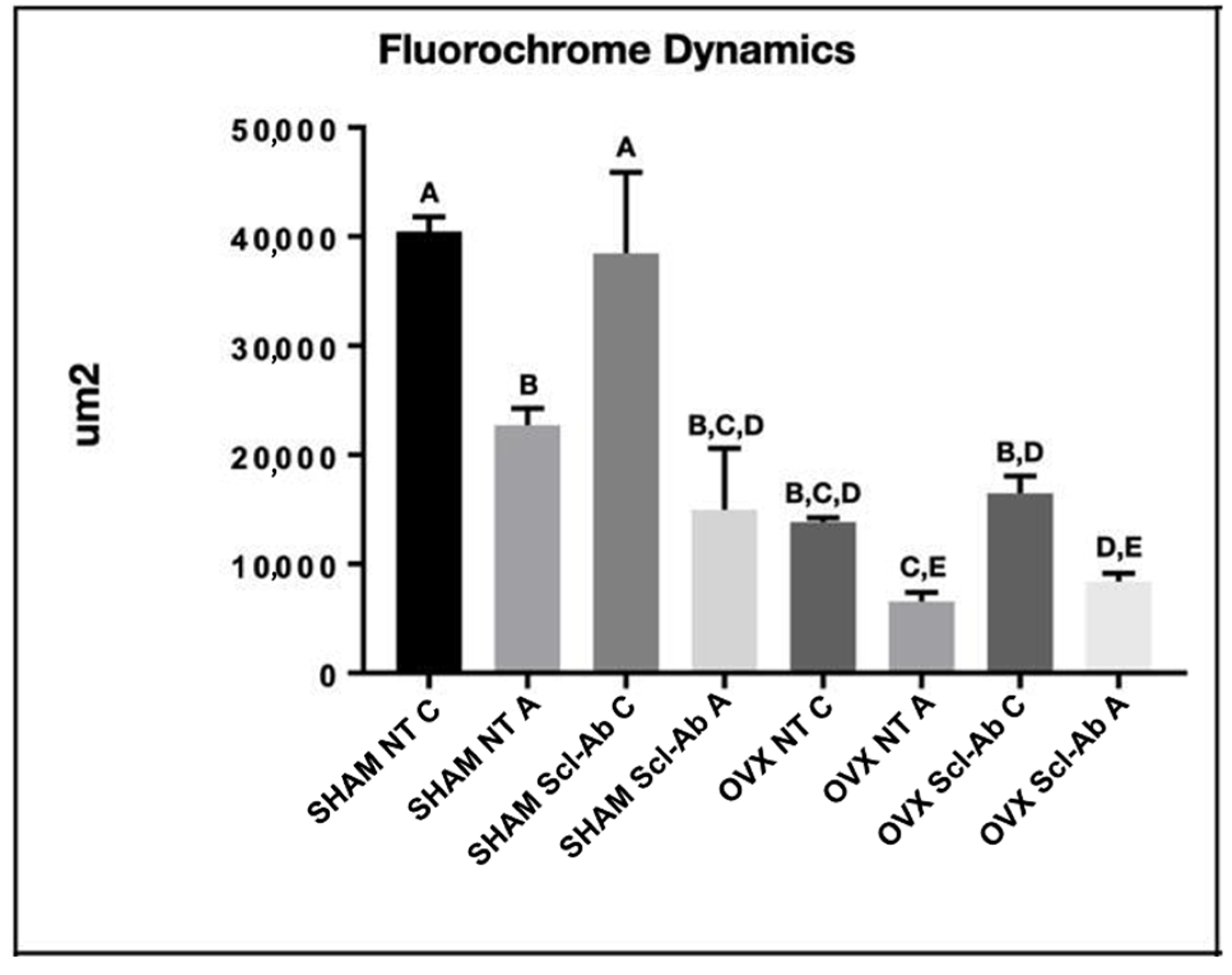
3.4. Laser Confocal Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amin, U.; McPartland, A.; O’Sullivan, M.; Silke, C. An overview of the management of osteoporosis in the aging female population. Women’s Health 2023, 19, 17455057231176655. [Google Scholar] [CrossRef] [PubMed]
- Klein-Nulend, J.; van Oers, R.F.; Bakker, A.D.; Bacabac, R.G. Bone cell mechanosensitivity, estrogen deficiency, and osteoporosis. J. Biomech. 2015, 48, 855–865. [Google Scholar]
- Duarte, N.D.; Mulinari-Santos, G.; Batista, F.R.S.; Gomes, M.B.; Monteiro, N.G.; Silva, A.C.E.D.; Gruber, R.; Lisboa-Filho, P.N.; Gomes-Ferreira, P.H.S.; Okamoto, R. Sonification of Deproteinized Bovine Bone Functionalized with Genistein Enhances Bone Repair in Peri-Implant Bone Defects in Ovariectomized Rats. J. Funct. Biomater. 2024, 15, 328. [Google Scholar] [CrossRef] [PubMed]
- Tella, S.H.; Gallagher, J.C. Prevention and treatment of postmenopausal osteoporosis. J. Steroid Biochem. Mol. Biol. 2014, 142, 155–170. [Google Scholar]
- Kostenuik, P.J.; Nguyen, H.Q.; McCabe, J.; Warmington, K.S.; Kurahara, C.; Sun, N.; Chen, C.; Li, L.; Cattley, R.C.; Van, G.; et al. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J. Bone Miner. Res. 2009, 24, 182–195. [Google Scholar]
- Neer, R.M.; Arnaud, C.D.; Zanchetta, J.R.; Prince, R.; Gaich, G.A.; Reginster, J.Y.; Hodsman, A.B.; Eriksen, E.F.; Ish-Shalom, S.; Genant, H.K.; et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 2001, 344, 1434–1441. [Google Scholar] [PubMed]
- Singh, S.; Dutta, S.; Khasbage, S.; Kumar, T.; Sachin, J.; Sharma, J.; Varthya, S.B. A systematic review and meta-analysis of efficacy and safety of Romosozumab in postmenopausal osteoporosis. Osteoporos. Int. 2022, 33, 1–12. [Google Scholar]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw: 2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar]
- Anastasilakis, A.D.; Pepe, J.; Napoli, N.; Palermo, A.; Magopoulos, C.; Khan, A.A.; Zillikens, M.C.; Body, J.J. Osteonecrosis of the Jaw and Antiresorptive Agents in Benign and Malignant Diseases: A Critical Review Organized by the ECTS. J. Clin. Endocrinol. Metab. 2022, 107, 1441–1460. [Google Scholar] [CrossRef]
- Fabre, S.; Funck-Brentano, T.; Cohen-Solal, M. Anti-Sclerostin Antibodies in Osteoporosis and Other Bone Diseases. J. Clin. Med. 2020, 9, 3439. [Google Scholar] [CrossRef]
