Can the Novel Photon-Counting CT Scan Accurately Predict Aortic Wall Thickness? Preliminary Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Outcomes
2.3. Statistical Analyses
2.4. Data Availability
3. Results
3.1. Patients’ Characteristics
3.2. Aortic Wall Thickness Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howard, D.P.J.; Sideso, E.; Handa, A.; Rothwell, P.M. Incidence, risk factors, outcome and projected future burden of acute aortic dissection. Ann. Cardiothorac. Surg. 2014, 3, 278–284. [Google Scholar]
- Plonek, T.; Zak, M.; Burzynska, K.; Rylski, B.; Gozdzik, A.; Kustrzycki, W.; Beyersdorf, F.; Jasinski, M.; Filipiak, J. The combined impact of mechanical factors on the wall stress of the human ascending aorta—A finite elements study. BMC Cardiovasc. Disord. 2017, 17, 297. [Google Scholar] [CrossRef]
- Guo, D.-C.; Papke, C.L.; He, R.; Milewicz, D.M. Pathogenesis of Thoracic and Abdominal Aortic Aneurysms. Ann. N. Y. Acad. Sci. 2006, 1085, 339–352. [Google Scholar] [CrossRef]
- Surman, T.L.; Abrahams, J.M.; Manavis, J.; Finnie, J.; O’Rourke, D.; Reynolds, K.J.; Edwards, J.; Worthington, M.G.; Beltrame, J. Histological regional analysis of the aortic root and thoracic ascending aorta: A complete analysis of aneurysms from root to arch. J. Cardiothorac. Surg. 2021, 16, 255. [Google Scholar] [CrossRef]
- McClure, R.S.; Brogly, S.B.; Lajkosz, K.; Payne, D.; Hall, S.F.; Johnson, A.P. Epidemiology and management of thoracic aortic dissections and thoracic aortic aneurysms in Ontario, Canada: A population-based study. J. Thorac. Cardiovasc. Surg. 2018, 155, 2254–2264.e4. [Google Scholar] [CrossRef]
