Transcranial Direct Current Stimulation Can Modulate Brain Complexity and Connectivity in Children with Autism Spectrum Disorder: Insights from Entropy Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. tDCS Interventions
2.3. Data Acquisition
2.4. Data Preprocessing
2.5. Entropy Method
2.5.1. Sample Entropy
2.5.2. Multiscale Sample Entropy
2.5.3. Phase Transfer Entropy
2.6. Statistical Analysis
3. Results
3.1. Comparison of Differences Between the ASD Group and the TD Group
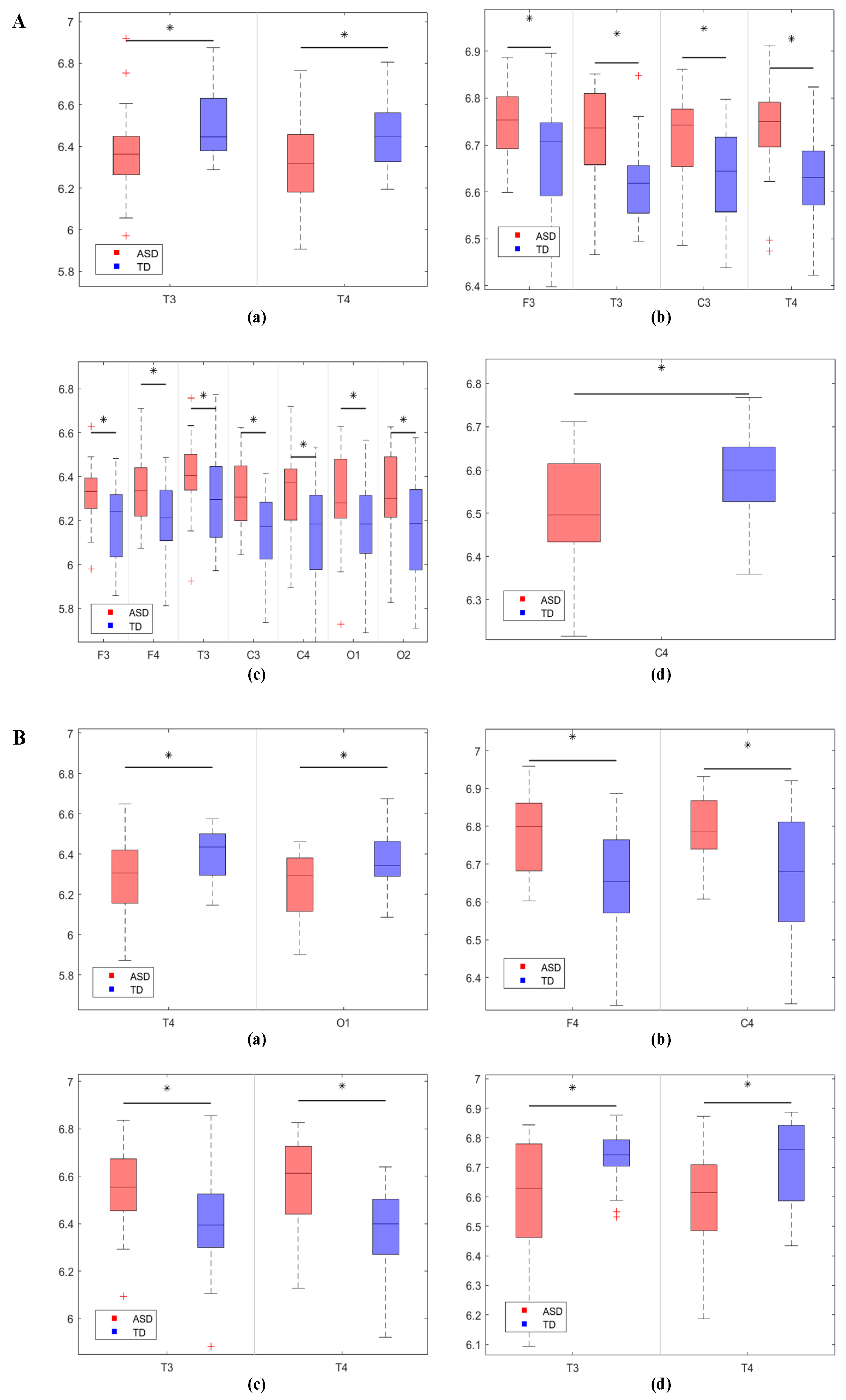
3.1.1. Comparison of EEG Complexity Results Between the Two Groups of Children
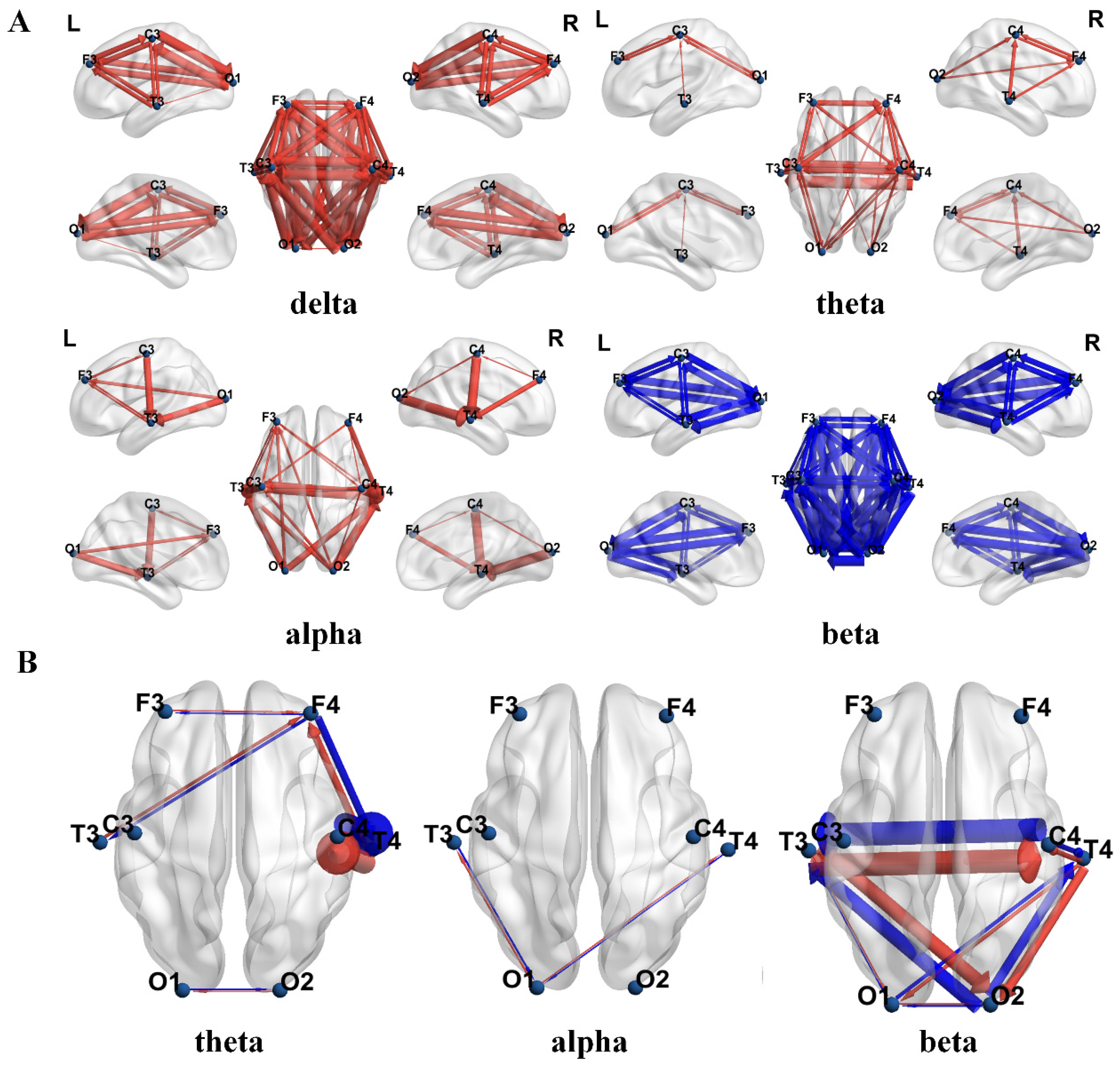
3.1.2. Comparison of EEG Effective Connectivity Results Between the Two Groups of Children
3.2. Comparison of Pre- and Post-tDCS Differences
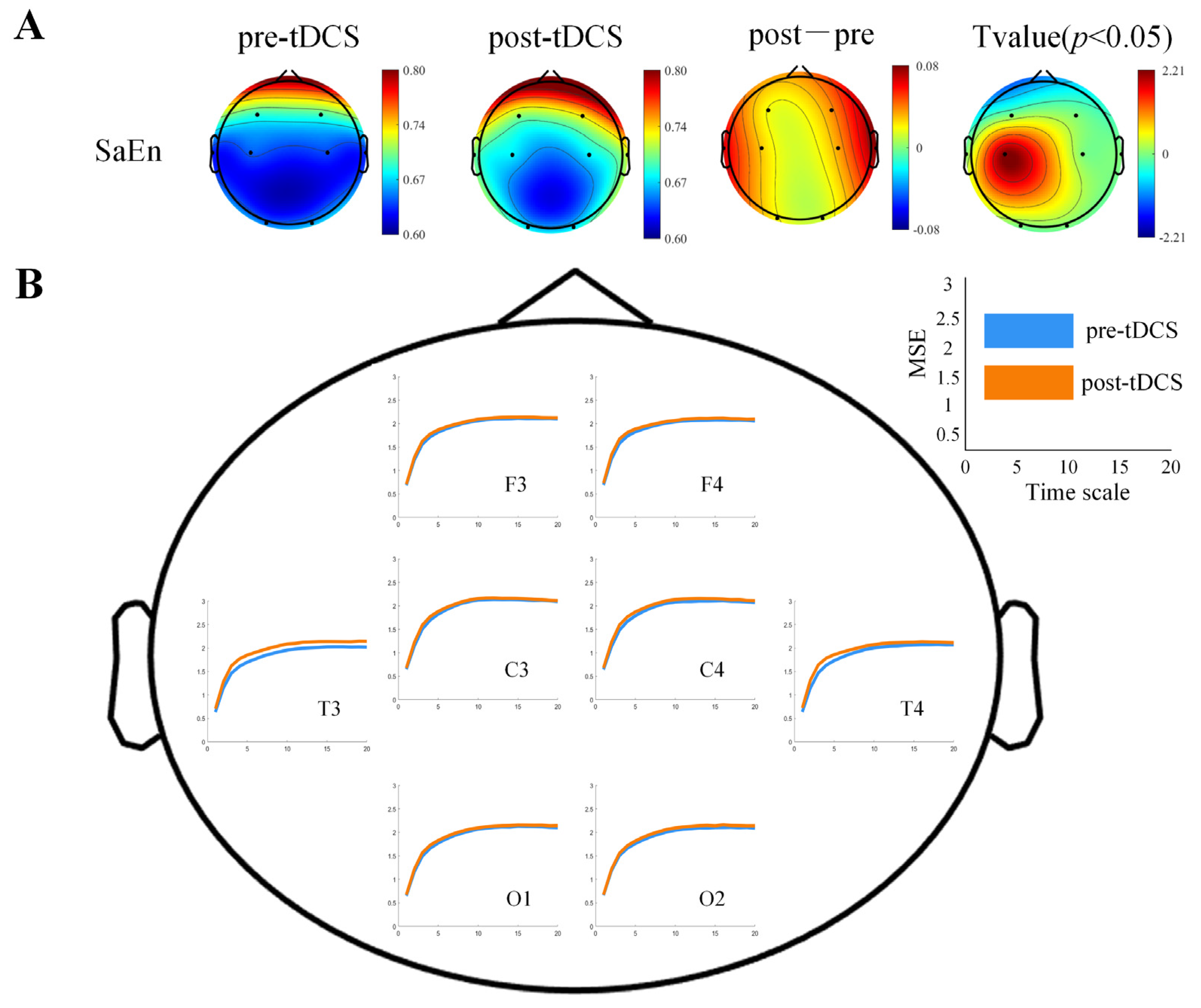
3.2.1. Differences in Brain Complexity Results
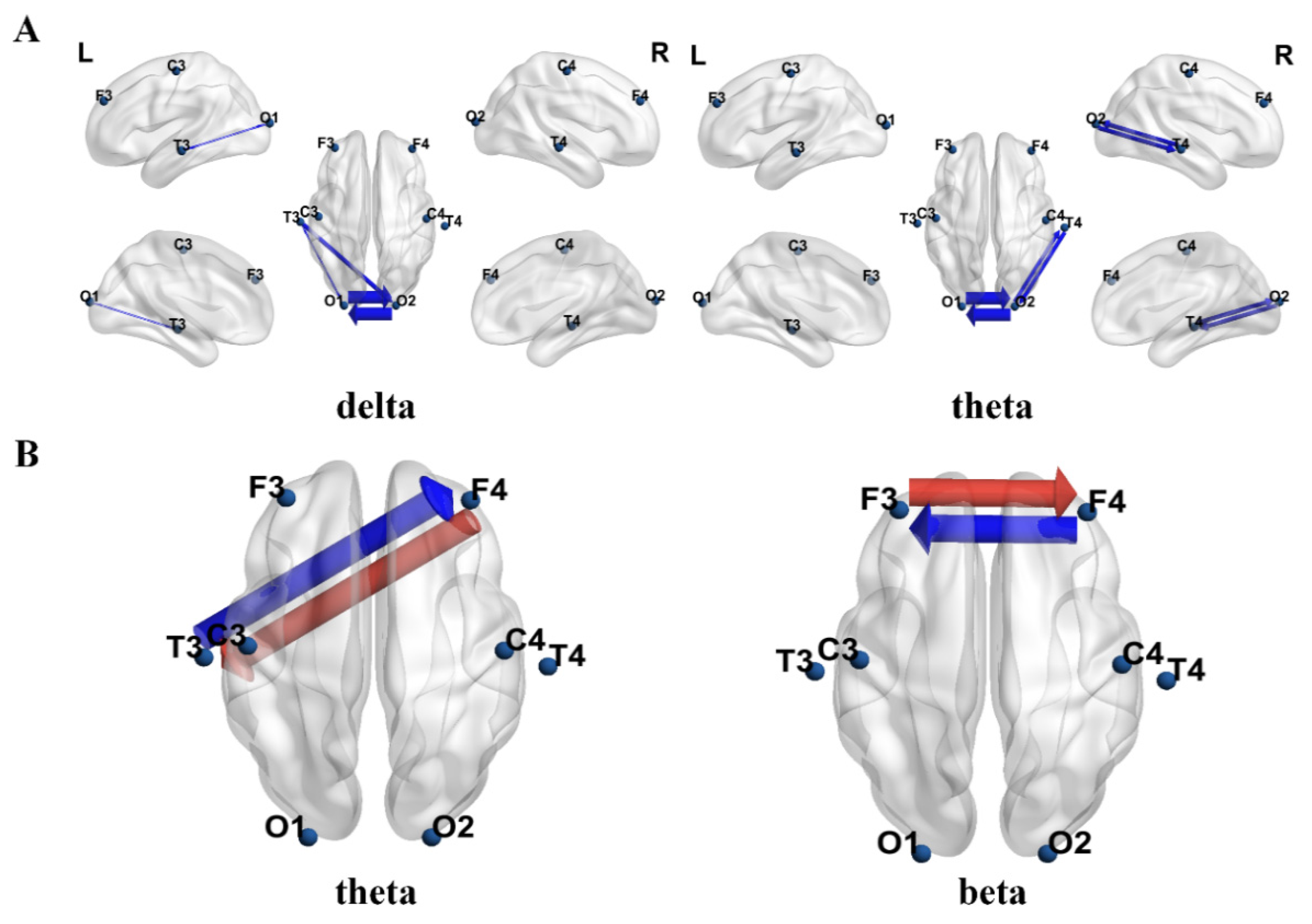
3.2.2. Differences in Effective Connectivity Results
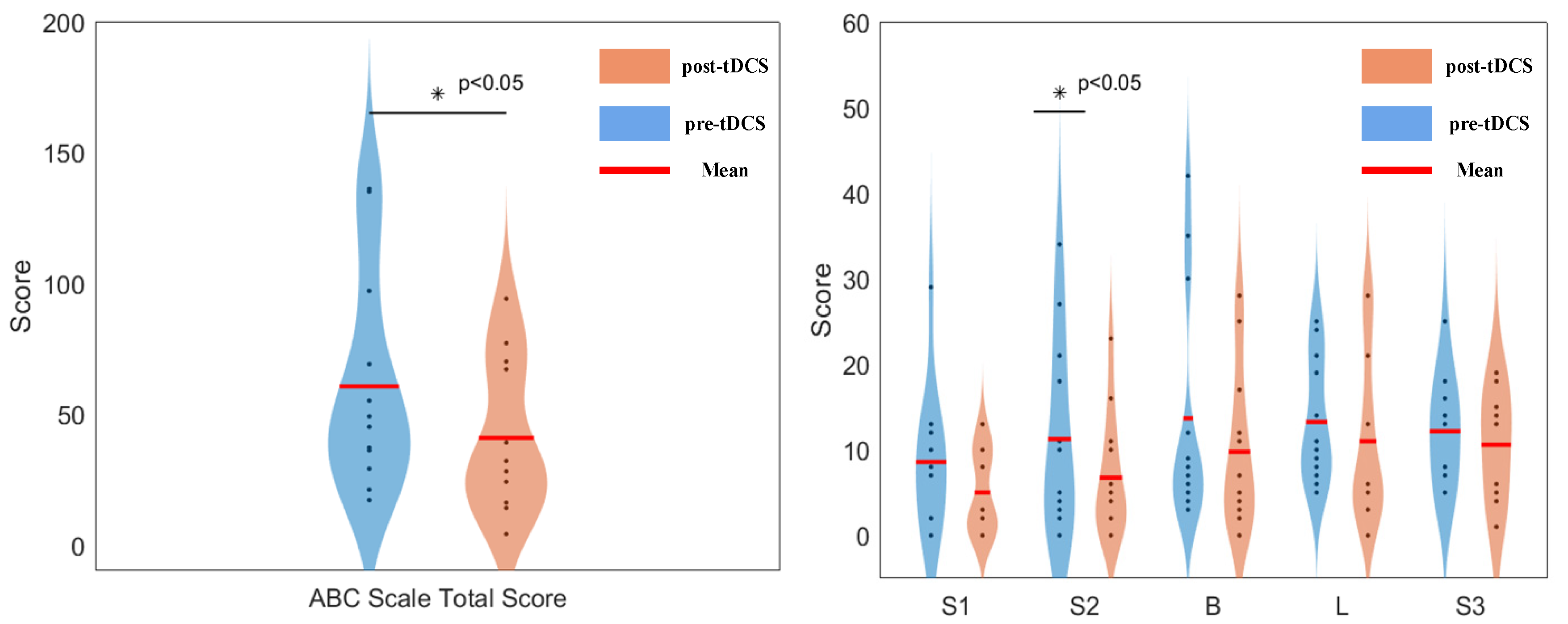
3.3. Scale Evaluation Results
4. Discussion
4.1. Brain Complexity and Effective Connectivity Abnormalities Between Two Groups
4.2. The Effects of tDCS on Brain Complexity and Effective Connectivity in Children with ASD
4.3. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, X.; Qiu, Z.; Zhang, D. Recent research progress in autism spectrum disorder. Neurosci. Bull. 2017, 33, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Teplan, M. Fundamentals of EEG measurement. Meas. Sci. Rev. 2002, 2, 1–11. [Google Scholar]