- McClung, M.R.; Grauer, A.; Boonen, S.; Bolognese, M.A.; Brown, J.P.; Diez-Perez, A.; Langdahl, B.L.; Reginster, J.Y.; Zanchetta, J.R.; Wasserman, S.M.; et al. Romosozumab in Postmenopausal Women with Low Bone Mineral Density. N. Engl. J. Med. 2014, 370, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Shibli, J.A.; Naddeo, V.; Cotrim, K.C.; Kalil, E.C.; de Avila, E.D.; Faot, F.; Faverani, L.P.; Souza, J.G.S.; Fernandes, J.C.H.; Fernandes, G.V.O. Osteoporosis’ effects on dental implants osseointegration and survival rate: A systematic review of clinical studies. Quintessence Int. 2025, 56, 206–216. [Google Scholar]
- de Medeiros, F.C.F.L.; Kudo, G.A.H.; Leme, B.G.; Saraiva, P.P.; Verri, F.R.; Honório, H.M.; Pellizzer, E.P.; Santiago Junior, J.F. Dental implants in patients with osteoporosis: A systematic review with meta-analysis. Int. J. Oral Maxillofac. Surg. 2018, 47, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.; Schuder, K.; Hof, M.; Heuberer, S.; Seemann, R.; Dvorak, G. Does osteoporosis influence the marginal peri-implant bone level in female patients? A cross-sectional study in a matched collective. Clin. Implant Dent. Relat. Res. 2017, 19, 616–623. [Google Scholar] [CrossRef]
- Inoue, B.K.N.; Paludetto, L.V.; Monteiro, N.G.; Batista, F.R.d.S.; Kitagawa, I.L.; da Silva, R.S.; Antoniali, C.; Lisboa Filho, P.N.; Okamoto, R. Synergic Action of Systemic Risedronate and Local Rutherpy in Peri-implantar Repair of Ovariectomized Rats: Biomechanical and Molecular Analysis. Int. J. Mol. Sci. 2023, 24, 16153. [Google Scholar] [CrossRef]
- de Oliveira, D.; de Oliveira Puttini, I.; Silva Gomes-Ferreira, P.H.; Palin, L.P.; Matsumoto, M.A.; Okamoto, R. Effect of intermittent teriparatide (PTH 1-34) on the alveolar healing process in orchiectomized rats. Clin. Oral Investig. 2019, 23, 2313–2322. [Google Scholar] [CrossRef]
- de Oliveira Puttini, I.; Gomes-Ferreira, P.H.D.S.; de Oliveira, D.; Hassumi, J.S.; Gonçalves, P.Z.; Okamoto, R. Teriparatide improves alveolar bone modeling after tooth extraction in orchiectomized rats. Arch. Oral Biol. 2019, 102, 147–154. [Google Scholar] [CrossRef]
- Gomes-Ferreira, P.H.S.; de Oliveira, D.; Frigério, P.B.; de Souza Batista, F.R.; Grandfield, K.; Okamoto, R. Teriparatide improves microarchitectural characteristics of peri-implant bone in orchiectomized rats. Osteoporos. Int. 2020, 31, 1807–1815. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLOS Biol. 2020, 18, e3000410. [Google Scholar]