- Berretta, P.; Patel, H.J.; Gleason, T.G.; Sundt, T.M.; Myrmel, T.; Desai, N.; Korach, A.; Panza, A.; Bavaria, J.; Khoynezhad, A.; et al. IRAD experience on surgical type A acute dissection patients: Results and predictors of mortality. Ann. Cardiothorac. Surg. 2016, 5, 346–351. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Black, J.H.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, 24. [Google Scholar] [CrossRef]
- Czerny, M.; Grabenwöger, M.; Berger, T.; Aboyans, V.; Della Corte, A.; Chen, E.P.; Desai, N.D.; Dumfarth, J.; Elefteriades, J.A.; Etz, C.D.; et al. EACTS/STS Guidelines for diagnosing and treating acute and chronic syndromes of the aortic organ. Eur. J. Cardio-Thoracic Surg. 2024, 65, ezad426. [Google Scholar] [CrossRef]
- Pape, L.A.; Tsai, T.T.; Isselbacher, E.M.; Oh, J.K.; O’Gara, P.T.; Evangelista, A.; Fattori, R.; Meinhardt, G.; Trimarchi, S.; Bossone, E.; et al. Aortic diameter ≥5.5 cm is not a good predictor of type A aortic dissection: Observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007, 116, 1120–1127. [Google Scholar] [CrossRef]
- Rylski, B.; Hoffmann, I.; Beyersdorf, F.; Suedkamp, M.; Siepe, M.; Nitsch, B.; Blettner, M.; Borger, M.A.; Weigang, E. Acute aortic dissection type A: Age-related management and outcomes reported in the German Registry for Acute Aortic Dissection Type A (GERAADA) of over 2000 patients. Ann. Surg. 2014, 259, 598–604. [Google Scholar] [CrossRef]
- Papakonstantinou, N.A.; Rorris, F.-P. Elective replacement of the ascending aorta: Is the 5.5-cm threshold appropriate? The insidious, small aorta. Eur. J. Cardio-Thoracic Surg. 2021, 59, 554–561. [Google Scholar] [CrossRef]
- Sartoretti, T.; Wildberger, J.E.; Flohr, T.; Alkadhi, H. Photon-counting detector CT: Early clinical experience review. Br. J. Radiol. 2023, 96, 20220544. [Google Scholar] [CrossRef] [PubMed]