- González, J.J.; Alba, G.; Mañas, S. Assessment of ADHD through electroencephalographic measures of functional connectivity. In ADHD—New Directions Diagnosis Treatment; Intech Open: London, UK, 2017; pp. 35–54. [Google Scholar]
- Azami, H.; Faes, L.; Escudero, J.; Humeau-Heurtier, A.; Silva, L.E. Entropy analysis of univariate biomedical signals: Review and comparison of methods. In Frontiers in Entropy Across the Disciplines: Panorama of Entropy: Theory, Computation, and Applications; World Scientific: Singapore, 2023; pp. 233–286. [Google Scholar]
- Jui, S.J.J.; Deo, R.C.; Barua, P.D.; Devi, A.; Soar, J.; Acharya, U.R. Application of entropy for automated detection of neurological disorders with electroencephalogram signals: A review of the last decade (2012–2022). IEEE Access 2023, 11, 71905–71924. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.H.; Li, L. Diagnosing autism spectrum disorder using brain entropy: A fast entropy method. Comput. Methods ProgramsBiomed. 2020, 190, 105240. [Google Scholar] [CrossRef]
- Kang, J.; Chen, H.; Li, X.; Li, X. EEG entropy analysis in autistic children. J. Clin. Neurosci. 2019, 62, 199–206. [Google Scholar] [CrossRef]
- Hadoush, H.; Alafeef, M.; Abdulhay, E. Brain complexity in children with mild and severe autism spectrum disorders: Analysis of multiscale entropy in EEG. Brain Topogr. 2019, 32, 914–921. [Google Scholar] [CrossRef]