- Long, J.A.; Evans, H.M. The Oestrus Cycle in the Rat and Its Related Phenomena, 1st ed.; University of California Press: Berkeley, CA, USA, 1922. [Google Scholar]
- Pan, H.; Han, J.J.; Park, Y.D.; Cho, T.H.; Hwang, S.J. Effect of sustained release of rhBMP-2 from dried and wet hyaluronic acid hydrogel carriers compared with direct dip coating of rhBMP-2 on peri-implant osteogenesis of dental implants in canine mandibles. J. Craniomaxillofac. Surg. 2016, 44, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Mulinari-Santos, G.; de Souza Batista, F.R.; Kirchweger, F.; Tangl, S.; Gruber, R.; Okamoto, R. Losartan reverses impaired osseointegration in spontaneously hypertensive rats. Clin. Oral Implants Res. 2018, 29, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Frigério, P.B.; de Moura, J.; Pitol-Palin, L.; Monteiro, N.G.; Mourão, C.F.; Shibli, J.A.; Okamoto, R. Combination of a Synthetic Bioceramic Associated with a Polydioxanone-Based Membrane as an Alternative to Autogenous Bone Grafting. Biomimetics 2024, 9, 284. [Google Scholar] [CrossRef]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2012, 28, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.; Cardaropoli, G.; Suzuki, M.; Lemons, J.E. Histomorphometric evaluation of a nano thickness bioceramic deposition on endosseous implants: A study in dogs. Clin. Implant Dent. Relat. Res. 2009, 11, 292–302. [Google Scholar] [CrossRef]
- Mello, A.; Hong, Z.; Rossi, A.M.; Luan, L.; Farina, M.; Querido, W.; Eon, J.; Terra, J.; Balasundaram, G.; Webster, T.; et al. Osteoblast proliferation on hydroxyapatite thin coatings produced by right angle magnetron sputtering. Biomed. Mater. 2007, 2, 67–77. [Google Scholar] [CrossRef]
- Misch, C.E. Density of bone: Effect on treatment plans, surgical approach, healing, and progressive bone loading. Int. J. Oral Implantol. 1990, 6, 23–31. [Google Scholar]
- Murray-Curtis, L.; Grusovin, M.G.; Coulthard, P.; Worthington, H.V. Interventions for replacing missing teeth: Different types of dental implants. Cochrane Database Syst. Rev. 2007, 17, CD003815. [Google Scholar]
- de Oliveira Fernandes, G.V.; Malta Santos, N.B.; Correia de Sousa, M.F.; Fernandes, J.C.H. Liquid Platelet-Rich Fibrin Coating Implant Surface to Enhance Osseointegration: A Double-Blinded, Randomized Split-Mouth Trial with 1-Year Follow-up. Int. J. Oral Maxillofac. Implants. 2022, 37, 159–170. [Google Scholar] [CrossRef]