- Dangelmaier, J.; Bar-Ness, D.; Daerr, H.; Muenzel, D.; Si-Mohamed, S.; Ehn, S.; Fingerle, A.A.; Kimm, M.A.; Kopp, F.K.; Boussel, L.; et al. Experimental feasibility of spectral photon-counting computed tomography with two contrast agents for the detection of endoleaks following endovascular aortic repair. Eur. Radiol. 2018, 28, 3318–3325. [Google Scholar] [CrossRef]
- Rajendran, K.; Petersilka, M.; Henning, A.; Shanblatt, E.R.; Schmidt, B.; Flohr, T.G.; Ferrero, A.; Baffour, F.; Diehn, F.E.; Yu, L.; et al. First Clinical Photon-counting Detector CT System: Technical Evaluation. Radiology 2022, 303, 130–138. [Google Scholar] [CrossRef]
- Euler, A.; Higashigaito, K.; Mergen, V.; Sartoretti, T.; Zanini, B.; Schmidt, B.; Flohr, T.G.; Ulzheimer, S.; Eberhard, M.; Alkadhi, H. High-Pitch Photon-Counting Detector Computed Tomography Angiography of the Aorta: Intraindividual Comparison to Energy-Integrating Detector Computed Tomography at Equal Radiation Dose. Investig. Radiol. 2022, 57, 115–121. [Google Scholar] [CrossRef]
- Carkeet, A.; Goh, Y.T. Confidence and coverage for Bland-Altman limits of agreement and their approximate confidence intervals. Stat. Methods Med. Res. 2018, 27, 1559–1574. [Google Scholar] [CrossRef]
- Zumbo, B.D.; Hubley, A.M. A note on misconceptions concerning prospective and retrospective power. J. R. Stat. Soc. Ser. D Stat. 1998, 47, 385–388. [Google Scholar] [CrossRef]
- Davies, R.R.; Goldstein, L.J.; Coady, M.A.; Tittle, S.L.; Rizzo, J.A.; Kopf, G.S.; Elefteriades, J.A. Yearly rupture or dissection rates for thoracic aortic aneurysms: Simple prediction based on size. Ann. Thorac. Surg. 2002, 73, 17–18. [Google Scholar] [CrossRef]