- Catarino, A.; Churches, O.; Baron-Cohen, S.; Andrade, A.; Ring, H. Atypical EEG complexity in autism spectrum conditions: A multiscale entropy analysis. Clin. Neurophysiol. 2011, 122, 2375–2383. [Google Scholar] [CrossRef]
- Wibral, M.; Vicente, R.; Lindner, M. Transfer entropy in neuroscience. In Directed Information Measures in Neuroscience; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–36. [Google Scholar]
- Granger, C.W.J. Investigating causal relations by econometric models and cross-spectral methods. Econom. J. Econom. Soc. 1969, 37, 424–438. [Google Scholar] [CrossRef]
- Friston, K.J.; Harrison, L.; Penny, W. Dynamic causal modelling. Neuroimage 2003, 19, 1273–1302. [Google Scholar] [CrossRef]
- Vicente, R.; Wibral, M.; Lindner, M.; Pipa, G. Transfer entropy—A model-free measure of effective connectivity for the neurosciences. J. Comput. Neurosci. 2011, 30, 45–67. [Google Scholar] [CrossRef]
- Lobier, M.; Siebenhühner, F.; Palva, S.; Palva, J.M. Phase transfer entropy: A novel phase-based measure for directed connectivity in networks coupled by oscillatory interactions. Neuroimage 2014, 85, 853–872. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.G.; Feng, C.; Zhou, Z.R.; Cao, W.Y.; He, D.J.; Jiang, Z.L.; Lin, F. Imbalanced gamma-band functional brain networks of autism spectrum disorders. Neuroscience 2022, 498, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Luckhardt, C.; Boxhoorn, S.; Schütz, M.; Fann, N.; Freitag, C.M. Brain stimulation by tDCS as treatment option in autism spectrum disorder—A systematic literature review. Prog. Brain Res. 2021, 264, 233–257. [Google Scholar] [PubMed]