- Bonato, R.S.; Fernandes, G.V.O.; Calasans-Maia, M.D.; Mello, A.; Rossi, A.M.; Carreira, A.C.O.; Sogayar, M.C.; Granjeiro, J.M. The Influence of rhBMP-7 Associated with Nanometric Hydroxyapatite Coatings Titanium Implant on the Osseointegration: A Pre-Clinical Study. Polymers 2022, 14, 4030. [Google Scholar] [CrossRef] [PubMed]
- Marini, F.; Giusti, F.; Palmini, G.; Brandi, M.L. Role of Wnt signaling and sclerostin in bone and as therapeutic targets in skeletal disorders. Osteoporos. Int. 2023, 34, 213–238. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Nie, F.; Zhang, W.; Cui, Q.; Fu, Y.; Li, H.; Zhang, J. Kaempferol promotes proliferation and osteogenic differentiation of periodontal ligament stem cells via Wnt/β-catenin signaling pathway. Life Sci. 2020, 258, 118143. [Google Scholar] [PubMed]
- Cui, X.; Zhang, Y.; Wang, J.; Huang, C.; Wang, Y.; Yang, H.; Liu, W.; Wang, T.; Wang, D.; Wang, G.; et al. Strontium modulates osteogenic activity of bone cement composed of bioactive borosilicate glass particles by activating Wnt/β-catenin signaling pathway. Bioact. Mater. 2021, 6, 2643–2645. [Google Scholar]
- Hu, L.; Chen, W.; Qian, A.; Li, Y.P. Wnt/β-catenin signaling components and mechanisms in bone formation, homeostasis, and disease. Bone Res. 2024, 12, 39. [Google Scholar]
- Virdi, A.S.; Liu, M.; Sena, K.; Maletich, J.; McNulty, M.; Ke, H.Z.; Sumner, D.R. Sclerostin antibody increases bone volume and enhances implant fixation in a rat model. J. Bone Joint. Surg. Am. 2012, 94, 1670–1680. [Google Scholar]
- Yu, S.H.; Hao, J.; Fretwurst, T.; Liu, M.; Kostenuik, P.; Giannobile, W.V.; Jin, Q. Sclerostin-Neutralizing Antibody Enhances Bone Regeneration Around Oral Implants. Tissue Eng. Part A 2018, 24, 1672–1679. [Google Scholar] [CrossRef]
- Zamai, R.S.; Corrêa, M.G.; Ribeiro, F.V.; Cirano, F.R.; Casati, M.Z.; Messora, M.R.; Pimentel, S.P. Does resveratrol favor peri-implant bone repair in rats with ovariectomy-induced osteoporosis? Gene expression, counter-torque and micro-CT analysis. Braz. Oral Res. 2023, 37, e003. [Google Scholar]
- Yao, Y.; Kauffmann, F.; Maekawa, S.; Sarment, L.V.; Sugai, J.V.; Schmiedeler, C.A.; Doherty, E.J.; Holdsworth, G.; Kostenuik, P.J.; Giannobile, W.V. Sclerostin antibody stimulates periodontal regeneration in large alveolar bone defects. Sci. Rep. 2020, 10, 16217. [Google Scholar] [CrossRef]