- Wu, J.; Zafar, M.A.; Liu, Y.; Chen, J.F.; Li, Y.; Ziganshin, B.A.; Ellauzi, H.; Mukherjee, S.K.; Rizzo, J.A.; Elefteriades, J.A. Fate of the unoperated ascending thoracic aortic aneurysm: Three-decade experience from the Aortic Institute at Yale University. Eur. Heart J. 2023, 44, 4579–4588. [Google Scholar] [CrossRef]
- Tran, T.; Sundaram, C.P.; Bahler, C.D.; Eble, J.N.; Grignon, D.J.; Monn, M.F.; Simper, N.B.; Cheng, L. Correcting the Shrinkage Effects of Formalin Fixation and Tissue Processing for Renal Tumors: Toward Standardization of Pathological Reporting of Tumor Size. J. Cancer 2015, 6, 759–766. [Google Scholar] [CrossRef]
- Hołda, M.K.; Klimek-Piotrowska, W.; Koziej, M.; Piątek, K.; Hołda, J. Influence of different fixation protocols on the preservation and dimensions of cardiac tissue. J. Anat. 2016, 229, 334–340. [Google Scholar] [CrossRef]
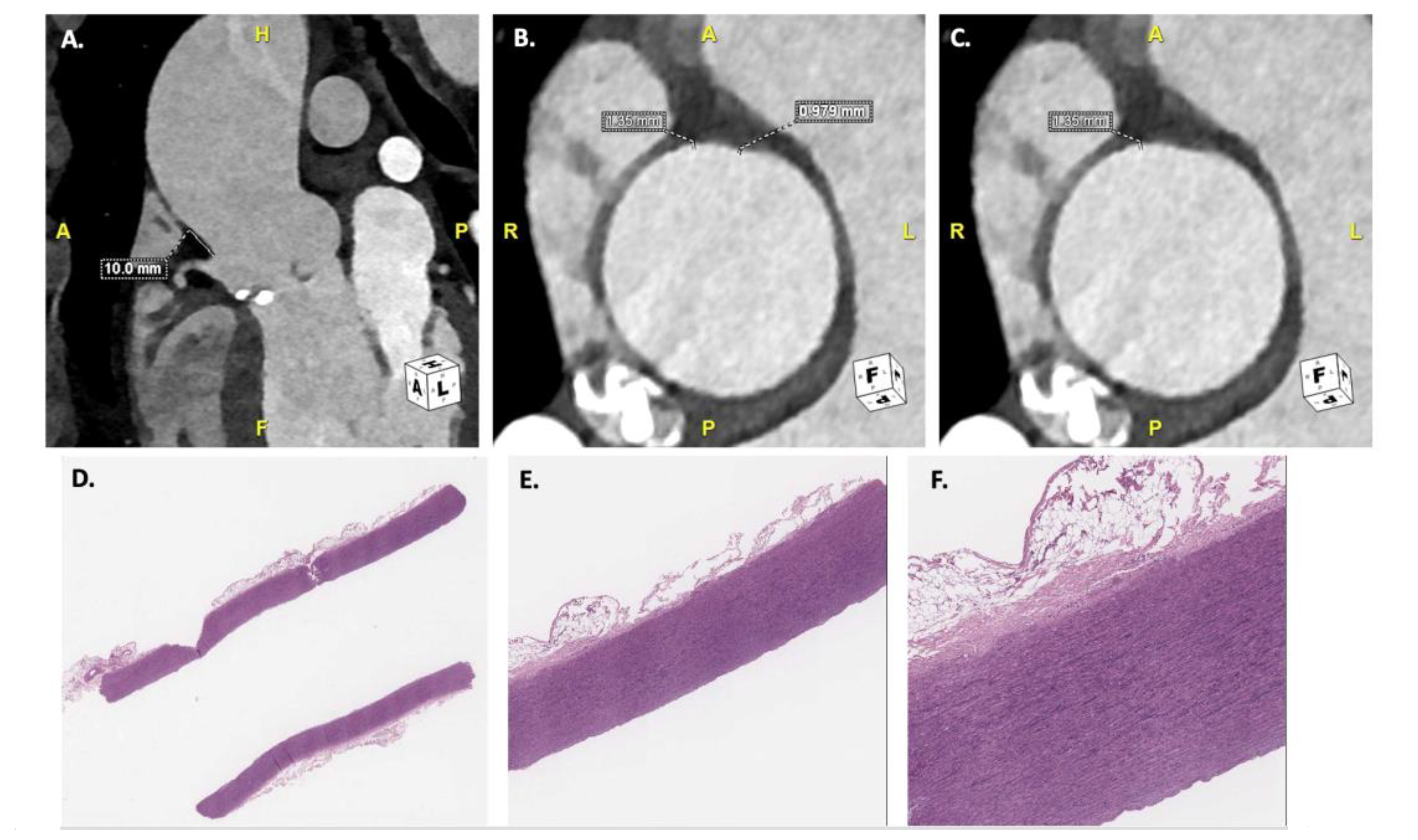
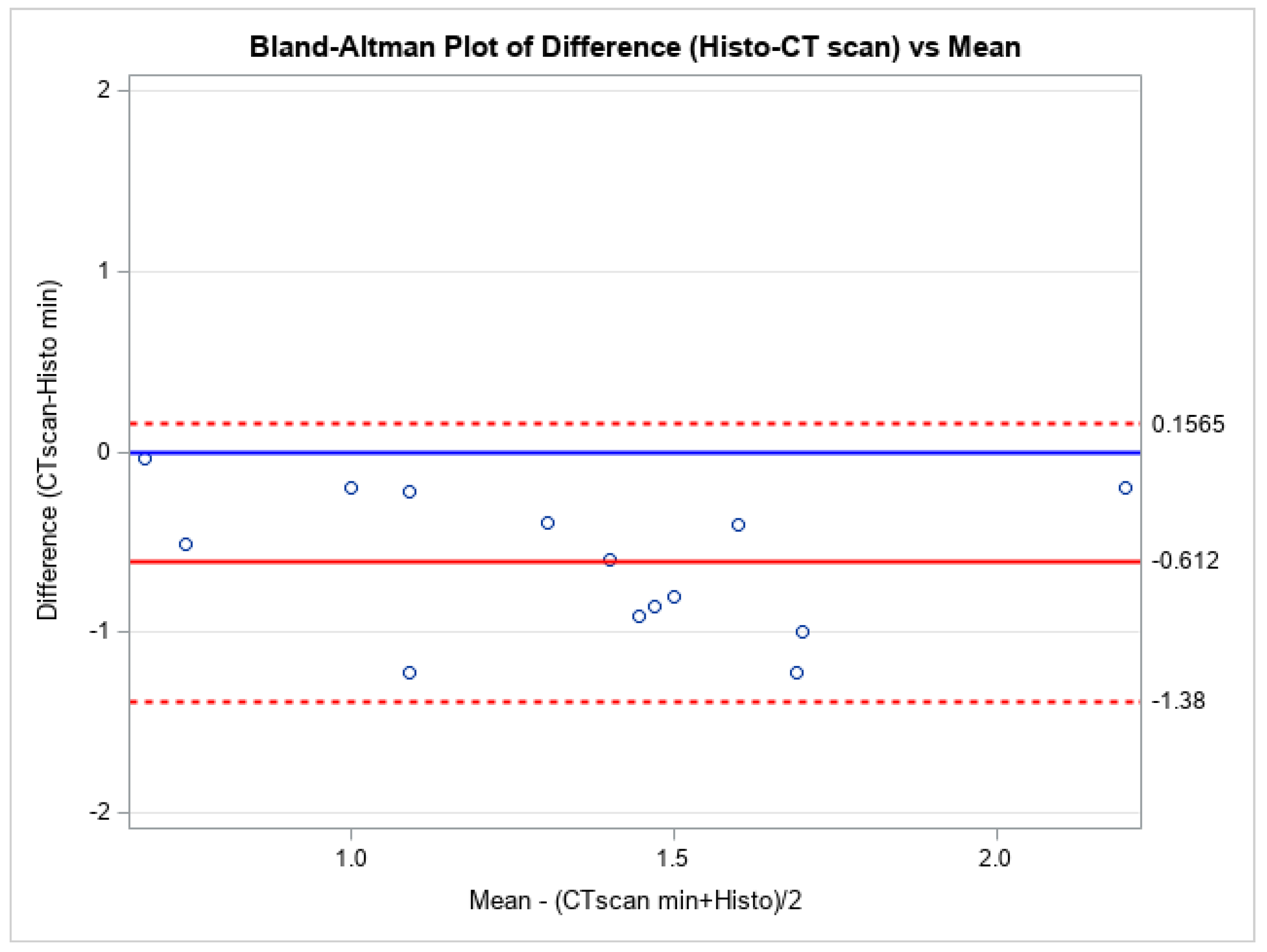
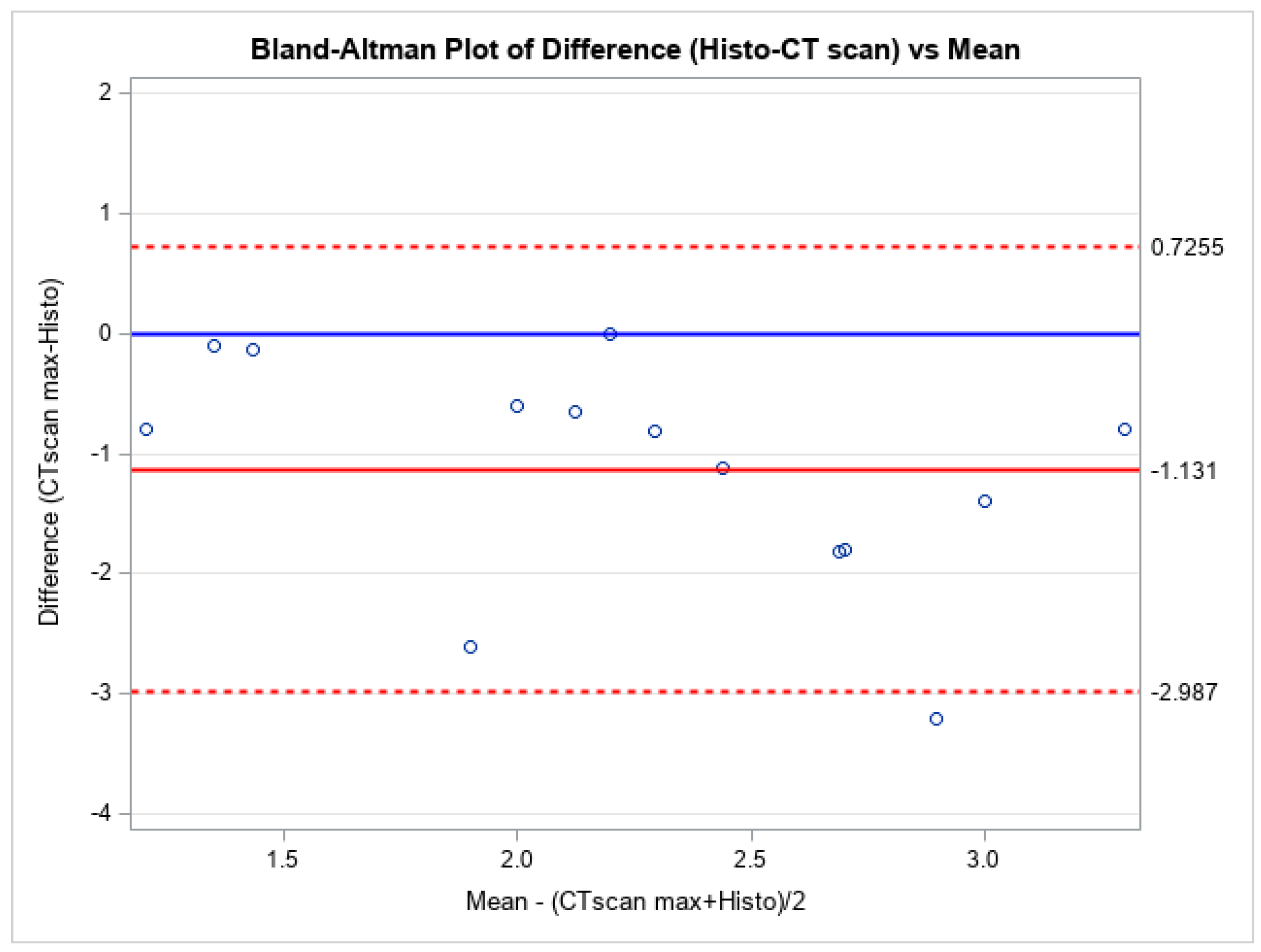
| Characteristics | N (%) or Mean ± STD − Median [IQR] |
|---|---|
| Patients’ characteristics | |
| Age (years) | 47.57 ± 10.98 − 46 [39–58] |
| Gender (M) | 11 (78.57) |
| BMI (kg/m2) | 24.16 ± 2.75 − 24.30 [22.80–25.90] |
| BSA (m2) | 1.85 ± 0.20 − 1.86 [1.75–2.01] |
| EuroSCORE (%) | 1.12 ± 0.35 − 1 [0.96–1.30] |
| Risk Factors | |
| Hypertension | 8 (57.14) |
| CKD | 2 (14.29) |
| Dyslipidemia | 3 (21.43) |
| DM | 1 (7.14) |
| OSAS + CPAP | 1 (7.14) |
| Family history aortic disease | 3 (21.43) |
| Current smoker | 4 (28.57) |
| Former smoker | 3 (21.43) |
| NHYA | |
| I | 11 (78.57) |
| II | 3 (21.43) |
| Pathology | |
| Ascending aortic aneurysm | 8 (57.14) |