- Hameed, M.Q.; Dhamne, S.C.; Gersner, R.; Kaye, H.L.; Oberman, L.M.; Pascual-Leone, A.; Rotenberg, A. Transcranial magnetic and direct current stimulation in children. Curr. Neurol. Neurosci. Rep. 2017, 17, 1–15. [Google Scholar] [CrossRef]
- Hadoush, H.; Nazzal, M.; Almasri, N.A.; Khalil, H.; Alafeef, M. Therapeutic effects of bilateral anodal transcranial direct current stimulation on prefrontal and motor cortical areas in children with autism spectrum disorders: A pilot study. Autism Res. 2020, 13, 828–836. [Google Scholar] [CrossRef]
- Hadoush, H.; Hadoush, A. Modulation of resting-state brain complexity after bilateral cerebellar anodal transcranial direct current stimulation in children with autism spectrum disorders: A randomized controlled trial study. Cerebellum 2023, 22, 1109–1117. [Google Scholar] [CrossRef]
- Kang, J.; Cai, E.; Han, J.; Tong, Z.; Li, X.; Sokhadze, E.M.; Casanova, M.F.; Ouyang, G.; Li, X. Transcranial direct current stimulation (tDCS) can modulate EEG complexity of children with autism spectrum disorder. Front. Neurosci. 2018, 12, 201. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Zeng, K.; Chen, D.; Ouyang, G.; Wang, L.; Liu, X.; Li, X. An EEMD-ICA approach to enhancing artifact rejection for noisy multivariate neural data. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 24, 630–638. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef]