- Maeng, L.Y.; Cover, K.K.; Landau, A.J.; Milad, M.R.; Lebron-Milad, K. Protocol for studying extinction of conditioned fear in naturally cycling female rats. J. Vis. Exp. 2015, 96, 52202. [Google Scholar]
- Mardas, N.; Busetti, J.; de Figueiredo, J.A.; Mezzomo, L.A.; Scarparo, R.K.; Donos, N. Guided bone regeneration in osteoporotic conditions following treatment with zoledronic acid. Clin. Oral Implant. Res. 2017, 28, 362–371. [Google Scholar]


| Gene | Gene Reference | Gene Code | Forward Primer, 5′→3′ | Reverse Primer, 5′→3′ |
|---|---|---|---|---|
| OPG | NM_057149.2 | Rn00563499_m1 | GCACTCCTGGTGTTCTTGGA | TTTGGTCCCAGGCAAACTGT |
| RANKL | NM_057149.1 | Rn00589289_m1 | CGAGCGCAGATGGATCCTAA | GAGCCACGAACCTTCCATCA |
| IBSP | NM_012587.2 | Rn00561414_m1 | GTACCGGCCACGCTACTTTC | ATCTCCAGCCTTCTTGGGTAGC |
| OCN | NM_013414.1 | Rn00566386_g1 | CTCTGAGTCTGACAAAGCCTTCAT | GTAGCGCCGGAGTCTATTCA |
| ALP | NM_031144.3 | Rn00564931_m1 | GAGGAACGGATCTCGGGGTA | ATGAGTTGGTAAGGCAGGGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes-Ferreira, P.H.S.; Frigério, P.B.; Duarte, N.D.; Moura, J.d.; Monteiro, N.G.; Fabris, A.L.d.S.; Okamoto, R. Evaluation of Peri-Implant Bone Repair in Ovariectomized Rats Submitted to the Implant Placement Functionalized with Anti-Sclerostin. Bioengineering 2025, 12, 358. https://doi.org/10.3390/bioengineering12040358
Gomes-Ferreira PHS, Frigério PB, Duarte ND, Moura Jd, Monteiro NG, Fabris ALdS, Okamoto R. Evaluation of Peri-Implant Bone Repair in Ovariectomized Rats Submitted to the Implant Placement Functionalized with Anti-Sclerostin. Bioengineering. 2025; 12(4):358. https://doi.org/10.3390/bioengineering12040358
Chicago/Turabian StyleGomes-Ferreira, Pedro Henrique Silva, Paula Buzo Frigério, Nathália Dantas Duarte, Juliana de Moura, Naara Gabriela Monteiro, André Luis da Silva Fabris, and Roberta Okamoto. 2025. "Evaluation of Peri-Implant Bone Repair in Ovariectomized Rats Submitted to the Implant Placement Functionalized with Anti-Sclerostin" Bioengineering 12, no. 4: 358. https://doi.org/10.3390/bioengineering12040358
APA StyleGomes-Ferreira, P. H. S., Frigério, P. B., Duarte, N. D., Moura, J. d., Monteiro, N. G., Fabris, A. L. d. S., & Okamoto, R. (2025). Evaluation of Peri-Implant Bone Repair in Ovariectomized Rats Submitted to the Implant Placement Functionalized with Anti-Sclerostin. Bioengineering, 12(4), 358. https://doi.org/10.3390/bioengineering12040358