| Root aneurysm | 5 (35.71) |
| No aneurysm | 2 (14.29) |
| Medical therapy | |
| ACE-inhibitors | 2 (14.29) |
| ARBs | 4 (28.57) |
| Ca-antagonist | 2 (14.29) |
| Cardioaspirin | 2 (14.29) |
| β-blockers | 5 (35.71) |
| Diuretics | 1 (7.14) |
| Characteristics | N (%) or Mean ± STD − Median [IQR] |
|---|---|
| Echocardiography | |
| LVEDD (mm) | 50.36 ± 7.57 − 48.50 [44–54] |
| LVEDV (mL) | 144.79 ± 52.95 − 138 [96–156] |
| LVEF (%) | 59.07 ± 7.28 − 58.50 [53–65] |
| RVEDD (mm) | 34.93 ± 2.53 − 35 [34–37] |
| TAPSE (mm) | 23.38 ± 2.81 − 23.50 [21–25] |
| AR | |
| 0–1+ | 6 (42.86) |
| 2+ | 1 (7.14) |
| 3–4+ | 7 (50) |
| AS | |
| 0–1+ | 10 (71.43) |
| 2+ | 1 (7.14) |
| 3–4+ | 3 (21.43) |
| AV Gmed (mmHg) | 16.79 ± 17.11 − 7 [5–28] |
| AV Gmax (mmHg) | 25.86 ± 24.96 − 13 [9–44] |
| Aortic root (mm | 41.86 ± 9.41 − 39.50 [37–48] |
| Ascending aorta (mm) | 45.07 ± 7.92 − 44.50 [40–49] |
| CT scan | |
| Aortic root (mm) | 40.71 ± 10.12 − 39.50 [35–44] |
| STJ (mm) | 39.36 ± 7.42 − 36.50 [35–45] |
| Ascending aorta (mm) | 44.89 ± 6.91 − 45.50 [42–51] |
| Aortic arch (mm) | 27.93 ± 3.89 − 28 [25–30] |
| Descending thoracic aorta (mm) | 25.39 ± 3.69 − 25.50 [22–26] |
| Characteristics | N (%) or Mean ± STD − Median [IQR] |
|---|---|
| Intraop AV inspection | |
| Bicuspid | 8 (57.14) |
| Tricuspid | 6 (42.86) |
| Surgical procedure | |
| AVR | 10 (71.43) |
| AVr | 1 (7.14) |
| Bioprosthesis | 1 (7.14) |
| Bentall | 4 (28.57) |
| T. David | 2 (14.29) |
| Wheat | 5 (35.71) |
| Surgical times | |
| CPB (min) | 85.07 ± 25.78 − 84 [64–103] |
| XCT (min) | 71.50 ± 22.80 − 71.50 [53–85] |
| Measurements | Histology | Photon-Counting CT Scan |
|---|---|---|
| Minimum (mm) | 1.66 ± 0.50 − 1.75 [1.20–1.90] | 1.05 ± 0.40 − 1.06 [0.90–1.11] |