- Yentes, J.M.; Hunt, N.; Schmid, K.K.; Kaipust, J.P.; McGrath, D.; Stergiou, N. The appropriate use of approximate entropy and sample entropy with short data sets. Ann. Biomed. Eng. 2013, 41, 349–365. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.-K. Multiscale entropy analysis of biological signals. Phys. Rev. E 2005, 71, 021906. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, T. Measuring information transfer. Phys. Rev. Lett. 2000, 85, 461. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, M.; Pikovsky, A.; Kurths, J.; Schäfer, C.; Tass, P.A. Phase synchronization: From theory to data analysis. In Handbook of Biological Physics; North-Holland Publishing: Amsterdam, The Netherlands, 2001; Volume 4, pp. 279–321. [Google Scholar]
- Chavez, M.; Besserve, M.; Adam, C.; Martinerie, J. Towards a proper estimation of phase synchronization from time series. Neurosci. Methods 2006, 154, 149–160. [Google Scholar] [CrossRef]
- Hasanzadeh, F.; Mohebbi, M.; Rostami, R. Graph theory analysis of directed functional brain networks in major depressive disorder based on EEG signal. J. Neural Eng. 2020, 17, 026010. [Google Scholar] [CrossRef]
- Scott, D.W. Multivariate Density Estimation; Wiley and Sons, Inc.: Hoboken, NJ, USA, 1992. [Google Scholar]
- Hillebrand, A.; Tewarie, P.; van Dellen, E.; Yu, M.; Carbo, E.W.; Douw, L.; Gouw, A.A.; van Straaten, E.C.; Stam, C.J. Direction of information flow in large-scale resting-state networks is frequency-dependent. Proc. Natl. Acad. Sci. USA 2016, 113, 3867–3872. [Google Scholar] [CrossRef]
- Ekhlasi, A.; Nasrabadi, A.M.; Mohammadi, M.R. Direction of information flow between brain regions in ADHD and healthy children based on EEG by using directed phase transfer entropy. Cogn. Neurodyn. 2021, 15, 975–986. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Y.; Chen, D.; Li, C.; Qiu, Y.; Wang, J. Altered electroencephalogram complexity in autistic children shown by the multiscale entropy approach. Neuroreport 2017, 28, 169–173. [Google Scholar] [CrossRef]
- Handayani, N.; Asyrafi, H.; Khotimah, S.N. EEG complexity in children with autism spectrum disorders: A multiscale entropy analysis. AIP Conf. Proc. 2024, 3210, 020003. [Google Scholar]
- Orekhova, E.V.; Elsabbagh, M.; Jones, E.J.; Dawson, G.; Charman, T.; Johnson, M.H. EEG hyper-connectivity in high-risk infants is associated with later autism. J. Neurodev. Disord. 2014, 6, 40. [Google Scholar] [CrossRef]
- Neuper, C.; Pfurtscheller, G. Event-related dynamics of cortical rhythms: Frequency-specific features and functional correlates. Int. J. Psychophysiol. 2001, 43, 41–58. [Google Scholar] [CrossRef]