| Maximum (mm) | 2.82 ± 0.95 − 2.85 [2.20–3.60] | 1.69 ± 0.59 − 1.79 [1.30–1.89] |
| Measurements | α | β | Interpretation |
|---|---|---|---|
| Minimum | 0.06 [−0.92−0.67] | 0.58 [0.22–1.24] | Measurements are similar |
| Maximum | 0.63 [−0.84–1.26] | 0.45 [0.17–1.01] | Measurements are similar |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sala, A.; de Vincentiis, C.; Grimaldi, F.; Rubino, B.; Cirami, M.; Perillo, N.; Vitale, R.; Cardani, R.; Boveri, S.; Conti, M.; et al. Can the Novel Photon-Counting CT Scan Accurately Predict Aortic Wall Thickness? Preliminary Results. Bioengineering 2025, 12, 306. https://doi.org/10.3390/bioengineering12030306
Sala A, de Vincentiis C, Grimaldi F, Rubino B, Cirami M, Perillo N, Vitale R, Cardani R, Boveri S, Conti M, et al. Can the Novel Photon-Counting CT Scan Accurately Predict Aortic Wall Thickness? Preliminary Results. Bioengineering. 2025; 12(3):306. https://doi.org/10.3390/bioengineering12030306
Chicago/Turabian StyleSala, Alessandra, Carlo de Vincentiis, Francesco Grimaldi, Barbara Rubino, Manuela Cirami, Noemi Perillo, Renato Vitale, Rosanna Cardani, Sara Boveri, Michele Conti, and et al. 2025. "Can the Novel Photon-Counting CT Scan Accurately Predict Aortic Wall Thickness? Preliminary Results" Bioengineering 12, no. 3: 306. https://doi.org/10.3390/bioengineering12030306
APA StyleSala, A., de Vincentiis, C., Grimaldi, F., Rubino, B., Cirami, M., Perillo, N., Vitale, R., Cardani, R., Boveri, S., Conti, M., & Spagnolo, P. (2025). Can the Novel Photon-Counting CT Scan Accurately Predict Aortic Wall Thickness? Preliminary Results. Bioengineering, 12(3), 306. https://doi.org/10.3390/bioengineering12030306