- Gogolla, N.; LeBlanc, J.J.; Quast, K.B.; Südhof, T.C.; Fagiolini, M.; Hensch, T.K. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J. Neurodev. Disord. 2009, 1, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Yizhar, O.; Fenno, L.E.; Prigge, M.; Schneider, F.; Davidson, T.J.; O’shea, D.J.; Sohal, V.S.; Goshen, I.; Finkelstein, J.; Paz, J.T.; et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011, 477, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Ellis, C.; Leech, R.; Voytek, B.; Garces, P.; Jones, E.; Buitelaar, J.; Loth, E.; dos Santos, F.P.; Amil, A.F.; et al. From mechanisms to markers: Novel noninvasive EEG proxy markers of the neural excitation and inhibition system in humans. Transl. Psychiatry 2022, 12, 467. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.; Cowley, A.B.; Alfaori, Q.; Larremore, D.B.; Restrepo, J.G.; Shew, W.L. Robust entropy requires strong and balanced excitatory and inhibitory synapses. Chaos Interdiscip. J. Nonlinear Sci. 2018, 28, 103115. [Google Scholar] [CrossRef]


| Electrode | ASD Group | TD Group | MSE p-Value |
|---|---|---|---|
| F3 | 1.7920 | 1.9258 | 0.0035 * |
| F4 | 1.7814 | 1.9362 | 0.0003 * |
| T3 | 1.7474 | 1.8786 | 0.0293 * |
| C3 | 1.8183 | 1.9660 | 0.0012 * |
| C4 | 1.7906 | 1.9524 | 0.0012 * |
| T4 | 1.7631 | 1.9165 | 0.002 * |
| O1 | 1.7810 | 1.9190 | 0.0004 * |
| O2 | 1.7798 | 1.9134 | 0.0033 * |
| Electrode | pre-tDCS | post-tDCS | MSE p-Value |
|---|---|---|---|
| F3 | 1.8966 | 1.9310 | 0.261 |
| F4 | 1.8812 | 1.9252 | 0.354 |
| T3 | 1.8003 | 1.9267 | 0.215 |
| C3 | 1.9101 | 1.9475 | 0.234 |
| C4 | 1.8820 | 1.9390 | 0.151 |
| T4 | 1.8381 | 1.9235 | 0.172 |
| O1 | 1.8862 | 1.9253 | 0.277 |
| O2 | 1.8740 | 1.9203 | 0.252 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Hao, P.; Gu, H.; Liu, Y.; Li, X.; Geng, X. Transcranial Direct Current Stimulation Can Modulate Brain Complexity and Connectivity in Children with Autism Spectrum Disorder: Insights from Entropy Analysis. Bioengineering 2025, 12, 283. https://doi.org/10.3390/bioengineering12030283
Kang J, Hao P, Gu H, Liu Y, Li X, Geng X. Transcranial Direct Current Stimulation Can Modulate Brain Complexity and Connectivity in Children with Autism Spectrum Disorder: Insights from Entropy Analysis. Bioengineering. 2025; 12(3):283. https://doi.org/10.3390/bioengineering12030283
Chicago/Turabian StyleKang, Jiannan, Pengfei Hao, Haiyan Gu, Yukun Liu, Xiaoli Li, and Xinling Geng. 2025. "Transcranial Direct Current Stimulation Can Modulate Brain Complexity and Connectivity in Children with Autism Spectrum Disorder: Insights from Entropy Analysis" Bioengineering 12, no. 3: 283. https://doi.org/10.3390/bioengineering12030283
APA StyleKang, J., Hao, P., Gu, H., Liu, Y., Li, X., & Geng, X. (2025). Transcranial Direct Current Stimulation Can Modulate Brain Complexity and Connectivity in Children with Autism Spectrum Disorder: Insights from Entropy Analysis. Bioengineering, 12(3), 283. https://doi.org/10.3390/bioengineering12030283







